Abstract
Background and Purpose
Hyperglycemia is an important diagnostic differential and has been reported to cause focal neurological deficits masquerading as stroke. Discussion of hyperglycemia as a stroke mimic has been sparse in the era of diffusion weighted imaging, but remains an important mimic.
Case Summary
A 67 year-old right-handed woman with presented with lethargy, global aphasia, left eye deviation and right hemiparesis. She received IV t-PA for left MCA syndrome and transferred for possible intervention. Initial labs showed a glucose 825mg/dL. MRI/MRA brain was negative for acute stroke with patent vessels, but abnormalities on MRperfusion. The patient was admitted and treated with medical resuscitation including IV fluids and an insulin drip. After normoglycemia was achieved the patient's neurological deficits resolved. EEG on day one of hospitalization showed left hemispheric slowing that subsequently normalized on continuous recording.
Conclusion
We report a case of hyperglycemia clinically mimicking a left MCA syndrome reversed with medical management possibly explained by metabolic demand-blood flow coupling of inactive tissue rather than hypoperfused tissue at risk of infarction.
Key Words: Stroke, Hyperglycemia, Stroke mimic
INTRODUCTION
Hyperglycemia has long been known to cause significant neurological disorders including chorea, seizures, as well as the spectrum of altered mental status from delirium to coma. Despite having been described as an important differential, there are very few cases reported of hyperglycemia causing acute focal neurological deficits, conforming to a vascular distribution.
Here we present a case of a nonketotic hyperglycemia presenting as left middle-cerebral artery syndrome for which the patient received IV t-PA.
SUMMARY OF CASE
A 67 year-old right-handed woman with a past medical history of non-insulin dependent diabetes, hypertension, hyperlipidemia, and a previous right external capsule lacunar stroke was at her nursing home, had eaten an early dinner and was in her normal state of health. She subsequently developed difficulty with speech. She was taken to a local hospital where she was initially found to have an NIHSS of 32 including global aphasia, left gaze deviation, and right hemiplegia consistent with a left MCA syndrome.
Initial labs were only significant for hyperglycemia of 825mg/dL. CT brain was negative for hemorrhage and IV t-PA was given before 3 hours of symptom onset. The patient was transferred to our institution with an initial glucose upon presentation of 410mg/dL. Neurologic exam demonstrated that the patient was lethargic, globally aphasic, with left gaze deviation, right homonymous hemianopsia and right hemiparesis with limited sensation for an NIHSS of 17 consistent with left MCA syndrome.
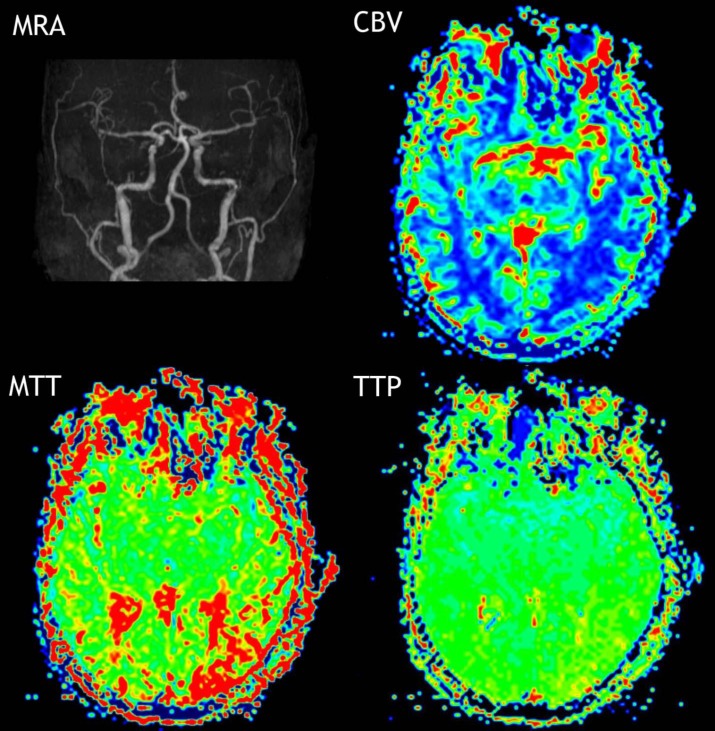
Initial MRI/MRA/MR perfusion performed at six hours after symptom onset was negative for diffusion restriction or large vessel occlusion, but demonstrated a small area within the left posterior temporal lobe of increased time to peak and mean transit time with normal cerebral blood volume. At this time we continued medical resuscitation with IV fluids, nicardipine IV drip for blood pressure control, and an insulin drip.
Serum glucose was 376mg/dL and two hours later 165mg/dL. Her exam significantly improved in that she became less somnolent and responded to voice. Over the next hours she began to move all extremities, and her aphasia and gaze deviation began to resolve.
On day one of hospitalization EEG demonstrated left hemispheric slowing without epileptiform discharges which normalized on continuous recording on day two with normal wakefulness and sleep. Repeat MRI with DWI on day five did not demonstrate any evidence of stroke or other etiology consistent with her presentation of a left MCA syndrome. Carotid ultrasound, 2D echocardiogram, and holter monitoring were unremarkable.
She returned to her baseline without any neurological deficits after 24 hours of admission.
DISCUSSION
Neurological impairment in the setting of hyperglycemic states with or without ketoacidosis has long been known. [1, 2] Through the 1980s there were many articles written about neurological manifestations of hyperglycemia with most of these cases involving a combination of coma or seizures without ketoacidosis. [3–5]
Focal neurological deficits are often reported in these case series including aphasia, homonymous hemianopsia, hemiparesis, or hyperreflexia, but usually in the setting of post-ictal Todd’s paresis or where post-ictal state could not be ruled out. [1, 5] In some of these early cases with diabetic coma and focal neurological signsintravascular thrombosis and brain ischemia later was found at autopsy. [6] Subsequent discussion of focal neurological presentations has been sparse after the advent diffusion weighted imaging.
It is unlikely that our patient presented with a seizure or post-ictal Todds paralysis. The noticing of speech difficulty and the subsequent acute deterioration is uncharacteristic of seizures. Nursing home staff monitored her at onset and did not report tonic-clonic movements, incontinence, tongue-biting, or trauma. Also, importantly her exam correlated with correction of her serum glucose level.
The patient’s EEG findings are non-specific and likely secondary to her hyperglycemia. Maccario and others noted that in their hyperglycemic patient with EEG slowing in the affected hemisphere was seen without clinical evidence of seizures. [5] In studies of the effects of hyperglycemia on EEGs it has been demonstrated that slowing can be a result of hyperglycemia and that the slowing seen associated with hyperglycemic states may take days to resolve. [7]
The MR perfusion demonstrated an area within the left posterior temporal lobe of increased time to peak (TTP) and mean transit time (MTT) with normal cerebral blood volume (CBV). Seizure is a metabolically active state which generates increased perfusion on perfusion imaging. [8, 9] However, there are reports of postictal hyerperfusion and hypoperfusion mimicking ischemia. [10–13] In all cases of postictal hyperfusion or hypoferfusion there was increased or decreased CBV, which was not seen in our case (see Figure I). Moreover, the area of concern on perfusion imaging was relatively small and was not consistent with patient’s gross deficits.
Figure I. Patent vasculature based on MRA. MR perfusion demonstrated a small area within the left posterior temporal lobe of increased time to peak and mean transit time with normal cerebral blood volume.
This disconnect between normal CBV with increased TTP and MTP with loss of function is consistent with flow-metabolism coupling, blood flow adjusting to energy demands. In our patient’s case hyperglycemia was associated with temporary neuronal dysfunction in the left hemisphere based on EEG slowing in the absence of vascular compromise. The lower perfusion reflects lower demand from metabolically inactive tissue rather than hypoperfused tissue at risk of infarction.
Limitations include presuming that there was decreased energy demand based on EEG slowing rather than direct measurement of rate of oxygen metabolism. How hyperglycemia causes decreased energy demand and laterality are unclear. Nonetheless, this case demonstrates that hyperglycemia is still a relevant mimic of acute ischemic stroke even in the era of rapid assessment for thrombolytics.
In conclusion, we report a case of hyperglycemia mimicking a left MCA syndrome without evidence of seizure reversed with medical management. We contend this stroke mimic could be explained by metabolic demand-blood flow coupling.
ACKNOWLEDGEMENTS, COMPETING INTERESTS, AND FUNDING
The authors have no competing interests or disclosures.
References
- Sament S, Schwartz MB. Severe diabetic stupor without ketosis. S Afr Med J. 1957;31:893–4. [PubMed] [Google Scholar]
- Young E, Bradley RF. Cerebral edema with irreversible coma in severe diabetic ketoacidosis. N Engl J Med. 1967;276:665–9. doi: 10.1056/NEJM196703232761204. [DOI] [PubMed] [Google Scholar]
- Halmos PB, Nelson JK, Lowry RC. Hyperosmolar non-ketoacidotic coma in diabetes. Lancet. 1966;1:675–9. doi: 10.1016/s0140-6736(66)91626-6. [DOI] [PubMed] [Google Scholar]
- Maccario M. Neurological dysfunction associated with nonketotic hyperglycemia. Arch Neurol. 1968;19:525–34. doi: 10.1001/archneur.1968.00480050095009. [DOI] [PubMed] [Google Scholar]
- Maccario M, Messis CP, Vastola EF. Focal Seizures as a Manifestation of Hyperglycemia without Ketoacidosis. A Report of Seven Cases with Review of the Literature. Neurology. 1965;15:195–206. doi: 10.1212/wnl.15.3.195. [DOI] [PubMed] [Google Scholar]
- Norris JW, Hachinski VC. Misdiagnosis of stroke. Lancet. 1982;1:328–31. doi: 10.1016/s0140-6736(82)91580-x. [DOI] [PubMed] [Google Scholar]
- Schomer . Niedermeyer’s Electroencephalography: BasicPrinciples, Clinical Applications, and Related Fields. 6. Philadelphia: Lippincott Williams &Wilkins; 2010. [Google Scholar]
- Hedna VS, Shukla PP, Waters MF. Seizure Mimicking Stroke: Role of CT Perfusion. J Clin Imaging Sci. 2012;2:32. doi: 10.4103/2156-7514.97728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royter VL, Waters MF. Stroke vs. status epilepticus. A case report utilizing CT perfusion. J Neurol Sci. 2008;266:174–6. doi: 10.1016/j.jns.2007.08.038. [DOI] [PubMed] [Google Scholar]
- Hassan AE, et al. Regional cerebral hyperperfusion associated with postictal paresis. J Vasc Interv Neurol. 2012;5:40–2. [PMC free article] [PubMed] [Google Scholar]
- Masterson K, Vargas MI, Delavelle J. Postictal deficit mimicking stroke: role of perfusion CT. J Neuroradiol. 2009;36:48–51. doi: 10.1016/j.neurad.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Mathews MS, et al. Local cortical hypoperfusion imaged with CT perfusion during postictal Todd's paresis. Neuroradiology. 2008;50:397–401. doi: 10.1007/s00234-008-0362-1. [DOI] [PubMed] [Google Scholar]
- Rupprecht S, et al. Hemispheric hypoperfusion in postictal paresis mimics early brain ischemia. Epilepsy Res. 2010;89:355–9. doi: 10.1016/j.eplepsyres.2010.02.009. [DOI] [PubMed] [Google Scholar]