Abstract
Background
Early life stress (ELS) can compromise development, with higher amounts of adversity linked to behavior problems. To understand this linkage, a growing body of research has examined two brain regions involved with socio-emotional functioning-the amygdala and hippocampus. Yet empirical studies have reported increases, decreases, and also no differences within human and non-human animal samples exposed to different forms of ELS. Divergence in findings may stem from methodological factors and/or non-linear effects of ELS.
Methods
We completed rigorous hand-tracing of the amygdala and hippocampus in three samples of children who suffered different forms of ELS (i.e., physical abuse, early neglect, or low SES). In addition, interview-based measures of cumulative life stress were also collected with children and their parents or guardians. These same measures were also collected in a fourth sample of comparison children who had not suffered any of these forms of ELS.
Results
Smaller amygdala volumes were found for children exposed to these different forms of ELS. Smaller hippocampal volumes were also noted for children who suffered physical abuse or from low SES-households. Smaller amygdala and hippocampal volumes were also associated with greater cumulative stress exposure and also behavior problems. Hippocampal volumes partially mediated the relationship between ELS and greater behavior problems.
Conclusions
This study suggests ELS may shape the development of brain areas involved with emotion processing and regulation in similar ways. Differences in the amygdala and hippocampus may be a shared diathesis for later negative outcomes related to ELS.
Keywords: stress, amygdala, hippocampus, early life stress, development, neuroimaging, chronic stress, emotion, medial temporal lobe, limbic system, neural plasticity, abuse, neglect, poverty
Increasingly, it is clear that early life stress (ELS) can compromise development, with research linking experiences such as child maltreatment or chronic poverty with behavior problems, such as aggressive and oppositional behavior (1). Such problems are associated with substantial financial costs and sow the seeds for later psychopathology (2–4). To make inroads in conceptualizing, studying, and treating these problem behaviors, recent work has focused on neurobiological risks (5–8). However, this research has not strongly focused on ELS. This gap is a major limitation since these behaviors often emerge after exposure to varying forms of ELS (9–25). To date, there have been very few investigations on the neurobiology of ELS and behavior problems. These limited investigations have focused on brain regions involved in emotion processing and regulation such as the prefrontal cortex (PFC), hippocampus, and amygdala (26). Consensus has begun to materialize regarding ELS and the PFC, with a number of studies reporting differences in this brain region after ELS (27–28). However, similar agreement does not exist for the hippocampus and amygdala, with inconsistent results even being reported in meta-analyses on the neurobiological effects of trauma (29–30). Resolving these inconsistencies is essential to understanding neural alterations associated with ELS and behavior problems.
Divergence in these findings is perhaps not surprising when one considers that past human studies of ELS often rely on “natural experiments” focused on samples exposed to stressful experiences. These retrospective designs though informative, have many significant limitations including the lack of random assignment. Working with multiple groups of children exposed to different forms of adversity is one fruitful way to overcome these limitations and has important advantages over past studies. First, limitations related to unobserved or unmeasured characteristics of specific stressful experiences can be minimized. For example, physical abuse is associated with familial poverty throughout development, more so than early neglect during institutionalization (31–32). Finding brain differences in both samples may indicate common neurobiological diatheses. Second, the timing, chronicity, and scope of stress may differ greatly between groups; however, the behavioral end-state (behavior problems) is similar across populations. As an example, children who experience early neglect commonly experience unresponsive caregiving and an overall dearth of individualized care and attention (33). Children who have been victims of physical abuse, in contrast, may interact with parents often but these experiences may involve excessive physical aggression directed at them (34). Examining different groups exposed to different forms of ELS is a powerful way to understand whether similar or unique patterns of neurobiological alterations put individuals at risk for behavior problems.
Past research implicates the amygdala and hippocampus in basic socio-emotional functioning, making them candidate brain regions for understanding behavior problems following ELS. The hippocampus is involved in learning, memory, and the neuroendocrine response to stress (35–36). The amygdala is central to emotional and social information processing, with damage to this area leading to problems in evaluating the significance of social stimuli (37–38). However, major inconsistencies have emerged in research examining these structures in human and non-human samples exposed to stress (39).
Chronic stress causes reductions in dendritic spines and apoptosis of hippocampal neurons in adult non-human animals (40–42). In humans, one form of ELS, child maltreatment, is consistently related to smaller hippocampi in adults (30,43–44). Earlier in development while the hippocampus is still changing, these findings are less clear. Smaller hippocampi have been reported in children living in poverty (45–47) and those exposed to ELS such as parental separation or loss (48). However, no differences in hippocampi have been found in non-human primates separated from their parents (49), human children exposed to early neglect and later adopted into enriched environments (50–53), or human children who suffered abuse before being diagnosed with Post-Traumatic Stress Disorder (PTSD; Refs.54–57).
For the amygdala, volumetric increases such as dendritic arborization in amygdala nuclei have been reported in adult rodents exposed to stress (58–61). Yet structural neuroimaging studies examining amygdala volumes in humans have been inconclusive. In children exposed to early neglect, research reports have noted larger amygdalae (50–51) but also no differences (52–53). Child poverty has been associated with larger (46) and also smaller (47) amygdalae. Smaller amygdalae (62) and also no differences (54–57) have been found in adolescents who suffered child maltreatment. Of note, many previous investigations in humans (45–46,51,55–56) have had a large age range of participants (e.g., 5–15 years of age). This is particularly important, as amygdala development appears to be non-linear in nature (63–64).
Divergence in results may also be due to methodological factors, such as MRI acquisition parameters or amygdala and hippocampal quantification procedures (65). For example, a review of amygdala quantification found the range of volumes was between 1050-mm3 to 3880-mm3, suggesting great variance in how researchers label these regions (66). Automated quantification of the hippocampus and amygdala also may be adding to inconsistencies in research findings. Methods such as Freesurfer yield high variability and low validity for regions like the amygdala (67–68), often changing study results (69; also see Supplemental Materials). To resolve prior discrepancies, highly valid and reliable measures of the amygdala and hippocampus are needed across different groups exposed to different forms of ELS.
In addition to methodological factors, the effects of stress on the medial temporal lobe (MTL) may be non-linear with different types of volumetric alterations depending on the timing and chronicity of stress (70–72). Our understanding of the effects of ELS on the MTL has been primarily informed by non-human animal models employing chronic immobilization stress (CIS), though other non-human animal paradigms exist (73). Though informative, CIS models may be hard to translate to human samples, particularly in how to understand the long-term neurobiological sequelae of ELS. For example, research suggests the amygdala may adapt and function differently after increased dendritic arborization. CIS leads to enlargement of amygdala volumes (58–61) and also amygdala hyperactivity (74–75). McEwen (70) noted parallels between these findings and patterns of brain alterations in humans during initial episodes of major depression where larger volumes and increased functional activity of the amygdala have been noted (77–78). McEwen further suggested that this hyperactivity might give way to eventual shrinkage, citing reports of smaller amygdalae after repeated depressive episodes (79). Similar ideas have been advanced and supported in research focusing on the amygdala and autism where volumetric overgrowths have been reported early in development but smaller volumes have been noted later in life (72,80–81). In further support of this idea, recent work employing CIS found a single, prolonged stressor actually caused apoptosis of amygdala cells (82).
Based upon this corpus of evidence, ELS may result in an initial increase in amygdala volume along with increases in activity and excitatory neurochemistry. Such speculation fits with three research reports finding higher amygdala activity in children who suffered ELS (83–85). Over time, this excessive functional activity may lead to a loss of neurons (70,74). Individuals exposed to greater amounts of stress or exhibiting greater levels of impairments may therefore have smaller volumes caused by this hypotrophy. In regards to the hippocampus, stress is theorized to be accompanied by a glucocorticoid cascade causing smaller hippocampi over time. Initial data suggests that hippocampal alterations may “reverse” over time, with previously detected differences not present after stress-free periods. Differences in the amygdala are, however, seen even after stress-free periods in non-human animals (86). Such models help in understanding non-linear patterns seen in other trauma-exposed populations (87) along with inconsistencies seen in previous research. For example, recent work by Mehta and colleagues (50) found larger amygdalae in children exposed to early neglect (a type of ELS); however, these investigators found the amount of early neglect to which these same children were exposed was actually related to smaller amygdalae.
For this study, we examined different forms of ELS, employing the same quantification procedures for the MTL for children who suffered early neglect, physical abuse, or who were from low socioeconomic status (SES) households. This approach allowed us to examine whether similar patterns of volumetric changes might be occurring with different forms of ELS and whether this may be a shared diathesis for behavior problems. To gain a greater understanding of how ELS might affect the brain and behavior, we also collected rigorous measures of cumulative stress exposure. Such data allow us to robustly probe the level of cumulative stress to which each child was exposed during development.
Based on theoretical models positing non-linear effects of stress, we postulated that all three forms of ELS would lead to smaller volumes in the amygdala. This idea is motivated by the extant literature reviewed above and theoretical models of non-linear changes in the amygdala after early increased dendritic arborization (70). In addition, we predicted that greater cumulative stress exposure would be associated with smaller amygdalae and, in turn, that smaller amygdalae would be associated with more behavior problems. Finally, we theorized that smaller amygdalae would help account for the contribution of cumulative stress exposure to individual differences in behavior problems. We postulated similar hypotheses for the hippocampus.
Methods
Subjects
T1-weighted MRI images were collected using a 3-Tesla GE SIGNA MRI scanner (additional information in Supplemental materials) for 128 children (61 females; Mean Age: 141.9 months; SD:+/−20.45; Range 108.23–178.70 months). These children comprised three different ELS risk groups: children who experienced early caregiving neglect while living in institutions for orphaned or abandoned children, children from low SES households, and children who were victims of physical abuse. Each group was recruited to allow for examination of different types of ELS. Similar data also were collected from comparison children not exposed to ELS. Informed consent from the parents/guardians of all children and then informed assent from all child participants were obtained in compliance with the University of Wisconsin-Madison Institutional Review Board (IRB). The IRB also approved all study procedures.
To understand the effects of drastic environmental change after ELS, thirty-six participants who were internationally adopted from institutions for orphaned or abandoned children after suffering neglect (21 female; Mean Age:139.34 months; SD:+/−20.2) were recruited for this study. These participants spent an average of 29.52 (SD:+/−16.681) months in institutional care, with a range from 3–64 months and a median of 33.0 months. These children were on average 38.08 (SD:+/−22.69) months when they were adopted, with a median of 35.0 months and a range of 3–92 months. These children had environments that changed drastically after they were adopted into normative family settings.
To represent the effects of exposure to extremely volatile emotional caregiving, thirty-one participants who suffered physical abuse (11 female; Mean Age:144.13 months; SD:+/−19.72) were recruited for this study. This sample was identified in one of two ways: (a)children whose parents scored at least 20 on the physical abuse subscale of the Conflict Tactics Scale Parent-Child Version (PC-CTS;88), a measure of parental aggression towards their children, and/or (b)had substantiated cases of physical abuse on record with the Dane County Department of Human Services.
To understand how pervasive environmental stress and lack of enrichment in the absence of overt parental aggression can influence the brain, twenty participants from low SES households (14 female; Mean Age:146.24 months; SD:+/−20.15) were recruited. Low SES was defined using the Hollingshead’s two-factor index (89), with children from low SES households having parents that were unskilled employees with a high-school education or less (additional information in the Supplemental Materials).
Forty-one participants were comparison children from middle-class SES households with no history of maltreatment (15 female; Mean Age:140.46; SD:+/−21.57 months ). To qualify as comparisons, children were required to have scores <12 on the PC-CTS and to have a Hollingshead’s index score above 50. Sample demographics are shown in Supplemental Table S1.
Pubertal examination
To control for possible influences of puberty on the MTL, all children completed a physical examination with Tanner’s staging (90–91, see Supplemental Materials). Children from low SES households exhibited more advanced pubertal development than comparison children from middle class SES households (t=3.54, p<.001). No differences in pubertal maturation were noted for children exposed to early neglect (t=.145, p=.885) or who suffered physical abuse (t=1.39, p=.168) when compared to children from middle class SES households. There were no group differences in age in months (p’s<.3). Group means and standard deviations are shown in Supplemental Table S1.
Amygdala and Hippocampal Volume of interest (VOI) Drawing
VOI drawing of the amygdala was based on Ref.(71). Hippocampal VOIs were traced based on the criteria detailed in (92) and informed by relevant brain atlases (93–94). Extensive detail regarding tracing procedures and anatomical boundaries is available in the Supplemental Materials. All tracing was carried out by raters blind to group, yielding highly reliable (interrater intraclass correlation (ICC)=0.95 amygdala volumes;0.93 hippocampal volumes) and high spatial reliability (mean intersection/union=0.84 amygdala, n=13;0.86 hippocampus, n=12). Example tracings are shown in the Supplemental Materials.
Assessment of Behavior Problems
The behavioral problems section of the Youth Life Stress Interview (YLSI; Refs. 95–96) was used to assess behavior problems. Advanced graduate-level researchers conducted all interviews. A series of probes was administered to elicit information from children and parents regarding children’s behavior problems at school (e.g., problems with teachers, disciplinary actions related to disruptive behavior). A panel of 3–6 trained raters who did not interact with the family then used a 5-point scale based on separate parent and child reports. Interviewers were trained on filtering out a participant’s subjective responses to probes (e.g., child’s affect) during discussion with this rating team. Once parent and child reports were scored individually, a consensual rating was assigned integrating information from both informants. Higher scores reflected more serious behavior problems. For example, a score of 1.5 reflects a child who was rarely in trouble at school whereas a score of 4 reflects a child who received frequent detentions at school and was often sent to the principal. High reliability has previously been achieved for ratings measuring functioning in different life domains derived from the YLSI (ICC=0.96; Refs.96–97).
Assessment of Cumulative Life Stress
To assess cumulative life stress, interviewers administered the lifetime adversity section of the YLSI separately to children and their parents. This module of the interview assessed a child’s exposure to severe negative life events and circumstances across their lifetime, excluding events within one year to distinguish recent life stressors. General and specific probes were employed to assess a child’s exposure to particularly stressful events and circumstances (e.g., death of close family members, severe chronic illness of close family members). Semi-structured follow-up questions were then asked to assess the event’s context (e.g., timing, duration).
An interviewer elicited objective information about the impact of stressors and then provided this information to an independent rating team with no knowledge of the child’s subjective state. Integrating across parent and child reports, the independent rating team (of 3–6 members) provided a consensual rating on a 10-point scale that reflected the overall level of cumulative life stress. This rating incorporated a detailed consideration of the context of events and the impact on an individual child’s life, rather than simply reflecting the number of stressors. For example, death of a relative receives a uniform score within many stress checklist approaches, but the YLSI differentiates a death of a relative who played a major role in the child’s life vs. a relative with infrequent contact and little involvement with the child (98). Specific examples from our study are detailed in the Supplemental Materials. Of important note, the scores not only reflect the occurrence of particular stressors but also an objective assessment of the degree of impact of each stressor on the child (e.g., long-term consequences). This rating system has high reliability and validity (ICC=0.99; Ref.97).
Results
To examine whether specific forms of ELS were associated with amygdala or hippocampal differences, three separate linear regression models were used to compare children who suffered different forms of ELS (i.e., physical abuse, early neglect, low SES) with comparison children who had not suffered ELS. Such an approach has been employed and recommended by other research groups (99–100). Right and left volumes for each structure were entered separately into linear regressions as dependent variables. Total gray matter, sex, pubertal stage, and group (dummy-coded) were entered as independent variables. In addition, SES was included as a covariate in analyses involving children who had suffered physical abuse or early neglect. Analyses controlling for age are detailed in the Supplemental Materials.
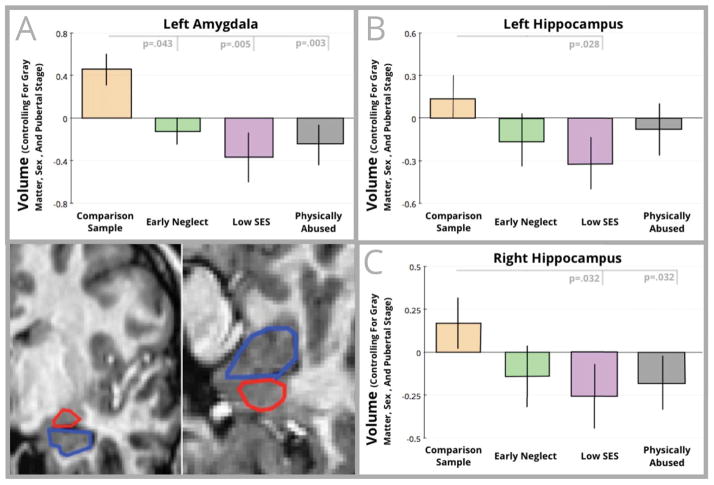
After controlling for puberty, children who suffered early neglect (t=−2.058, p=.043) and children from low SES households (t=−2.927, p=.005) had smaller left amygdalae relative to comparison children. Smaller left (t=−2.257, p=.028) and right (t=−2.205, p=.032) hippocampi were also found for children from low SES households relative to comparison children. Children who suffered physical abuse had smaller left amygdalae (t=−3.107, p=.003) and smaller right hippocampi (t=−2.193, p=.032), relative to comparison children. These differences are shown in Figure 1.
Figure 1.
Volumetric comparisons for the left amygdala (panel A) and hippocampus (Left hippocampus shown in Panel B; Right hippocampus in Panel C) are shown in this figure. For each graph, standardized residuals controlling for total gray matter, pubertal stage, and sex are shown on the vertical axis, while group is shown on the horizontal axis. In the bottom corner of the figure are example hand-tracings of the amygdala (outlined in red) and hippocampus (outlined in blue).
MTL, Cumulative Life Stress, and Behavior Problems
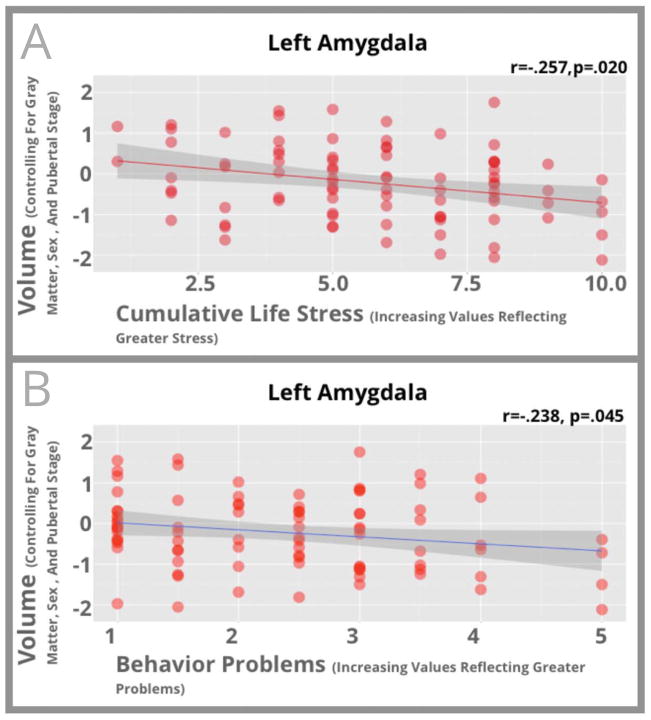
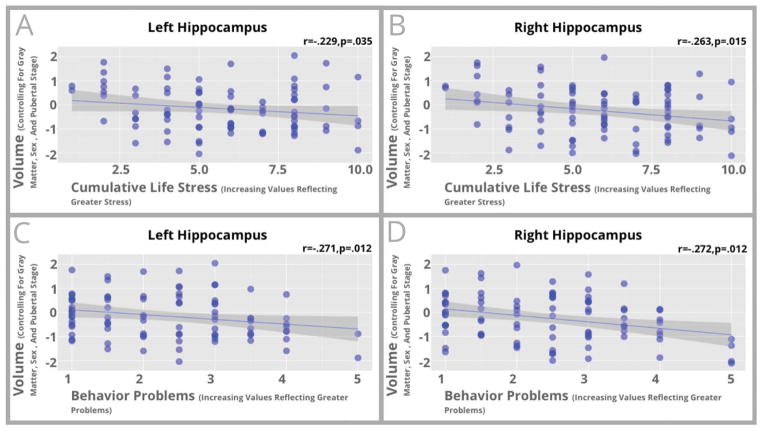
Because similar patterns of volumetric differences were found in the analyses detailed above, we collapsed across the three ELS groups and examined correlations between level of cumulative life stress and amygdala and hippocampal volumes to gain greater statistical power. For children exposed to any form of ELS, higher levels of cumulative stress were associated with smaller volumes in the left amygdala (r=−.257, p=.020) and the hippocampus (Left r=−.229, p=.035; Right r=−.263, p=.015). These relationships are shown in Figures 2–3. Similar associations were seen if comparison controls were included these analyses (Left-Amygdala r=−.316, p<.001; Left-Hippocampus r=−.313, p<.001; Right-Hippocampus r=−.340, p<.001; Supplemental Figure S3).
Figure 2.
Scatterplots between left amygdala volume and cumulative stress exposure (Panel A) and behavioral problems (Panel B) for participants who had suffered ELS are shown in this figure. Standardized residuals of amygdala volume controlling for total gray matter, pubertal stage, and sex are shown on the vertical axis while cumulative stress exposure (Panel A) or behavioral problems (Panel B) is shown on the horizontal axis.
Figure 3.
Scatterplots between hippocampal volume and cumulative stress exposure (Left hippocampus shown in Panel A; Right hippocampus in Panel B) and behavioral problems (Left hippocampus shown in Panel C; Right hippocampus in Panel D) for participants who had suffered ELS are shown in this figure. Standardized residuals of hippocampal volume controlling for total gray matter, pubertal stage, and sex are shown on the vertical axis while cumulative stress exposure (Panels A and B) or behavioral problems (Panels C and D) is shown on the horizontal axis.
Next, we examined correlations between MTL volumes and behavior problems in children exposed to ELS. Greater behavior problems such as disobeying rules were associated with smaller left amygdala volumes (r=−.238, p=.045) and smaller hippocampal volumes (Left r=−.271, p=.012; Right r=−.272, p=.012). These associations are shown in Figures 2–3. Similar associations were again seen if comparison controls were included in analyses (Left-Amygdala r=−.211, p=.019; Left-Hippocampus r=−.284, p=.001; Right-Hippocampus r=−.289, p=.001; Supplemental Figure S4). Descriptive statistics on ELS and behavior problems are noted in Supplemental Materials.
MTL Mediation of ELS and Behavior Problems
After finding these associations, we next sought to investigate whether individual differences in the MTL mediated the effects of ELS on behavior problems (using Sobel tests, Ref.101). These tests revealed that hippocampal volumes (Left-Hippocampus Z=2.032, p=.042; Right-Hippocampus Z=2.051, SE=0.013, p=.040) partially mediated the association between ELS and behavior problems1. No such association was found for the amygdala (p’s>.22).
Discussion
The goal of this study was to understand if ELS was associated with volumetric differences in the amygdala and hippocampus, two important MTL structures involved with socio-emotional functioning. By working with groups of children exposed to different forms of ELS, we additionally sought to overcome limitations of past research studies such as unobserved or unmeasured characteristics of specific stressful experiences. Rigorous hand-tracing methods revealed that each form of ELS presently investigated was associated with differences in amygdala and, to some extent, hippocampal volumes. Smaller amygdalae were observed in children exposed to physical abuse, early neglect, and from low SES households when compared to children who had not suffered such early adversities. In regards to the hippocampus, smaller volumes were observed in children exposed to physical abuse and children from low SES households relative to comparison children.
Our results fit with some previous findings, but also stand in contrast to some of the extant literature. For the amygdala, smaller volumes in children who have suffered physical abuse mirror recent results in a similar-age sample who suffered this ELS (62). In regards to early neglect, our results are in contrast to previous null results and reports showing larger amygdalae in similar samples. Additionally, we found smaller amygdalae in children living in low SES households, which fits with results with (47) but is counter to (46). Our results for the hippocampus fit well with the extant literature. Unlike the amygdala, hippocampal alterations after stress are typically only unidirectional, with smaller volumes being commonly reported. We found smaller hippocampi in children who suffered physical abuse and also children from low SES households, which fits with past reports (45–47). Unique to our work, we found that greater cumulative stress exposure was associated with smaller volumes in both the amygdala and hippocampus. In turn, smaller volumes in these structures were associated with behavior problems. Of note, individual differences in hippocampal volumes partially mediated the contribution of ELS to increased levels of behavior problems.
In considering inconsistencies in past research, it should be noted that our sample had a more narrow age range and had a larger sample size than previous reports. In regards to age range, many past studies have had samples that spanned from early childhood into late adolescence (e.g., Ref.51: 5.22–15.76 years; Ref.56: 4.9–17.0 years). In regards to the range of ELS in this study, the amount of some forms of ELS may be higher than past work. For example, Tottenham and co-workers (51) reported larger amygdalae in children who suffered early neglect; however this report’s sample had experienced a shorter period of caregiving neglect than our participants (Ref.51: placement in institution at 2.7 months on average; average age of adoption:18.8 months). Differences in institutional duration may be one possible explanation why larger volumes were previously noted (51). In an older sample of children who experienced early neglect with periods of deprivation similar to our sample, Mehta and colleagues (50) report results similar to ours. These investigators found a negative correlation with time spent in institutions, with those experiencing longer periods of neglect having smaller amygdalae. Additionally, the use of less rigorous anatomical methods in previous research may, in part, be driving inconsistencies in the past literature. For example as noted in our Supplemental Materials, all associations with the amygdala are non-significant when employing automated segmentation methods.
Thinking broadly, we believe our results for the amygdala fit into a non-linear model of amygdala alterations after ELS. Compelling data exists that ELS is associated with volumetric increases in the amygdala (as evinced by Refs.50–51,60–61) and also increased amygdala activity (83–85). Preliminary data also suggest ELS is related to increased excitation and cell death (74–75,82). With greater stress or if examined later in development, reductions in volume are expected. The smaller volumes across the multiple samples we examined, we believe, provide indirect support for this latter idea. Great caution however must be used when inferring developmental patterns from cross-sectional studies: only longitudinal research can truly validate such a model of amygdala development after ELS. This non-linear model, does however have implications for cross-sectional studies that distinguish it from a model of amygdala hyperfunction. The integrated structural and functional alterations in the amygdala may help us understand individual differences in risk and resilience to behavior problems (and also different forms of psychopathology) seen after ELS.
Of important note, there are potential limitations of the study design. Our data are based on a single MRI scan. It is possible that brain development is simply delayed in children who were subjected to high levels of cumulative life stress. Volumetric differences could “equalize” over time. This may be particularly true of the hippocampus, where research has demonstrated reversibility in volumetric differences if given a “stress-free” period (60). Related to this idea, we did not find any differences in the hippocampus for children who suffered early neglect and then had an enriched (and potentially less stressful) environment after adoption. In future work, we hope to assess other structural and functional properties of the amygdala and hippocampus through the use of longitudinal functional MRI and magnetic resonance spectroscopy (102).
This study demonstrates adverse early experience is associated with structural differences in the MTL. These results are particularly important because ELS has been linked with psychopathology later in life where this brain circuit may play a central role (103–104). Overall, children who suffered ELS had volumetric alterations in the amygdala and hippocampus. Individual differences in MTL structures were, in turn, associated with behavior problems, particularly for the hippocampus. This research also has implications for basic science, by increasing understanding of how post-natal experience shapes brain and behavioral development. Stressful experiences with different onsets, severities, and chronicities may all similarly impact neurobiological circuitry related to behavior problems. Further research is needed to uncover if critical and sensitive periods exist for these processes.
Supplementary Material
Acknowledgments
We thank Andrew Alexander, Michael Anderle, Patrick Bauer, Aaron Cohn, and Johnna Dorshorst for help with data collection and Andrew Fox, Terrence Oakes, and Nicole Strang for helpful discussions. This work was supported by the US National Institute of Mental Health (Grants MH61285, MH68858 to SDP and Grants P50-MH84051 and MH43454 to RJD), a US National Institute of Drug Abuse Fellowship (DA028087 to JLH), and a core grant to the Waisman Center IDDRC from NICHD (P30-HD03352). EAS was at the University of Wisconsin-Madison at the time the data were collected and salary support was provided by MH077687 to EAS.
Footnotes
The relationship between cumulative life stress and behavior problems was still significant when hippocampal volumes were included in regression analyses (Life Stress t=3.7,p<.001).
Financial Disclosures: All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shonkoff JP, Phillips DA, editors. From neurons to neighborhoods: The science of early childhood development. National Academies Press; 2000. [PubMed] [Google Scholar]
- 2.Belfer ML. Child and adolescent mental disorders: the magnitude of the problem across the globe. Journal of Child Psychology and Psychiatry. 2008;49(3):226–236. doi: 10.1111/j.1469-7610.2007.01855.x. [DOI] [PubMed] [Google Scholar]
- 3.Reef J, Diamantopoulou S, van Meurs I, Verhulst FC, van der Ende J. Developmental trajectories of child to adolescent externalizing behavior and adult DSM-IV disorder: results of a 24-year longitudinal study. Social Psychiatry And Psychiatric Epidemiology. 2011;46(12):1233–1241. doi: 10.1007/s00127-010-0297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott S, Knapp M, Henderson J, Maughan B. Financial cost of social exclusion: follow up study of antisocial children into adulthood. BMJ: British Medical Journal. 2001;323(7306):191. doi: 10.1136/bmj.323.7306.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ameis SH, Ducharme S, Albaugh MD, Hudziak JJ, Botteron KN, Lepage C, Karama S. Cortical thickness, cortico-amygdalar networks, and externalizing behaviors in healthy children. Biological psychiatry. 2014;75(1):65–72. doi: 10.1016/j.biopsych.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Beauchaine TP, Gatzke-Kopp LM. Instantiating the multiple levels of analysis perspective in a program of study on externalizing behavior. Development And Psychopathology. 2012;24(03):1003–1018. doi: 10.1017/S0954579412000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy F. Internalizing versus externalizing comorbidity: neural circuit hypothesis. Australian and New Zealand Journal of Psychiatry. 2010;44(5):399–409. doi: 10.3109/00048670903559585. [DOI] [PubMed] [Google Scholar]
- 8.Patrick CJ, Durbin CE, Moser JS. Reconceptualizing antisocial deviance in neurobehavioral terms. Development And Psychopathology. 2012;24(3):1047–1071. doi: 10.1017/S0954579412000533. [DOI] [PubMed] [Google Scholar]
- 9.Fergusson DM, Horwood LJ. Resilience to childhood adversity: Results of a 21-year study. Resilience And Vulnerability: Adaptation in the Context of Childhood Adversities. 2003:130–155. [Google Scholar]
- 10.Hicks BM, South SC, DiRago AC, Iacono WG, McGue M. Environmental adversity and increasing genetic risk for externalizing disorders. Archives of General Psychiatry. 2009;66(6):640–648. doi: 10.1001/archgenpsychiatry.2008.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaffee SR, Caspi A, Moffitt TE, Taylor A. Physical maltreatment victim to antisocial child: evidence of an environmentally mediated process. Journal of Abnormal Psychology. 2004;113(1):44–55. doi: 10.1037/0021-843X.113.1.44. [DOI] [PubMed] [Google Scholar]
- 12.Jaffee SR, Caspi A, Moffitt TE, Polo-Tomás M, Taylor A. Individual, family, and neighborhood factors distinguish resilient from non-resilient maltreated children: A cumulative stressors model. Child Abuse & Neglect. 2007;31(3):231–253. doi: 10.1016/j.chiabu.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lansford JE, Dodge KA, Pettit GS, Bates JE, Crozier J, Kaplow J. A 12-year prospective study of the long-term effects of early child physical maltreatment on psychological, behavioral, and academic problems in adolescence. Archives of Pediatrics & Adolescent Medicine. 2002;156(8):824–830. doi: 10.1001/archpedi.156.8.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lansford JE, Miller-Johnson S, Berlin LJ, Dodge KA, Bates JE, Pettit GS. Early Physical Abuse and Later Violent Delinquency: A Prospective Longitudinal Study. Child Maltreatment. 2007;12(3):233–245. doi: 10.1177/1077559507301841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawk B, McCall RB. CBCL behavior problems of post-institutionalized international adoptees. Clinical Child and Family Psychology review. 2010;13(2):199–211. doi: 10.1007/s10567-010-0068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merz EC, Mccall RB. Behavior Problems in Children Adopted from Psychosocially Depriving Institutions. Journal of Abnormal Child Psychology. 2010;38(4):459–470. doi: 10.1007/s10802-009-9383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiik KL, Loman MM, Van Ryzin MJ, Armstrong JM, Essex MJ, Pollak SD, Gunnar MR. Behavioral and emotional symptoms of post-institutionalized children in middle childhood. Journal of Child Psychology and Psychiatry. 2010;52(1):56–63. doi: 10.1111/j.1469-7610.2010.02294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley RH, Corwyn RF. Socioeconomic status and child development. Annual Review of Psychology. 2002;53(1):371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- 19.Brooks-Gunn J, Duncan GJ. The Effects of Poverty on Children. The Future of Children. 1997:55–71. [PubMed] [Google Scholar]
- 20.McLoyd VC. Socioeconomic disadvantage and child development. American Psychologist. 1998;53(2):185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- 21.Barnow S, Schuckit MA, Lucht M, John U, Freyberger HJ. The importance of a positive family history of alcoholism, parental rejection and emotional warmth, behavioral problems and peer substance use for alcohol problems in teenagers: a path analysis. Journal of Studies on Alcohol and Drugs. 2002;63(3):305–315. doi: 10.15288/jsa.2002.63.305. [DOI] [PubMed] [Google Scholar]
- 22.Briggs-Gowan MJ, Carter AS, Clark R, Augustyn M, McCarthy KJ, Ford JD. Exposure to potentially traumatic events in early childhood: differential links to emergent psychopathology. Journal of Child Psychology and Psychiatry. 2010;51(10):1132–1140. doi: 10.1111/j.1469-7610.2010.02256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Essex MJ, Shirtcliff EA, Burk LR, Ruttle PL, Klein MH, Slattery MJ, Armstrong JM. Influence of early life stress on later hypothalamic–pituitary–adrenal axis functioning and its covariation with mental health symptoms: a study of the allostatic process from childhood into adolescence. Development and Psychopathology. 2011;23(4):1039–1058. doi: 10.1017/S0954579411000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovallo WR, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS. Lifetime adversity leads to blunted stress axis reactivity: studies from the Oklahoma Family Health Patterns Project. Biological psychiatry. 2012;71(4):344–349. doi: 10.1016/j.biopsych.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maughan B, McCarthy G. Childhood adversities and psychosocial disorders. British Medical Bulletin. 1997;53(1):156–169. doi: 10.1093/oxfordjournals.bmb.a011597. [DOI] [PubMed] [Google Scholar]
- 26.Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology. 2011;214(1):55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10(6):410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lupien SJ, Mcewen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 29.Karl A, Schaefer M, Malta LS, Dörfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neuroscience and Biobehavioral Reviews. 2006;30(7):1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Woon FL, Hedges DW. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: A meta-analysis. Hippocampus. 2008;18(8):729–736. doi: 10.1002/hipo.20437. [DOI] [PubMed] [Google Scholar]
- 31.Drake B, Pandey S. Understanding the relationship between neighborhood poverty and specific types of child maltreatment. Child Abuse & Neglect. 1996;20(11):1003–1018. doi: 10.1016/0145-2134(96)00091-9. [DOI] [PubMed] [Google Scholar]
- 32.Hellerstedt WL, Madsen NJ, Gunnar MR, Grotevant HD, Lee RM, Johnson DE. The international adoption project: population-based surveillance of Minnesota parents who adopted children internationally. Maternal and Child Health Journal. 2008;12(2):162–171. doi: 10.1007/s10995-007-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rutter M. Developmental Catch-up, and Deficit, Following Adoption after Severe Global Early Privation. Journal of Child Psychology and Psychiatry. 1998;39(4):465–476. [PubMed] [Google Scholar]
- 34.Bousha DM, Twentyman CT. Mother–child interactional style in abuse, neglect, and control groups: Naturalistic observations in the home. Journal of Abnormal Psychology. 1984;93(1):106–114. doi: 10.1037//0021-843x.93.1.106. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocrine Reviews. 1991;12(2):118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 36.Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behavioral and Neural biology. 1993;60(1):9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- 37.Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15(9):5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aggleton JP, Young AW. The enigma of the amygdala: On its contribution to human emotion. In: Lane RD, Nadel L, editors. Series in affective science. New York, NY, US: Oxford University Press; 2000. pp. 106–128. [Google Scholar]
- 39.Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in Human Neuroscience. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conrad CD, Magariños AM, LeDoux JE, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behavioral neuroscience. 1999;113(5):902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 41.Lambert KG, Buckelew SK, Staffiso-Sandoz G, Gaffga S, Carpenter W, Fisher J, Kinsley CH. Activity-stress induces atrophy of apical dendrites of hippocampal pyramidal neurons in male rats. Physiology & behavior. 1998;65(1):43–49. doi: 10.1016/s0031-9384(98)00114-0. [DOI] [PubMed] [Google Scholar]
- 42.Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69(1):89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- 43.Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. The Journal of Neuropsychiatry and Clinical Neurosciences. 2008;20(3):292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proceedings of the National Academy of Sciences. 2012;109(9):E563–72. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanson JL, Chandra A, Wolfe BL, Pollak SD. Association between income and the hippocampus. PLoS ONE. 2011;6(5):e18712. doi: 10.1371/journal.pone.0018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain. Developmental science. 2012;15(4):516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, Barch D. The Effects of Poverty on Childhood Brain Development: The Mediating Effect of Caregiving and Stressful Life Events. JAMA Pediatrics. 2013;167(12):1135–1142. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao U, Chen LA, Bidesi AS, Shad MU, Thomas MA, Hammen CL. Hippocampal changes associated with early-life adversity and vulnerability to depression. Biological Psychiatry. 2010;67(4):357–364. doi: 10.1016/j.biopsych.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spinelli S, Chefer S, Suomi SJ, Higley JD, Barr CS, Stein E. Early-life stress induces long-term morphologic changes in primate brain. Archives of General Psychiatry. 2009;66(6):658–665. doi: 10.1001/archgenpsychiatry.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SCR, Rutter M, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: The English and Romanian Adoptees Study Pilot. Journal of Child Psychology and Psychiatry. 2009;50(8):943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 51.Tottenham N, Hare TA, Quinn BT, Mccarry TW, Nurse M, Gilhooly T, Millner A, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, Nelson CA. Variation in neural development as a result of exposure to institutionalization early in childhood. Proceedings of the National Academy of Sciences. 2012;109(32):12927–12932. doi: 10.1073/pnas.1200041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, Nelson CA. Widespread Reductions in Cortical Thickness Following Severe Early-Life Deprivation: A Neurodevelopmental Pathway to Attention-Deficit/Hyperactivity Disorder. Biological psychiatry. 2013 doi: 10.1016/j.biopsych.2013.08.016. Available online 3 October 2013. http://dx.doi.org/10.1016/j.biopsych.2013.08.016. [DOI] [PMC free article] [PubMed]
- 54.Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, Reiss AL. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biological psychiatry. 2001;50(12):943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- 55.De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, Frustaci K, et al. A.E. Bennett Research Award. Developmental traumatology. Part II: Brain development. Biological Psychiatry. 1999;45(10):1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 56.De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, Moritz G. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biological Psychiatry. 2002;52(11):1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- 57.De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biological Psychiatry. 2001;50(4):305–309. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- 58.Mitra R, Jadhav S, Mcewen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(26):9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Padival MA, Blume SR, Rosenkranz JA. Repeated restraint stress exerts different impact on structure of neurons in the lateral and basal nuclei of the amygdala. Neuroscience. 2013;246:230–242. doi: 10.1016/j.neuroscience.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143(2):387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Edmiston EE, Wang F, Mazure CM, Guiney J, Sinha R, Mayes LC, Blumberg HP. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Archives of pediatrics & adolescent medicine. 2011;165(12):1069–1077. doi: 10.1001/archpediatrics.2011.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Østby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. The Journal of Neuroscience. 2009;29(38):11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goddings AL, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore SJ. The influence of puberty on subcortical brain development. NeuroImage. 2014;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brierley B, Shaw P, David AS. The human amygdala: a systematic review and meta-analysis of volumetric magnetic resonance imaging. Brain research Brain research reviews. 2002;39(1):84–105. doi: 10.1016/s0165-0173(02)00160-1. [DOI] [PubMed] [Google Scholar]
- 66.Teipel SJ, Ewers M, Wolf S, Jessen F, Kölsch H, Arlt S, Luckhaus C, et al. Multicentre variability of MRI-based medial temporal lobe volumetry in Alzheimer’s disease. Psychiatry Research: Neuroimaging. 2010;182(3):244–250. doi: 10.1016/j.pscychresns.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 67.Hanson JL, Suh JW, Nacewicz BM, Sutterer MJ, Cayo AA, Stodola DE, et al. Robust Automated Amygdala Segmentation via Multi-Atlas Diffeomorphic Registration. Frontiers in Neuroscience. 2012;6:166. doi: 10.3389/fnins.2012.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morey RA, Petty CM, Xu Y, Hayes JP, Ii HRW, Lewis DV, et al. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. NeuroImage. 2009;45(3):855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dewey J, Hana G, Russell T, Price J, McCaffrey D, Harezlak J, et al. Reliability and validity of MRI-based automated volumetry software relative to auto-assisted manual measurement of subcortical structures in HIV-infected patients from a multisite study. Neuroimage. 2010;51:1334–1344. doi: 10.1016/j.neuroimage.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism: clinical and experimental. 2005;54(5 Suppl 1):20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 71.Nacewicz BM, Dalton KM, Johnstone T, Long MT, McAuliff EM, Oakes TR, Alexander AL, et al. Amygdala volume and nonverbal social impairment in adolescent and adult males with autism. Archives of General Psychiatry. 2006;63(12):1417–1428. doi: 10.1001/archpsyc.63.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schumann CM, Amaral DG. Stereological estimation of the number of neurons in the human amygdaloid complex. The Journal of Comparative Neurology. 2005;491(4):320–329. doi: 10.1002/cne.20704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmidt MV, Wang XD, Meijer OC. Early life stress paradigms in rodents: potential animal models of depression? Psychopharmacology. 2011;214(1):131–140. doi: 10.1007/s00213-010-2096-0. [DOI] [PubMed] [Google Scholar]
- 74.Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biological Psychiatry. 2010;67(12):1128–1136. doi: 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Padival M, Quinette D, Rosenkranz JA. Effects of repeated stress on excitatory drive of basal amygdala neurons in vivo. Neuropsychopharmacology. 2013;38(9):1748–62. doi: 10.1038/npp.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McEwen BS. Mood disorders and allostatic load. Biological Psychiatry. 2003;54(3):200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 77.Frodl T, Meisenzahl EM, Zetzsche T, Born C, Jäger M, Groll C, Möller HJ. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biological Psychiatry. 2003;53(4):338–344. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- 78.Siegle GJ, Konecky RO, Thase ME, Carter CS. Relationships between Amygdala Volume and Activity during Emotional Information Processing Tasks in Depressed and Never-Depressed Individuals. Annals of the New York Academy of Sciences. 2003;985(1):481–484. doi: 10.1111/j.1749-6632.2003.tb07105.x. [DOI] [PubMed] [Google Scholar]
- 79.Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9(9):2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- 80.Mosconi MW, Cody-Hazlett H, Poe MD, Gerig G, Gimpel-Smith R, Piven J. Longitudinal study of amygdala volume and joint attention in 2-to 4-year-old children with autism. Archives of general psychiatry. 2009;66(5):509–516. doi: 10.1001/archgenpsychiatry.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim JE, Lyoo IK, Estes AM, Renshaw PF, Shaw DW, Friedman SD, Dager SR. Laterobasal amygdalar enlargement in 6-to 7-year-old children with autism spectrum disorder. Archives of general psychiatry. 2010;67(11):1187–1197. doi: 10.1001/archgenpsychiatry.2010.148. [DOI] [PubMed] [Google Scholar]
- 82.Ding J, Han F, Shi Y. Single-prolonged stress induces apoptosis in the amygdala in a rat model of post-traumatic stress disorder. Journal of Psychiatric Research. 2010;44(1):48–55. doi: 10.1016/j.jpsychires.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 83.Maheu FS, Dozier M, Guyer AE, Mandell D, Peloso E, Poeth K, Ernst M. A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cognitive, Affective, & Behavioral Neuroscience. 2010;10(1):34–49. doi: 10.3758/CABN.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA, Viding E. Heightened neural reactivity to threat in child victims of family violence. Current Biology. 2011;21(23):R947–R948. doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 85.Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Developmental science. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128(4):667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 87.Kuo JR, Kaloupek DG, Woodward SH. Amygdala Volume in Combat-Exposed Veterans With and Without Posttraumatic Stress Disorder: A Cross-sectional Study. Archives of General Psychiatry. 2012;69(10):1080–1086. doi: 10.1001/archgenpsychiatry.2012.73. [DOI] [PubMed] [Google Scholar]
- 88.Straus MA, Hamby SL, Finkelhor D, Moore DW, Runyan D. Identification of child maltreatment with the Parent-Child Conflict Tactics Scales: development and psychometric data for a national sample of American parents. Child Abuse & Neglect. 1998;22(4):249–270. doi: 10.1016/s0145-2134(97)00174-9. [DOI] [PubMed] [Google Scholar]
- 89.Hollingshead AB. Two Factor Index of Social Position. Mimeo. New Haven, Connecticut: Yale University; 1957. [Google Scholar]
- 90.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Archives of disease in childhood. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Archives of disease in childhood. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rusch BD, Abercrombie HC, Oakes TR, Schaefer SM, Davidson RJ. Hippocampal morphometry in depressed patients and control subjects: relations to anxiety symptoms. Biological Psychiatry. 2001;50(12):960–964. doi: 10.1016/s0006-3223(01)01248-3. [DOI] [PubMed] [Google Scholar]
- 93.Duvernoy HM. The human brain stem and cerebellum: surface, structure, vascularization, and three-dimensional sectional anatomy with MRI. Spring-Verlag; 1995. [Google Scholar]
- 94.Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. San Diego, Calif: Academic Press; 1997. [Google Scholar]
- 95.Rudolph KD, Flynn M. Childhood adversity and youth depression: Influence of gender and pubertal status. Development and Psychopathology. 2007;19(02):1–34. doi: 10.1017/S0954579407070241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rudolph KD, Hammen C. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: a transactional perspective. Child Development. 1999;70(3):660–677. doi: 10.1111/1467-8624.00048. [DOI] [PubMed] [Google Scholar]
- 97.Rudolph KD, Hammen C, Burge D, Lindberg N, Herzberg D, Daley SE. Toward an interpersonal life-stress model of depression: the developmental context of stress generation. Development and Psychopathology. 2000;12(2):215–234. doi: 10.1017/s0954579400002066. [DOI] [PubMed] [Google Scholar]
- 98.Wethington E, Brown G, Kessler R. Interview measurement of stressful life events. In: Cohen S, Underwood G, Kessler R, editors. Measuring Stress. New York: Oxford University Press; 1995. [Google Scholar]
- 99.Roberts JA, Scott KA. Interpreting Assessment Data of Internationally Adopted Children. Topics in Language Disorders. 2009;29(1):82–99. [Google Scholar]
- 100.Rutter MM, Dunn JJ, Plomin RR, Simonoff EE, Pickles AA, Maughan BB, Ormel JJ, et al. Integrating nature and nurture: implications of person-environment correlations and interactions for developmental psychopathology. Development and Psychopathology. 1997;9(2):335–364. doi: 10.1017/s0954579497002083. [DOI] [PubMed] [Google Scholar]
- 101.Sobel ME. Some new results on indirect effects and their standard errors in covariance structure models. In: Tuma N, editor. Sociological Methodology. Washington, DC: American Sociological Association; 1986. pp. 159–186. [Google Scholar]
- 102.Nacewicz BM, Angelos L, Dalton KM, Fischer R, Anderle MJ, Alexander AL, Davidson RJ. Reliable non-invasive measurement of human neurochemistry using proton spectroscopy wit. 2012 doi: 10.1016/j.neuroimage.2011.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jamieson E, Duku EK, et al. Childhood abuse and lifetime psychopathology h an anatomically defined amygdala-specific voxel. NeuroImage. 2001;59(3):2548–2559. doi: 10.1016/j.neuroimage.2011.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Drevets WC. Functional neuroimaging studies of depression: the anatomy of melancholia. Annual review of medicine. 1998;49:341–361. doi: 10.1146/annurev.med.49.1.341. [DOI] [PubMed] [Google Scholar]
- 104.MacMillan HL, Fleming JE, Streiner DL, Lin E, Boyle M. H in a community sample. The American journal of psychiatry. 158(11):1878–1883. doi: 10.1176/appi.ajp.158.11.1878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.