Summary
Foot ulcers are a severe complication of diabetic patients resulting from nerve and tendon pathologic alterations. In diabetic patients the tendons are thicker, shorter and have increased stiffness. We examined C57BL/KsJ (BKS.Cg-Dock7m +/+ Leprdb/J) (db/db) mice tendons to determine whether they are an animal model for human diabetic tendon changes. We hypothesized that the Achilles tendons of db/db diabetic mice would be thicker, stiffer, fail at lower loads and stresses, and have degenerative changes compared to control mice. Biomechanical and histologic analyses of the Achilles tendons of 16 week old db/db and control male mice were performed. There was a significant increase in tendon diameter and significant decreases in maximum load, tensile stress, stiffness and elastic modulus in tendons from diabetic mice compared to controls. Mild degenerative and neutrophil infiltration was observed near the tendon insertions on the calcaneous in 25% of db/db mice. In summary, hyper-glycemia and obesity lead to severe changes in db/db mice will be a useful model to examine mechanisms for tendon alterations.
Keywords: biomechanics, db/db, diabetes, mice, pathology, tendon
Introduction
The World Health Organization estimates that diabetes affects more than 370 million people worldwide, including 60 million in the European region. According to the CDC 25.8 million Americans have diabetes with over 1.8 million cases reported annually1. Diabetes leads to numerous secondary disease conditions including but not limited to retinopathy, neuropathy, poor healing, cardiovascular disease, renal failure, and foot ulcers. Although typically not fatal, one of the more debilitating consequences of diabetes is the development of foot ulcers. The incidence of foot ulcers in diabetic patients ranges from 2.5 to 10.7% and those requiring amputation from 0.25–1.8%2. Although the cause of the ulcers is poorly understood, it is believed to be a combination of neurologic deterioration in the extremities and alteration of tendon structure. The gastroc soleus tendon (Achilles tendon) from diabetic humans are thicker, shorter and have increased stiffness3,4. The combined alterations in proprioception and tendon mechanics leads to an altered gait described as an early Windlass mechanism maintained throughout the entire gait cycle3. Treatment of ulcers once they have developed has limitations as amputation is unfortunately still the resolution in many patients. Prevention or treatment of the underlying tendon injury prior to ulcer development would be a significant improvement for these diabetic patients.
Rodents have been used to model many diseases found in diabetic humans. In this manuscript the potential for using mice to model the diabetic tendon changes was investigated. One of the commonly used mouse models of type 2 diabetes mellitus is the db/db mouse. Db/db mice have a genetic mutation in the leptin gene receptor leading to early onset of diabetes by 6 weeks of age5. Db/db mice are insulin resistant, have increased blood glucose, and are obese. Characterization of tendon alterations in diabetic mice has been limited to one previous publication on the ob/ob mouse6. In that study the histologic features of the Achilles tendon of ob/ob mice revealed tenocyte degeneration, chondrocyte metaplasia in the tendon, vascular proliferation, and tendon insertional ruptures6.
The gold standard for assessing tendon function is the measurement of biomechanical properties. To our knowledge, the structural and material mechanical properties of tendons from db/db or other diabetic mice have not been examined. In this study we investigated the biomechanical properties and histology of the Achilles tendon. We hypothesized that the tendons of db/db mice would be thicker and exhibit diminished structural (stiffness, maximum load) and material (modulus, maximum stress) properties compared to non-diabetic control mice. We also hypothesized that there would be degenerative histologic changes in the Achilles tendon.
Materials and methods
Mice
Male db/db [5 week old; background strain C57BL/KsJ (BKS.Cg-Dock7m +/+ Leprdb/J)] (n=16) and age-matched non-diabetic lean control mice (n=18) were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were housed in shoebox cages with wood chips (Sani-chips) in a temperature controlled room (22–23° C) with 12:12 h light cycle for 11 weeks prior to analysis. Mice were free from pathogenic agents including ectromelia virus, epizootic diarrhea of infant mice virus, lymphocytic choriomeningitis virus, Mycoplasma pulmonis, mouse adenovirus strain 1 and 2, mouse hepatitis virus, mouse parvovirus, minute virus of mice, polyoma virus, pneumonia virus of mice, reovirus type 3, Theiler’s murine encephalomyelitis virus, sendai virus, endoparasites and ectoparasites. Mice were euthanized at 16 weeks of age and tissues collected for analysis. All experimental procedures were approved by the Wright State University Institutional Animal Care and Use Committee. This study meets the ethical standards of the Journal7.
Glucose measurements
Prior to euthanasia at 16 weeks of age, glucose and weight measurements were conducted on all mice. For glucose measurements, blood samples were taken from a cut made on the tip of the tail and the glucose concentration determined using a Free Style Lite® Blood Glucose Monitor (Abbott Diabetes Care, CA).
Biomechanical testing
Both hind limbs of 5 db/db and 6 wild type mice were harvested and stored at ≤ −65°C until use. Specimens were thawed in a refrigerator overnight prior to testing. The skin was removed and the Achilles tendon with proximal (gastrocnemius muscle) and distal (calcaneous) attachments was isolated. Tendon diameter (medial-lateral) was measured using a laser micrometer (Model LS7030-MT; Keyence, Elmwood Park, NJ). Following an established procedure8, a custom grip comprised of a brass block (12.7 × 12.7 × 23.7 mm) with a hollow conical shape was used to hold the calcaneous during testing. The tendon was threaded through a small opening (1.75 mm diameter) in the block using a suture. The intramuscular gastrocnemius and soleus tendon fibers were then secured in a pneumatic grip mounted to a 50 N load cell on a materials testing system (ElectroPuls E3000; Instron, Norwood, MA). Initial specimen length was measured using digital calipers with the specimen clamped in the upper grip and brass block grip hanging from the other tendon end (0.196N force while hanging). The brass block was then secured in another pneumatic grip fixed to the load frame. The tendon was manually preloaded to 0.5 N to remove slack and then loaded in tension at a rate of 0.1 mm/sec until tendon failure. No specimen preconditioning beyond the preload was performed. Specimen hydration was maintained throughout testing via periodic misting with physiologic saline.
Tendon structural properties were calculated from the load-displacement data obtained from mechanical testing. Maximum load was defined as the load at which the tendon failed, and stiffness was calculated as the slope of the linear region of the load-displacement curve. Additionally, material properties were calculated by converting the load-displacement data into stress-strain data using the measured tendon dimensions. Stress was calculated as the force divided by cross-sectional area. Maximum stress was defined as the stress at tendon failure and tensile strain was defined as the corresponding strain at failure. Elastic modulus was calculated as the slope of the linear region of the stress-strain curve. For stiffness and elastic modulus calculations, the linear portion of the curve was defined in a region between 20 and 80% from the peak load (for load-displacement curves) or peak stress (for stress-strain curves). The corresponding x-y coordinates at these two points were used to draw a line tangent to the curve, and the slope of this line is reported as the stiffness or elastic moduli, respectively. Both structural and material properties describe the behavior of the tendons under loading, with the material properties allowing more exact comparisons of tendons independent of specimen size and shape.
Histology
Both right and left Achilles tendons were collected from 6 db/db and 6 wild type control C57BL/6 mice. In addition, the left Achilles tendon was harvested after euthanasia from 5 db/db and 6 wild type mice. The tendons were fixed in 10% neutral buffered formalin, decalcified in Cal-Rite (Richard Allan Scientific, Kalamazoo, MI) solution for 48 hours, dehydrated through a gradient of alcohols and xylene, and embedded in paraffin. Slides were sectioned at 5 µm, and stained with eosin and hematoxylin by AML Laboratories (Baltimore, MD). Three step serial sections 150 µm apart were examined. The tendon mid-substance and insertion were examined for alterations in cell density, inflammatory cell infiltration, and extracellular matrix organization. Changes were scored subjectively as no differences, mild, moderate or severe changes. Qualitative scoring was performed blinded to group and was performed by one reviewer to maintain consistency.
Statistics
Biomechanical specimens from the two hindlimbs within an animal were considered to be replicates. A two-tailed ANOVA confirmed that there were no differences in any biomechanical property due to hindlimb side (p≥0.118 for all biomechanical parameters). Therefore, the biomechanical values for left and right tendons were averaged for each animal. All data were determined to be normally distributed by Shapiro-Wilk or Kolmogorov-Smirnov tests. A one-tailed Student’s T-test analysis was used to detect statistical differences in these biomechanical data with significance level set at p<0.05. Results are presented as the mean ± standard deviation. Additional analysis included a Pearson’s correlation to examine relationships between glucose, body weight and bio-mechanical properties. Significance was set at p<0.05.
Results
Db/db mice (n=5) and control lean mice (n=6) were examined for weight, glucose levels, and tendon bio-mechanical properties. At 16 weeks, body weight was increased for all db/db mice compared to controls (39.7±1.6 g and 27.3±3.9 g respectively, p<0.001). Blood glucose levels in db/db mice were significantly elevated compared to control mice (576±45 mg/dl and 135±9 mg/dl respectively, p<0.001).
Tendon failure occurred at least 1 mm from the grip edges in all cases, and no slippage from the grips was observed during testing. Maximum load (p=0.010), elastic modulus (p=0.001), maximum stress (p=0.001), and stiffness (p=0.004) were significantly decreased in the db/db mice compared to controls (Tab. 1). Tendon diameter (p=0.001) was significantly increased in the db/db mice compared to controls (Tab. 1). No significant difference was detected in tensile strain (Tab. 1). A Pearson’s correlation was conducted to compare relationship between glucose and biomechanical properties, and between body weight and biomechanical properties. There were no significant relationships for any comparison, however two comparisons had values that approached significance (maximum load and body weight: R= −0.76, p=0.06; and tendon diameter and body weight: R=−0.83, p=0.08).
Table 1.
Structural and material properties of control and db/db mice (mean ± SD).
| Length (mm) | Diameter (mm) | Maximum Load (N) | Stiffness (N/mm) | Tensile stress (MPa) | Tensile strain (mm/mm) | Elastic modulus (MPa) | |
|---|---|---|---|---|---|---|---|
| Control | 4.4±0.9 | 1.2±0.1 | 8.1±0.6 | 3.9±0.7 | 7.8±1.4 | 0.6±0.1 | 16.0±3.7 |
| db/db | 4.4±1.0 | 1.4±0.1 | 6.6±1.0 | 2.6±0.4 | 4.6±1.0 | 0.7±0.2 | 8.0±1.1 |
| p value | N/A | 0.001 | 0.010 | 0.004 | 0.001 | 0.144 | 0.001 |
| Power | N/A | 0.986 | 0.838 | 0.925 | 0.989 | 0.274 | 0.995 |
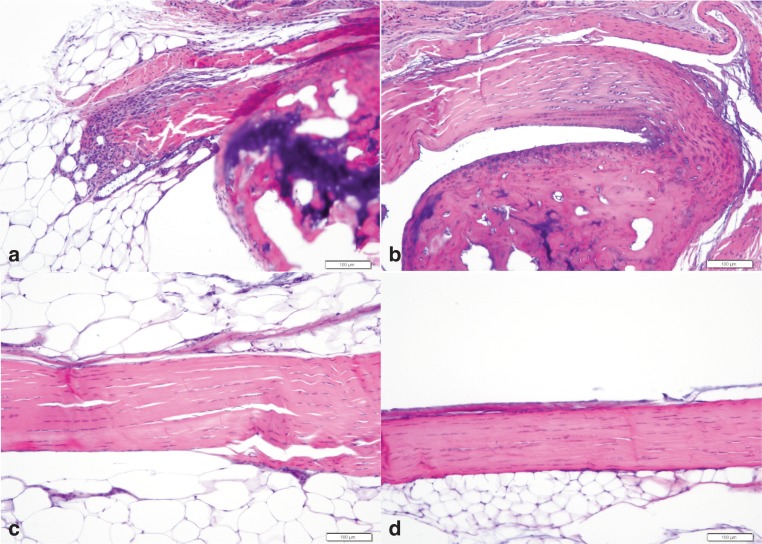
Longitudinal sections of the Achilles tendon were examined for pathologic changes. Unfortunately sections of the insertion were difficult to obtain in several mice because of sectioning difficulties. Pathologic changes were observed at or near the insertion site in 3 of 12 db/db Achilles tendons and 0 of 10 wildtype tendons (Fig. 1a, b). The histologic alteration in all 3 db/db tendons were characterized by mild neutrophil infiltration, mild disorganization of the collagen fibers, mild increased basophilia of the tenocytes, and mild increased nuclear size/rounding. These pathologic changes are consistent with degenerative tendon. In addition, in 1 db/db and 1 wild type mouse there was mild neutrophil infiltration in the fat pad adjacent to the tendon. Nearly all mice had analyzable sections which included the Achilles tendon midstubstance and origin. There were no histologic changes in the midsubstance or origin of the Achilles tendon in any of the 15 db/db tendons or 16 wild type tendons examined (Fig. 1c, d).
Figure 1.
Representative Achilles tendon histologic photographs of db/db (a, c) and wild type (b, d) C57Bl/6J mice. Figure (a) and (b) are at the calcaneus insertion and figures (c) and (d) are of the midsubstance. Note the mild neutrophil infiltration and disruption of the normal architecture in figure a. H&E, bar= 100µ.
Discussion
Results showed that the Achilles tendon of db/db mice at 16 weeks of age had inferior biomechanical properties compared to control mice. There was a significant decrease in maximum load, maximum stress, elastic modulus and stiffness, and an increase in tendon diameter in the diabetic mice. Alterations in tendon histology were also observed in 25% of the db/db mice but there were no histologic changes in the mid-substance. Interestingly though, in contrast to the histologic observations of insertional tendon changes, the tendons primarily failed in the mid-tissue and not at the insertion. Our observed failure mode indicates that the tendons were weakest within the soft tissue.
Limitations of the study were few. The initial tendon length was used to determine strain rather than the preloaded length of the tendon in the mechanical testing fixture. This may lead to overestimation of strain. However, this is believed to be consistent between both the control and db/db Achilles tendon so did not influence the observed differences. Furthermore, the reported strain is a bulk property calculated from grip-to-grip measurements as local strain measurement was not performed. The authors realize the accuracy limitations of this approach, but since no specimen slippage was observed in the singular clamped end this approach yielded a valid approximation of specimen behavior. We also only examined mice at one age. We would hypothesize that older db/db mice will likely have further alterations as advanced glycation end-products further impact the collagen in the tendon, however, that has not been examined.
Histologic lesions correlated well with the histologic findings of Ji et al. who observed degenerative Achilles tendon alterations at the insertion site in the ob/ob mice6. One aspect observed in the ob/ob mice that was not seen in the db/db mice was the presence of microruptures. This difference may be due to the age of the mouse (12 weeks versus 16 weeks), the sex of the mouse (not identified in the manuscript by Ji et al.), different genotype (ob/ob versus db/db), and number of animals examined (this also was not identified in the Ji et al. manuscript)6.
Although there are no other biomechanical studies in diabetic mice to our knowledge, our results are also consistent with data accumulated from rat diabetic models. Diabetes in rats is commonly induced with an injection of streptozotocin to destroy β islet cells9,10. This quickly leads to insulin deficiency and a non-controlled diabetic state consistent with type 1 diabetes. In one study, the biomechanical properties of the Achilles tendon of rats (Wistar lineage) 10 weeks after streptozotocin induced diabetes were examined1. Diabetic rats had decreased elastic modulus, and higher maximum strain compared with controls9. Similar studies were conducted by another group in Lewis rats in which the rats were euthanized 12 and 19 days following diabetes induction. The rat patellar tendon had a reduced Young’s modulus, but other parameters were not affected10. The lack of effect in the parameters was speculated to be due to the short time after diabetes development. These same rats also were used to analyze repair ability of the supraspinatus tendon11. The enthesis of diabetic animals had a significantly reduced ultimate load-to-failure and stiffness compared with control animals. One of the short comings of streptozotocin use is the relatively short length of time that animals can be followed after diabetes induction, cytotoxic effect on other cells in addition to the beta islet cells, and potential re-growth of beta islet cells12.
In addition to the rat study in vitro analysis of rabbit Achilles tendons have been performed. New Zealand white rabbit Achilles tendon were exposed to ribose in the media compared with control media for 8 weeks. Biomechanical testing of the glycated tendons (those exposed to ribose) showed increased maximum load, stress, strain, modulus, and toughness13. This data demonstrates the ability of glycogen to effect tendon biomechanics even in vitro further supporting the role of advanced glycation end-products in Achilles tendon alterations.
The initiating cause of diabetic foot ulcers in humans is multimodal. A combination of neurologic, infectious, structural, vascular and physiologic changes resulting from the diabetes are believed to play a role14,15. For example, epidemiologic studies in diabetics with foot ulcers show the prevalence of microvascular and macrovascular complications is about 46% and 65%, respectively. Further, among those with a lower extremity amputation, the prevalence of microvascular and macrovascular complications is about 46% and 76%, respectively5. The vascular change is speculated to lead to poor oxygenation of the tendon and thus alteration of the extra-cellular matrix composition including collagen.
The other component of altered gait is the change in tendon and joint mobility. Diabetic patients without neuropathy were examined for alteration in tendon and muscle stretch distribution during walking. Tendon length changes were attenuated in diabetic patients compared to controls4. There is also increased thickness and stiffness of the Achilles tendon3,16, and decreased joint mobility17, which alters the biomechanics of the foot-ankle complex. Changes in tendon mechanical properties are caused by non-enzymatic glycosylation of collagen with advanced glycation end-products, which causes an increase in collagen crosslinks17. Combined with the neurologic and vascular alterations, these changes are believed to all contribute to increasing susceptibility to foot ulcers.
Tendon stiffness is central to proper function, and how tendon structure relates to function is critical to understanding tendon pathology18. In humans, the Achilles tendon thickness is increased as a result of running in both over weight and normal weight adult males, and also in sedentary over weight adult males19. There is also an increase stiffness of tendons as a result of chronic diabetes3,16,20. Interestingly there was a reduced stiffness of Achilles tendons in the db/db mice, opposite of what was originally hypothesized. We also found that db/db mice tendons had significantly reduced elastic modulus. This difference between human diabetic patients who have increased tendon stiffness3,16,20 and reduced stiffness or modulus in rodent models9–11 including our study requires further analysis to determine the cause.
The results of this study show that the db/db mouse is a potential diabetic model for future studies to improve the tendon histologic and biomechanical alterations. Future studies will include determine the progression of the disease in db/db mice and analysis of therapeutic effects on tendon biomechanical, biochemical and histologic parameters.
References
- 1. http://www.cdc.gov/diabetes/pubs/estimates11.htm#1 accessed December 7, 2011.
- 2.Hunt DL. Diabetes: foot ulcers and amputations. Clin Evidence. 2011;8:1–43. 602. [PMC free article] [PubMed] [Google Scholar]
- 3.D’Ambrogi E, Giacomozzi C, Macellari V, Uccioli L. Abnormal foot function in diabetic patients: the altered onset of Windlass mechanism. Diabet Med. 2005;22:1713–1719. doi: 10.1111/j.1464-5491.2005.01699.x. [DOI] [PubMed] [Google Scholar]
- 4.Cronin NJ, Peltonen J, Ishikawa M, et al. Achilles tendon length changes during walking in long-term diabetes patients. Clin Biomech. 2010;25:476–482. doi: 10.1016/j.clinbiomech.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Coleman DL, Hummel KP. Hyperinsulinemia in pre-weaning diabetes (db) mice. Diabetologia. 1974;10(Suppl):607–610. doi: 10.1007/BF01221993. [DOI] [PubMed] [Google Scholar]
- 6.Ji J, Wang Z, Shi D, Gao X, Jiang Q. Pathologic changes of Achilles tendon in leptin-deficient mice. Rheumatol Int. 2010;30:489–493. doi: 10.1007/s00296-009-1001-9. [DOI] [PubMed] [Google Scholar]
- 7.Padulo J, Oliva F, Frizziero A, Maffulli N. Muscles, Ligaments and Tendons Journal. Basic principles and recommendations in clinical and field science research. MLTJ. 2013;4:250–252. [PMC free article] [PubMed] [Google Scholar]
- 8.Probst A, Palmes D, Freise H, Langer M, Joist A, Spiegel HU. A New Clamping Technique for Biomechanical Testing of Tendons in Small Animals. J Investigative Surg. 2000;13:313–318. doi: 10.1080/089419300750059352. [DOI] [PubMed] [Google Scholar]
- 9.De Oliveira RR, de Lira KDS, de Castro Silveira PV, et al. Mechanical properties of Achilles tendon in rats induced to experimental diabetes. Ann Biomed Eng. 2011;39:1528–1534. doi: 10.1007/s10439-011-0247-z. [DOI] [PubMed] [Google Scholar]
- 10.Fox AJS, Bedi A, Deng XH, et al. Diabetes mellitus alters the mechanical properties of the native tendon in an experimental rat model. J Orth Res. 2011;29:880–885. doi: 10.1002/jor.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bedi A, Fox AJS, Harris PE, et al. Diabetes mellitus impairs tendon-bone healing after rotator cuff repair. J Shoulder Elbow Surg. 2010;19:978–988. doi: 10.1016/j.jse.2009.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srinivasan K, Ramarao P. Animal models in type 2 diabetes research: an overview. Indian J Med Res. 2007;125:451–472. [PubMed] [Google Scholar]
- 13.Reddy GK. Cross-linking in collagen by nonenzymatic glycation increases the matrix stiffness in rabbit Achilles tendon. Exp Diab Res. 2004;5:143–153. doi: 10.1080/15438600490277860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turns M. The diabetic foot: an overview of assessment and complications. Br J Nurs. 2011;20(15):S19–25. doi: 10.12968/bjon.2011.20.Sup8.S19. [DOI] [PubMed] [Google Scholar]
- 15.Wrobel JS, Najafi B. Diabetic foot biomechanics and gait dysfunction. J Diabetes Sci Tech. 2010;4:833–845. doi: 10.1177/193229681000400411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramirez LC, Raskin P. Diabetic foot tendinopathy: abnormalities in the flexor plantar tendons in patients with diabetes mellitus. J Diabetes Complications. 1998;12:337–339. doi: 10.1016/s1056-8727(98)00024-5. [DOI] [PubMed] [Google Scholar]
- 17.Abate M, Schiavone C, Pelotti P, Salini V. Limited joint mobility (LJM) in elderly subjects with type II diabetes mellitus. Arh Gerontology Geriatrics. 2011;53:135–140. doi: 10.1016/j.archger.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Rigozzi S, Müller R, Snedeker JG. Collagen fibril morphology and mechanical properties of the Achilles tendon in two inbred mouse strains. J Anat. 2010;216:724–731. doi: 10.1111/j.1469-7580.2010.01225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abate M, Oliva F, Schiavone C, Salini V. Achilles tendinopathy in amateur runners: role of adiposity (Tendinopathies and obesity) MLTJ. 2012;2(1):44–48. [PMC free article] [PubMed] [Google Scholar]
- 20.Gefen A, Megido-Ravid M, Azariah M, Itzchak Y, Arcan M. Integration of plantar soft tissue stiffness measurements in routine MRI of the diabetic foot. Clin Biomech. 2001;16:921–925. doi: 10.1016/s0268-0033(01)00074-2. [DOI] [PubMed] [Google Scholar]