Abstract
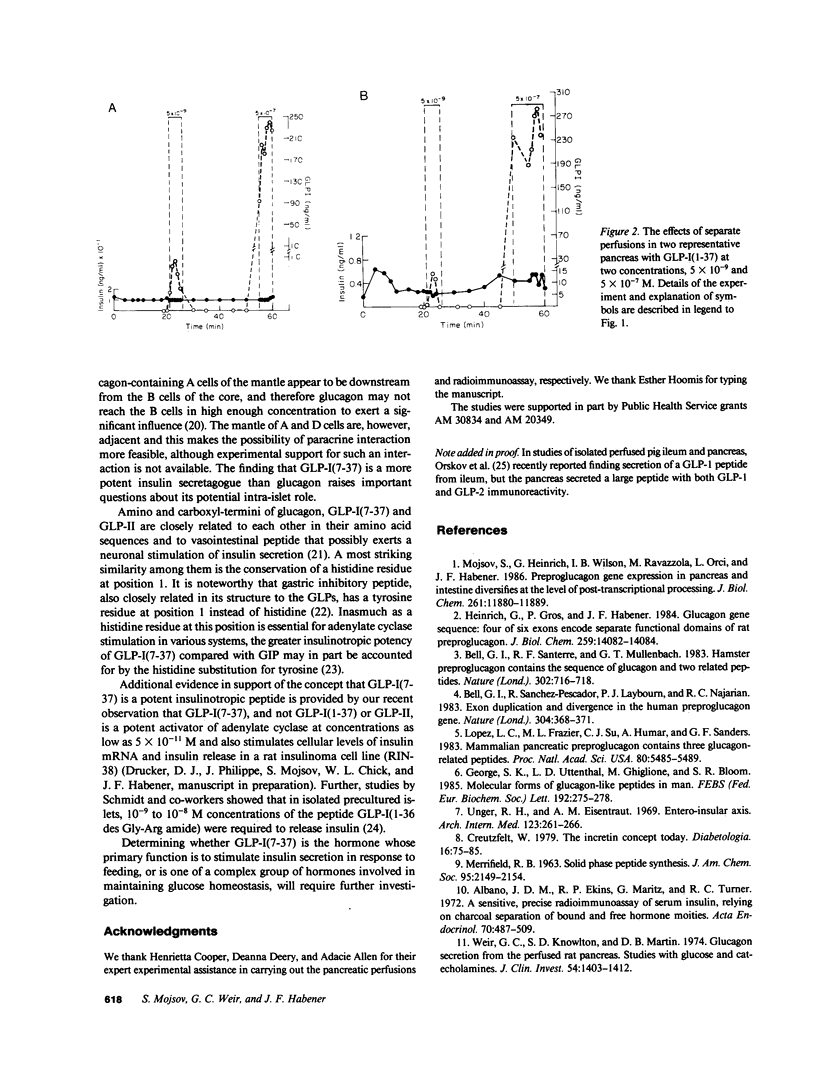
Insulin secretion is controlled by a complex set of factors that include not only glucose but amino acids, catecholamines, and intestinal hormones. We report that a novel glucagon-like peptide, co-encoded with glucagon in the glucagon gene is a potent insulinotropic factor. The glucagon gene encodes a proglucagon that contains in its sequence glucagon and additional glucagon-like peptides (GLPs). These GLPs are liberated from proglucagon in both the pancreas and intestines. GLP-I exists in at least two forms: 37 amino acids GLP-I(1-37), and 31 amino acids, GLP-I(7-37). We studied the effects of synthetic GLP-Is on insulin secretion in the isolated perfused rat pancreas. In the presence of 6.6 mM glucose, GLP-I(7-37) is a potent stimulator of insulin secretion at concentrations as low as 5 X 10(-11) M (3- to 10-fold increases over basal). GLP-I(1-37) had no effect on insulin secretion even at concentrations as high as 5 X 10(-7) M. The earlier demonstration of specific liberation of GLP-I(7-37) in the intestine and pancreas, and the magnitude of the insulinotropic effect at such low concentrations, suggest that GLP-I(7-37) participates in the physiological regulation of insulin secretion.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano J. D., Ekins R. P., Maritz G., Turner R. C. A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol (Copenh) 1972 Jul;70(3):487–509. doi: 10.1530/acta.0.0700487. [DOI] [PubMed] [Google Scholar]
- Andersen D. K., Elahi D., Brown J. C., Tobin J. D., Andres R. Oral glucose augmentation of insulin secretion. Interactions of gastric inhibitory polypeptide with ambient glucose and insulin levels. J Clin Invest. 1978 Jul;62(1):152–161. doi: 10.1172/JCI109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Sanchez-Pescador R., Laybourn P. J., Najarian R. C. Exon duplication and divergence in the human preproglucagon gene. 1983 Jul 28-Aug 3Nature. 304(5924):368–371. doi: 10.1038/304368a0. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Santerre R. F., Mullenbach G. T. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature. 1983 Apr 21;302(5910):716–718. doi: 10.1038/302716a0. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S., Orci L. New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes. 1982 Oct;31(10):883–889. doi: 10.2337/diab.31.10.883. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W. The incretin concept today. Diabetologia. 1979 Feb;16(2):75–85. doi: 10.1007/BF01225454. [DOI] [PubMed] [Google Scholar]
- Dupre J., Ross S. A., Watson D., Brown J. C. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab. 1973 Nov;37(5):826–828. doi: 10.1210/jcem-37-5-826. [DOI] [PubMed] [Google Scholar]
- George S. K., Uttenthal L. O., Ghiglione M., Bloom S. R. Molecular forms of glucagon-like peptides in man. FEBS Lett. 1985 Nov 18;192(2):275–278. doi: 10.1016/0014-5793(85)80124-1. [DOI] [PubMed] [Google Scholar]
- Heinrich G., Gros P., Habener J. F. Glucagon gene sequence. Four of six exons encode separate functional domains of rat pre-proglucagon. J Biol Chem. 1984 Nov 25;259(22):14082–14087. [PubMed] [Google Scholar]
- Kawai K., Ipp E., Orci L., Perrelet A., Unger R. H. Circulating somatostatin acts on the islets of Langerhans by way of a somatostatin-poor compartment. Science. 1982 Oct 29;218(4571):477–478. doi: 10.1126/science.6126931. [DOI] [PubMed] [Google Scholar]
- Lopez L. C., Frazier M. L., Su C. J., Kumar A., Saunders G. F. Mammalian pancreatic preproglucagon contains three glucagon-related peptides. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5485–5489. doi: 10.1073/pnas.80.18.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojsov S., Heinrich G., Wilson I. B., Ravazzola M., Orci L., Habener J. F. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem. 1986 Sep 5;261(25):11880–11889. [PubMed] [Google Scholar]
- Moody A. J., Thim L., Valverde I. The isolation and sequencing of human gastric inhibitory peptide (GIP). FEBS Lett. 1984 Jul 9;172(2):142–148. doi: 10.1016/0014-5793(84)81114-x. [DOI] [PubMed] [Google Scholar]
- Orskov C., Holst J. J., Knuhtsen S., Baldissera F. G., Poulsen S. S., Nielsen O. V. Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology. 1986 Oct;119(4):1467–1475. doi: 10.1210/endo-119-4-1467. [DOI] [PubMed] [Google Scholar]
- Pandol S. J., Seifert H., Thomas M. W., Rivier J., Vale W. Growth hormone-releasing factor stimulates pancreatic enzyme secretion. Science. 1984 Jul 20;225(4659):326–328. doi: 10.1126/science.6204379. [DOI] [PubMed] [Google Scholar]
- Pederson R. A., Brown J. C. The insulinotropic action of gastric inhibitory polypeptide in the perfused isolated rat pancreas. Endocrinology. 1976 Sep;99(3):780–785. doi: 10.1210/endo-99-3-780. [DOI] [PubMed] [Google Scholar]
- Penhos J. C., Wu C. H., Basabe J. C., Lopez N., Wolff F. W. A rat pancreas-small gut preparation for the study of intestinal factor(s) and insulin release. Diabetes. 1969 Nov;18(11):733–738. doi: 10.2337/diab.18.11.733. [DOI] [PubMed] [Google Scholar]
- Samols E., Marri G., Marks V. Interrelationship of glucagon, insulin and glucose. The insulinogenic effect of glucagon. Diabetes. 1966 Dec;15(12):855–866. doi: 10.2337/diab.15.12.855. [DOI] [PubMed] [Google Scholar]
- Schebalin M., Said S. I., Makhlouf G. M. Stimulation of insulin and glucagon secretion by vasoactive intestinal peptide. Am J Physiol. 1977 Feb;232(2):E197–E200. doi: 10.1152/ajpendo.1977.232.2.E197. [DOI] [PubMed] [Google Scholar]
- Schmidt W. E., Siegel E. G., Creutzfeldt W. Glucagon-like peptide-1 but not glucagon-like peptide-2 stimulates insulin release from isolated rat pancreatic islets. Diabetologia. 1985 Sep;28(9):704–707. doi: 10.1007/BF00291980. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Eisentraut A. M. Entero-insular axis. Arch Intern Med. 1969 Mar;123(3):261–266. [PubMed] [Google Scholar]
- Unger R. H., Orci L. Possible roles of the pancreatic D-cell in the normal and diabetic states. Diabetes. 1977 Mar;26(3):241–244. doi: 10.2337/diab.26.3.241. [DOI] [PubMed] [Google Scholar]
- Weir G. C., Knowlton S. D., Martin D. B. Glucagon secretion from the perfused rat pancreas. Studies with glucose and catecholamines. J Clin Invest. 1974 Dec;54(6):1403–1412. doi: 10.1172/JCI107887. [DOI] [PMC free article] [PubMed] [Google Scholar]