Abstract
Nephrotic syndrome (NS) is a clinical condition with a high degree of morbidity and mortality, caused by failure of the glomerular filtration barrier, resulting in massive proteinuria. Our current diagnostic, prognostic and therapeutic decisions in NS are largely based upon clinical or histological patterns such as “focal segmental glomerulosclerosis” or “steroid sensitive”. Yet these descriptive classifications lack the precision to explain the physiologic origins and clinical heterogeneity observed in this syndrome. A more precise definition of NS is required to identify mechanisms of disease and capture various clinical trajectories. An integrative genomics approach to NS applies bioinformatics and computational methods to comprehensive experimental, molecular and clinical data for holistic disease definition. A unique aspect is analysis of data together to discover NS-associated molecules, pathways and networks. Integrating multidimensional datasets from the outset highlights how molecular lesions impact the entire individual. Data sets integrated range from mutation to gene expression, to histologic changes, to progression of chronic kidney disease (CKD). This review will introduce the tenets of integrative genomics and suggest how it can increase our understanding of NS from molecular and pathophysiological perspectives. A diverse group of genome-scale experiments are presented that have sought to define molecular signatures of NS. Finally, the Nephrotic Syndrome Study Network (NEPTUNE) will be introduced as an international, prospective cohort study of patients with NS that utilizes an integrated systems genomics approach from the outset. A major NEPTUNE goal is to achieve comprehensive disease definition from a genomics perspective and identify shared molecular drivers of disease.
Keywords: Transcriptome, Genome, Epigenome, Proteome, Nephrotic Syndrome, NEPTUNE
Learning objectives
The goal of this Educational Review is to describe the means by which integrative, systems genomics can aid in attaining a better molecular understanding of nephrotic syndrome (NS). We will first discuss the current challenges in classifying NS and introduce the general approach of integrative genomics. We will then review studies that have already used targeted and genome-wide molecular datasets to increase our understanding of NS. We will closely examine the recent investigation of APOL1 to illustrate how diverse datasets integrated across multiple research domains can uniquely contribute to our holistic understanding of a molecular target. We will conclude by introducing a current implementation of this approach, the Nephrotic Syndrome Study Network (NEPTUNE). We will describe how its design can uniquely aid in advancing our goal of improving our molecular classification of NS.
The challenge of classifying nephrotic syndrome
The nephrotic syndrome (NS) is a clinical constellation of edema, proteinuria, hypoalbuminemia and hyperlipidemia. The central pathology underlying this clinical syndrome is increased permeability of the glomerular filtration barrier to protein. Descriptive studies across multiple domains have helped characterize NS in children [1]. As classified by light microscopy, the predominant forms of primary NS in children are minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS) [2]. The major clinical classifications are steroid-sensitive (SSNS) versus steroid-resistant (SRNS). With these classifications, we base our current diagnostic, prognostic and therapeutic decisions [3]. Yet, we recognize that there is significant heterogeneity concealed within these broad classifications [4].
For example, there are subtypes within each of these clinical and histological groups (eg steroid-dependent NS, collapsing FSGS) as well as combinatorial classifications across groups (eg SRNS with MCD) [5]. The small percentage of identified contributory factors to primary NS are heterogeneous. They include rare mutations in podocyte genes [6], immunologic perturbations as indirectly supported by a number of clinical observations [7], or autoimmune irregularities [8] (in the case of membranous glomerulonephritis). Additional heterogeneity lies in the natural history of NS after onset [4]. Challenged by this complexity, clinicians and scientists have sought to classify NS in a way that could guide optimal clinical management.
Soon after a child’s initial presentation with NS, their pediatric nephrologist would ideally have the ability to explain why the NS occurred, predict the important clinical outcomes, and execute a rational treatment strategy customized for their patient’s underlying cause of disease. To achieve this, we clearly need to increase our mechanistic understanding of NS. Arguably the most promising efforts in this arena thus far have been in developing a molecular disease definition, ie taxonomy, of NS.
An integrative, systems genomics approach to NS
By more precisely defining the molecular mechanism of disease, we can more accurately diagnose disease, confer prognoses, and highlight abnormally functioning pathways and networks. Identifying disease-associated molecules also provides an opportunity to repurpose an existing medication or designing a novel therapeutic to target a specific molecular lesion. In oncology, molecular definition of various cancers has led to improved clinical care [9–12].
In NS, classification schema based on molecular profiling is also ongoing. Some of the most high profile of these subtypes thus far are monogenic forms of SRNS/FSGS [13], and FSGS associated with apolipoprotein L1 (APOL1) risk haplotypes in those of African ancestry [14]. Molecular profiles may serve to identify subpopulations of NS that share common pathways of activation or repression. Therapies targeted based on these profiles may improve our ability to rationally treat these conditions. These initial discoveries motivate us all to continue to discover novel NS-associated molecules. We can move beyond only studying single molecules at a time because of improvements in genomics technology and precipitous decreases in its cost. We can generate high quality, genome-wide genetic, epigenetic, transcriptomic and proteomic datasets from cohorts of patients with NS. This has opened up entirely new avenues of inquiry.
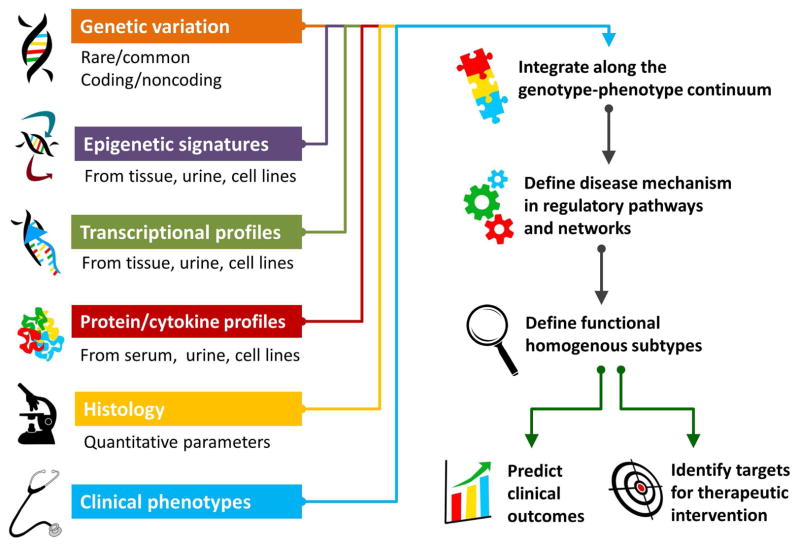
The process of applying bioinformatics and computational methods to large amounts of existing experimental data across multiple dimensions is a hallmark of an integrative, systems genomics approach to biomedical research [15,16] (Figure 1). This strategy attempts to contextualize molecular defects within the overall health of an individual. For patients with NS, the goal is to establish associations: 1) within their molecular datasets, and 2) between this molecular information and their comprehensive histological, biochemical, and clinical phenotypes [17–19]. An integrative genomics approach also seeks to define the molecular intermediary drivers responsible for disease onset or progression.
Figure 1. Schema for Integrative Genomics of NS.
To define the biologic mechanisms of NS, targeted and genome-scale molecular data is analyzed with histologic and clinical phenotypic data. NS is unique in that genomic data derived from kidney tissue and urine allows the study of cells and fluids directly impacted by this syndrome. A mechanistic understanding of NS allows us to identify functionally homogenous subtypes of NS, more accurately predict outcomes, and illuminate targets for precision intervention.
Analytic challenges of genome-scale data in glomerular disease
It is fair to say that the major challenge in this field has now shifted from creating genomics data to effectively analyzing it. The vast size of genome-wide data coming off so called “next generation” sequencing machines necessitates significant computational resources to process and store the data, and experts to do this work [20]. When cataloguing genomics variants, analytic pipelines must take into account variability resulting from technological factors such as different machines being used for sequencing, batch effects, or quality of the input material [21]. Variability due to biological factors unrelated to NS, such as ancestry [22], gender [23] or age [24], must also be considered. Medications can also impact levels of proteins, transcripts or metabolites [25,26]. Finally, once a robust call set is created and is ready for association testing, the large number of hypotheses being tested must be addressed through stringent p-value thresholds or false discovery rate cutoffs [27,28].
In association testing for patients with NS, it is also challenging to distinguish molecular signatures of the cause of the glomerular disease versus reactive signatures of this condition. Studying molecular data of these individuals longitudinally can help with this distinction. In addition, comparing samples between different forms of NS, or from healthy controls, can delineate proximal causes of disease versus common markers of kidney damage.
Fortunately, statistical geneticists, bioinformaticians and computational biologists continue to develop quality control metrics and statistical methodologies to address these challenges [29,30]. This is being done in the context of disease-specific and population-based cohorts. Yet, the complexity of these statistical approaches and technologies necessitates close collaboration with experts in these areas when conducting NS-specific genomics research.
We will now turn to a number of studies that are using genome-wide or targeted molecular datasets to gain a more precise molecular understanding of NS. A common theme underlying these diverse studies is their direct study of patient-derived samples. Some of these studies are defining the molecular landscape of the kidney in both health and disease. Others are identifying molecular signatures associated with particular clinical outcomes and attempting to unravel their underlying mechanisms of disease. As with all research methods, genome-scale research has its strengths and its limitations and we will discuss these.
Genetics
Historically, genetic discovery research of NS has studied families with SRNS and SSNS in order to discover rare mutations in single genes that cause this disease [13]. Rare, single nucleotide variants (SNV) (“mutations”) that, when present, cause SRNS have been found in more than 20 genes [6]. While greatly impactful for those affected, known monogenic forms of NS represent the minority of cases in NS. This is particularly true of those sporadically affected and with an older age of onset [31,32]. The spectrum of monogenic SRNS, and the various biological insights and clinical correlates associated with this NS subtype are comprehensively covered elsewhere [32,33]. Here, we will discuss studies that examine known SRNS genes in novel ways.
Next-generation sequencing (NGS) technologies allow investigators to economically sequence all the exons of sets of known monogenic SRNS genes in disease cohorts. This approach was recently done in subsets of patients from two European-based NS cohorts, the United Kingdom SRNS in Childhood Study [34] and PodoNet [31]. In the UK study, NGS was recently used to sequence, in parallel, 24 known SRNS-causing genes in a cohort of 36 sporadically affected children with congenital NS and SRNS. Bioinformatics-based functional prediction revealed that 70% of children with familial disease and 15% of sporadic cases had a definitely or probably pathogenic variant identified. PodoNet reported sequencing results of WT1, TRPC6, ACTN4, INF2 and NPHS2 in 227 adolescents (age 10–19) with sporadic or familial SRNS. They reported that 43% of those with familial disease had a causal mutation in one of these genes while only 10% of those with sporadic disease did. Ongoing phenotypic analysis of these patients will help to define the clinical impact for those who have monogenic SRNS. This could help guide decision-making, especially as it relates to choice of pharmacotherapy.
Groups have also studied whether there are also more common variants within known SRNS genes that can increase the risk of NS in a population. This has long been recognized with the R229Q variant of NPHS2 [35]. This polymorphism is present in ~3% of the European-American population, but can contribute to monogenic SRNS when present in compound heterozygosity with a rare, deleterious mutation in NPHS2 [22]. As such, the search for more common risk alleles has been undertaken for other known NS genes.
Prenyl (decaprenyl) diphosphate synthase, subunit 2 (PDDS2) is a known monogenic NS gene whose protein is involved in coenzyme Q10 biosynthesis [36]. A recent targeted association study genotyped 9 SNPs in PDDS2 in 377 patients with FSGS or collapsing glomerulopathy and 900 controls [37]. In European-Americans with FSGS, an individual with two copies of a common 6-SNP haplotype (“H2”) had a statistically significant 5.6x increased risk of FSGS. A recent study investigated African-Americans with non-diabetic end-stage renal disease (ESRD) and APOL1 risk alleles. They sought to discover common variants that statistically interacted with the APOL1 risk alleles to modulate risk for poor kidney outcomes [38]. They identified a common SNP in NPHS2 in African-American (minor allele frequency=15% in population) that interacted with APOL1. When this common SNP is inherited with two copies of the APOL1 risk alleles, individuals have a 50% decreased risk of ESRD and a decreased risk of proteinuria.
Finally, genome-wide association studies (GWAS) of cohorts of unrelated, affected subjects with NS can discover disease-associated loci. This approach can complement rare variant discovery. GWAS has effectively discovered novel risk loci associated with IgA nephropathy, membranous nephropathy and proteinuric kidney disease [39–41]. This has helped illuminate the biology of both of these conditions. GWAS of diabetic nephropathy due to Type 1 and Type 2 diabetes mellitus have occurred in multiple ethnicities, taking advantage of the large sample sizes due to the commonality of this disease [42]. The role of APOL1 variants in African-Americans with FSGS was discovered by secondary analysis of an admixture mapping linkage-disequilibrium genome scan [43,44]. With sufficient sample size or homogeneity of outcome tested, GWAS of additional forms of NS or in pediatric populations may identify meaningful risk loci.
However, many biologically relevant pathways or molecules that could be potential biomarkers or therapeutic targets may not be detectable by studies that compare genetic variation to clinical phenotype. Studies of molecular intermediates and their association with clinical outcomes, independent of genetic variation, are also needed to understand the mechanisms of NS. Below, we will discuss NS-relevant studies of some of these molecular intermediates. We can consider how to relate these NS-associated intermediates to underlying genetic variation. Similar approaches have contributed to our integrated, systems genomics approach to NS [18,19,45,46].
Transcriptomics
Transcriptomics, also referred to as gene-expression profiling, refers to the study of the sets of RNA transcripts produced in an organism [47]. This can be studied at an organ- (kidney), structure- (glomerulus), or cell- (podocyte) specific manner, and at various developmental and disease states. Efforts have been directed towards genome-wide transcriptional profiling of the glomerulus or podocyte in healthy and disease states.
Cataloguing the cell and tissue-specific transcriptomes
Genome-wide expression profiling can define the constituents of a specific transcriptome. Pertinent to NS, transcriptional profiles have been generated for human glomeruli and tubulointerstitium [48,49], transformed human podocyte [50], mesangial [51], and endothelial [52] cell lines, as well as the podocytes of developing and adult mice [53,54]. With this information, abundance or rarity of particular transcripts can be quantified. These profiles can also be compared to expression levels in non-kidney tissues [48,55,56].
One novel downstream study used the expression characteristics of known podocyte-specific proteins to predict cell-lineage specificity of other uncharacterized genes [57]. This in silico prediction tool was able to predict podocyte-enriched transcripts. Together, this information can provide cell- and tissue-specific targets for further investigation [58].
Classifying glomerular disease though transcriptional profiling
Gene expression profiling can also catalogue transcripts that are differentially expressed in among different histologic subtypes of NS. For example, one study generated expression profiles from the glomeruli of 21 patients with biopsy-proven MCD, classic FSGS and collapsing FSGS. They discovered 316 differentially expressed genes that were able to separate the two forms of FSGS from MCD and normal biopsy tissue [59]. Reich et al exposed primary human kidney tubular epithelial cells to albumin and discovered 231 genes differentially expressed after exposure [60]. By measuring kidney biopsy-based expression levels of only an 11-transcript subset of these genes, they were able to discriminate cases of IgAN from controls.
Correlations between transcriptional profile and other outcome measures
Associations can also be discovered between gene expression and other clinical phenotypes such as proteinuria [60], estimated GFR decline over time [48], cross-sectional eGFR [61] and steroid responsiveness [62]. For example, an expression profile of 30 genes was derived from an animal model of chronic kidney disease (CKD). In human tubulointerstitial tissue, this profile correlated with eGFR with an r2=0.53 (p<0.001). It could also classify individuals as Stage I/II CKD vs Stage III/IV/V with a positive predictive value of 83% [61].
This approach does not directly translate to a clinical test. However, further work can try to understand why these 30 genes correlate with eGFR. This could illuminate novel biology that is modifiable. In addition, studies could be done to see if a gene expression profile such as this does a better job in predicting endpoints as compared to serum creatinine measurements.
Eight transcripts known to be relevant to podocyte function were measured in glomerular biopsy tissue from 81 patients with glomerular disease. Measuring the ratio of two of these transcripts, podocin and synaptopodin, could distinguish FSGS from MCD [62]. Wiggins and colleagues explored this approach further by profiling podocin, nephrin and other podocyte mRNAs in urinary cells in patients from the nephrology clinic [63,64]. They demonstrated a strong correlation of urinary mRNA levels with eGFR, progression of disease, and response to therapy. This relationship was specific to glomerular disease and was not seen in polycystic kidney disease subjects.
In addition to their use as biomarkers, disease-associated transcripts can also illuminate novel biology for downstream bioinformatic or functional analysis. In the study that found 316 genes that were differentially expressed in FSGS vs MCD, gene ontology analysis was utilized to determine biological pathways specific to FSGS [59]. In FSGS, over-represented functions were related to “development,” “differentiation and morphogenesis,” “cell motility and migration,” “cytoskeletal organization,” and “signal transduction.” These processes support our current understanding of the pathogenesis of FSGS and reveal novel pathways for additional exploration.
As mentioned above, a set of 231 genes were differentially expressed in renal tubular cells in response to proteinuria, and a subset of 11 of these genes was found to distinguish proteinuric glomerulopathies from control biopsies [60]. From this 11-gene signature, a transcriptional network was constructed using natural language processing tools. This identified early growth response protein 1 (EGR1) as the central node that linked to the other 10 transcripts. This led to examination of the promoter regions of these 11 genes and identified a common EGR1 promoter region upstream of most of the 10 genes. This suggests that these genes may be co-regulated in tubular cells in response to albumin.
Proteomics
In addition to examining DNA variation and gene expression, investigators have also performed large scale or candidate-based proteomics studies of patients with NS. We will focus here on urinary proteomic studies. Most urine proteomic research has focused on free protein existing in the urine. Biomarkers for acute kidney allograft rejection [65] and diabetic nephropathy [66] have been found. Various methods can be used to remove non-specific proteins, or to enrich for proteins of interest, such as glycoproteins. Urine also contains exosomes (or exosome-like vesicles). These are small, membrane-bound vesicles derived from all epithelial cell types facing the urinary lumen, from podocyte to bladder [67,68]. Evaluating urinary proteins is a noninvasive way to assess the state of the glomeruli.
Urinary protein profiles from small cohorts can differentiate between nephrotic and control urine, as well as between the urine of those with SSNS versus SRNS [69,70]. Studies in small numbers of patients have also been employed in the hopes of discovering proteins specific for a whole range of glomerulopathies. This includes IgA nephropathy (IgAN), membranous nephropathy (MN), FSGS, systemic lupus erythematous (SLE), membranoproliferative glomerulonephritis (MPGN) and anti-glomerular basement membrane glomerulonephritis [71]. However, none of these studies have been cross-validated.
Capillary electrophoresis coupled to mass spectrometry (CE-MS) was used in an attempt to classify the urine of subjects as either being healthy or having CKD [72]. More than 1,200 molecular weight peptides were isolated and analyzed from the urine of 379 controls and 230 patients with CKD of diverse causes. After various filtering steps, 273 peptides were used in a classification model to distinguish controls from those with kidney disease. Replication in an independent cohort with controls and CKD revealed a sensitivity of 86% and specificity of 100%.
Glycoprotein enrichment was used to identify glycosylated urinary proteins that differed in 6 patients with CKD versus 6 controls. Using liquid chromatography electrospray ionization and tandem mass spectrometry analysis, 23 glycoproteins were found to be differentially expressed in CKD [73]. Using standard gene ontology analyses, the top three biologic processes enriched were “immune-stress response,” “acute-phase and inflammatory response,” and “regulation of hemostasis, platelet degranulation and coagulation.”
Major efforts are being directed towards defining the full complement of peptides represented in urinary exosomes and identifying those that can act as biomarkers. One human study of urinary exosomes reported 1160 distinct proteins present [68]. Many were specific to renal tubular cells and podocytes. A recent study isolated the specific subfraction of exosomes that were glomerular membrane vesicles (GMV). They then analyzed the GMV proteome in seven healthy individuals [74]. Using liquid chromatography and tandem MS, 1830 proteins were identified. This included known monogenic NS proteins, those known to be involved in glomerular biology, and other novel peptides. In three individuals with glomerular disease, they identified 5657 proteins. This included a subset of proteins of known importance (NPHS1, TRCP6, PLA2R1), which were not present in GMV of control individuals.
In a targeted manner, investigators measured WT1 levels from urinary exosomes isolated from 25 children with FSGS and SSNS to determine if this could be associated with disease classification or treatment responsiveness [75]. They found significantly elevated WT1 levels in patients with FSGS as compared to both those with SSNS and healthy volunteers. Levels were also significantly decreased in children in remission versus those with active disease.
Future studies examining both candidate exosomal proteins and the whole exosomal proteome in relation to glomerular disease will shed further light on its utility in molecular profiling. The Human Kidney and Urine Proteome Project (HKUPP) as part of the Human Proteome Project has set out to facilitate studies of the human renal proteome in health and disease, see http://www.hkupp.org/ for details.
Epigenomics
The epigenome consists of heritable changes across the chromosome that result in a stably inherited phenotype without changes in DNA sequence [76]. These changes differ across cell types and in different states of health and disease. Novel methods are permitting investigators to map epigenetic processes in both a genome-wide and targeted manner [76,77] [78]. We will briefly focus on a few recent, kidney-specific epigenomic studies.
The role of micro-RNAs have been studied extensively in relation to glomerular disease. Multiple studies in animal models have shown that perturbations in miRNA homeostasis can result in glomerular disease, including sclerosis, hypertrophy, collapse of glomeruli, and foot process effacement [79–82]. A recent study that profiled 754 serum and urine miRNA in 159 children with NS reported that a six miRNA profile distinguished cases from controls and active disease from remission status [83]. A recent experimental study in mice showed that over expression of miR-193A results in FSGS [84]. miR-193A levels were then measured from the glomeruli of 90 controls and subjects with glomerular disease. Those with FSGS had statistically significant increased levels of miR-193A as compared to healthy controls and those with non-FSGS glomerulopathy.
Another exciting area of study is defining glomerular- or tubulointerstitial-specific promoters, enhancers, repressors and transcription factor binding sites. Identifying these functional, non-coding regions of the genome is a first step to understanding how these epigenetic regulatory regions differ in health and disease-states. Early studies in this area performed genome-wide cytosine methylation studies of kidney tubular tissue of healthy individuals and those with CKD [85,86]. One of these studies reported that enhancer regions and transcription factor binding sites are differentially methylated in health and CKD. Both reported gene sets that were significantly differentially methylated in CKD versus controls. Future methylation studies of glomerular DNA and genome-wide studies of other epigenetic marks using compartment-specific kidney tissue will further reveal the importance of these processes in NS.
Genomics and histopathology
A histologic classification of NS that integrates molecular information will yield a mechanistic-oriented disease classification. This could result in more accurate diagnostic classification and improved prediction of treatment response [49]. Molecular profiling to complement and improve existing histologic classification schemes has already begun.
Interstitial fibrosis quantification is predictive of outcome in both renal transplantation and native disease [87,88]. Maluf et al [89] observed a distinctive gene expression pattern in kidney allografts with tubular atrophy and interstitial fibrosis versus normal tissue. These genes were related to immune response, inflammation and matrix deposition. Henger et al [90] demonstrated a gene expression fingerprint separating fibrotic from inflammatory damage in hydronephrotic kidneys. This revealed a stringent correlation with disease progression.
Well-established classification schemes for biopsy analysis of native glomerular diseases [88,91,92] and renal transplant [93] exist and are ideal for integration with molecular profiling. Transcriptomic profiling of glomeruli from patients with NS revealed an over-representation of differentially expressed genes associated with developmental processes in classic and collapsing FSGS versus normal controls and MCD [59]. This indicates the importance of reactivation of developmental programs in the pathogenesis of FSGS. Thus a gene expression-based categorization of disease will yield a more precise evaluation of the pathogenesis behind the visible histopathologic alterations.
The APOL1 investigation as an example of integrative genomics
Examining current research efforts around APOL1 illustrates how many of the approaches described above can be combined to help elucidate the role of a previously unknown molecule in glomerular disease (Figure 2).
Figure 2. Studying APOL1 using integrative genomics.
We detail a series of integrative genomics analyses that could aid both in functionally defining APOL1’s mechanism of disease association and in measuring its impact on clinical outcomes. This “bottoms-up” analytic approach can be applied to any candidate molecule.
In 2010, secondary analysis of a cohort of unrelated, sporadically affected African-Americans with FSGS identified two common, non-synonymous variants within APOL1 that were associated with greatly increased risk of disease [43]. Of note, for a number of compelling reasons, the initial GWAS of this cohort identified MYH9 as the causal gene within this locus on chromosome 22 [44]. Subsequent studies confirmed that it was APOL1 variants that drove this association, and that these variants were specific to those in African ancestry. Over the ensuing 4 years, APOL1 has been studied from multiple perspectives. The clinical impact of these variants has been studied on a patient and population level. Work is ongoing to gain a deep understanding of the biologic mechanisms underlying its pathogenicity in NS.
From a clinical epidemiology perspective, this association was subsequently replicated in other populations of African-Americans with FSGS, with even stronger associations seen in HIV-associated nephropathy (HIVAN) [94–96]. Early studies in the transplant population indicate that the increased risk of FSGS follows the kidney from the APOL1 risk allele positive donor rather than being driven by the risk allele status of the recipient [97,98].
Plasma levels of APOL1 are not found to correlate with CKD [99]. This further suggests an intra-renal effect of APOL1. Finally, a current study analyzed African-Americans with CKD from two existing studies and demonstrated that APOL1 risk variants were associated with worse renal outcomes, independent of the underlying cause of CKD [100].
In parallel are attempts to experimentally dissect the biological mechanisms underlying the association of APOL1 with NS. APOL1 has been localized only to the podocyte in the glomerulus, the proximal tubule epithelium, and the arteriolar endothelium [101]. It has similar distributions in health and disease independent of the known risk variants. A question remains as to what extent APOL1 is transcribed in the kidney versus being taken up from glomerular or arteriolar filtration. The fact that the APOL1 gene is unique to humans and a few non-human primates has hindered the community’s ability to use animal models for functional characterization. However, efforts to develop suitable experimental systems are ongoing.
Thus, harnessing existing and novel data from diverse sources has permitted us to rapidly gain knowledge of the impact of APOL1 on NS and to experimentally study the molecular function of APOL1 in the kidney. This strategy can be expanded for APOL1 as well as generalized moving forward for other candidate molecules. Embedding these methods within a longitudinal study of individuals with NS can enhance the clinical relevance of an integrated genomics approach to NS.
The Nephrotic Syndrome Study Network (NEPTUNE)
In this review, we have highlighted targeted and genome-wide studies that are defining molecular subtypes of NS that share particular biological mechanisms or clinical behavior. One caveat to most of the findings described thus far is their relatively small sample size, lack of replication in independent cohorts, and cross-sectional nature of the findings. This is due, in part, to the early stage of many of these technologies and the relative rarity of these conditions. In addition, the prospective collection of well-phenotyped patients with paired biosamples is financially and logistically challenging. The establishment of the Nephrotic Syndrome Study Network (NEPTUNE) can aid in addressing these challenges through integrative molecular profiling in a large, prospective cohort [102].
NEPTUNE is one of 17 Rare Disease Clinical Research Networks funded by the NIH Office of Rare Disease Research. Its major objective is to establish a collaborative, integrated, cost-effective investigational infrastructure to conduct prospective clinical and translational research in incident patients with suspected FSGS, MCD and MN. Each subject recruited undergoes a renal biopsy as part of their clinical evaluation for fixed proteinuria and has an extra core collected for research.
A core concept of NEPTUNE is that the clinical heterogeneity observed in primary NS is a result of it being a molecularly heterogeneous syndrome currently grouped together based on common histopathologic findings. NEPTUNE hypothesizes that discovering and studying NS-associated molecular markers will allow us to move away from solely histopathologic based diagnoses. By molecularly defining this disease, improved care can be achieved through increased precision.
Currently made up of 21 clinical centers in the United States and Canada, NEPTUNE has enrolled 528 patients as of Jan 1, 2014. From each enrolled patient, blood, renal biopsy tissue and urine, along with highly detailed demographic and clinical data, is being collected. Patients are followed over a minimum of five years. The primary outcomes are change in urinary protein excretion and change in renal function. Clinical investigators utilize traditional epidemiologic approaches to better understand NS. Randomized control trials can leverage the clinical research infrastructure of NEPTUNE.
The collection of a research renal biopsy core is one of the more unique features of NEPTUNE [103]. This allows state-of-the-art digital histopathology, tissue- and disease-specific molecular profiling, and immunohistochemistry to be performed. This is then integrated with clinical and other genomics data from the same individual. Gene expression data is generated in a tissue compartment-specific manner (Affymetrix and RNA-Sequencing (RNA-Seq)). RNA-Seq uses NGS technology and allows detection of common and rare transcripts on an isoform-specific level [47].
An integrated systems biology approach is applied to genomics and clinical data to identify biomarkers that: 1) can group patients into specific subtypes, and 2) correlate with the primary outcomes. Currently, exome chip genotyping, whole genome sequencing at 4x depth, and deep exonic sequencing of 20 known monogenic FSGS genes and ~2500 expressed in the podocyte are being performed on the enrolled subjects. Expression quantitative trait loci studies using biopsy tissue allows identification of gene expression changes associated with genetic differences in subjects with NS. In addition, direct association studies between genomic variants and clinical outcomes are studied.
Transcriptomic, proteomic and metabolomic data will also be collected from blood and urine samples. Urinary transcriptomic data will allow investigators to determine whether these non-invasive measures of gene expression are as effective in predicting outcome as intra-renal gene expression. Proteomic and metabolomics data will enable investigators to define correlations between gene variation, gene expression, protein expression and clinical parameters.
The creation of NEPTUNE is, in part, an actualization of the many large-scale molecular investigations that have been described above. NEPTUNE’s goal is to establish these data and sample sets to serve as a resource to the NS research community. Data and samples are accessed by a broad spectrum of ancillary studies. See www.neptune-study.org for details on access.
Conclusion
To improve the health of these patients living with NS, we are challenged to discover and characterize novel molecules and pathways critical in its pathogenesis and natural history. Integrative genomics is poised to fit in well with established investigative approaches (eg molecular biology, mouse genetics, clinical epidemiology) to achieve this. In the future, we may be able to use these molecular signatures in classification schema for our patients. They may be based on genetic variation, up- or down-regulation of specific signaling pathways or networks, or epigenetic changes. Given that these classifications would be based on a greater biologic understanding of disease, defining patients in this way could improve specificity and precision of our diagnoses and treatment plans. The infrastructure of NEPTUNE and its large group of investigators of diverse expertise forms a starting point to pursue this particular strategy.
Acknowledgments
Expert graphical by Dr. Joseph Laycock is highly appreciated.
MS is supported by 1K08-DK100662-01 and the U54DK083912 Nephrotic Syndrome Study Network Consortium
JBH is supported by 1K08-DK088944-04 and the NephCure-ASN Foundation for Kidney Research
MK is supported by U54DK083912 Nephrotic Syndrome Study Network Consortium and P30 DK081943 George M. O’Brien Kidney Research Core Center at the University of Michigan
The Nephrotic Syndrome Study Network Consortium (NEPTUNE; U54-DK-083912) is a part of the National Institutes of Health (NIH) Rare Disease Clinical Research Network (RDCRN), supported through a collaboration between the Office of Rare Diseases
Research (ORDR), NCATS, and the National Institute of Diabetes and Digestive and Kidney Diseases
References
- 1.The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr. 1981;98:561–564. doi: 10.1016/s0022-3476(81)80760-3. [DOI] [PubMed] [Google Scholar]
- 2.Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet. 2003;362:629–639. doi: 10.1016/S0140-6736(03)14184-0. [DOI] [PubMed] [Google Scholar]
- 3.Gipson DS, Massengill SF, Yao L, Nagaraj S, Smoyer WE, Mahan JD, Wigfall D, Miles P, Powell L, Lin JJ, Trachtman H, Greenbaum LA. Management of childhood onset nephrotic syndrome. Pediatrics. 2009;124:747–757. doi: 10.1542/peds.2008-1559. [DOI] [PubMed] [Google Scholar]
- 4.Greenbaum LA, Benndorf R, Smoyer WE. Childhood nephrotic syndrome--current and future therapies. Nat Rev Nephrol. 2012;8:445–458. doi: 10.1038/nrneph.2012.115. [DOI] [PubMed] [Google Scholar]
- 5.Kiffel J, Rahimzada Y, Trachtman H. Focal segmental glomerulosclerosis and chronic kidney disease in pediatric patients. Adv Chronic Kidney Dis. 2011;18:332–338. doi: 10.1053/j.ackd.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hildebrandt F. Genetic kidney diseases. Lancet. 2010;375:1287–1295. doi: 10.1016/S0140-6736(10)60236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarthy ET, Sharma M, Savin VJ. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2010;5:2115–2121. doi: 10.2215/CJN.03800609. [DOI] [PubMed] [Google Scholar]
- 8.Ronco P, Debiec H. Pathogenesis of membranous nephropathy: recent advances and future challenges. Nat Rev Nephrol. 2012;8:203–213. doi: 10.1038/nrneph.2012.35. [DOI] [PubMed] [Google Scholar]
- 9.Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Varambally S, Kurachi K, Pienta KJ, Rubin MA, Chinnaiyan AM. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412:822–826. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- 10.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, Ashworth A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 11.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 12.Decock A, Ongenaert M, Hoebeeck J, De Preter K, Van Peer G, Van Criekinge W, Ladenstein R, Schulte JH, Noguera R, Stallings RL, Van Damme A, Laureys G, Vermeulen J, Van Maerken T, Speleman F, Vandesompele J. Genome-wide promoter methylation analysis in neuroblastoma identifies prognostic methylation biomarkers. Genome Biol. 2012;13:R95. doi: 10.1186/gb-2012-13-10-r95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benoit G, Machuca E, Antignac C. Hereditary nephrotic syndrome: a systematic approach for genetic testing and a review of associated podocyte gene mutations. Pediatr Nephrol. 2010;25:1621–1632. doi: 10.1007/s00467-010-1495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollak MR, Genovese G, Friedman DJ. APOL1 and kidney disease. Current opinion in nephrology and hypertension. 2012;21:179–182. doi: 10.1097/MNH.0b013e32835012ab. [DOI] [PubMed] [Google Scholar]
- 15.Greene CS, Troyanskaya OG. Integrative systems biology for data-driven knowledge discovery. Semin Nephrol. 2010;30:443–454. doi: 10.1016/j.semnephrol.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawkins RD, Hon GC, Ren B. Next-generation genomics: an integrative approach. Nat Rev Genet. 2010;11:476–486. doi: 10.1038/nrg2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin Y, Ratnam K, Chuang PY, Fan Y, Zhong Y, Dai Y, Mazloom AR, Chen EY, D’Agati V, Xiong H, Ross MJ, Chen N, Ma’ayan A, He JC. A systems approach identifies HIPK2 as a key regulator of kidney fibrosis. Nat Med. 2012;18:580–588. doi: 10.1038/nm.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martini S, Nair V, Patel SR, Eichinger F, Nelson RG, Weil EJ, Pezzolesi MG, Krolewski AS, Randolph A, Keller BJ, Werner T, Kretzler M. From single nucleotide polymorphism to transcriptional mechanism: a model for FRMD3 in diabetic nephropathy. Diabetes. 2013;62:2605–2612. doi: 10.2337/db12-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keller BJ, Martini S, Sedor JR, Kretzler M. A systems view of genetics in chronic kidney disease. Kidney Int. 2012;81:14–21. doi: 10.1038/ki.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mardis ER. The $1,000 genome, the $100,000 analysis? Genome Med. 2010;2:84. doi: 10.1186/gm205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28:882–883. doi: 10.1093/bioinformatics/bts034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tory K, Menyhard DK, Woerner S, Nevo F, Gribouval O, Kerti A, Stráner P, Arrondel C, Cong EH, Tulassay T, Mollet G, Perczel A, Antignac C. Mutation-dependent recessive inheritance of NPHS2-associated steroid-resistant nephrotic syndrome. Nat Genet. 2014;46:299–304. doi: 10.1038/ng.2898. [DOI] [PubMed] [Google Scholar]
- 23.Kurina LM, Weiss LA, Graves SW, Parry R, Williams GH, Abney M, Ober C. Sex differences in the genetic basis of morning serum cortisol levels: genome-wide screen identifies two novel loci specific to women. J Clin Endocrinol Metab. 2005;90:4747–4752. doi: 10.1210/jc.2005-0384. [DOI] [PubMed] [Google Scholar]
- 24.Wheeler HE, Metter EJ, Tanaka T, Absher D, Higgins J, Zahn JM, Wilhelmy J, Davis RW, Singleton A, Myers RM, Ferrucci L, Kim SK. Sequential use of transcriptional profiling, expression quantitative trait mapping, and gene association implicates MMP20 in human kidney aging. PLoS Genet. 2009;5:e1000685. doi: 10.1371/journal.pgen.1000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kainz A, Wilflingseder J, Mitterbauer C, Haller M, Burghuber C, Perco P, Langer RM, Heinze G, Oberbauer R. Steroid pretreatment of organ donors to prevent postischemic renal allograft failure: a randomized, controlled trial. Ann Intern Med. 2010;153:222–230. doi: 10.7326/0003-4819-153-4-201008170-00003. [DOI] [PubMed] [Google Scholar]
- 26.Liang Y, Kelemen A. Bayesian models and meta analysis for multiple tissue gene expression data following corticosteroid administration. BMC Bioinformatics. 2008;9:354. doi: 10.1186/1471-2105-9-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantor RM, Lange K, Sinsheimer JS. Prioritizing GWAS results: A review of statistical methods and recommendations for their application. Am J Hum Genet. 2010;86:6–22. doi: 10.1016/j.ajhg.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mechanic LE, Chen HS, Amos CI, Chatterjee N, Cox NJ, Divi RL, Fan R, Harris EL, Jacobs K, Kraft P, Leal SM, McAllister K, Moore JH, Paltoo DN, Province MA, Ramos EM, Ritchie MD, Roeder K, Schaid DJ, Stephens M, Thomas DC, Weinberg CR, Witte JS, Zhang S, Zöllner S, Feuer EJ, Gillanders EM. Next generation analytic tools for large scale genetic epidemiology studies of complex diseases. Genet Epidemiol. 2012;36:22–35. doi: 10.1002/gepi.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Awwad HM, Kirsch SH, Geisel J, Obeid R. Measurement of concentrations of whole blood levels of choline, betaine, and dimethylglycine and their relations to plasma levels. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;957:41–45. doi: 10.1016/j.jchromb.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 31.Lipska BS, Iatropoulos P, Maranta R, Caridi G, Ozaltin F, Anarat A, Balat A, Gellermann J, Trautmann A, Erdogan O, Saeed B, Emre S, Bogdanovic R, Azocar M, Balasz-Chmielewska I, Benetti E, Caliskan S, Mir S, Melk A, Ertan P, Baskin E, Jardim H, Davitaia T, Wasilewska A, Drozdz D, Szczepanska M, Jankauskiene A, Higuita LM, Ardissino G, Ozkaya O, Kuzma-Mroczkowska E, Soylemezoglu O, Ranchin B, Medynska A, Tkaczyk M, Peco-Antic A, Akil I, Jarmolinski T, Firszt-Adamczyk A, Dusek J, Simonetti GD, Gok F, Gheissari A, Emma F, Krmar RT, Fischbach M, Printza N, Simkova E, Mele C, Ghiggeri GM, Schaefer F PodoNet Consortium. Genetic screening in adolescents with steroid-resistant nephrotic syndrome. Kidney Int. 2013;84:206–213. doi: 10.1038/ki.2013.93. [DOI] [PubMed] [Google Scholar]
- 32.Rood IM, Deegens JK, Wetzels JF. Genetic causes of focal segmental glomerulosclerosis: implications for clinical practice. Nephrol Dial Transplant. 2012;27:882–890. doi: 10.1093/ndt/gfr771. [DOI] [PubMed] [Google Scholar]
- 33.Saleem MA. New developments in steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2013;28:699–709. doi: 10.1007/s00467-012-2239-0. [DOI] [PubMed] [Google Scholar]
- 34.McCarthy HJ, Bierzynska A, Wherlock M, Ognjanovic M, Kerecuk L, Hegde S, Feather S, Gilbert RD, Krischock L, Jones C, Sinha MD, Webb NJ, Christian M, Williams MM, Marks S, Koziell A, Welsh GI, Saleem MA RADAR the UK SRNS Study Group. Simultaneous Sequencing of 24 Genes Associated with Steroid-Resistant Nephrotic Syndrome. Clin J Am Soc Nephrol. 2013;8:637–648. doi: 10.2215/CJN.07200712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Machuca E, Hummel A, Nevo F, Dantal J, Martinez F, Al-Sabban E, Baudouin V, Abel L, Grünfeld JP, Antignac C. Clinical and epidemiological assessment of steroid-resistant nephrotic syndrome associated with the NPHS2 R229Q variant. Kidney Int. 2009;75:727–735. doi: 10.1038/ki.2008.650. [DOI] [PubMed] [Google Scholar]
- 36.Peng M, Falk MJ, Haase VH, King R, Polyak E, Selak M, Yudkoff M, Hancock WW, Meade R, Saiki R, Lunceford AL, Clarke CF, Gasser DL. Primary coenzyme Q deficiency in Pdss2 mutant mice causes isolated renal disease. PLoS Genet. 2008;4:e1000061. doi: 10.1371/journal.pgen.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gasser DL, Winkler CA, Peng M, An P, McKenzie LM, Kirk GD, Shi Y, Xie LX, Marbois BN, Clarke CF, Kopp JB. Focal segmental glomerulosclerosis is associated with a PDSS2 haplotype and, independently, with a decreased content of coenzyme Q10. Am J Physiol Renal Physiol. 2013;305:F1228–1238. doi: 10.1152/ajprenal.00143.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Divers J, Palmer ND, Lu L, Langefeld CD, Rocco MV, Hicks PJ, Murea M, Ma L, Bowden DW, Freedman BI. Gene-gene interactions in APOL1-associated nephropathy. Nephrol Dial Transplant. 2013;29:587–594. doi: 10.1093/ndt/gft423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gharavi AG, Kiryluk K, Choi M, Li Y, Hou P, Xie J, Sanna-Cherchi S, Men CJ, Julian BA, Wyatt RJ, Novak J, He JC, Wang H, Lv J, Zhu L, Wang W, Wang Z, Yasuno K, Gunel M, Mane S, Umlauf S, Tikhonova I, Beerman I, Savoldi S, Magistroni R, Ghiggeri GM, Bodria M, Lugani F, Ravani P, Ponticelli C, Allegri L, Boscutti G, Frasca G, Amore A, Peruzzi L, Coppo R, Izzi C, Viola BF, Prati E, Salvadori M, Mignani R, Gesualdo L, Bertinetto F, Mesiano P, Amoroso A, Scolari F, Chen N, Zhang H, Lifton RP. Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet. 2011;43:321–327. doi: 10.1038/ng.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanescu HC, Arcos-Burgos M, Medlar A, Bockenhauer D, Kottgen A, Dragomirescu L, Voinescu C, Patel N, Pearce K, Hubank M, Stephens HA, Laundy V, Padmanabhan S, Zawadzka A, Hofstra JM, Coenen MJ, den Heijer M, Kiemeney LA, Bacq-Daian D, Stengel B, Powis SH, Brenchley P, Feehally J, Rees AJ, Debiec H, Wetzels JF, Ronco P, Mathieson PW, Kleta R. Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med. 2011;364:616–626. doi: 10.1056/NEJMoa1009742. [DOI] [PubMed] [Google Scholar]
- 41.Okamoto K, Tokunaga K, Doi K, Fujita T, Suzuki H, Katoh T, Watanabe T, Nishida N, Mabuchi A, Takahashi A, Kubo M, Maeda S, Nakamura Y, Noiri E. Common variation in GPC5 is associated with acquired nephrotic syndrome. Nat Genet. 2011;43:459–463. doi: 10.1038/ng.792. [DOI] [PubMed] [Google Scholar]
- 42.Gu HF, Brismar K. Genetic association studies in diabetic nephropathy. Curr Diabetes Rev. 2012;8:336–344. doi: 10.2174/157339912802083522. [DOI] [PubMed] [Google Scholar]
- 43.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kopp JB, Smith MW, Nelson GW, Johnson RC, Freedman BI, Bowden DW, Oleksyk T, McKenzie LM, Kajiyama H, Ahuja TS, Berns JS, Briggs W, Cho ME, Dart RA, Kimmel PL, Korbet SM, Michel DM, Mokrzycki MH, Schelling JR, Simon E, Trachtman H, Vlahov D, Winkler CA. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40:1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keller B, Martini S, Sedor J, Kretzler M. Linking variants from genome-wide association analysis to function via transcriptional network analysis. Semin Nephrol. 2010;30:177–184. doi: 10.1016/j.semnephrol.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He JC, Chuang PY, Ma’ayan A, Iyengar R. Systems biology of kidney diseases. Kidney Int. 2012;81:22–39. doi: 10.1038/ki.2011.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen CD, Frach K, Schlondorff D, Kretzler M. Quantitative gene expression analysis in renal biopsies: a novel protocol for a high-throughput multicenter application. Kidney Int. 2002;61:133–140. doi: 10.1046/j.1523-1755.2002.00113.x. [DOI] [PubMed] [Google Scholar]
- 49.Yasuda Y, Cohen CD, Henger A, Kretzler M. Gene expression profiling analysis in nephrology: towards molecular definition of renal disease. Clin Exp Nephrol. 2006;10:91–98. doi: 10.1007/s10157-006-0421-z. [DOI] [PubMed] [Google Scholar]
- 50.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- 51.Sarrab RM, Lennon R, Ni L, Wherlock MD, Welsh GI, Saleem MA. Establishment of conditionally immortalised human glomerular mesangial cells in culture, with unique migratory properties. Am J Physiol Renal Physiol. 2011;301:F1131–1138. doi: 10.1152/ajprenal.00589.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Satchell SC, Tasman CH, Singh A, Ni L, Geelen J, von Ruhland CJ, O’Hare MJ, Saleem MA, van den Heuvel LP, Mathieson PW. Conditionally immortalized human glomerular endothelial cells expressing fenestrations in response to VEGF. Kidney Int. 2006;69:1633–1640. doi: 10.1038/sj.ki.5000277. [DOI] [PubMed] [Google Scholar]
- 53.Boerries M, Grahammer F, Eiselein S, Buck M, Meyer C, Goedel M, Bechtel W, Zschiedrich S, Pfeifer D, Laloë D, Arrondel C, Gonçalves S, Krüger M, Harvey SJ, Busch H, Dengjel J, Huber TB. Molecular fingerprinting of the podocyte reveals novel gene and protein regulatory networks. Kidney Int. 2013;83:1052–1064. doi: 10.1038/ki.2012.487. [DOI] [PubMed] [Google Scholar]
- 54.Brunskill EW, Georgas K, Rumballe B, Little MH, Potter SS. Defining the molecular character of the developing and adult kidney podocyte. PloS One. 2011;6:e24640. doi: 10.1371/journal.pone.0024640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lindenmeyer MT, Eichinger F, Sen K, Anders HJ, Edenhofer I, Mattinzoli D, Kretzler M, Rastaldi MP, Cohen CD. Systematic analysis of a novel human renal glomerulus-enriched gene expression dataset. PloS One. 2010;5:e11545. doi: 10.1371/journal.pone.0011545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cuellar LM, Fujinaka H, Yamamoto K, Miyamoto M, Tasaki M, Zhao L, Tamer I, Yaoita E, Yoshida Y, Yamamoto T. Identification and localization of novel genes preferentially expressed in human kidney glomerulus. Nephrology. 2009;14:94–104. doi: 10.1111/j.1440-1797.2008.01009.x. [DOI] [PubMed] [Google Scholar]
- 57.Ju W, Greene CS, Eichinger F, Nair V, Hodgin JB, Bitzer M, Lee YS, Zhu Q, Kehata M, Li M, Jiang S, Rastaldi MP, Cohen CD, Troyanskaya OG, Kretzler M. Defining cell-type specificity at the transcriptional level in human disease. Genome Res. 2013;23:1862–1873. doi: 10.1101/gr.155697.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishibori Y, Katayama K, Parikka M, Oddsson A, Nukui M, Hultenby K, Wernerson A, He B, Ebarasi L, Raschperger E, Norlin J, Uhlén M, Patrakka J, Betsholtz C, Tryggvason K. Glcci1 deficiency leads to proteinuria. J Am Soc Nephrol. 2011;22:2037–2046. doi: 10.1681/ASN.2010111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hodgin JB, Borczuk AC, Nasr SH, Markowitz GS, Nair V, Martini S, Eichinger F, Vining C, Berthier CC, Kretzler M, D’Agati VD. A molecular profile of focal segmental glomerulosclerosis from formalin-fixed, paraffin-embedded tissue. Am J Pathol. 2010;177:1674–1686. doi: 10.2353/ajpath.2010.090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reich HN, Tritchler D, Cattran DC, Herzenberg AM, Eichinger F, Boucherot A, Henger A, Berthier CC, Nair V, Cohen CD, Scholey JW, Kretzler M. A molecular signature of proteinuria in glomerulonephritis. PloS One. 2010;5:e13451. doi: 10.1371/journal.pone.0013451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ju W, Eichinger F, Bitzer M, Oh J, McWeeney S, Berthier CC, Shedden K, Cohen CD, Henger A, Krick S, Kopp JB, Stoeckert CJ, Jr, Dikman S, Schröppel B, Thomas DB, Schlondorff D, Kretzler M, Böttinger EP. Renal gene and protein expression signatures for prediction of kidney disease progression. Am J Pathol. 2009;174:2073–2085. doi: 10.2353/ajpath.2009.080888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmid H, Henger A, Cohen CD, Frach K, Gröne HJ, Schlöndorff D, Kretzler M. Gene expression profiles of podocyte-associated molecules as diagnostic markers in acquired proteinuric diseases. J Am Soc Nephrol. 2003;14:2958–2966. doi: 10.1097/01.asn.0000090745.85482.06. [DOI] [PubMed] [Google Scholar]
- 63.Fukuda A, Wickman LT, Venkatareddy MP, Wang SQ, Chowdhury MA, Wiggins JE, Shedden KA, Wiggins RC. Urine podocin:nephrin mRNA ratio (PNR) as a podocyte stress biomarker. Nephrol Dial Transplant. 2012;27:4079–4087. doi: 10.1093/ndt/gfs313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wickman L, Afshinnia F, Wang SQ, Yang Y, Wang F, Chowdhury M, Graham D, Hawkins J, Nishizono R, Tanzer M, Wiggins J, Escobar GA, Rovin B, Song P, Gipson D, Kershaw D, Wiggins RC. Urine Podocyte mRNAs, Proteinuria, and Progression in Human Glomerular Diseases. J Am Soc Nephrol. 2013;24:2081–2095. doi: 10.1681/ASN.2013020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ling XB, Sigdel TK, Lau K, Ying L, Lau I, Schilling J, Sarwal MM. Integrative urinary peptidomics in renal transplantation identifies biomarkers for acute rejection. J Am Soc Nephrol. 2010;21:646–653. doi: 10.1681/ASN.2009080876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang H, Guan G, Zhang R, Liu G, Cheng J, Hou X, Cui Y. Identification of urinary soluble E-cadherin as a novel biomarker for diabetic nephropathy. Diabetes Metab Res Rev. 2009;25:232–241. doi: 10.1002/dmrr.940. [DOI] [PubMed] [Google Scholar]
- 67.Knepper MA, Pisitkun T. Exosomes in urine: who would have thought...? Kidney Int. 2007;72:1043–1045. doi: 10.1038/sj.ki.5002510. [DOI] [PubMed] [Google Scholar]
- 68.Gonzales PA, Pisitkun T, Hoffert JD, Tchapyjnikov D, Star RA, Kleta R, Wang NS, Knepper MA. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol. 2009;20:363–379. doi: 10.1681/ASN.2008040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woroniecki RP, Orlova TN, Mendelev N, Shatat IF, Hailpern SM, Kaskel FJ, Goligorsky MS, O’Riordan E. Urinary proteome of steroid-sensitive and steroid-resistant idiopathic nephrotic syndrome of childhood. Am J Nephrol. 2006;26:258–267. doi: 10.1159/000093814. [DOI] [PubMed] [Google Scholar]
- 70.Khurana M, Traum AZ, Aivado M, Wells MP, Guerrero M, Grall F, Libermann TA, Schachter AD. Urine proteomic profiling of pediatric nephrotic syndrome. Pediatr Nephrol. 2006;21:1257–1265. doi: 10.1007/s00467-006-0165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reinhardt CP, Germain MJ, Groman EV, Mulhern JG, Kumar R, Vaccaro DE. Functional immunoassay technology (FIT), a new approach for measuring physiological functions: application of FIT to measure glomerular filtration rate (GFR) Am J Physiol Renal Physiol. 2008;295:F1583–1588. doi: 10.1152/ajprenal.90354.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Good DM, Zurbig P, Argiles A, Bauer HW, Behrens G, Coon JJ, Dakna M, Decramer S, Delles C, Dominiczak AF, Ehrich JH, Eitner F, Fliser D, Frommberger M, Ganser A, Girolami MA, Golovko I, Gwinner W, Haubitz M, Herget-Rosenthal S, Jankowski J, Jahn H, Jerums G, Julian BA, Kellmann M, Kliem V, Kolch W, Krolewski AS, Luppi M, Massy Z, Melter M, Neusüss C, Novak J, Peter K, Rossing K, Rupprecht H, Schanstra JP, Schiffer E, Stolzenburg JU, Tarnow L, Theodorescu D, Thongboonkerd V, Vanholder R, Weissinger EM, Mischak H, Schmitt-Kopplin P. Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol Cell Proteomics. 2010;9:2424–2437. doi: 10.1074/mcp.M110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vivekanandan-Giri A, Slocum JL, Buller CL, Basrur V, Ju W, Pop-Busui R, Lubman DM, Kretzler M, Pennathur S. Urine glycoprotein profile reveals novel markers for chronic kidney disease. Int J Proteomics. 2011;2011:214715. doi: 10.1155/2011/214715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hogan MC, Johnson KL, Zenka RM, Cristine Charlesworth M, Madden BJ, Mahoney DW, Oberg AL, Huang BQ, Leontovich AA, Nesbitt LL, Bakeberg JL, McCormick DJ, Robert Bergen H, Ward CJ. Subfractionation, characterization, and in-depth proteomic analysis of glomerular membrane vesicles in human urine. Kidney Int. 2013;85:1225–1237. doi: 10.1038/ki.2013.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou H, Kajiyama H, Tsuji T, Hu X, Leelahavanichkul A, Vento S, Frank R, Kopp JB, Trachtman H, Star RA, Yuen PS. Urinary exosomal Wilms’ tumor-1 as a potential biomarker for podocyte injury. Am J Physiol Renal Physiol. 2013;305:F553–559. doi: 10.1152/ajprenal.00056.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mohtat D, Susztak K. Fine tuning gene expression: the epigenome. Semin Nephrol. 2010;30:468–476. doi: 10.1016/j.semnephrol.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thiagarajan RD, Cloonan N, Gardiner BB, Mercer TR, Kolle G, Nourbakhsh E, Wani S, Tang D, Krishnan K, Georgas KM, Rumballe BA, Chiu HS, Steen JA, Mattick JS, Little MH, Grimmond SM. Refining transcriptional programs in kidney development by integration of deep RNA-sequencing and array-based spatial profiling. BMC Genomics. 2011;12:441. doi: 10.1186/1471-2164-12-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Woroniecki R, Gaikwad AB, Susztak K. Fetal environment, epigenetics, and pediatric renal disease. Pediatr Nephrol. 2011;26:705–711. doi: 10.1007/s00467-010-1714-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harvey SJ, Jarad G, Cunningham J, Goldberg S, Schermer B, Harfe BD, McManus MT, Benzing T, Miner JH. Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease. J Am Soc Nephrol. 2008;19:2150–2158. doi: 10.1681/ASN.2008020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shi S, Yu L, Chiu C, Sun Y, Chen J, Khitrov G, Merkenschlager M, Holzman LB, Zhang W, Mundel P, Bottinger EP. Podocyte-selective deletion of dicer induces proteinuria and glomerulosclerosis. J Am Soc Nephrol. 2008;19:2159–2169. doi: 10.1681/ASN.2008030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhdanova O, Srivastava S, Di L, Li Z, Tchelebi L, Dworkin S, Johnstone DB, Zavadil J, Chong MM, Littman DR, Holzman LB, Barisoni L, Skolnik EY. The inducible deletion of Drosha and microRNAs in mature podocytes results in a collapsing glomerulopathy. Kidney Int. 2011;80:719–730. doi: 10.1038/ki.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ho J, Ng KH, Rosen S, Dostal A, Gregory RI, Kreidberg JA. Podocyte-specific loss of functional microRNAs leads to rapid glomerular and tubular injury. J Am Soc Nephrol. 2008;19:2069–2075. doi: 10.1681/ASN.2008020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luo Y, Wang C, Chen X, Zhong T, Cai X, Chen S, Shi Y, Hu J, Guan X, Xia Z, Wang J, Zen K, Zhang CY, Zhang C. Increased Serum and Urinary MicroRNAs in Children with Idiopathic Nephrotic Syndrome. Clin Chem. 2013;59:658–666. doi: 10.1373/clinchem.2012.195297. [DOI] [PubMed] [Google Scholar]
- 84.Gebeshuber CA, Kornauth C, Dong L, Sierig R, Seibler J, Reiss M, Tauber S, Bilban M, Wang S, Kain R, Böhmig GA, Moeller MJ, Gröne HJ, Englert C, Martinez J, Kerjaschki D. Focal segmental glomerulosclerosis is induced by microRNA-193a and its downregulation of WT1. Nat Med. 2013;19:481–487. doi: 10.1038/nm.3142. [DOI] [PubMed] [Google Scholar]
- 85.Smyth LJ, McKay GJ, Maxwell AP, McKnight AJ. DNA hypermethylation and DNA hypomethylation is present at different loci in chronic kidney disease. Epigenetics. 2013;9:366–376. doi: 10.4161/epi.27161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ko YA, Mohtat D, Suzuki M, Park AS, Izquierdo MC, Han SY, Kang HM, Si H, Hostetter T, Pullman JM, Fazzari M, Verma A, Zheng D, Greally JM, Susztak K. Cytosine methylation changes in enhancer regions of core pro-fibrotic genes characterize kidney fibrosis development. Genome Biol. 2013;14:R108. doi: 10.1186/gb-2013-14-10-r108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grimm PC, Nickerson P, Gough J, McKenna R, Jeffery J, Birk P, Rush DN. Quantitation of allograft fibrosis and chronic allograft nephropathy. Pediatr Transplant. 1999;3:257–270. doi: 10.1034/j.1399-3046.1999.00044.x. [DOI] [PubMed] [Google Scholar]
- 88.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D’Agati V, D’Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 89.Maluf DG, Mas VR, Archer KJ, Yanek K, Gibney EM, King AL, Cotterell A, Fisher RA, Posner MP. Molecular pathways involved in loss of kidney graft function with tubular atrophy and interstitial fibrosis. Mol Med. 2008;14:276–285. doi: 10.2119/2007-00111.Maluf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Henger A, Kretzler M, Doran P, Bonrouhi M, Schmid H, Kiss E, Cohen CD, Madden S, Porubsky S, Gröne EF, Schlöndorff D, Nelson PJ, Gröne HJ. Gene expression fingerprints in human tubulointerstitial inflammation and fibrosis as prognostic markers of disease progression. Kidney Int. 2004;65:904–917. doi: 10.1111/j.1523-1755.2004.00499.x. [DOI] [PubMed] [Google Scholar]
- 91.D’Agati VD, Fogo AB, Bruijn JA, Jennette JC. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis. 2004;43:368–382. doi: 10.1053/j.ajkd.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 92.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241–250. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 93.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 94.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22:2129–2137. doi: 10.1681/ASN.2011040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, Bekele E, Bradman N, Wasser WG, Behar DM, Skorecki K. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128:345–350. doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Papeta N, Kiryluk K, Patel A, Sterken R, Kacak N, Snyder HJ, Imus PH, Mhatre AN, Lawani AK, Julian BA, Wyatt RJ, Novak J, Wyatt CM, Ross MJ, Winston JA, Klotman ME, Cohen DJ, Appel GB, D’Agati VD, Klotman PE, Gharavi AG. APOL1 variants increase risk for FSGS and HIVAN but not IgA nephropathy. J Am Soc Nephrol. 2011;22:1991–1996. doi: 10.1681/ASN.2011040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee BT, Kumar V, Williams TA, Abdi R, Bernhardy A, Dyer C, Conte S, Genovese G, Ross MD, Friedman DJ, Gaston R, Milford E, Pollak MR, Chandraker A. The APOL1 genotype of African American kidney transplant recipients does not impact 5-year allograft survival. Am J Transplant. 2012;12:1924–1928. doi: 10.1111/j.1600-6143.2012.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reeves-Daniel AM, DePalma JA, Bleyer AJ, Rocco MV, Murea M, Adams PL, Langefeld CD, Bowden DW, Hicks PJ, Stratta RJ, Lin JJ, Kiger DF, Gautreaux MD, Divers J, Freedman BI. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11:1025–1030. doi: 10.1111/j.1600-6143.2011.03513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bruggeman LA, O’Toole JF, Ross MD, Madhavan SM, Smurzynski M, et al. Plasma Apolipoprotein L1 Levels Do Not Correlate with CKD. J Am Soc Nephrol. 2013 doi: 10.1681/ASN.2013070700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, Feldman HI, Parekh RS, Kusek JW, Greene TH, Fink JC, Anderson AH, Choi MJ, Wright JT, Jr, Lash JP, Freedman BI, Ojo A, Winkler CA, Raj DS, Kopp JB, He J, Jensvold NG, Tao K, Lipkowitz MS, Appel LJ AASK Study Investigators; CRIC Study Investigators. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369:2183–2196. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Madhavan SM, O’Toole JF, Konieczkowski M, Ganesan S, Bruggeman LA, Sedor JR. APOL1 localization in normal kidney and nondiabetic kidney disease. J Am Soc Nephrol. 2011;22:2119–2128. doi: 10.1681/ASN.2011010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gadegbeku CA, Gipson DS, Holzman LB, Ojo AO, Song PX, Barisoni L, Sampson MG, Kopp JB, Lemley KV, Nelson PJ, Lienczewski CC, Adler SG, Appel GB, Cattran DC, Choi MJ, Contreras G, Dell KM, Fervenza FC, Gibson KL, Greenbaum LA, Hernandez JD, Hewitt SM, Hingorani SR, Hladunewich M, Hogan MC, Hogan SL, Kaskel FJ, Lieske JC, Meyers KE, Nachman PH, Nast CC, Neu AM, Reich HN, Sedor JR, Sethna CB, Trachtman H, Tuttle KR, Zhdanova O, Zilleruelo GE, Kretzler M. Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int. 2013;83:749–756. doi: 10.1038/ki.2012.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barisoni L, Nast CC, Jennette JC, Hodgin JB, Herzenberg AM, Lemley KV, Conway CM, Kopp JB, Kretzler M, Lienczewski C, Avila-Casado C, Bagnasco S, Sethi S, Tomaszewski J, Gasim AH, Hewitt SM. Digital Pathology Evaluation in the Multicenter Nephrotic Syndrome Study Network (NEPTUNE) Clin J Am Soc Nephrol. 2013;8:1449–1459. doi: 10.2215/CJN.08370812. [DOI] [PMC free article] [PubMed] [Google Scholar]