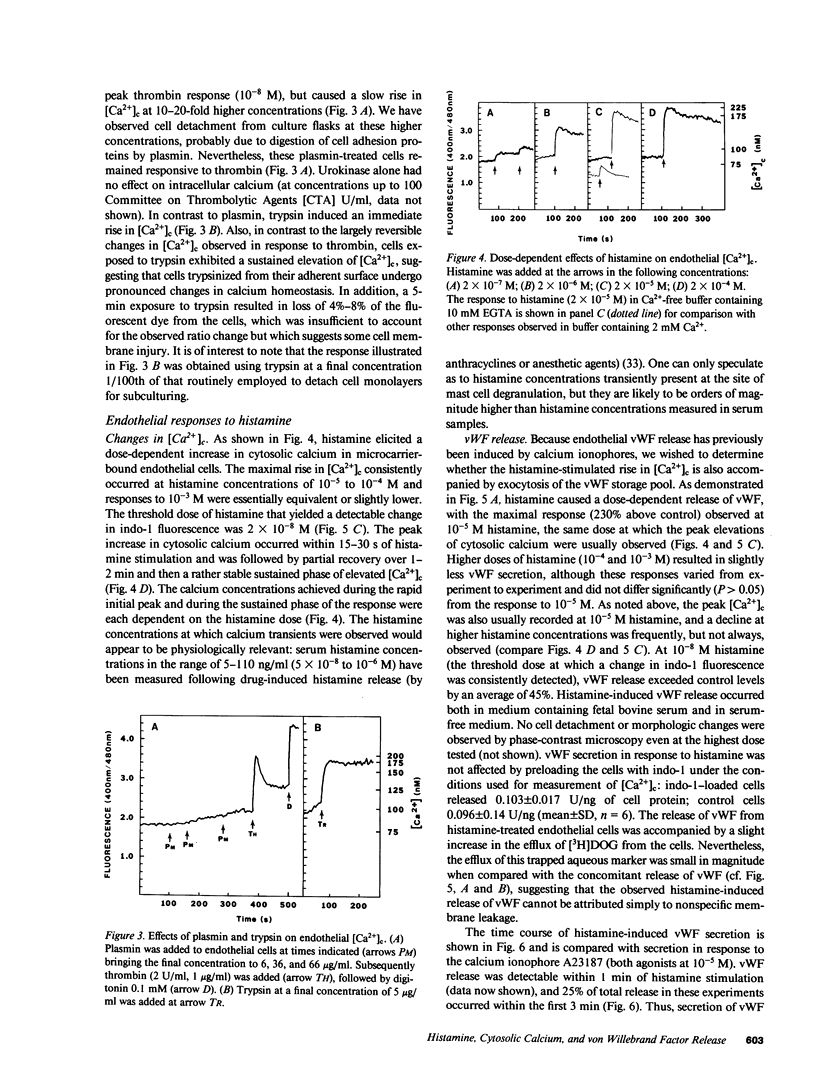
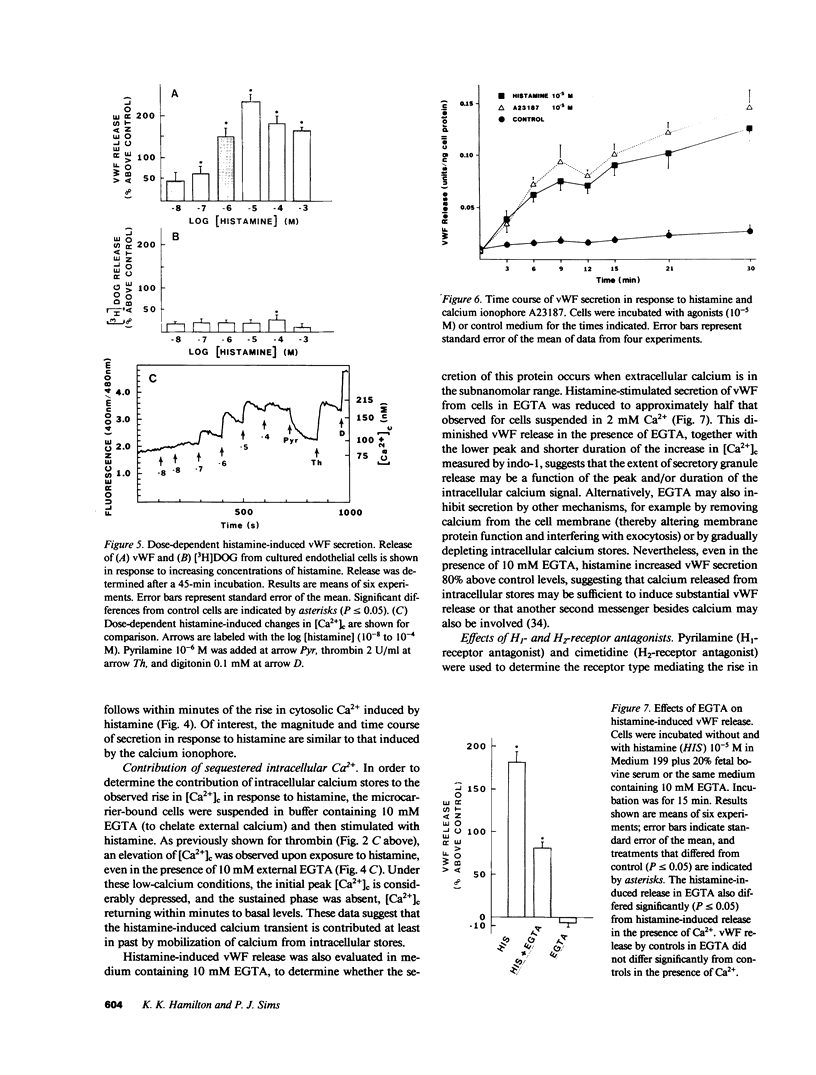
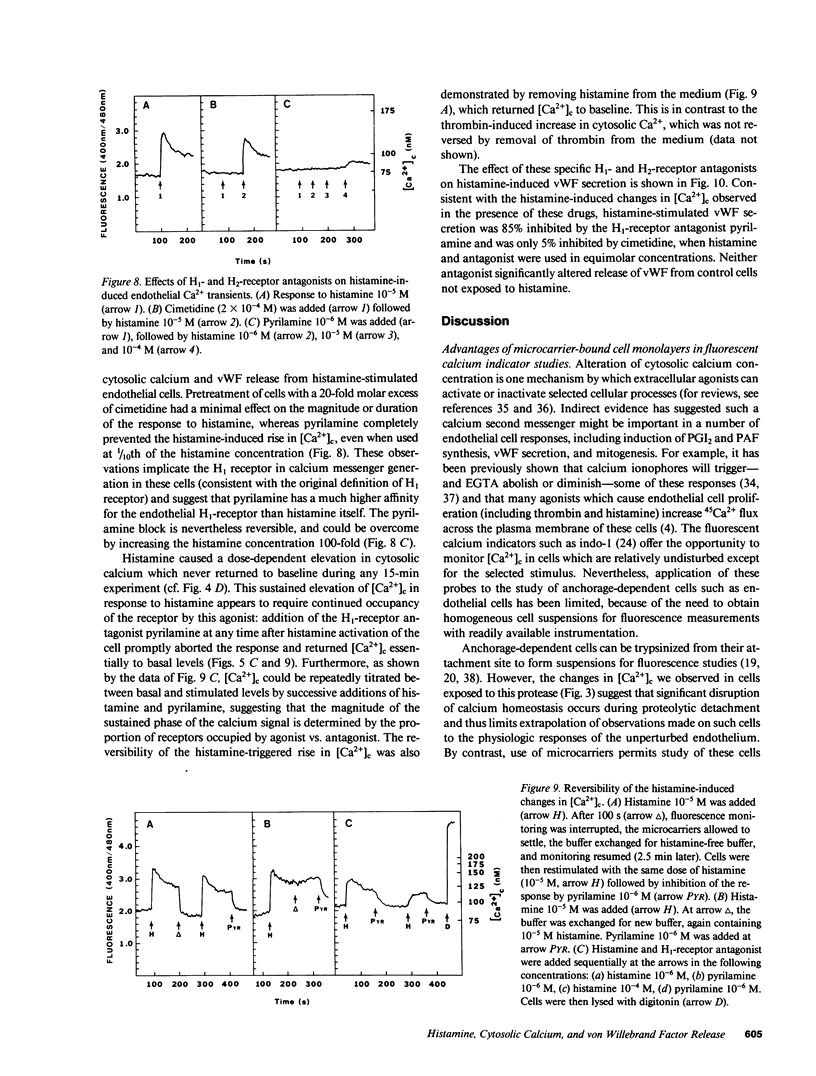
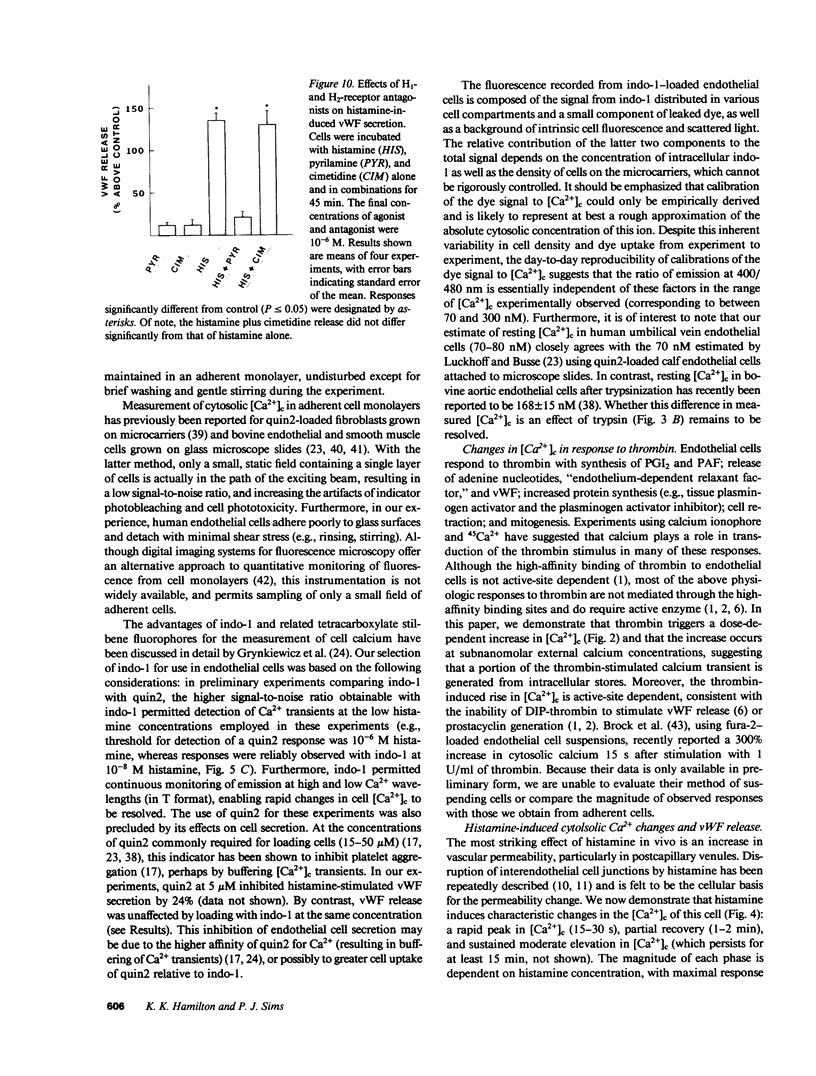
Abstract
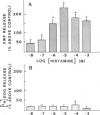
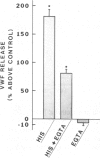
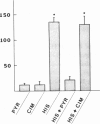
A method for measuring fluorescence in anchored monolayers of human endothelial cells is described and used to demonstrate changes in the cytosolic free-calcium concentration ([Ca2+]c) in these cells exposed to histamine and thrombin; some endothelial responses to both agonists (e.g., mitogenesis) have been suggested to be Ca2+-mediated. Umbilical vein endothelial cells were cultured on microcarriers and loaded with the Ca2+ indicator, indo-1. Enzymatic cell detachment was avoided by monitoring the indo-1 fluorescence ratio (400/480 nm) of a stirred suspension of cell-covered microcarriers. Basal [Ca2+]c was estimated to be 70-80 nM. Thrombin induced a transient dose-dependent increase in [Ca2+]c, which was active-site dependent. Histamine stimulated a dose-dependent increase in [Ca2+]c, which was reversed by removal of histamine and inhibited competitively by the H1-receptor antagonist pyrilamine, but not by the H2-receptor antagonist cimetidine. Furthermore, histamine induced a dose-dependent secretion of von Willebrand factor, which paralleled the rise in [Ca2+]c and was similarly blocked by the H1-receptor antagonist, and which may contribute to platelet deposition at sites of inflammation.
Full text
PDF

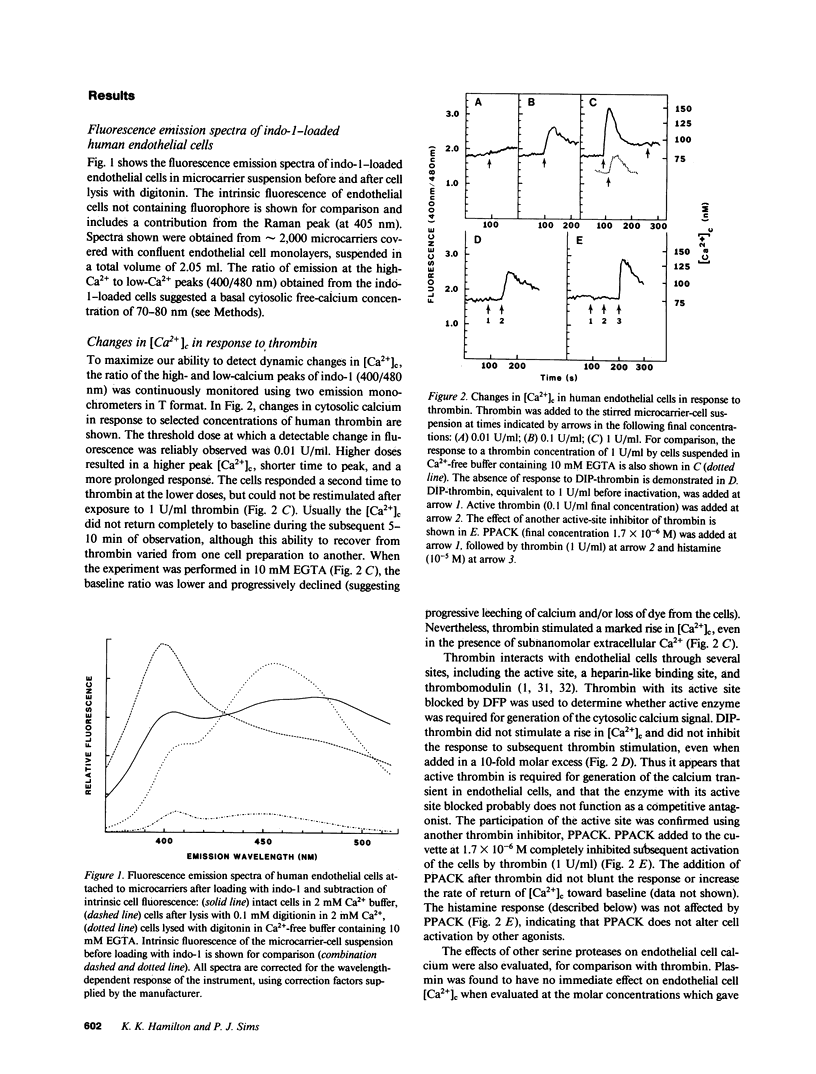


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler S. K., Taylor P. Mobilization of intracellular calcium by alpha 1-adrenergic receptor activation in muscle cell monolayers. J Biol Chem. 1986 May 5;261(13):5866–5871. [PubMed] [Google Scholar]
- Andreoli S. P., Baehner R. L., Bergstein J. M. In vitro detection of endothelial cell damage using 2-deoxy-D-3H-glucose: comparison with chromium 51, 3H-leucine, 3H-adenine, and lactate dehydrogenase. J Lab Clin Med. 1985 Sep;106(3):253–261. [PubMed] [Google Scholar]
- Bauer P. T., Machovich R., Arányi P., Büki K. G., Csonka E., Horváth I. Mechanism of thrombin binding to endothelial cells. Blood. 1983 Feb;61(2):368–372. [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Brock T. A., Gimbrone M. A., Jr Platelet activating factors alters calcium homeostasis in cultured vascular endothelial cells. Am J Physiol. 1986 Jun;250(6 Pt 2):H1086–H1092. doi: 10.1152/ajpheart.1986.250.6.H1086. [DOI] [PubMed] [Google Scholar]
- Capponi A. M., Lew P. D., Vallotton M. B. Cytosolic free calcium levels in monolayers of cultured rat aortic smooth muscle cells. Effects of angiotensin II and vasopressin. J Biol Chem. 1985 Jul 5;260(13):7836–7842. [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Berthon B., Exton J. H. Changes in free cytosolic Ca2+ in hepatocytes following alpha 1-adrenergic stimulation. Studies on Quin-2-loaded hepatocytes. J Biol Chem. 1983 Jul 25;258(14):8769–8773. [PubMed] [Google Scholar]
- D'Amore P., Shepro D. Stimulation of growth and calcium influx in cultured, bovine, aortic endothelial cells by platelets and vasoactive substances. J Cell Physiol. 1977 Aug;92(2):177–183. doi: 10.1002/jcp.1040920206. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Role of calcium and phosphoinositides in the actions of certain hormones and neurotransmitters. J Clin Invest. 1985 Jun;75(6):1753–1757. doi: 10.1172/JCI111886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fishman D. J., Jones P. K., Menitove J. E., Ratnoff O. D., Everson B. Detection of the carrier state for classic hemophilia using an enzyme-linked immunosorbent assay (ELISA). Blood. 1982 Jun;59(6):1163–1168. [PubMed] [Google Scholar]
- Fukuo K., Morimoto S., Koh E., Yukawa S., Tsuchiya H., Imanaka S., Yamamoto H., Onishi T., Kumahara Y. Effects of prostaglandins on the cytosolic free calcium concentration in vascular smooth muscle cells. Biochem Biophys Res Commun. 1986 Apr 14;136(1):247–252. doi: 10.1016/0006-291x(86)90901-0. [DOI] [PubMed] [Google Scholar]
- Galdal K. S., Evensen S. A., Høglund A. S., Nilsen E. Actin pools and actin microfilament organization in cultured human endothelial cells after exposure to thrombin. Br J Haematol. 1984 Dec;58(4):617–625. doi: 10.1111/j.1365-2141.1984.tb06108.x. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr Culture of vascular endothelium. Prog Hemost Thromb. 1976;3:1–28. [PubMed] [Google Scholar]
- Griendling K. K., Rittenhouse S. E., Brock T. A., Ekstein L. S., Gimbrone M. A., Jr, Alexander R. W. Sustained diacylglycerol formation from inositol phospholipids in angiotensin II-stimulated vascular smooth muscle cells. J Biol Chem. 1986 May 5;261(13):5901–5906. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Heltianu C., Simionescu M., Simionescu N. Histamine receptors of the microvascular endothelium revealed in situ with a histamine-ferritin conjugate: characteristic high-affinity binding sites in venules. J Cell Biol. 1982 May;93(2):357–364. doi: 10.1083/jcb.93.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdijk W. P., Sakariassen K. S., Nievelstein P. F., Sixma J. J. Role of factor VIII-von Willebrand factor and fibronectin in the interaction of platelets in flowing blood with monomeric and fibrillar human collagen types I and III. J Clin Invest. 1985 Feb;75(2):531–540. doi: 10.1172/JCI111729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafferji S. S., Michell R. H. Stimulation of phosphatidylinositol turnover by histamine, 5-hydroxytryptamine and adrenaline in the longitudinal smooth muscle of guinea pig ileum. Biochem Pharmacol. 1976 Jun 15;25(12):1429–1430. doi: 10.1016/0006-2952(76)90115-5. [DOI] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Rabinovitch P. S., Martin P. J., Beatty P. G., Hansen J. A. Distinct patterns of transmembrane calcium flux and intracellular calcium mobilization after differentiation antigen cluster 2 (E rosette receptor) or 3 (T3) stimulation of human lymphocytes. J Clin Invest. 1986 Apr;77(4):1224–1232. doi: 10.1172/JCI112425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killackey J. J., Johnston M. G., Movat H. Z. Increased permeability of microcarrier-cultured endothelial monolayers in response to histamine and thrombin. A model for the in vitro study of increased vasopermeability. Am J Pathol. 1986 Jan;122(1):50–61. [PMC free article] [PubMed] [Google Scholar]
- Kruskal B. A., Keith C. H., Maxfield F. R. Thyrotropin-releasing hormone-induced changes in intracellular [Ca2+] measured by microspectrofluorometry on individual quin2-loaded cells. J Cell Biol. 1984 Sep;99(3):1167–1172. doi: 10.1083/jcb.99.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. D., Harlan J. M., Harker L. A., Joseph M. L., Counts R. B. Thrombin-mediated release of factor VIII antigen from human umbilical vein endothelial cells in culture. Blood. 1982 Aug;60(2):531–534. [PubMed] [Google Scholar]
- Loesberg C., Gonsalves M. D., Zandbergen J., Willems C., van Aken W. G., Stel H. V., Van Mourik J. A., De Groot P. G. The effect of calcium on the secretion of factor VIII-related antigen by cultured human endothelial cells. Biochim Biophys Acta. 1983 Sep 22;763(2):160–168. doi: 10.1016/0167-4889(83)90039-3. [DOI] [PubMed] [Google Scholar]
- Lollar P., Owen W. G. Active-site-dependent, thrombin-induced release of adenine nucleotides from cultured human endothelial cells. Ann N Y Acad Sci. 1981;370:51–56. doi: 10.1111/j.1749-6632.1981.tb29720.x. [DOI] [PubMed] [Google Scholar]
- Lollar P., Owen W. G. Evidence that the effects of thrombin on arachidonate metabolism in cultured human endothelial cells are not mediated by a high affinity receptor. J Biol Chem. 1980 Sep 10;255(17):8031–8034. [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Increased free calcium in endothelial cells under stimulation with adenine nucleotides. J Cell Physiol. 1986 Mar;126(3):414–420. doi: 10.1002/jcp.1041260312. [DOI] [PubMed] [Google Scholar]
- Mayhan W. G., Joyner W. L. The effect of altering the external calcium concentration and a calcium channel blocker, verapamil, on microvascular leaky sites and dextran clearance in the hamster cheek pouch. Microvasc Res. 1984 Sep;28(2):159–179. doi: 10.1016/0026-2862(84)90015-3. [DOI] [PubMed] [Google Scholar]
- McIntyre T. M., Zimmerman G. A., Satoh K., Prescott S. M. Cultured endothelial cells synthesize both platelet-activating factor and prostacyclin in response to histamine, bradykinin, and adenosine triphosphate. J Clin Invest. 1985 Jul;76(1):271–280. doi: 10.1172/JCI111957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. D., Metcalfe J. C., Smith G. A., Hesketh T. R., Taylor M. V. Some mitogens cause rapid increases in free calcium in fibroblasts. FEBS Lett. 1984 Apr 24;169(2):189–193. doi: 10.1016/0014-5793(84)80316-6. [DOI] [PubMed] [Google Scholar]
- Patterson M. K., Jr Measurement of growth and viability of cells in culture. Methods Enzymol. 1979;58:141–152. doi: 10.1016/s0076-6879(79)58132-4. [DOI] [PubMed] [Google Scholar]
- Rao G. H., Peller J. D., White J. G. Measurement of ionized calcium in blood platelets with a new generation calcium indicator. Biochem Biophys Res Commun. 1985 Oct 30;132(2):652–657. doi: 10.1016/0006-291x(85)91182-9. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. The calcium messenger system (2). N Engl J Med. 1986 May 1;314(18):1164–1170. doi: 10.1056/NEJM198605013141807. [DOI] [PubMed] [Google Scholar]
- Shimada K., Ozawa T. Evidence that cell surface heparan sulfate is involved in the high affinity thrombin binding to cultured porcine aortic endothelial cells. J Clin Invest. 1985 Apr;75(4):1308–1316. doi: 10.1172/JCI111831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensjö E., Grega G. J. Evidence for endothelial cell-mediated regulation of macromolecular permeability by postcapillary venules. Fed Proc. 1986 Feb;45(2):89–95. [PubMed] [Google Scholar]
- Teitelbaum I., Berl T. Effects of calcium on vasopressin-mediated cyclic adenosine monophosphate formation in cultured rat inner medullary collecting tubule cells. Evidence for the role of intracellular calcium. J Clin Invest. 1986 May;77(5):1574–1583. doi: 10.1172/JCI112473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turitto V. T., Weiss H. J., Baumgartner H. R. Platelet interaction with rabbit subendothelium in von Willebrand's disease: altered thrombus formation distinct from defective platelet adhesion. J Clin Invest. 1984 Nov;74(5):1730–1741. doi: 10.1172/JCI111591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade M. H., Trosko J. E., Schindler M. A fluorescence photobleaching assay of gap junction-mediated communication between human cells. Science. 1986 Apr 25;232(4749):525–528. doi: 10.1126/science.3961495. [DOI] [PubMed] [Google Scholar]
- Weksler B. B., Ley C. W., Jaffe E. A. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A 23187. J Clin Invest. 1978 Nov;62(5):923–930. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welles S. L., Shepro D., Hechtman H. B. Vasoactive amines modulate actin cables (stress fibers) and surface area in cultured bovine endothelium. J Cell Physiol. 1985 Jun;123(3):337–342. doi: 10.1002/jcp.1041230307. [DOI] [PubMed] [Google Scholar]
- Wolff A. A., Levi R. Histamine and cardiac arrhythmias. Circ Res. 1986 Jan;58(1):1–16. doi: 10.1161/01.res.58.1.1. [DOI] [PubMed] [Google Scholar]
- de Groot P. G., Gonsalves M. D., Loesberg C., van Buul-Wortelboer M. F., van Aken W. G., van Mourik J. A. Thrombin-induced release of von Willebrand factor from endothelial cells is mediated by phospholipid methylation. Prostacyclin synthesis is independent of phospholipid methylation. J Biol Chem. 1984 Nov 10;259(21):13329–13333. [PubMed] [Google Scholar]