Abstract
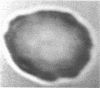
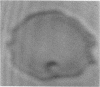
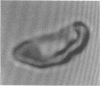
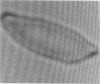
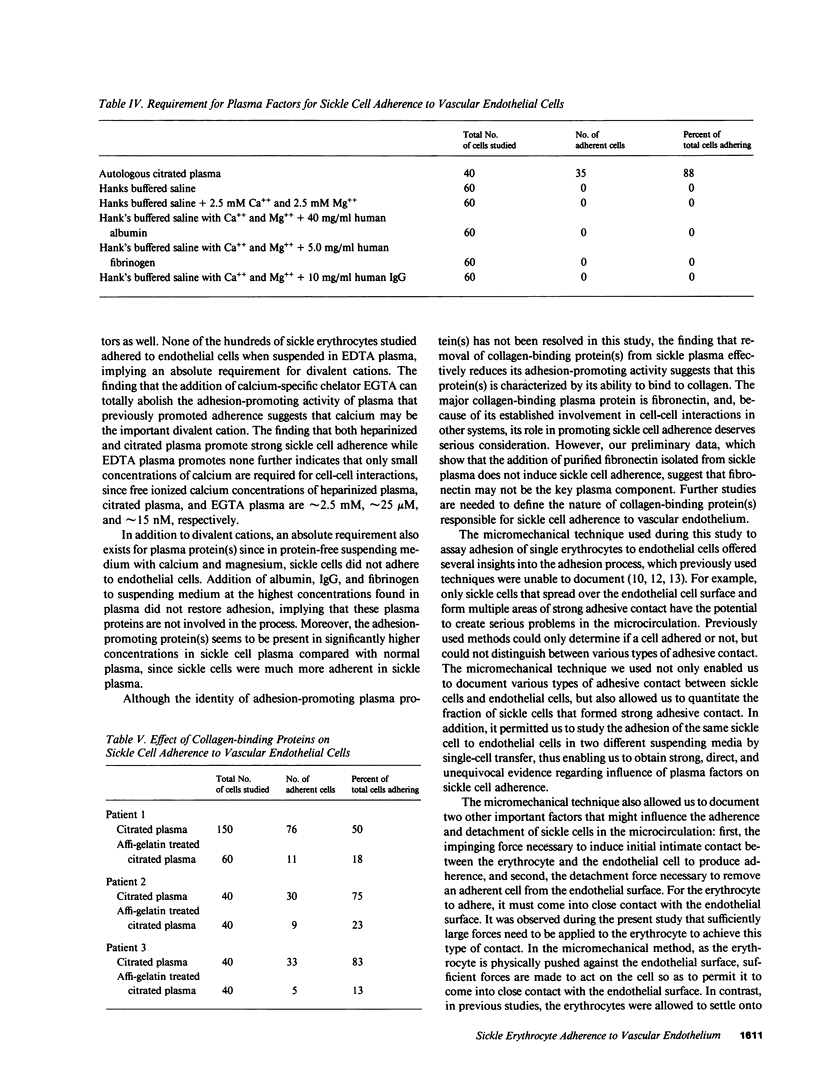
Increased adherence of sickle erythrocytes to vascular endothelium has been suggested by Hebbel and his colleagues to play a role in vasocclusive events of sickle cell disease. To define the role of cell membrane changes and plasma factors in cell adherence, a micropipette technique previously developed by us to obtain a direct, quantitative measure of cell adherence was used to evaluate the adhesivity of different morphologic classes of sickle cells to endothelial cells in various suspending media. Irregularly shaped, deformable sickle cells were four- to fivefold more adherent than discoid sickle cells, whereas rigid irreversibly sickled cells were least adherent. Sickle erythrocytes adhered to endothelial cells when suspended in autologous citrated or heparinized plasma but were totally nonadherent when suspended in autologous EDTA plasma. Removal of the divalent cation chelator and addition of calcium to EDTA plasma restored its ability to promote adhesion, implying an absolute requirement for divalent cations in sickle cell adherence. Sickle cells also did not adhere to endothelial cells in protein-free media containing divalent cations, suggesting an additional requirement for plasma proteins. Removal of collagen-binding proteins from citrated sickle plasma resulted in a three- to fivefold reduction in its ability to promote cell adhesion, suggesting an important role for these plasma proteins in sickle cell adherence. The results of this study imply that sickle cell adherence to vascular endothelium is a complex process in which temporal changes in the numbers of cells identified to be most adhesive and the plasma concentration of protein(s) involved in the adhesive process determine the extent of in vivo sickle cell adherence.
Full text
PDF

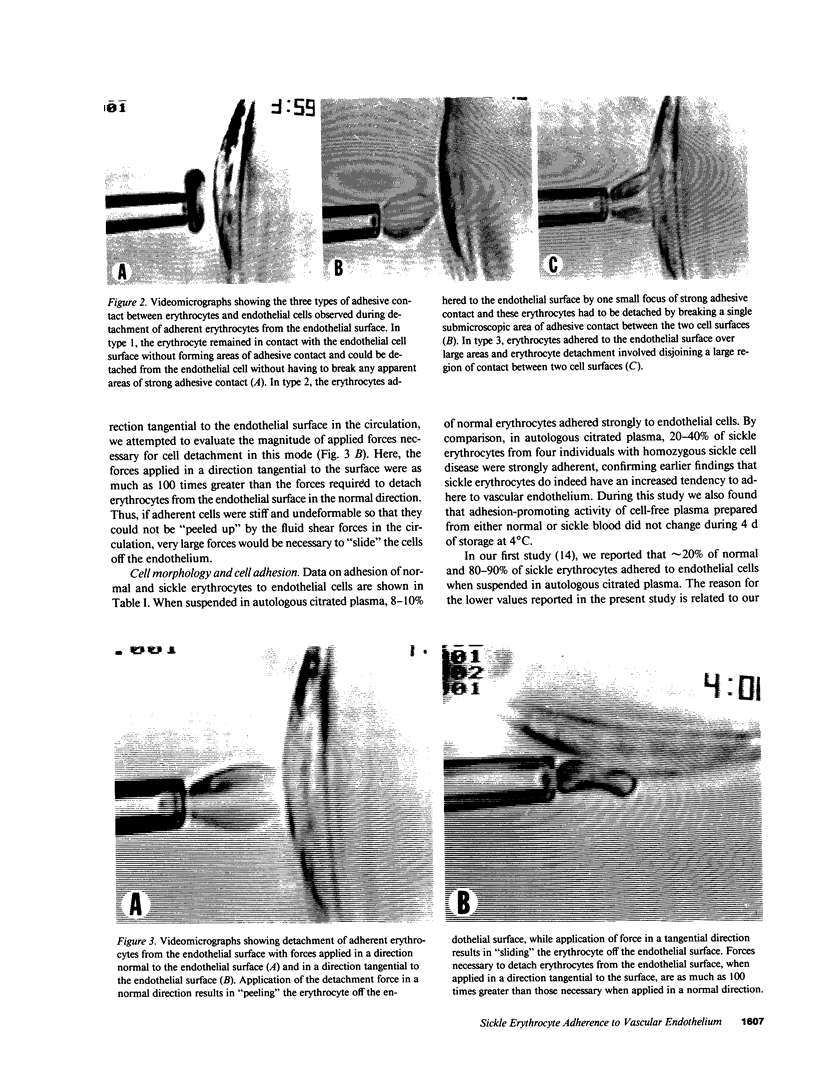

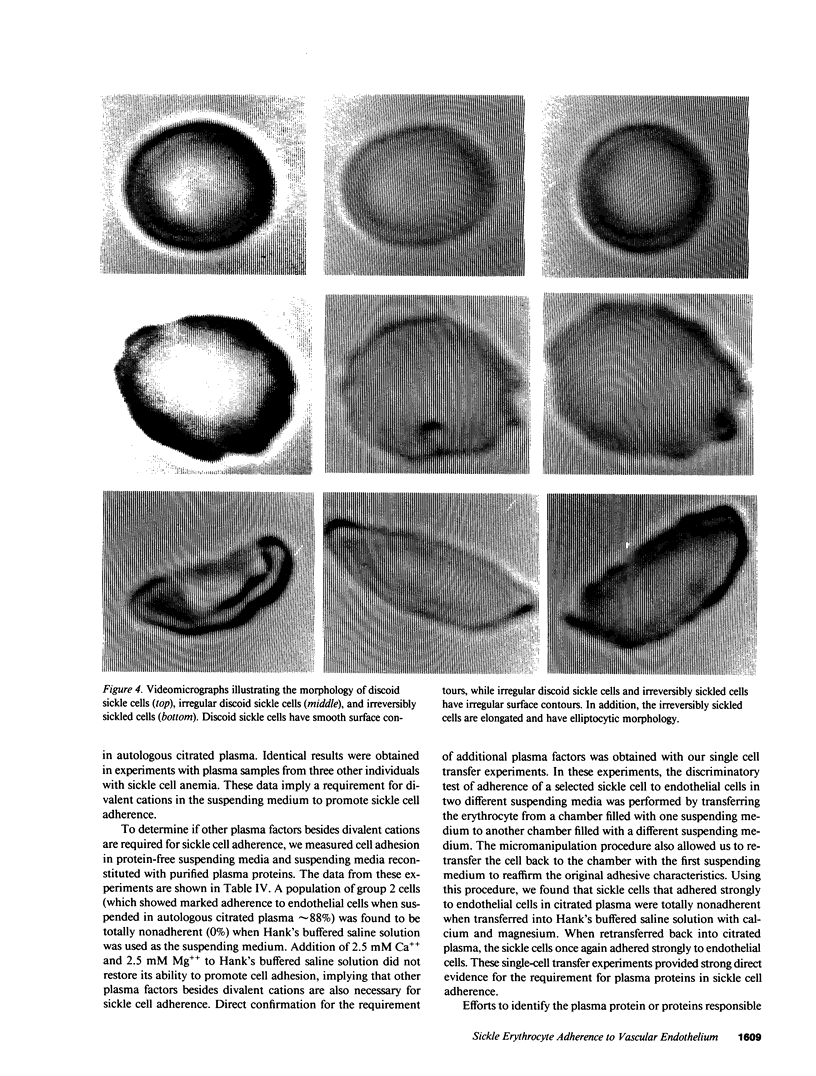
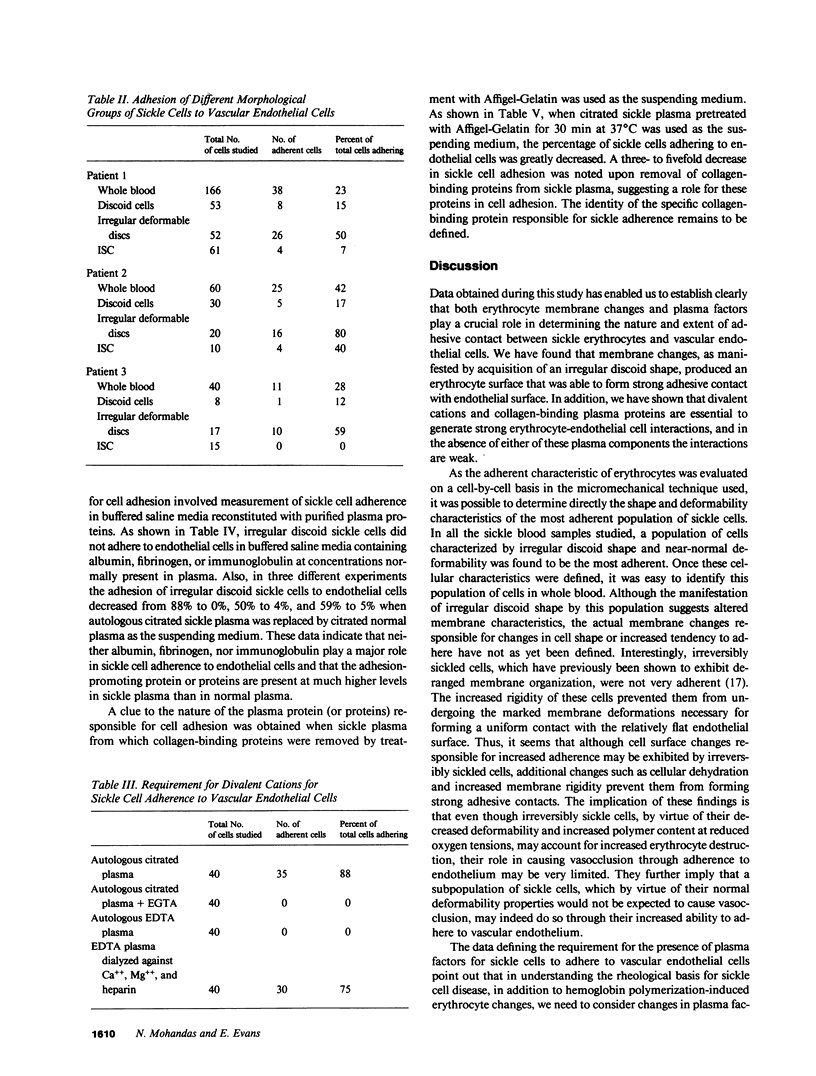

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DIGGS L. W. The crisis in sickle cell anemia; hematologic studies. Am J Clin Pathol. 1956 Oct;26(10):1109–1118. doi: 10.1093/ajcp/26.10.1109. [DOI] [PubMed] [Google Scholar]
- Dean J., Schechter A. N. Sickle-cell anemia: molecular and cellular bases of therapeutic approaches (first of three parts). N Engl J Med. 1978 Oct 5;299(14):752–763. doi: 10.1056/NEJM197810052991405. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Bialecki H., Greenburg G. Purification of the fibroblast growth factor activity from bovine brain. J Biol Chem. 1978 May 25;253(10):3736–3743. [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Bialecki H., Zetter B. R. Factors involved in the modulation of cell proliferation in vivo and in vitro: the role of fibroblast and epidermal growth factors in the proliferative response of mammalian cells. In Vitro. 1978 Jan;14(1):85–118. doi: 10.1007/BF02618177. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P., Boogaerts M. A., Eaton J. W., Steinberg M. H. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N Engl J Med. 1980 May 1;302(18):992–995. doi: 10.1056/NEJM198005013021803. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P., Moldow C. F., Steinberg M. H. Modulation of erythrocyte-endothelial interactions and the vasocclusive severity of sickling disorders. Blood. 1981 Nov;58(5):947–952. [PubMed] [Google Scholar]
- Hebbel R. P., Yamada O., Moldow C. F., Jacob H. S., White J. G., Eaton J. W. Abnormal adherence of sickle erythrocytes to cultured vascular endothelium: possible mechanism for microvascular occlusion in sickle cell disease. J Clin Invest. 1980 Jan;65(1):154–160. doi: 10.1172/JCI109646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover R., Rubin R., Wise G., Warren R. Adhesion of normal and sickle erythrocytes to endothelial monolayer cultures. Blood. 1979 Oct;54(4):872–876. [PubMed] [Google Scholar]
- Klug P. P., Lessin L. S., Radice P. Rheological aspects of sickle cell disease. Arch Intern Med. 1974 Apr;133(4):577–590. [PubMed] [Google Scholar]
- Lux S. E., John K. M., Karnovsky M. J. Irreversible deformation of the spectrin-actin lattice in irreversibly sickled cells. J Clin Invest. 1976 Oct;58(4):955–963. doi: 10.1172/JCI108549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy P. R., Sherman A. S. Irreversibly sickled cells and red cell survival in sickle cell anemia: a study with both DF32P and 51CR. Am J Med. 1978 Feb;64(2):253–258. doi: 10.1016/0002-9343(78)90053-0. [DOI] [PubMed] [Google Scholar]
- Mohandas N., Evans E. Adherence of sickle erythrocytes to vascular endothelial cells: requirement for both cell membrane changes and plasma factors. Blood. 1984 Jul;64(1):282–287. [PubMed] [Google Scholar]
- Noguchi C. T., Schechter A. N. The intracellular polymerization of sickle hemoglobin and its relevance to sickle cell disease. Blood. 1981 Dec;58(6):1057–1068. [PubMed] [Google Scholar]
- Richardson S. G., Matthews K. B., Stuart J., Geddes A. M., Wilcox R. M. Serial changes in coagulation and viscosity during sickle-cell crisis. Br J Haematol. 1979 Jan;41(1):95–103. doi: 10.1111/j.1365-2141.1979.tb03685.x. [DOI] [PubMed] [Google Scholar]
- Rickles F. R., O'Leary D. S. Role of coagulation system in pathophysiology of sickle cell disease. Arch Intern Med. 1974 Apr;133(4):635–641. [PubMed] [Google Scholar]
- Serjeant G. R., Serjeant B. E., Milner P. F. The irreversibly sickled cell; a determinant of haemolysis in sickle cell anaemia. Br J Haematol. 1969 Dec;17(6):527–533. doi: 10.1111/j.1365-2141.1969.tb01403.x. [DOI] [PubMed] [Google Scholar]
- Steinberg M. H., Dreiling B. J., Lovell W. J. Sickle cell anemia: erythrokinetics, blood volumes, and a study of possible determinants of severity. Am J Hematol. 1977;2(1):17–23. doi: 10.1002/ajh.2830020103. [DOI] [PubMed] [Google Scholar]
- Steinberg M. H., Dreiling B. J., Morrison F. S., Necheles T. F. Mild sickle cell disease. Clinical and laboratory studies. JAMA. 1973 Apr 16;224(3):317–321. [PubMed] [Google Scholar]