Abstract
Chronic lymphocytic leukemia (CLL) remains incurable, but over the past decade there have been major advances in the understanding of the pathophysiology of CLL and in the treatment of this disease. This has led to greatly increased response rates and durations of response, as well as improved survival. CLL is a disease of the elderly and not all patients are eligible for the aggressive upfront chemoimmunotherapy regimens that are resulting in improved response rates and survival, so what is the optimal treatment approach for more frail elderly patients? It is highly likely that our treatment approaches will continue to evolve as the results of ongoing clinical trials are released. The age range of patients involved in clinical trials is not representative of this disease, and more research is required in patients who are representative of the majority of CLL patients seen in practice before we will see outcome improvements in these more elderly and often more frail patient populations.
Keywords: chronic lymphocytic leukemia, clinical trials, comorbidity, elderly, treatment
Improvement in outcome for patients with chronic lymphocytic leukemia
There have been major advances in the treatment of previously untreated chronic lymphocytic leukemia (CLL). In contrast to most other types of leukemia, CLL is not treated upon diagnosis, but upon progression to symptomatic disease [1]. This approach is based on the results of randomized trials that failed to show any benefit of early treatment with chemotherapy [2]. However, all of these studies were performed in an era when alkylator agents, especially chlorambucil, were the treatment of choice. A series of randomized trials performed over the past decade have demonstrated that we have moved from a setting where palliation of symptoms was the goal of treatment to one where attainment of complete response (CR) and improvement in overall survival is the aim [3–7], and we have seen the transition from single-agent alkylator-based therapies to nucleoside analogues, combination chemotherapy and, most recently, chemoimmunotherapy (e.g., combining monoclonal antibodies such as rituximab with chemotherapy such as fludarabine plus cyclophosphamide [FCR]) [8]. As a result, CR rates have improved from 7% (chlorambucil) to 44–70% with FCR [7,9], and chemoimmunotherapy is now the treatment of choice.
Incidence & age distribution in CLL
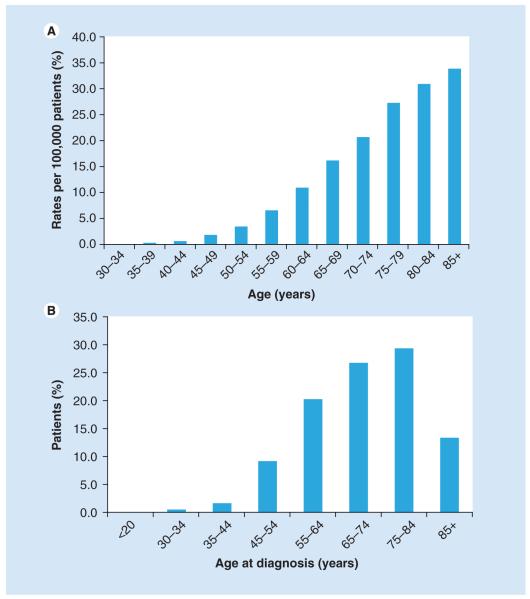
Chronic lymphocytic leukemia was diagnosed in 15,490 people (9200 men and 6290 women) in the USA in 2009 [101]. CLL is a disease of the elderly, with a median age at diagnosis of 72 years and median age at death of 79 years, with the age-adjusted incidence increasing with age (Figure 1A). Almost 70% of CLL patients are older than 65 years at the time of diagnosis; less than 2% are younger than 45 years; 9.1% are between 45 and 54 years of age; 19.3% are between 55 and 64 years of age; 26.5% are between 65 and 74 years of age; 30.0% are between 75 and 84 years of age; and 13.2% are 85 years of age or older (Figure 1B). CLL is extremely heterogeneous in its clinical course, with some patients living for decades with no need for treatment for their disease while others have a rapidly aggressive clinical course.
Figure 1. Chronic lymphocytic leukemia is a disease of the elderly.
(A) Increasing age-adjusted incidence in chronic lymphocytic leukemia. (B) Percentage of patients by age category at time of diagnosis of chronic lymphocytic leukemia.
The International Workshop on Chronic Lymphocytic Leukemia has revised the diagnostic criteria for CLL to require 5000 or more CLL phenotype B cells per mm3 [1]. Lymphocytosis with fewer than 5000 CLL-phenotype B cells per mm3 and an absence of symptoms of CLL is defined as monoclonal B-cell lymphocytosis (MBL). In a study to assess the incidence of MBL and to determine the likelihood of progression to CLL, 1520 subjects who were 62–80 years of age, and who had normal blood counts and no history of cancer were examined [10]. A CLL-phenotype MBL was detected in 78 individuals (5.1%) and increased with age, while non-CLL-phenotype MBL (i.e., light-chain-restricted CD19+ B cells with no CD5 expression and strong CD20 expression) was identified in 27 individuals (1.8%). In most subjects, the absolute B-cell count was within the normal range. Among 2228 subjects referred for lymphocytosis review who had a current or previous lymphocyte count above 4000 per mm3, CLL-phenotype MBL was detected in 309 (13.9%), CLL was diagnosed in 1031 (46.3%), and a non-CLL B-cell abnormality or reactive lymphocytosis was seen in 888 individuals (39.9%). During a median follow-up of 6.7 years, progressive CLL (characterized by lymphadenopathy, splenomegaly, anemia, thrombocytopenia, lymphocyte doubling time <6 months, persistent infection or drenching night sweats) developed in 15% (28 out of 185) of subjects with CLL-phenotype MBL with an initial lymphocyte count of more than 4000 per mm3. The annual risk of developing CLL requiring chemotherapy among subjects with CLL-phenotype MBL presenting with lymphocytosis was 1–2%, which is similar to the rate of progression to myeloma seen in patients with monoclonal gammopathy of undetermined significance.
The goal of therapy has been to maintain the best quality of life and treat only when patients become symptomatic from their disease [1]. For the majority of patients this means following a ‘watch and wait’ approach to determine the rate of progression of the disease and assess for the development of symptoms. This means that the age of requirement of first treatment is considerably older than the age of presentation. Older patients have more comorbidity, with a mean comorbidity of 4.2 in patients over the age of 75 years [11]. Advancing age is associated with increased vulnerability to age-related health problems; more comorbidity, which results in increasing adverse treatment effects; and increased frailty, defined as a multisystem reduction in physiological capacity, resulting in increased vulnerability to small environmental challenges. The question of comorbidities in CLL is underinvestigated, but in over 1000 newly diagnosed CLL patients evaluated at the Mayo Clinic (MN, USA), 89% had one or more comorbidities, with 46% having at least one major morbidity (Figure 2)[12]. A number of factors affect decision making in the elderly CLL patient. These include uncertainty regarding optimum treatments as older patients are under-represented in clinical trials, poor quality of clinic–patient communication, patient and family factors that impact on how individuals come to a decision about and comply with treatment, and negative cultural and social stereotyping of older age by many clinicians. Elderly patients are vastly under-represented in clinical trials, most likely since they may not have a sufficiently good performance status to tolerate aggressive chemoimmunotherapy approaches.
Figure 2. Most patients with chronic lymphocytic leukemia have comorbidities.
Data taken from [12].
Outcome of older patients in clinical trials
A relatively small number of trials have examined the impact of advanced age on outcome in CLL. The Leukemia Research Fund CLL4 study was a randomized clinical trial in the UK comparing treatment with chlorambucil, fludarabine, or fludarabine and chlorambucil [6]. Among the patients enrolled in this trial, 30% were over the age of 70 years, but there must have been considerable patient selection bias here, since oncologists had to be prepared to treat these patients with combination chemotherapy if they were to have been randomized to that arm. It is therefore likely that only patients with sufficiently good performance status to tolerate the more aggressive regimens were enrolled. The quality of life of these patients was assessed at study entry and fatigue was the greatest problem. Patients who were older than 70 years of age had significantly poorer physical functions [13]. In all age groups, good responses and overall responses were highest with fludarabine plus cyclophosphamide and lowest with chlorambucil, and there was no evidence of benefit for fludarabine over chlorambucil.
Few trials have been designed with enrolment of older patients specifically, but one such study was the German CLL study group (GCLLSG) CLL5 trial [14]. The advance in the CLL5 study design is that both study arms were deemed tolerable for the intended patient population, and therefore the results are likely to be more applicable to the more elderly patients seen in practice. This multicenter Phase III trial enrolled patients older than 65 years and compared first-line therapy with fludarabine with chlorambucil. A total of 193 patients with a median age of 70 years were randomized to receive fludarabine (25 mg/m2 for 5 days intravenously, every 28 days, for six courses) or chlorambucil (0.4 mg/kg bodyweight with increase to 0.8 mg/kg, every 15 days, for 12 months). The results demonstrated that although fludarabine resulted in a significantly higher overall response (72 vs 51%; p = 0.003) and CR (7 vs 0%; p = 0.011) rate, there was no difference in progression-free survival (19 months with fludarabine vs 18 months with chlorambucil; p = 0.7) or overall survival (46 months with fludarabine vs 64 months in the chlorambucil arm; p = 0.15), as shown in TABle 1. The aim of treatment is to improve the quality of life of patients, and measures of quality of life are critical in interpreting clinical trials, particularly in the elderly. A quality-of-life assessment was incorporated into the CLL5 study, although it is disappointing that only a minority of patients completed it. If these more elderly frail patients are to have an improved outcome, then alternative approaches have to be taken.
Table 1.
Outcome of the German Chronic Lymphocytic Leukemia Study CLL5
| Chemotherapy | CR (%) | ORR (%) | PFS (months) | OS (%) | |
|---|---|---|---|---|---|
| CLL5 | Fludarabine | 7 | 72 | 19 | 46 |
| Elderly patients | Chlorambucil | 0 | 51 | 18 | 46 |
CLL: Chronic lymphocytic leukemia; CR: Complete remission; ORR: Overall response rate; OS: Overall survival; PFS: Progression-free survival.
Data taken from [4].
Importance of performance status in the treatment of elderly patients
It is clear that the performance status is more important than the chronological age of the patient, and it is extremely important to assess the patient’s comorbidities and fitness before recommending treatment. Comorbidity is frequent in CLL and frequently limits treatment in older cancer patients, but oncologists have perhaps not paid sufficient attention to comorbidity in making treatment decisions (Box 1). Several different methods are used to assess the fitness of patients. To date, there is no standard measure of the comorbidity burden available for these patients, but among the instruments designed to detect and quantify comorbidity, there are three scales that are potentially applicable to the CLL population. The Sorror version of the Charlson Index (CI) rates 19 diseases and can generate an age/comorbidity index [15]. The Cumulative Illness Rating Scale (CIRS) rates 13 body systems on a five-point pathophysiology severity scale to assess comorbidities in different organ systems (TABle 2), and the index of coexisting disease (ICED) measures the disease severity of 14 categories of disease and assesses disability [16]. To assess the performance of two comorbidity scales and their relationship with functional status, the CIRS-Geriatric (CIRS-G) was compared with the CI in 203 patients who received a comprehensive geriatric assessment (CGA) in a Senior Adult Oncology Program (SAOP) Study [17]. The study assessed variability, reliability, correlation with Eastern Cooperative Oncology Group performance status, activities of daily living, and instrumental activities of daily living, and the relative weight of comorbidity versus tumor stage in correlation with functional status. The median age of the patients assessed was 75 years (range: 63–91 years); 64% of patients scored 0 on the CI scale versus 6% on the CIRS-G. The correlation between the CI and CIRS-G was fair (p = 0.25–0.39). Otherwise, there was low or no correlation between comorbidity and functional status across the measures. Tumor stage was not correlated with functional status. The authors concluded that comorbidity needs to be assessed independently from functional status and both the CI and CIRS-G scales are reliable tools for use in trials of older cancer patients. The International Society of Geriatric Oncology Chemotherapy Taskforce published consensus recommendations on chemotherapy in the elderly [18–20]. The authors concluded that there is a lack of evidence-based data with regard to chemotherapy and that consensus recommendations had to be made on the basis of inadequate data.
Box 1. Oncology versus geriatric assessment of patient characteristics.
Oncology
Age
Performance status
Geriatric medicine
Functional status (e.g., ADL, iADL, aADL)
Depression (e.g., geriatric depression scale)
Dementia (e.g., mini-mental state examination)
Mobility (e.g., timed up and go)
Nutrition (e.g., mini-nutritional status)
Social circumstances
Comorbidity (e.g., Charlson or CIRS score)
Polypharmacy
aADL: Advance activities of daily living; ADL: Activities of daily living; CIRS: Cumulative illness rating score; iADL: Instrumental activities of daily living.
Table 2.
Cumulative Illness Rating Score
| Organ System | If illness/impairment present, specify: | Score |
|---|---|---|
| Heart | [ ] | |
| Blood pressure | [ ] | |
| Vascular | [ ] | |
| Respiratory | [ ] | |
| Ear/nose/throat | [ ] | |
| Upper gastrointestinal | [ ] | |
| Lower gastrointestinal | [ ] | |
| Liver | [ ] | |
| Renal | [ ] | |
| Genitourinary | [ ] | |
| Musculoskeletal | [ ] | |
| Endocrine/metabolic | [ ] | |
| Neurological | [ ] | |
| Psychiatric | [ ] | |
| Total score | [ ] |
Please insert the appropriate grade of illness impairment.
The GCLLSG has taken a lead in this area with respect to treatment in CLL, and has incorporated physiologic assessment to guide the choice of chemotherapy in any given patient. The GCLLSG assess suitability for treatment approaches using the CIRS. Those with a CIRS score less than 6 are deemed suitable for standard treatment. Patients with a CIRS score of 6 or greater are assessed on their suitability to receive chemotherapy. Those who are suitable are eligible for reduced treatment and those who are not eligible for treatment are suitable for supportive care. The relationship between comorbidity and drug pharmacokinetics is unknown and requires further study. Of major importance, the basis for enrollment in ongoing GCLLSG studies is not the age of the patient, but whether they fit into the ‘go’, ‘slow-go’ or ‘no’ categories (Figure 3).
Figure 3.

Comorbidity is the major factor determining treatment in studies from the German Chronic Lymphocytic Leukemia Study Group.
Treatment options for CLL patients with comorbidities
Chemoimmunotherapy using the FCR regimen is the standard of care for the treatment of CLL patients who are able to tolerate this therapy. For patients who cannot tolerate such treatment approaches, alternative treatments are required, although some studies have examined the use of this combination using lower doses [21]. Chlorambucil was the first effective agent used in the treatment of CLL. Chlorambucil is rapidly absorbed from the GI tract and peak plasma concentrations occur within 1 h of ingestion. There has been great variability in dosage and schedule of administration, but the two most commonly used approaches are low-dose continuous therapy using a dose of 0.08 mg/kg (usual dose: 4–8 mg orally) or pulsed intermittent dosage of 0.8 mg/kg (usual dose: 40–80 mg) given in a single dose orally every 3–4 weeks. The drug has fallen out of fashion in the USA, but continues to be used widely in Europe, and the results of the CLL5 trial suggest it still has a role to play in patients with decreased performance status. Fludarabine is now available as an oral preparation in most countries. It can be used alone or in combination. A problem with the use of purine analogs in the elderly is that the drug must be used cautiously and at reduced doses in those with impaired renal function, and a reduced glomerular filtration rate is often the rate-limiting factor for more elderly patients entering into clinical trials incorporating fludarabine-based combinations. Ongoing clinical trials are assessing the addition of monoclonal antibodies, including rituximab or ofatumumab [22], to chlorambucil compared with chlorambucil alone. Additional agents being assessed in clinical trials in this patient population include bendamustine alone and in combination with rituximab [23], lenalidomide [24], the PI3K inhibitor CAL101 and the BCL-2 inhibitor ABT263. Design of clinical trials specifically targeting those older CLL patients who have comorbidity and will not be eligible for standard approaches is clearly a major step forward in the development of evidence-based optimal treatment approaches for the majority of patients with CLL.
Expert commentary & five-year view
There has been considerable progress made over the last decade in the understanding of the molecular pathophysiology of CLL, and this has led to the identification of a large number of biomarkers that have important prognostic significance in this disease. Attempts are being made to identify the role of these biomarkers in determining the optimal treatment approach in individual patients, rather than a ‘one treatment fits all’ approach. The most notable example of how this is now being put into practice is the appreciation that those patients with 17p deletion or p53 mutations will not respond well to standard therapies and that patients with p53 deletions or mutations should have alternative front-line treatment incorporating agents, such as alemtuzumab, that have activity in the absence of functional p53.
Despite the fact that CLL is a disease of the elderly, and that age is itself an important prognostic marker, until recently there has been insufficient attention paid to the specific management of patients based upon age at need for first treatment. In 5 years, the results of a larger number of studies that assess treatment in patients with a poor performance status will be available, providing evidence of the efficacy of novel agents in studies specifically designed to address the utility of these agents in this group of patients. As these agents become approved for use, there will be a greater choice of front-line therapy for CLL. What is now clear is that it is not only age but performance status that impacts on the tolerability and applicability of treatment in CLL. Therefore, treatment approaches must take into account the performance status of patients. Once a decision has been made to alter treatment based upon any marker, there must be some objective assessment. This can readily be done and it is no longer acceptable to alter treatment based solely on the subjective assessment of the treating oncologist.
Therefore, 5 years hence, once a decision is made to begin treatment in a patient with CLL, an objective assessment will be made of the suitability of that patient for that treatment, based not only on the molecular profile of the disease, but also on the suitability of the patient to receive such therapy. Entry for clinical trials will require formal assessment of performance and as more work is carried out in this area, there will be greater confidence in the use of tools to assess performance status, and new tools will be developed to refine this. There will continue to be clinical trials that are designed specifically for CLL patients based upon performance status, allowing the entry of more CLL patients into clinical trials. It is likely that as new agents emerge with lower toxicity profiles, such trials will include the utility of earlier treatment in elderly patients with CLL. The biggest difference will probably be that in 5 years time, elderly patients with CLL will no longer be ignored by the academic community.
Key issues.
There have been major advances in the treatment of chronic lymphocytic leukemia (CLL) over the past decade, and approaches are now being developed to evaluate if we can direct therapy to individual patients based upon the molecular risk features of their disease.
Comorbidity and not age is the limiting factor in the use of chemoimmunotherapy approaches in CLL.
Elderly patients with comorbidities are vastly under-represented in clinical trials in CLL, and more trials are needed in this population.
A number of tools are available to assess comorbidity in CLL.
The validation of these tools in CLL is being undertaken in CLL studies that specifically target patients who would not be considered fit for standard treatment approaches.
A number of promising agents are entering clinical trials in the frail CLL patient population.
Acknowledgments
John G Gribben has received honoraria for consultancy and for speaking from Roche, Celgene, Biogen and Mundipharma, and funding from the NIH for the CLL Research Consortium Program Grant (NCI PO1 CA81538)
Footnotes
Financial & competing interests disclosure The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute – Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chemotherapeutic options in chronic lymphocytic leukemia: a meta-analysis of the randomized trials. CLL Trialists’ Collaborative Group. J. Natl Cancer Inst. 1999;91(10):861–868. doi: 10.1093/jnci/91.10.861. [DOI] [PubMed] [Google Scholar]
- 3.Rai KR, Peterson BL, Appelbaum FR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N. Engl. J. Med. 2000;343(24):1750–1757. doi: 10.1056/NEJM200012143432402. [DOI] [PubMed] [Google Scholar]
- 4.Eichhorst BF, Busch R, Hopfinger G, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood. 2006;107(3):885–891. doi: 10.1182/blood-2005-06-2395. [DOI] [PubMed] [Google Scholar]
- 5.Flinn IW, Neuberg DS, Grever MR, et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. J. Clin. Oncol. 2007;25(7):793–798. doi: 10.1200/JCO.2006.08.0762. [DOI] [PubMed] [Google Scholar]
- 6.Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet. 2007;370(9583):230–239. doi: 10.1016/S0140-6736(07)61125-8. [DOI] [PubMed] [Google Scholar]
- 7.Hallek M, Fingerle-Rowson G, Fink AM, et al. First-line treatment with fludarabine (F), cyclophosphamide (C), and rituximab (R) (FCR) improves overall survival (OS) in previously untreated patients (pts) with advanced chronic lymphocytic leukemia (CLL): results of a randomized Phase III trial on behalf of an international group of investigators and the German CLL Study Group. Blood. 2009;114:535. •• Abstract of the first study to show a survival advantage for frontline chemotherapy in chronic lymphocytic leukemia. The use of chemoimmunotherapy improves overall survival compared with combination chemotherapy alone.
- 8.Gribben JG. How I treat CLL up front. Blood. 2010;115(2):187–197. doi: 10.1182/blood-2009-08-207126. • Reviews an upfront treatment approach in chronic lymphocytic leukemia.
- 9.Tam CS, O’Brien S, Wierda W, et al. Long term results of the fludarabine, cyclophosphamide and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112(4):975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rawstron AC, Bennett FL, O’Connor SJ, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N. Engl. J. Med. 2008;359(6):575–583. doi: 10.1056/NEJMoa075290. [DOI] [PubMed] [Google Scholar]
- 11.Yancik R. Cancer burden in the aged: an epidemiologic and demographic overview. Cancer. 1997;80(7):1273–1283. [PubMed] [Google Scholar]
- 12.Thurmes P, Call T, Slager S, et al. Comorbid conditions and survival in unselected, newly diagnosed patients with chronic lymphocytic leukemia. Leuk. Lymphoma. 2008;49(1):49–56. doi: 10.1080/10428190701724785. [DOI] [PubMed] [Google Scholar]
- 13.Else M, Smith AG, Cocks K, et al. Patients’ experience of chronic lymphocytic leukaemia: baseline health-related quality of life results from the LRF CLL4 trial. Br. J. Haematol. 2008;143(5):690–697. doi: 10.1111/j.1365-2141.2008.07407.x. [DOI] [PubMed] [Google Scholar]
- 14.Eichhorst BF, Busch R, Stilgenbauer S, et al. First line therapy with fludarabine compared to chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114(16):3382–3391. doi: 10.1182/blood-2009-02-206185. •• This study enrolled only older patients with comorbidities. Its importance lies in the fact that it starts to redress the balance in designing trials in patients who are more representative of those seen in clinical practice.
- 15.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity. A critical review of available methods. J. Clin. Epidemiol. 2003;56(3):221–229. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 17.Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J. Clin. Oncol. 1998;16(4):1582–1587. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 18.Lichtman SM, Balducci L, Aapro M. Geriatric oncology: a field coming of age. J. Clin. Oncol. 2007;25(14):1821–1823. doi: 10.1200/JCO.2007.10.6567. [DOI] [PubMed] [Google Scholar]
- 19.Lichtman SM, Wildiers H, Chatelut E, et al. International Society of Geriatric Oncology Chemotherapy Taskforce: evaluation of chemotherapy in older patients – an analysis of the medical literature. J. Clin. Oncol. 2007;25(14):1832–1843. doi: 10.1200/JCO.2007.10.6583. •• Increases the awareness of the need for special considerations in planning oncology treatment for the elderly.
- 20.Lichtman SM, Wildiers H, Launay-Vacher V, Steer C, Chatelut E, Aapro M. International Society of Geriatric Oncology (SIOG) recommendations for the adjustment of dosing in elderly cancer patients with renal insufficiency. Eur. J. Cancer. 2007;43(1):14–34. doi: 10.1016/j.ejca.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Foon KA, Boyiadzis M, Land SR, et al. Chemoimmunotherapy with low-dose fludarabine and cyclophosphamide and high dose rituximab in previously untreated patients with chronic lymphocytic leukemia. J. Clin. Oncol. 2009;27(4):498–503. doi: 10.1200/JCO.2008.17.2619. [DOI] [PubMed] [Google Scholar]
- 22.Wierda WG, Kipps TJ, Mayer J, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J. Clin. Oncol. 2010;28(10):1749–1755. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheson BD, Wendtner CM, Pieper A, et al. Optimal use of bendamustine in chronic lymphocytic leukemia, non-Hodgkin lymphomas, and multiple myeloma: treatment recommendations from an international consensus panel. Clin. Lymphoma Myeloma Leuk. 2010;10(1):21–27. doi: 10.3816/CLML.2010.n.002. [DOI] [PubMed] [Google Scholar]
- 24.Ferrajoli A, Lee BN, Schlette EJ, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111(11):5291–5297. doi: 10.1182/blood-2007-12-130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.Age-adjusted incidence of CLL in the USA. http://seer.cancer.gov/statfacts/html/clyl.html.
- 102.Percentage of patients in age groups at presentation of CLL in the USA. http://seer.cancer.gov/faststats/selections.php.