Abstract
This study asks whether arterial blood ionized calcium concentration (Ca++) can regulate the serum level of 1,25-dihydroxy-vitamin D3 [1,25(OH)2D3] independently of serum phosphorus and parathyroid hormone (PTH). We infused either PTH (bovine 1-34, 10 U/kg body wt/h) or saline into awake and unrestrained rats for 24 h, through a chronic indwelling catheter. PTH raised total serum calcium and arterial blood ionized calcium, yet serum 1,25(OH)2D3 fell from 35 +/- 6 (mean +/- SEM, n = 10) with saline to 12 +/- 3 pg/ml (n = 11, P less than 0.005 vs. saline). To determine if the decrease in serum 1,25(OH)2D3 was due to the elevated Ca++, we infused PTH into other rats for 24 h, along with varying amounts of EGTA. Infusion of PTH + 0.67 micron/min EGTA reduced Ca++, and 1,25(OH)2D3 rose to 90 +/- 33 (P less than 0.02 vs. PTH alone). PTH + 1.00 micron/min EGTA lowered Ca++ more, and 1,25(OH)2D3 increased to 148 +/- 29 (P less than 0.01 vs. saline or PTH alone). PTH + 1.33 micron/min EGTA lowered Ca++ below values seen with saline or PTH alone, and 1,25(OH)2D3 rose to 267 +/- 46 (P less than 0.003 vs. all other groups). Thus, during PTH infusion lowering Ca++ with EGTA raised 1,25(OH)2D3 progressively. There were no differences in serum phosphorus concentration or in arterial blood pH in any group infused with PTH. The log of serum 1,25(OH)2D3 was correlated inversely with Ca++ in all four groups infused with PTH (r = -0.737, n = 31, P less than 0.001), and also when the saline group was included (r = -0.677, n = 41, P less than 0.001). The results of this study indicate that serum 1,25(OH)2D3 may be regulated by Ca++ independent of PTH and serum phosphorus levels in the rat. Since 1,25(OH)2D3 regulates gastrointestinal calcium absorption, there may be direct feedback control of 1,25(OH)2D3, by its regulated ion, Ca++.
Full text
PDF
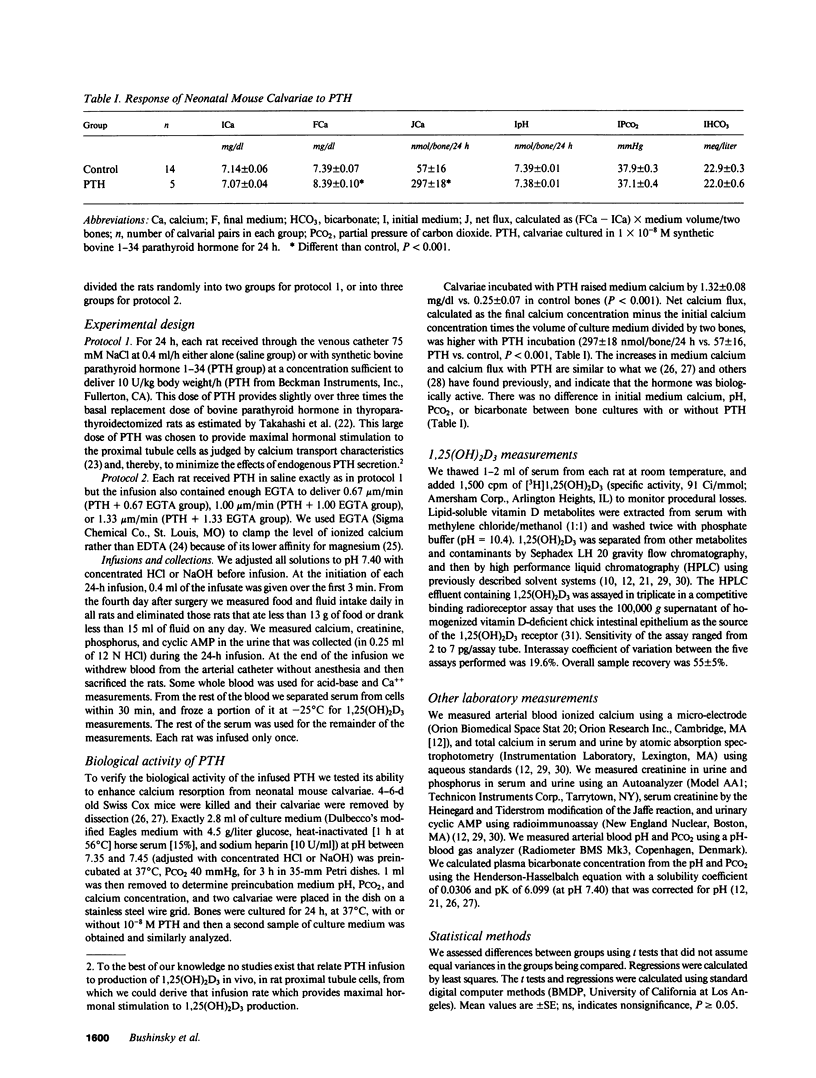

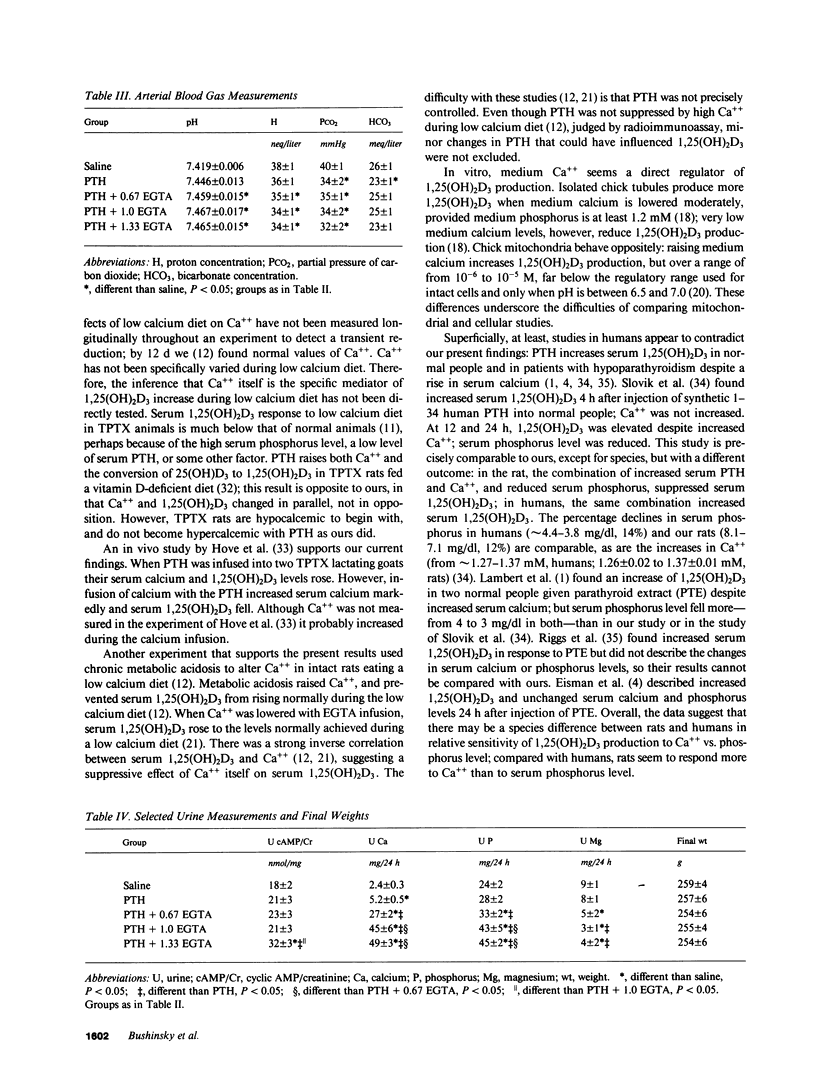


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin L. A., Heath H., 3rd, Go V. L. Regulation of calcitonin secretion in normal man by changes of serum calcium within the physiologic range. J Clin Invest. 1979 Dec;64(6):1721–1724. doi: 10.1172/JCI109636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D. D., Murphy E. W., Rasmussen H. The ionic control of 1,25-dihydroxyvitamin D3 synthesis in isolated chick renal mitochondria. The role of calcium as influenced by inorganic phosphate and hydrogen-ion. J Clin Invest. 1975 Feb;55(2):299–304. doi: 10.1172/JCI107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D. D., Rasmussen H. The ionic control of 1,25-dihydroxyvitamin D3 production in isolated chick renal tubules. J Clin Invest. 1975 Feb;55(2):292–298. doi: 10.1172/JCI107932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle I. T., Gray R. W., DeLuca H. F. Regulation by calcium of in vivo synthesis of 1,25-dihydroxycholecalciferol and 21,25-dihydroxycholecalciferol. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2131–2134. doi: 10.1073/pnas.68.9.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadus A. E., Horst R. L., Lang R., Littledike E. T., Rasmussen H. The importance of circulating 1,25-dihydroxyvitamin D in the pathogenesis of hypercalciuria and renal-stone formation in primary hyperparathyroidism. N Engl J Med. 1980 Feb 21;302(8):421–426. doi: 10.1056/NEJM198002213020801. [DOI] [PubMed] [Google Scholar]
- Broadus A. E., Magee J. S., Mallette L. E., Horst R. L., Lang R., Jensen P. S., Gertner J. M., Baron R. A detailed evaluation of oral phosphate therapy in selected patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 1983 May;56(5):953–961. doi: 10.1210/jcem-56-5-953. [DOI] [PubMed] [Google Scholar]
- Bushinsky D. A., Favus M. J., Coe F. L. Elevated 1,25(OH)2D3, intestinal absorption, and renal mineral conservation in male rats. Am J Physiol. 1984 Feb;246(2 Pt 2):F140–F145. doi: 10.1152/ajprenal.1984.246.2.F140. [DOI] [PubMed] [Google Scholar]
- Bushinsky D. A., Favus M. J., Coe F. L. Mechanism of chronic hypocalciuria with chlorthalidone: reduced calcium absorption. Am J Physiol. 1984 Nov;247(5 Pt 2):F746–F752. doi: 10.1152/ajprenal.1984.247.5.F746. [DOI] [PubMed] [Google Scholar]
- Bushinsky D. A., Favus M. J., Schneider A. B., Sen P. K., Sherwood L. M., Coe F. L. Effects of metabolic acidosis on PTH and 1,25(OH)2D3 response to low calcium diet. Am J Physiol. 1982 Dec;243(6):F570–F575. doi: 10.1152/ajprenal.1982.243.6.F570. [DOI] [PubMed] [Google Scholar]
- Bushinsky D. A., Goldring J. M., Coe F. L. Cellular contribution to pH-mediated calcium flux in neonatal mouse calvariae. Am J Physiol. 1985 Jun;248(6 Pt 2):F785–F789. doi: 10.1152/ajprenal.1985.248.6.F785. [DOI] [PubMed] [Google Scholar]
- Bushinsky D. A., Krieger N. S., Geisser D. I., Grossman E. B., Coe F. L. Effects of pH on bone calcium and proton fluxes in vitro. Am J Physiol. 1983 Aug;245(2):F204–F209. doi: 10.1152/ajprenal.1983.245.2.F204. [DOI] [PubMed] [Google Scholar]
- Bushinsky D. A., Riera G. S., Favus M. J., Coe F. L. Response of serum 1,25(OH)2D3 to variation of ionized calcium during chronic acidosis. Am J Physiol. 1985 Sep;249(3 Pt 2):F361–F365. doi: 10.1152/ajprenal.1985.249.3.F361. [DOI] [PubMed] [Google Scholar]
- Coe F. L., Bushinsky D. A. Pathophysiology of hypercalciuria. Am J Physiol. 1984 Jul;247(1 Pt 2):F1–13. doi: 10.1152/ajprenal.1984.247.1.F1. [DOI] [PubMed] [Google Scholar]
- Colston K. W., Evans I. M., Galante L., MacIntyre I., Moss D. W. Regulation of vitamin D metabolism: factors influencing the rate of formation of 1,25-dihydroxycholecalciferol by kidney homogenates. Biochem J. 1973 Jul;134(3):817–820. [PMC free article] [PubMed] [Google Scholar]
- Eisman J. A., Hamstra A. J., Kream B. E., DeLuca H. F. A sensitive, precise, and convenient method for determination of 1,25-dihydroxyvitamin D in human plasma. Arch Biochem Biophys. 1976 Sep;176(1):235–243. doi: 10.1016/0003-9861(76)90161-2. [DOI] [PubMed] [Google Scholar]
- Eisman J. A., Wark J. D., Prince R. L., Moseley J. M. Modulation of plasma 1,25-dihydroxyvitamin D in man by stimulation and suppression tests. Lancet. 1979 Nov 3;2(8149):931–933. doi: 10.1016/s0140-6736(79)92624-2. [DOI] [PubMed] [Google Scholar]
- Favus M. J., Coe F. L., Kathpalia S. C., Porat A., Sen P. K., Sherwood L. M. Effects of chlorothiazide on 1,25-dihydroxyvitamin D3, parathyroid hormone, and intestinal calcium absorption in the rat. Am J Physiol. 1982 Jun;242(6):G575–G581. doi: 10.1152/ajpgi.1982.242.6.G575. [DOI] [PubMed] [Google Scholar]
- Favus M. J. Factors that influence absorption and secretion of calcium in the small intestine and colon. Am J Physiol. 1985 Feb;248(2 Pt 1):G147–G157. doi: 10.1152/ajpgi.1985.248.2.G147. [DOI] [PubMed] [Google Scholar]
- Favus M. J., Walling M. W., Kimberg D. V. Effects of dietary calcium restriction and chronic thyroparathyroidectomy on the metabolism of (3H)25-hydroxyvitamin D3 and the active transport of calcium by rat intestine. J Clin Invest. 1974 Apr;53(4):1139–1148. doi: 10.1172/JCI107652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J., Heath H., 3rd The "calcium clamp": effect of constant hypocalcemia on parathyroid hormone secretion. Am J Physiol. 1981 Jun;240(6):E649–E655. doi: 10.1152/ajpendo.1981.240.6.E649. [DOI] [PubMed] [Google Scholar]
- Garabedian M., Holick M. F., Deluca H. F., Boyle I. T. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R. W. Control of plasma 1,25-(OH)2-vitamin D concentrations by calcium and phosphorus in the rat: effects of hypophysectomy. Calcif Tissue Int. 1981;33(5):485–488. doi: 10.1007/BF02409478. [DOI] [PubMed] [Google Scholar]
- Gray R. W., Wilz D. R., Caldas A. E., Lemann J., Jr The importance of phosphate in regulating plasma 1,25-(OH)2-vitamin D levels in humans: studies in healthy subjects in calcium-stone formers and in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 1977 Aug;45(2):299–306. doi: 10.1210/jcem-45-2-299. [DOI] [PubMed] [Google Scholar]
- Henry H. L., Midgett R. J., Norman A. W. Regulation of 25-hydroxyvitamin D3-1-hydroxylase in vivo. J Biol Chem. 1974 Dec 10;249(23):7584–7592. [PubMed] [Google Scholar]
- Horiuchi N., Suda T., Takahashi H., Shimazawa E., Ogata E. In vivo evidence for the intermediary role of 3',5'-cyclic AMP in parathyroid hormone-induced stimulation of 1alpha,25-dihydroxyvitamin D3 synthesis in rats. Endocrinology. 1977 Sep;101(3):969–974. doi: 10.1210/endo-101-3-969. [DOI] [PubMed] [Google Scholar]
- Hove K., Horst R. L., Littledike E. T., Beitz D. C. Infusions of parathyroid hormone in ruminants: hypercalcemia and reduced plasma 1,25-dihydroxyvitamin D concentrations. Endocrinology. 1984 Mar;114(3):897–903. doi: 10.1210/endo-114-3-897. [DOI] [PubMed] [Google Scholar]
- Hughes M. R., Brumbaugh P. F., Hussler M. R., Wergedal J. E., Baylink D. J. Regulation of serum 1alpha,25-dihydroxyvitamin D3 by calcium and phosphate in the rat. Science. 1975 Nov 7;190(4214):578–580. doi: 10.1126/science.1188357. [DOI] [PubMed] [Google Scholar]
- Kalu D. N., Miyasaki B. C., Foster G. V. Effects of ethylenediaminetetraacetate (EDTA) on urinary excretion of hydroxyproline, calcium and phosphorus in the rat. Calcif Tissue Res. 1974;16(1):1–12. doi: 10.1007/BF02008209. [DOI] [PubMed] [Google Scholar]
- Kaplan R. A., Haussler M. R., Deftos L. J., Bone H., Pak C. Y. The role of 1 alpha, 25-dihydroxyvitamin D in the mediation of intestinal hyperabsorption of calcium in primary hyperparathyroidism and absorptive hypercalciuria. J Clin Invest. 1977 May;59(5):756–760. doi: 10.1172/JCI108696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert P. W., Hollis B. W., Bell N. H., Epstein S. Demonstration of a lack of change in serum 1 alpha,25-dihydroxyvitamin D in response to parathyroid extract in pseudohypoparathyroidism. J Clin Invest. 1980 Oct;66(4):782–791. doi: 10.1172/JCI109916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins R. G., Colston K. W., Galante L. S., MacAuley S. J., Evans I. M., MacIntyre I. Regulation of vitamin-D metabolism without parathyroid hormone. Lancet. 1973 Aug 11;2(7824):289–291. doi: 10.1016/s0140-6736(73)90794-0. [DOI] [PubMed] [Google Scholar]
- Omdahl J. L. Control of kidney 25-hydroxyvitamin D3 metabolism. Strontium and the involvement of parathyroid hormone. Arch Biochem Biophys. 1977 Nov;184(1):172–178. doi: 10.1016/0003-9861(77)90339-3. [DOI] [PubMed] [Google Scholar]
- Omdahl J. L. Interaction of the parathyroid and 1,25-dihydroxyvitamin D3 in the control of renal 25-hydroxyvitamin D3 metabolism. J Biol Chem. 1978 Dec 10;253(23):8474–8478. [PubMed] [Google Scholar]
- Riggs B. L., Hamstra A., DeLuca H. F. Assessment of 25-hydroxyvitamin D 1 alpha-hydroxylase reserve in postmenopausal osteoporosis by administration of parathyroid extract. J Clin Endocrinol Metab. 1981 Oct;53(4):833–835. doi: 10.1210/jcem-53-4-833. [DOI] [PubMed] [Google Scholar]
- Slovik D. M., Adams J. S., Neer R. M., Holick M. F., Potts J. T., Jr Deficient production of 1,25-dihydroxyvitamin D in elderly osteoporotic patients. N Engl J Med. 1981 Aug 13;305(7):372–374. doi: 10.1056/NEJM198108133050704. [DOI] [PubMed] [Google Scholar]
- Stern P. H., Krieger N. S. Comparison of fetal rat limb bones and neonatal mouse calvaria: effects of parathyroid hormone and 1,25-dihydroxyvitamin D3. Calcif Tissue Int. 1983;35(2):172–176. doi: 10.1007/BF02405027. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Shimazawa E., Horiuchi N., Suda T., Yamashita K., Ogata E. An estimation of the parathyroid hormone secretion rate in vitamin D deficient rats. Horm Metab Res. 1978 Mar;10(2):161–167. doi: 10.1055/s-0028-1093467. [DOI] [PubMed] [Google Scholar]
- Trechsel U., Bonjour J. P., Fleisch H. Regulation of the metabolism of 25-hydroxyvitamin D3 in primary cultures of chick kidney cells. J Clin Invest. 1979 Jul;64(1):206–217. doi: 10.1172/JCI109441. [DOI] [PMC free article] [PubMed] [Google Scholar]



