As the effectiveness of beta-agonists for treating asthma attacks has been established, numerous other supportive treatments for asthma attacks have also been investigated, such as systemic glucocorticoids and magnesium. Among these additional therapies, inhaled furosemide is of particular interest; several studies have evaluated the effects of prophylactic inhaled furosemide in attenuating bronchoconstriction and asthma attacks. To determine the efficacy of inhaled furosemide during asthma attacks, we performed a systematic review using the MEDLINE, EMBASE, Web of Science, and Cochrane Library databases from their inception through 14 March 2014. A meta-analysis was conducted by calculating the standardized mean difference from each study and integrating these means using a random effects model. In addition, subanalyses were performed in the studies that evaluated the peak expiratory flow rate and the forced expiratory volume in 1 second.
We identified six studies using double-blinded, randomized control trial designs that evaluated inhaled furosemide in conjunction with standard treatments in patients experiencing asthma attacks [1-6] (Figure 1); a total of 78 patients received inhaled furosemide and 79 patients received a placebo (Tables 1 and 2). The mean age of patients ranged from 8.4 to 47 years [3,6]. In two studies, patients were administered 40 mg inhaled furosemide [1,2]; in one study, patients were administered 20 mg inhaled furosemide [6]; and in the three studies that recruited children, patients were administered either 1.0 mg/kg [4,5] or 10 mg/m2 [3] inhaled furosemide.
Figure 1.
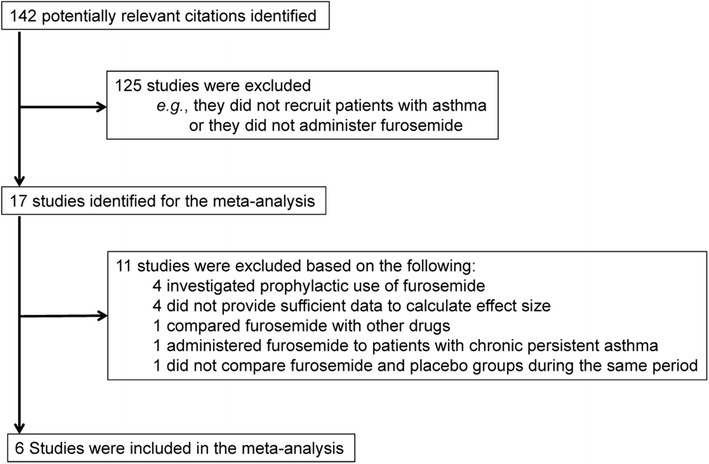
Study selection procedure.
Table 1.
Trial characteristics
| Furosemide group | Placebo group | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study (year) | Number of randomized patients | Number of patients completing study | Mean patient age (years) | Number of patients (male) | Mean patient age (years) | Number of patients (male) | Smoking | COPD | β-agonist dose | Inhaled furosemide dose | Expiratory airflow assessment time (minutes) | Spirometry measurement used | Severity | Hydrocortisone (mg) |
| Alshehri and colleagues, 2005 [5] | 39 | 39 | 8.4 | 19 (11) | 8.5 | 20 (9) | N/Aa | N/Aa | 0.15 mg/kg | 1.0 mg/kg | 30 | PEFR, FEV1.0 | Moderate | N/A |
| González-Sánchez and colleagues, 2002 [4] | 20 | 20 | 9.8 | 10 (7) | 10 | 10 (5) | N/Aa | N/Aa | 0.15 mg/kg | 1.0 mg/kg | 30, 60 | FEV1.0 | Mild or moderate | N/A |
| Nannini and colleagues, 1992 [1] | 20 | 16 | 31 | 7 (N/A) | 41 | 9 (N/A) | N/A | N/A | 2.5 mg | 40 mg | 15, 30 | PEFR | N/A | N/A |
| Nuhoğlu and colleagues, 2006 [3] | 32 | 32 | 8.6 | 16 (8) | 8.4 | 16 (12) | N/Aa | N/Aa | 0.15 mg/kg | 10 mg/m2 | N/A | PEFR | Mild or moderate | N/A |
| Ono and colleagues, 1997b [6] | 37 | 37 | 47 | 20 (7) | 41 | 17 (8) | N/A | Exclude | N/A | 20 mg | 30, 60 | PEFR, FEV1.0 | Mild to severe | 100 |
| Pendino and colleagues, 1998c [2] | 42 | 42 | 38 | 6 (N/A) | 34 | 8 (N/A) | Not >10 pack-years | Exclude | 2.5 mg | 40 mg | 15, 30 | PEFR | Mild or moderate | 300 |
COPD, chronic obstructive pulmonary disease; FEV1.0, forced expiratory volume in 1 second; N/A, not available; PEFR, peak expiratory flow rate. aSmoking and COPD histories were not available, although no smoking or COPD history was assumed because patients were children. bCombination treatment in all trials was simultaneous administration of a beta-agonist plus furosemide, except for Ono and colleagues, in which patients in both groups received hydrocortisone succinate and aminophylline, followed 30 minutes later by either furosemide or placebo. cOnly subgroup data (pertaining to patients whose exacerbations lasted <8 hours) were available.
Table 2.
Trial results
| Furosemide | Placebo | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEFR | FEV1.0 | PEFR | FEV 1.0 | |||||||||||||
| Study (year) | Baseline airflow | SD | Post-treatment airflow | SD | Baseline airflow | SD | Post-treatment airflow | SD | Baseline airflow | SD | Post-treatment airflow | SD | Baseline airflow | SD | Post-treatment airflow | SD |
| Alshehri and colleagues, 2005 [5] | 59.0 | 22.0 | 84.9 | 14.0 | 58.5 | 14.5 | 80.2 | 13.9 | 57.2 | 25.4 | 80.7 | 17.4 | 56.7 | 17.3 | 77.8 | 19.1 |
| Nuhoğlu and colleagues, 2006 [3] | 178 | 65.9 | 222 | 66.1 | N/A | N/A | 183 | 51.7 | 218 | 60.3 | N/A | N/A | ||||
| Baseline airflow | SD | Net airflow improvement above baseline | SD | Baseline airflow | SD | Net airflow improvement above baseline | SD | Baseline airflow | SD | Net airflow improvement above baseline | SD | Baseline airflow | SD | Net airflow improvement above baseline | SD | |
| González-Sánchez and colleagues, 2002 [4] | N/A | N/A | 0.820 | 0.460 | 0.910 | 0.067 | N/A | N/A | 0.850 | 0.340 | 0.980 | 0.078 | ||||
| Nannini and colleagues, 1992 [1] | 147 | 68.0 | 269 | 89.7 | N/A | N/A | 234 | 82.0 | 316 | 56.2 | N/A | N/A | ||||
| Ono and colleagues, 1997 [6] | 171 | 20.0 | 205 | 45.1 | 1.18 | 0.13 | 178 | 25.0 | 198 | 21.3 | 1.32 | 0.74 | N/A | |||
| Pendino and colleagues, 1998 [2] | 200 | 71.0 | 426 | 98.0 | N/A | N/A | 209 | 68.0 | 337 | 73.2 | N/A | N/A | ||||
FEV1.0, forced expiratory volume in 1 second; N/A, not available; PEFR, peak expiratory flow rate; SD, standard deviation.
Integrating the standardized mean difference in each study, a random effects model showed that inhaled furosemide had a significant positive effect on asthma attacks (Z = 2.70; 95% confidence interval, 0.14 to 0.85; P = 0.007) with a negligible heterogeneity (I2 = 16.82) (Figure 2 and Table 3). Subanalyses of the studies reporting the peak expiratory flow rate (Z = 2.23; P = 0.026; n = 68/70, inhaled furosemide/placebo) and the forced expiratory flow in 1 second (Z = 1.84; P = 0.066; n = 49/46, inhaled furosemide/placebo) values confirmed the significant effectiveness of inhaled furosemide for asthma attacks (Table 3). Jackknife sensitivity analyses confirmed the replicability of these findings (P <0.028) (Figure 3). No adverse events associated with furosemide inhalation were reported.
Figure 2.
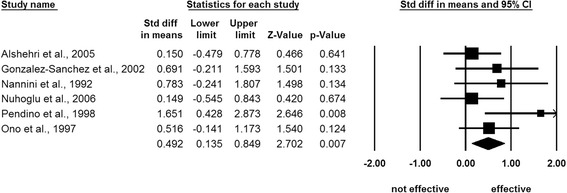
Meta-analysis of randomized clinical trial studies. A random effects model demonstrated significant effectiveness of inhaled furosemide for asthma attacks [1-6]. CI, confidence interval; Std, standard.
Table 3.
Meta-analyses of randomized controlled trials
| Number of studies | Furosemide group ( n ) | Placebo group ( n ) | Lower 95% CI | Upper 95% CI | Z value | P value | I 2 | |
|---|---|---|---|---|---|---|---|---|
| Whole studies | 6 | 78 | 79 | 0.14 | 0.85 | 2.70 | 0.007 | 16.8 |
| PEFR | 5 | 68 | 70 | 0.058 | 0.90 | 2.23 | 0.026 | 30.4 |
| FEV1.0 | 3 | 49 | 46 | –0.027 | 0.83 | 1.84 | 0.066 | 8.16 |
CI, confidence interval; FEV1.0, forced expiratory volume in 1 second; PEFR, peak expiratory flow rate.
Figure 3.

Jackknife sensitivity analysis, excluding one study at a time. All sensitivity analyses preserved the significant effectiveness of inhaled furosemide for asthma attacks [1-6]. CI, confidence interval; Std, standard.
These results thus reveal a statistically significant improvement in airflow obstruction with no evident adverse events when inhaled furosemide was used as an adjunctive treatment for acute asthma exacerbation. The present study provides evidence supporting the addition of inhaled furosemide to conventional treatment in clinical situations.
Acknowledgements
The authors are grateful to Dr Masao Iwagami (The London School of Hygiene & Tropical Medicine, London, UK) for his assistance.
Footnotes
Ryota Inokuchi, Ai Aoki and Yuta Aoki contributed equally.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RI and AA drafted the initial manuscript. YA contributed to the manuscript composition. RI and AA both independently screened the studies. YA performed the statistical analyses. NY critically reviewed the manuscript. All authors provided written consent for publication. All authors read and approved the final manuscript.
Contributor Information
Ryota Inokuchi, Email: inokuchi-icu@h.u-tokyo.ac.jp.
Ai Aoki, Email: sayran.n@gmail.com.
Yuta Aoki, Email: youyouryuta@gmail.com.
Naoki Yahagi, Email: yahagin-eme@h.u-tokyo.ac.jp.
References
- 1.Nannini L, Pendino J, Molfino N, Slutsky A. Inhaled furosemide and salbutamol in acute asthma. Am Rev Respir Dis. 1992;145:422. [Google Scholar]
- 2.Pendino JC, Nannini LJ, Chapman KR, Slutsky A, Molfino NA. Effect of inhaled furosemide in acute asthma. J Asthma. 1998;35:89–93. doi: 10.3109/02770909809055409. [DOI] [PubMed] [Google Scholar]
- 3.Nuhoğlu C, Yaşar Kiliç M, Ceran O. Effectiveness of nebulized furosemide added to nebulized salbutamol in children with acute asthma. Allergol Immunopathol (Madr) 2006;34:54–58. doi: 10.1157/13086747. [DOI] [PubMed] [Google Scholar]
- 4.González-Sánchez R, Trujillo-Hernández B, Huerta M, Vásquez C, Trujillo X. Furosemide plus albuterol compared with albuterol alone in children with acute asthma. Allergy Asthma Proc. 2002;23:181–184. [PubMed] [Google Scholar]
- 5.Alshehri M, Almegamesi T, Alfrayh A. Efficacy of nebulized furosemide in children with moderate attack of asthma. West Afr J Med. 2005;24:246–251. doi: 10.4314/wajm.v24i3.28207. [DOI] [PubMed] [Google Scholar]
- 6.Ono Y, Kondo T, Tanigaki T, Ohta Y. Furosemide given by inhalation ameliorates acute exacerbation of asthma. J Asthma. 1997;34:283–289. doi: 10.3109/02770909709067218. [DOI] [PubMed] [Google Scholar]


