Abstract
Introduction
There are no reliable markers of dysplasia in patients with incidentally discovered intraductal papillary mucinous neoplasms of the pancreas (IPMN). IPMN dysplasia may be associated with mucin protein (MUC) expression and histopathologic subtype. We hypothesize that MUC expression in cyst fluid and serum can identify lesions with high risk of malignancy.
Methods
Cyst fluid and serum were collected from 40 patients during pancreatectomy for IPMN between 2005 and 2009. Samples were grouped into low-risk (low-grade or moderate dysplasia, n = 21) and high-risk groups (high-grade dysplasia or carcinoma, n = 19). Mucin expression (MUC1, MUC2, MUC4, and MUC5AC) was assessed utilizing enzyme-linked immunosorbent assays.
Results
MUC2 and MUC4 cyst fluid concentrations were elevated in high-risk versus low-risk groups (10 ± 3.0 ng/ml vs. 4.4 ± 1.2 ng/ml, p = 0.03; 20.6 ± 10.6 ng/ml vs. 4.5 ± 1.4 ng/ml, p = 0.03, respectively). Corresponding serum samples revealed higher levels of MUC5AC in high-risk compared with low-risk patients (19.9 ± 9.3 ng/ml vs. 2.2 ± 1.1 ng/ml, p = 0.04). Histopathologic subtype was significantly associated with grade of dysplasia, and the intestinal subtype displayed increased MUC2 cyst fluid concentrations (13.8 ± 6.5 ng/ml vs. 4.1 ± 0.9 ng/ml, p = 0.02).
Conclusions
In this study, high-risk IPMN showed elevated cyst fluid concentrations of MUC2 and MUC4, and increased serum levels of MUC5AC. High-risk IPMN also displayed a distinct mucin expression profile in specific histologic subtypes. These data, if validated, may allow surgeons to more appropriately select patients for operative resection.
Identification of intraductal papillary mucinous neoplasms of the pancreas (IPMN) has increased in recent years, largely due to increased utilization of high-quality cross-sectional imaging.1,2 Management of IPMN has remained problematic due to an inability to determine the degree of dysplasia in those presenting with radiographically equivocal lesions.3
It is presumed that IPMN evolve from low-grade dysplasia to carcinoma; however, the frequency and timeframe for this progression remains unknown. Most surgeons would agree that IPMN with high-grade dysplasia or carcinoma should be resected, but in the absence of obvious radiographic findings, this pathologic information is generally not available preoperatively. Endoscopically obtained fine-needle aspiration (FNA) samples of cystic lesions have not been able to accurately predict levels of dysplasia nor confidently histotype lesions.4 Cyst fluid carcinoembryonic antigen (CEA) levels have been found to be predictive of mucinous lesions, but have not been found to correlate with degree of dysplasia.
Mucins (MUCs) are highly glycosylated proteins that lubricate and protect epithelial mucosa and have various roles in homeostasis and carcinogenesis.5,6 At least 19 human MUC genes have been distinguished by complementary DNA (cDNA) cloning, and immunohistochemical MUC expression patterns can distinguish the different histopathologic types of IPMN (gastric, intestinal, pancreatobiliary).7–18 The gastric subtype of IPMN consists of cells resembling gastric foveolae and on immunohistochemical staining typically expresses MUC5AC but not MUC1 or MUC2. This subtype of IPMN is almost uniformly low grade.19 The intestinal subtype of IPMN consists of cells resembling villous adenomas and typically expresses MUC2, and the pancreatobiliary subtype of IPMN consists of cells resembling biliary papillae and typically expresses MUC1. These subtypes of IPMN frequently contain high-grade dysplasia.20,21 We hypothesized that the differential expression of MUC proteins identified on immunohistochemical studies of tissue sections may be reflected in the cyst fluid and allow for development of a MUC panel predictive of cyst dysplasia.
Methods
Pancreatic cyst fluid aspirates and serum samples were prospectively collected at time of resection from 40 of 147 patients with IPMN between 2005 and 2009. Patients were preoperatively consented to an institutional review board (IRB) tissue collection protocol, and a waiver of authorization was obtained from the institutional IRB prior to data review. Serum samples were collected at time of preadmission testing, which generally occurred within 2 weeks of operation. Cyst fluid was obtained at time of resection. Resected specimens were immediately transported to the Memorial Sloan-Kettering Cancer Center (MSKCC) tumor procurement facility in the Department of Pathology, where cyst aspiration was performed by a surgeon, pathologist or technician. Cyst fluid was aspirated with an 18-21-gauge needle, divided into 500-μl aliquots, and stored at − 80°C. All analyses were performed on samples with no prior freeze–thaw cycles.
Samples were divided into low-risk (low-grade and moderate dysplasia) and high-risk groups (high-grade dysplasia and invasive carcinoma). All histopathology was independently reviewed and assessed by a single dedicated gastrointestinal (GI) pathologist (N.K.) for IPMN subtyping and to determine the degree of cyst dysplasia. Gastric subtype was defined by flat epithelium or thick finger-like papillae similar to gastric foveolae, apical cytoplasmic mucin, and basal nuclei. Intestinal subtype was characterized by well-formed villous papillae lined by cells with elongated, pseudostratified nuclei, resembling colonic villous adenomas. Pancreatobiliary subtype was defined by the presence of arborizing papillae similar to biliary papillary neoplasms, and cytologic atypia with enlarged hyperchromatic nuclei. Grading of dysplasia in IPMN was based on the most severe degree of dysplasia identified. Low-grade dysplasia was characterized by basal nuclei and intracellular mucin, whereas in moderate dysplasia there was full-thickness nuclear pseudostratification with mild to moderate nuclear atypia. High-grade dysplasia was distinguished by significant cytologic atypia, complex disorganized architecture, and tufting. In the setting of invasive carcinoma, tubular morphology was characterized by glandular structures with stromal invasion and a general lack of mucin, while colloid carcinoma contained > 80% mucin.
Expression of the mucins MUC1, MUC2, MUC4, and MUC5AC was quantified utilizing enzyme-linked immunosorbent assays (ELISAs; USCN-life, China). All samples were run according to manufacturer's guidelines. Cyst fluid viscosity was noted at time of ELISA and determined to be “thick” if the fluid was unable to be aspirated into a 200-μl pipette tip. Thick samples were diluted with phosphate-buffered saline (PBS); all others were run neat. Absorbance was read at 450 nm.
Differences between groups were assessed utilizing analysis of variance (ANOVA) and t test calculations. Values are expressed as mean ± standard error of the mean (SEM).
Results
Patient Demographics and Cyst Characteristics
This study included 27 women (67%) and 13 (33%) men. Invasive carcinoma was identified in five cysts, 14 had high-grade dysplasia (HGD), 15 had moderate dysplasia, and six had low-grade dysplasia. There were 19 “high-risk” and 21 “low-risk” cysts. Serum samples were available from 12 patients with high-risk cysts and 14 patients with low-risk cysts. Radiographic and histopathologic findings of the 40 patients are presented in Table 1. Average cyst diameter on radiology was 4.2 cm (range: 1.2–23 cm, median: 3.0 cm). Mean cyst diameter was 5.5 cm for high-risk cysts and 2.9 cm for low-risk cysts. Though cyst size was not a significant predictor of high-risk lesions as a continuous variable, when dichotomized at 4.0 cm, cyst diameter was associated with high-risk lesions (p = 0.02).
Table 1. Patient demographics.
| Sex | Degree of dysplasia | Histologic subtype | Duct type | Cyst diameter (cm) | |
|---|---|---|---|---|---|
| 1 | M | Carcinoma | Intestinal | Main | 2.5 |
| 2 | F | Carcinoma | Intestinal | Main | 6.7 |
| 3 | F | Carcinoma | Intestinal | Main | 18 |
| 4 | F | Carcinoma | Intestinal | Main | 2.5 |
| 5 | F | Carcinoma | Gastric and pancreatobiliary | Main | 10.0 |
| 6 | M | HGD | Intestinal | Main | 2.0 |
| 7 | M | HGD | Gastric and oncocytic | Branch | 2.9 |
| 8 | M | HGD | Intestinal | Branch and main | 2.0 |
| 9 | M | HGD | Oncocytic | Main | 4.5 |
| 10 | M | HGD | Gastric and pancreatobiliary | Main | 1.5 |
| 11 | M | HGD | Intestinal | Main | 3.0 |
| 12 | F | HGD | Gastric and focal pancreatobiliary | Branch and main | 2.6 |
| 13 | F | HGD | Gastric and pancreatobiliary | Branch and main | 2.3 |
| 14 | F | HGD | Gastric and pancreatobiliary | Branch and main | 4.5 |
| 15 | M | HGD | Gastric and focal pancreatobiliary | Branch and main | 5.9 |
| 16 | F | HGD | Intestinal and focal pancreatobiliary | Main | 7.0 |
| 17 | F | HGD | Gastric | Branch | 3.0 |
| 18 | M | HGD | Intestinal | Main | 23.0 |
| 19 | F | HGD | Gastric | Branch | 1.5 |
| 20 | F | Moderate | Gastric | Uncinate | 3.1 |
| 21 | F | Moderate | Gastric | Branch and main | 3.0 |
| 22 | F | Moderate | Gastric | Branch | 1.7 |
| 23 | F | Moderate | Gastric | Branch | 4.5 |
| 24 | M | Moderate | Gastric | Branch | 3 |
| 25 | F | Moderate | Gastric | Branch | 1.2 |
| 26 | F | Moderate | Gastric | Branch and main | 1.5 |
| 27 | F | Moderate | Gastric | Branch | 2.0 |
| 28 | M | Moderate | Gastric | Branch | 2.7 |
| 29 | F | Moderate | Gastric | Main | 3.9 |
| 30 | M | Moderate | Gastric | Branch | 2.5 |
| 31 | F | Moderate | Gastric | Branch | 6.0 |
| 32 | F | Moderate | Gastric | Branch and main | 3.5 |
| 33 | F | Moderate | Gastric | Branch | 2.4 |
| 34 | F | Moderate | Gastric | Branch | 4.0 |
| 35 | F | Low | Gastric | Branch | 3.4 |
| 36 | M | Low | Gastric | Branch | 1.5 |
| 37 | F | Low | Gastric | Branch and main | 3.5 |
| 38 | F | Low | Gastric | Branch | 4.0 |
| 39 | F | Low | Gastric | Branch | 2.2 |
| 40 | F | Low | Gastric | Branch | 2.0 |
F female, M male
The majority of cysts were branch duct IPMN (n = 19). Eighteen (95%) of the branch duct lesions were of gastric subtype and one contained oncocytic features. Three of 19 (16%) of branch duct cysts were in the high-risk group. There were 12 main duct lesions and 9 mixed (branch and main duct) cysts. All cysts with invasive disease were main duct IPMN, and main duct IPMN was associated with high-risk lesions (p = 0.03).
The majority of lesions (33/40, 83%) were radiographically equivocal without clear signs of an associated mass or nodularity. Solid components were seen in two cysts with invasive cancer and two cysts with high-grade dysplasia. Nodularity was observed in one cyst with invasive cancer, one cyst with high-grade dysplasia, and one cyst with low-grade dysplasia. There were no cysts with moderate dysplasia and a solid component or nodularity.
Histopathologic Subtype and Dysplasia
All 21 of the low-risk cysts (low-grade and moderate dysplasia) were of gastric histopathologic subtype. Of the 14 cysts with HGD, four were intestinal, six were pancreatobiliary, two were gastric, and two had oncocytic features. Of the five cysts with invasive carcinoma, four were of intestinal subtype and one was pancreatobiliary. All intestinal, pancreatobiliary, and oncocytic cysts also contained gastric epithelium. All of the intestinal and pancreatobiliary cysts (n = 15) were in the high-risk group (Table 2).
Table 2. Dysplasia correlated with histopathologic subtype.
| Degree of dysplasia | Histologic subtype | ||
|---|---|---|---|
|
| |||
| Intestinala | Pancreatobiliarya | Gastrica | |
| Carcinoma or high-grade dysplasia | 100% (8/8) | 100% (7/7) | 9% (2/23) |
| Low- or moderate-grade dysplasia | 0% | 0% | 91% (21/23) |
All cysts harboring the intestinal or pancreatobiliary subtype were found to be high-risk cysts containing high-grade dysplasia or invasive cancer. The majority of gastric cysts were low-risk cysts. All of the low-risk cysts were of gastric subtype
p < 0.05
Histopathologic Subtype and MUC Expression
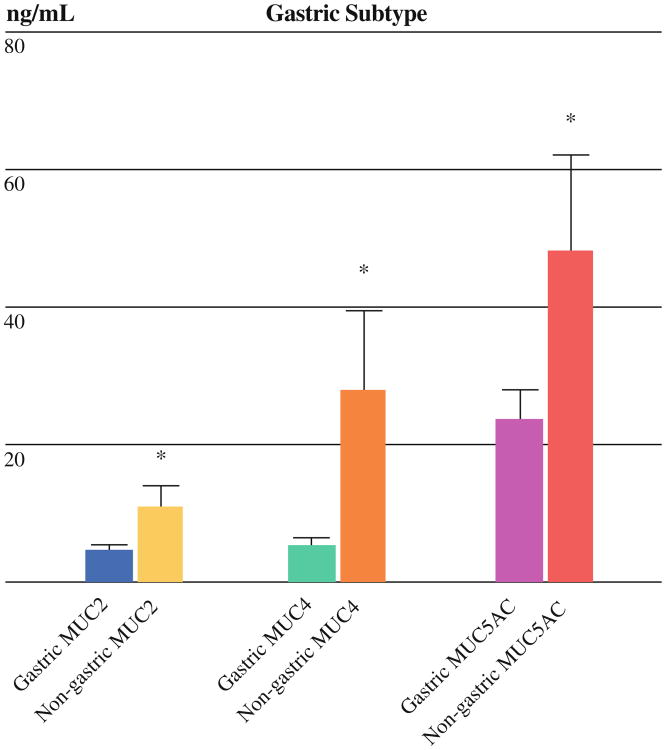
Gastric cysts (n = 23) expressed lower levels of MUC2 (4.3 ± 1.1 vs. 10.8 ± 3.3 ng/ml, p = 0.02), MUC4 (5.1 ± 1.5 vs. 27.6 ± 11.9 ng/ml, p = 0.02), and MUC5AC (23.5 ± 4.4 vs. 48.0 ± 14.1 ng/ml, p = 0.04) compared with nongastric cysts (n = 17) (Fig. 1). There was no difference in MUC1 expression, with very low or undetectable levels in the majority of cysts.
Fig. 1.
Gastric subtype correlated with MUC expression. Expression of MUCs 2, 4, and 5AC were significantly decreased in gastric (n = 23) compared with nongastric cysts (n = 17). * p < 0.05
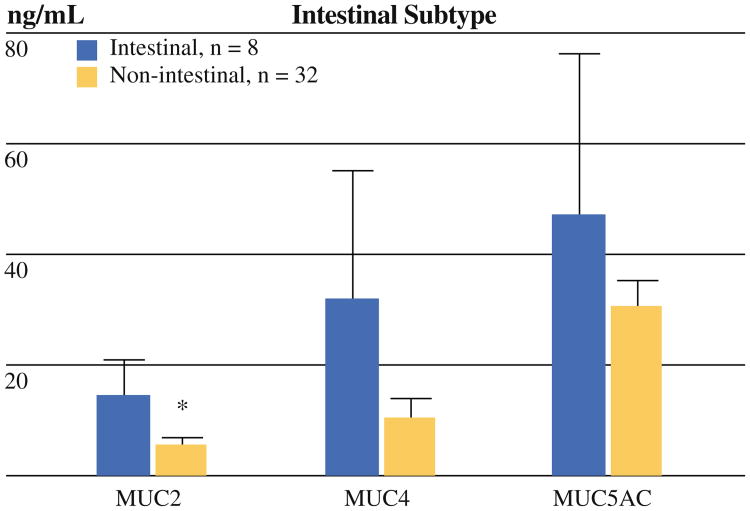
Intestinal cysts (n = 8) contained higher levels of MUC2 (13.8 ± 6.5 vs. 5.4 ± 1.1 ng/ml, p = 0.03) and tended to have increased levels of MUC4 (31.9 ± 23.2 vs. 10.3 ± 3.4 ng/ml, p = 0.054) compared with nonintestinal cysts (n = 32) (Fig. 2). Pancreatobiliary cysts were not significantly associated with a distinct mucin profile (n = 6).
Fig. 2.
Intestinal subtype correlated with MUC expression. Expression of MUC2 and MUC4 were increased in intestinal compared with nonintestinal cysts (p = 0.03* and 0.054, respectively)
Degree of Cyst Dysplasia and MUC Expression
MUC1 expression was very low in the cyst fluid across all groups of IPMN (mean 0.8 ng/ml), and there was no correlation between MUC1 level and degree of cyst dysplasia. MUC5AC expression was much higher than MUC1 across all IPMN groups (mean 33.9 ng/ml), but there was no association between level of dysplasia and MUC5AC expression.
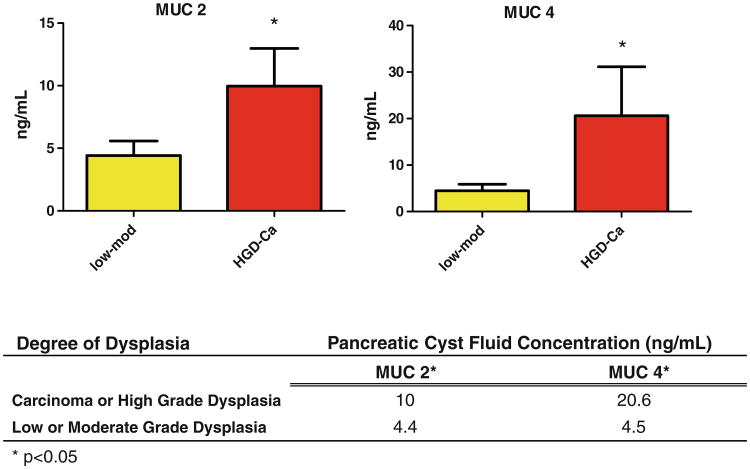
MUC2 and MUC4 levels tended to cluster by degree of dysplasia, with higher levels of expression in highly dysplastic and invasive cysts. High-risk cysts (n = 19) had increased expression of MUC2 (10.0 ± 3.0 vs. 4.4 ± 1.2 ng/ml, p = 0.03) and MUC4 (20.6 ± 10.6 vs. 4.5 ± 1.4 ng/ml, p = 0.03) compared with low-risk cysts (n = 21) (Fig. 3).
Fig. 3.
MUC2 and MUC4 expression by degree of cyst dysplasia. Levels of MUC2 and MUC4 were significantly elevated in high-risk cysts compared with low-risk cysts. Mod moderate dysplasia, HGD high-grade dysplasia, Ca invasive cancer
MUC Expression in Serum
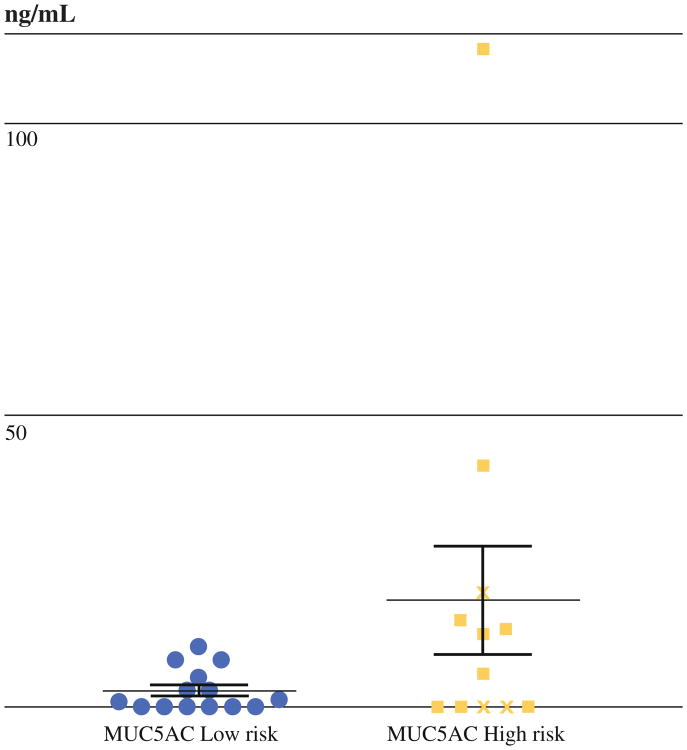
Serum levels of MUC5AC were not different between individual levels of cyst dysplasia, but they were elevated in patients with high-risk cysts compared with low-risk cysts (17.9 ± 9.3 vs. 2.2 ± 1.1 ng/ml, p = 0.025). There was no difference in serum levels of MUCs 1, 2 or 4 between the groups, and serum levels of MUCs were not associated with histopathologic subtype (Fig. 4).
Fig. 4.
Serum levels of MUC5AC. Serum levels of MUC5AC were elevated in patients with high-risk cysts compared with low-risk cysts (17.9 ± 9.3 vs. 2.2 ± 1.1 ng/ml, p = 0.025) in a small number of patients (high risk, n = 12, low risk, n = 14). Data points from the three serum samples of patients with invasive IPMN are denoted by crosses
Cyst Fluid Thickness and Level of Dysplasia
Ten of 40 cyst samples were determined to be thick (not able to be aspirated through a 200-μl pipette). Seven of these were from cysts with high-grade dysplasia, one from a cyst with low-grade dysplasia, and two from moderately dysplastic cysts. Presence of thick cyst fluid was associated with high-grade dysplasia (p = 0.007) with a positive predictive value of 70%.
Discussion
Current management guidelines for patients who present with incidentally discovered IPMN are based primarily upon radiographic findings. When the main pancreatic duct is dilated, high-grade dysplasia or invasive carcinoma has been reported to occur in approximately 50% of patients and resection is generally recommended.22,23 When patients present with isolated branch duct disease, the risk of high-grade dysplasia or invasive carcinoma appears to correlate with the presence of a solid component and cyst size. In the absence of a solid component and size <3 cm, the risk of high-grade IPMN is generally <5% and radiographic observation is warranted in selected patients.24,25 Better markers of dysplasia may allow for safe monitoring of these lesions even in the setting of radiographic concern, and identify high-risk lesions for resection with benign radiographic characteristics.
Elevated cyst fluid CEA levels have been shown to distinguish mucinous from nonmucinous cysts with an approximate 80% accuracy.26 Cyst fluid CEA, however, has not been shown to be a marker of dysplasia, and we have found that degree of CEA elevation is not associated with in situ or invasive IPMN.27,28
In the current study we have further demonstrated that the histopathologic subtype of IPMN is associated with the degree of dysplasia. Gastric cysts were more likely to be low-risk cysts (91% of all low-risk cysts), and intestinal and pancreatobiliary subtypes were more likely to be high-risk cysts (79% of all high-risk cysts). Histologic subtype correlated with cyst fluid MUC expression, with decreased MUC2, MUC4, and MUC5AC in gastric cysts and elevated MUC2 in intestinal cysts. Furthermore, elevated MUC2 and MUC4 cyst fluid concentrations were associated with high-grade dysplasia and carcinoma, and elevated serum MUC5AC was associated with high-risk cysts. Others have stained for mucins in IPMN and pancreatic juice; however, this is the only report we know of that quantifies MUC1, 2, 4, and 5AC in this setting.
Though it is not clear whether cysts transform from benign to malignant through a progression of histologic subtypes, or if they are phenotypically predisposed to malignant change, it has been recognized that the subtype of IPMN is associated with the degree of cyst dysplasia and distinct mucin staining profiles.10,19,29–31 The gastric subtype histologically resembles gastric foveolar cells, often contains low-grade dysplasia, and stains for MUC5AC.19 The intestinal subtype resembles villous intestinal neoplasms, often contains moderate to high-grade dysplasia, and stains for MUCs 2, 4, and 5AC.19 The pancreatobiliary subtype resembles biliary papillary neoplasms, often contains moderate or high-grade dysplasia, and stains for MUCs 1, 4, and 5AC.19,32 The two types of invasive cancer encountered in IPMN, tubular carcinoma and colloid carcinoma, differ histologically in the amount of mucin in the cysts, and typically stain for MUC1 and MUC2, respectively.31 Consistent with these observations, all of our low-grade and moderately dysplastic cysts were of the gastric subtype and all of the intestinal and pancreatobiliary subtypes were found in cysts with high-grade dysplasia or carcinoma. We found low levels of expression of all MUCs in the gastric subtype, and this correlated with branch duct phenotype, and low malignant potential, therefore implying that MUCs 2 and 4 may be increasingly expressed with higher levels of dysplasia.
MUC1 is a transmembrane signal transducer molecule. It is commonly expressed in PanIN-3 (not in 1 or 2) and ductal adenocarcinoma, and found only rarely in noninvasive, nonpancreatobiliary-type IPMN and colloid carcinoma. MUC1 overexpression, aberrant intracellular localization, and changes in glycosylation are associated with aggressive phenotype. Consistent with studies of cyst epithelium, we found cyst fluid MUC1 expression to be infrequent in IPMN.7 Since we found only minimal MUC1 cyst fluid expression in all histologic subtypes, it likely has minimal translocation or secretion into the cyst fluid, and is not a useful marker in this setting.
MUC2 is a secretory mucin prominent in the gut and secreted from goblet cells. It provides an insoluble mucous barrier and protects intestinal epithelium. It is not expressed in normal pancreatic tissue but is expressed in intestinal-type IPMN and colloid carcinoma, a less aggressive lesion that arises in association with IPMN.5,20,21 Similar to the findings of MUC2 staining in intestinal-type cysts, we found cyst fluid MUC2 to be a marker of the intestinal subtype, and further, have found it to be a marker of high-grade dysplasia/invasive cancer.7–14,32
MUC4 is a transmembrane ligand for ErbB-2 and is involved in cell–cell and cell–extracellular matrix interactions.33 It participates in tumor growth and metastases by directly altering cell properties and modulating ErbB-2 expression.34 It is not expressed in normal pancreas, may be expressed more frequently in high-grade and invasive IPMN, and has been associated with worse overall survival in pancreas cancer.5,21,35,36 Our data support that MUC4 is secreted into the cyst fluid and expressed at higher concentrations in high-risk cysts, consistent with studies that implicate MUC4 in the development of IPMN.37 Like MUC1, MUC4 is a membrane protein. However, whereas MUC1 acts as a docking protein, MUC4 is a receptor ligand, which may account for its higher level of expression.38 It is possible that an increase in MUC4 in high-risk cysts is reflective of activation of the ErbB-2 signaling pathway that may transform borderline cysts to a malignant phenotype.37
MUC5AC is a secretory mucin expressed on the gastric mucosa.39 It is not detected in normal pancreas, but is seen in all types of IPMN and PanINs, though the degree to which MUC5AC is expressed has not been confidently quantified.10,19,31,40 Our study is one of the first to quantify mucin concentrations, and consistent with other studies that immunohistochemically identified MUC5AC in cyst epithelium, we also found cyst fluid expression of MUC5AC in all histologic subtypes of IPMN and across all levels of dysplasia.21 There was no significant difference in cyst fluid MUC5AC levels between the groups. Though cyst fluid MUC5AC did not discriminate between high- and low-risk cysts, it did discriminate between gastric and nongastric cysts. However, serum levels of MUC5AC were elevated in high-risk cysts compared with low-risk cysts. Gastric subtype is a component of all cysts, and it is unclear why MUC5AC was more likely to be present in serum of patients with high-risk lesions. It is possible that MUC5AC is more likely to be secreted when the lesion becomes high grade, as was seen in the cyst fluid of intestinal and pancreaticobiliary cysts, but this remains to be elucidated. It is not clear why MUC 2 and 4 were not also differentially expressed in serum. Serum is easier than cyst fluid to acquire and would be the ideal medium to identify a biomarker of high-risk IPMN. The serum studies were performed on a small set of patients, and further studies need to be done to validate these findings, as they may corroborate that MUC5AC overexpression is an early event in IPMN progression.40
Thick cyst fluid is likely a reflection of higher mucin and glycoprotein expression. We measured the viscosity of cyst fluid using a simple test of whether it could be aspirated with a 200-ll pipette tip and found that thick samples were significantly associated with highly dysplastic cysts. It is likely that increased secretion of mucins into the cyst fluid accounts for this observation, and it may be that MUC2 and/or MUC4 are responsible for the increased viscosity, since they were independently found to be associated with high-risk cysts. Further studies evaluating cyst fluid viscosity need to be performed.
In conclusion, high-risk IPMN were associated with distinct histopathologic subtypes and mucin profiles. This study introduces cyst fluid MUC2 and MUC4, and serum MUC5AC, as potential biomarkers of dysplasia in IPMN worthy of further study. These data, if validated, may allow surgeons to more appropriately select patients for operative resection.
Footnotes
This paper was presented as an oral presentation at the Society of Surgical Oncology meeting March 5, 2010, St. Louis, MO.
References
- 1.Fernández-Del Castillo C, Targarona J, Thayer SP, et al. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427–34. doi: 10.1001/archsurg.138.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang XM, Mitchell DG, Dohke M, Holland GA, Parker L. Pancreatic cysts: Depiction on single-shot fast spin-echo MR images. Radiology. 2002;223:547–53. doi: 10.1148/radiol.2232010815. [DOI] [PubMed] [Google Scholar]
- 3.Sato N, Fukushima N, Maitra A, et al. Gene expression profiling identifies genes associated with invasive intraductal papillary mucinous neoplasms of the pancreas. Am J Pathol. 2004;164:903–14. doi: 10.1016/S0002-9440(10)63178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maker AV, Lee LS, Raut CP, Clancy TE, Swanson RS. Cytology from pancreatic cysts has marginal utility in surgical decision-making. Ann Surg Oncol. 2008;15:3187–92. doi: 10.1245/s10434-008-0110-0. [DOI] [PubMed] [Google Scholar]
- 5.Ringel J, Löhr M. The MUC gene family: their role in diagnosis and early detection of pancreatic cancer. Mol Cancer. 2003;2:9. doi: 10.1186/1476-4598-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strous GJ, Dekker J. Mucin-type glycoproteins. Crit Rev Biochem Mol Biol. 1992;27:57–92. doi: 10.3109/10409239209082559. [DOI] [PubMed] [Google Scholar]
- 7.Adsay NV, Merati K, Basturk O, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am J Surg Pathol. 2004;28:839–48. doi: 10.1097/00000478-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Lüttges J, Brocker V, Kremer B. Immunohistochemical mucin expression and DPC4 status in intraductal papillary mucinous tumors (IPMTs) of the pancreas. Pancreas. 2000;21:459. [Google Scholar]
- 9.Lüttges J, Feyerabend B, Buchelt T, Pacena M, Klöppel G. The mucin profile of noninvasive and invasive mucinous cystic neoplasms of the pancreas. Am J Surg Pathol. 2002;26:466–71. doi: 10.1097/00000478-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Lüttges J, Zamboni G, Longnecker D, Klöppel G. The immunohistochemical mucin expression pattern distinguishes different types of intraductal papillary mucinous neoplasms of the pancreas and determines their relationship to mucinous noncystic carcinoma and ductal adenocarcinoma. Am J Surg Pathol. 2001;25:942–8. doi: 10.1097/00000478-200107000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura A, Horinouchi M, Goto M, et al. New classification of pancreatic intraductal papillary-mucinous tumour by mucin expression: Its relationship with potential for malignancy. J Pathol. 2002;197:201–10. doi: 10.1002/path.1109. [DOI] [PubMed] [Google Scholar]
- 12.Yonezawa S, Horinouchi M, Osako M, et al. Gene expression of gastric type mucin (MUC5AC) in pancreatic tumors: Its relationship with the biological behavior of the tumor. Pathol Int. 1999;49:45–54. doi: 10.1046/j.1440-1827.1999.00823.x. [DOI] [PubMed] [Google Scholar]
- 13.Yonezawa S, Nakamura A, Horinouchi M, Sato E. The expression of several types of mucin is related to the biological behavior of pancreatic neoplasms. J Hepatobiliary Pancreat Surg. 2002;9:328–41. doi: 10.1007/s005340200037. [DOI] [PubMed] [Google Scholar]
- 14.Yonezawa S, Taira M, Osako M, et al. MUC-1 mucin expression in invasive areas of intraductal papillary mucinous tumors of the pancreas. Pathol Int. 1998;48:319–22. doi: 10.1111/j.1440-1827.1998.tb03913.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Zhao YH, Kalaslavadi TB, et al. Genome-wide search and identification of a novel gel-forming mucin MUC19/Muc19 in glandular tissues. Am J Respir Cell Mol Biol. 2004;30:155–65. doi: 10.1165/rcmb.2003-0103OC. [DOI] [PubMed] [Google Scholar]
- 16.Dekker J, Rossen JWA, Büller HA, Einerhand AWC. The MUC family: an obituary. Trends Biochem Sci. 2002;27:126–31. doi: 10.1016/s0968-0004(01)02052-7. [DOI] [PubMed] [Google Scholar]
- 17.Jass JR, Walsh MD. Altered mucin expression in the gastrointestinal tract: a review. J Cell Mol Med. 2001;5:327–51. doi: 10.1111/j.1582-4934.2001.tb00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor-Papadimitriou J, Burchell JM, Plunkett T, et al. MUC1 and the immunobiology of cancer. J Mammary Gland Biol Neoplasia. 2002;7:209–21. doi: 10.1023/a:1020360121451. [DOI] [PubMed] [Google Scholar]
- 19.Furukawa T, Klöppel G, Volkan Adsay N, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794–9. doi: 10.1007/s00428-005-0039-7. [DOI] [PubMed] [Google Scholar]
- 20.Ban S, Naitoh Y, Mino-Kenudson M, et al. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: its histopathologic difference between 2 major types. Am J Surg Pathol. 2006;30:1561–9. doi: 10.1097/01.pas.0000213305.98187.d4. [DOI] [PubMed] [Google Scholar]
- 21.Nagata K, Horinouchi M, Saitou M, et al. Mucin expression profile in pancreatic cancer and the precursor lesions. J Hepatobiliary Pancreat Surg. 2007;14:243–54. doi: 10.1007/s00534-006-1169-2. [DOI] [PubMed] [Google Scholar]
- 22.D'Angelica M, Brennan MF, Suriawinata AA, Klimstra D, Conlon KC. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg. 2004;239:400–8. doi: 10.1097/01.sla.0000114132.47816.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–97. doi: 10.1097/01.sla.0000128306.90650.aa. discussion 97–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen PJ, D'Angelica M, Gonen M, et al. A selective approach to the resection of cystic lesions of the pancreas: results from 539 consecutive patients. Ann Surg. 2006;244:572–9. doi: 10.1097/01.sla.0000237652.84466.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 26.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–6. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Belletrutti PJ, DiMaio CJ, Nagula S, et al. Pancreatic cyst fluid CEA concentration [1000 ng/ml is not an indicator of malignancy. Gastroenterology. 2010;138:S549. [Google Scholar]
- 28.Nagula S, Kennedy TJ, Schattner MA, et al. Performance characteristics of cyst fluid CEA analysis for the diagnosis of mucinous cysts of the pancreas. Gastroenterology. 2009;136:A148. doi: 10.1007/s11605-010-1281-0. [DOI] [PubMed] [Google Scholar]
- 29.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–87. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 30.Terris B, Dubois S, Buisine MP, et al. Mucin gene expression in intraductal papillary-mucinous pancreatic tumours and related lesions. J Pathol. 2002;197:632–7. doi: 10.1002/path.1146. [DOI] [PubMed] [Google Scholar]
- 31.Takaori K. Current understanding of precursors to pancreatic cancer. J Hepatobiliary Pancreat Surg. 2007;14:217–23. doi: 10.1007/s00534-006-1165-6. [DOI] [PubMed] [Google Scholar]
- 32.Hibi Y, Fukushima N, Tsuchida A, et al. Pancreatic juice cytology and subclassification of intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2007;34:197–204. doi: 10.1097/MPA.0b013e31802dea0. [DOI] [PubMed] [Google Scholar]
- 33.Carraway KL, Price-Schiavi SA, Komatsu M, Jepson S, Perez A, Carraway CA. Muc4/sialomucin complex in the mammary gland and breast cancer. J Mammary Gland Biol Neoplasia. 2001;6:323–37. doi: 10.1023/a:1011327708973. [DOI] [PubMed] [Google Scholar]
- 34.Singh AP, Moniaux N, Chauhan SC, Meza JL, Batra SK. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 2004;64:622–30. doi: 10.1158/0008-5472.can-03-2636. [DOI] [PubMed] [Google Scholar]
- 35.Grutzmann R, Foerder M, Alldinger I, et al. Gene expression profiles of microdissected pancreatic ductal adenocarcinoma. Virchows Arch. 2003;443:508–17. doi: 10.1007/s00428-003-0884-1. [DOI] [PubMed] [Google Scholar]
- 36.Iacobuzio-Donahue CA, Ashfaq R, Maitra A, et al. Highly expressed genes in pancreatic ductal adenocarcinomas: a comprehensive characterization and comparison of the transcription profiles obtained from three major technologies. Cancer Res. 2003;63:8614–22. [PubMed] [Google Scholar]
- 37.Kanno A, Satoh K, Kimura K, et al. The expression of MUC4 and MUC5AC is related to the biologic malignancy of intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2006;33:391–6. doi: 10.1097/01.mpa.0000236742.92606.c1. [DOI] [PubMed] [Google Scholar]
- 38.Ramsauer VP, Carraway CA, Salas PJ, Carraway KL. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, translocates ErbB2 to the apical surface in polarized epithelial cells. J Biol Chem. 2003;278:30142–7. doi: 10.1074/jbc.M303220200. [DOI] [PubMed] [Google Scholar]
- 39.Audie JP, Janin A, Porchet N, Copin MC, Gosselin B, Aubert JP. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J Histochem Cytochem. 1993;41:1479–85. doi: 10.1177/41.10.8245407. [DOI] [PubMed] [Google Scholar]
- 40.Kim GE, Bae H, Park H, et al. Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology. 2002;123:1052–60. doi: 10.1053/gast.2002.36018. [DOI] [PubMed] [Google Scholar]