Abstract
Background
Induction of labour is carried out for a variety of indications and using a range of pharmacological, mechanical and other methods. For women at low risk, some methods of induction of labour may be suitable for use in outpatient settings.
Objectives
To examine pharmacological and mechanical interventions to induce labour in outpatient settings in terms of feasibility, effectiveness, maternal satisfaction, healthcare costs and, where information is available, safety. The review complements existing reviews on labour induction examining effectiveness and safety.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (December 2009) and reference lists of retrieved studies.
Selection criteria
We included randomised controlled trials examining outpatient cervical ripening or induction of labour with pharmacological agents or mechanical methods.
Data collection and analysis
Two authors independently extracted data and assessed eligible papers for risk of bias. We checked all data after entry into review manager software.
Main results
We included 28 studies with 2616 women examining different methods of induction of labour where women received treatment at home or were sent home after initial treatment and monitoring in hospital.
Studies examined vaginal and intracervical PGE2, vaginal and oral misoprostol, isosorbide mononitrate, mifepristone, oestrogens, and acupuncture. Overall, the results demonstrate that outpatient induction of labour is feasible and that important adverse events are rare. There was no strong evidence that agents used to induce labour in outpatient settings had an impact (positive or negative) on maternal or neonatal health. There was some evidence that, compared to placebo or no treatment, induction agents reduced the need for further interventions to induce labour, and shortened the interval from intervention to birth. We were unable to pool results on outcomes relating to progress in labour as studies tended to measure a very broad range of outcomes.
There was no evidence that induction agents increased interventions in labour such as operative deliveries. Only two studies provided information on women’s views about the induction process, and overall there was very little information on the costs to health service providers of different methods of labour induction in outpatient settings.
Authors’ conclusions
Induction of labour in outpatient settings appears feasible. We do not have sufficient evidence to know which induction methods are preferred by women, or the interventions that are most effective and safe to use in outpatient settings.
Medical Subject Headings (MeSH): * Ambulatory Care; Acupuncture Therapy [methods]; Feasibility Studies; Labor, Induced [* methods]; Oxytocics; Randomized Controlled Trials as Topic
MeSH check words: Female, Humans, Pregnancy
BACKGROUND
Introduction
The number of women whose labours are induced has risen dramatically over the past two decades. Rates in the USA and the UK now exceed 20% of all births (Glantz 2003; Kirby 2004; NHS 2007). There is an enormous variation in reported induction rates and the reasons for this variability are not clear. In some units in the USA, up to half of all births follow induction of labour (Rayburn 2002). There is no convincing evidence that the increase in inductions has been associated with improvements in maternal, fetal or neonatal outcomes, and women who are induced tend to be less satisfied with their experience of childbirth (Shetty 2005). In this context, and with increasing pressure on healthcare resources, it is particularly important to address questions about how to provide safe induction of labour, in settings and ways that are acceptable to women, and in the most cost-effective way possible.
A number of pharmacological and mechanical methods of cervical ripening and induction of labour are available, and these have been the focus of a series of previous Cochrane reviews which share generic protocols (Hofmeyr 2000; Kelly 2001). In these reviews, the safety and effectiveness of different methods and agents have been examined, but less attention has been paid to the setting in which cervical ripening and induction of labour take place. In this review we will bring together some of the studies included in previous reviews, focusing specifically on those studies where labour induction has been carried out in outpatient settings. For most methods of induction, the number of trials carried out in outpatient settings is likely to be small, and here, the purpose of the review is mainly descriptive, examining issues such as feasibility, health service utilisation and women’s views about their care. For some interventions, there may be sufficient data to address questions of effectiveness and safety. In this way the review will complement existing ones rather than simply duplicating findings. A related review includes trials in which the same methods of ripening or induction have been compared in outpatient and hospital settings (Kelly 2009b).
Cervical ripening and induction of labour in outpatient settings
Induction of labour is carried out for a variety of indications and using a range of pharmacological, mechanical and other methods. The main indication for induction of labour is prolonged pregnancy, and there is evidence from a related Cochrane review (Gülmezoglu 2006) that for pregnancies that have continued beyond 41 weeks, induction of labour may reduce perinatal mortality. Other inductions are carried out on an individual basis. Most inductions of labour are carried out in inpatient settings. Outpatient procedures may not be safe for women with important risk factors, and some methods may only be feasible and safe in hospital, or in settings with specialised staff and facilities available. For example, outpatient induction is unlikely to be suitable for women with serious medical conditions or complications in the current pregnancy (Sawai 1995). Some women may be unsuitable for home care simply because they live at an unacceptable travelling distance from emergency care facilities.
Ideally, the agents or methods used for cervical ripening at home would achieve changes in the cervix similar to the normal physiological changes which promote the ‘spontaneous’ onset of labour, but without causing uterine contractions (Sawai 1995). Most methods for cervical ripening or induction of labour do have some undesirable side effects, including, on occasions, excessive uterine activity. The consequences of excessive uterine activity as a result of iatrogenic uterine hyperstimulation can be life-threatening for the mother and fetus.
Sometimes drugs to induce labour can only be administered by intravenous infusion or by repeated injections, or using specialist procedures that cannot easily be carried out in an outpatient setting. Drugs that can be taken orally, or procedures that are simple to perform, and require only limited monitoring, may lend themselves more readily for use in an outpatient setting. At least theoretically, outpatient induction may offer a number of advantages to women, clinical staff and providers of health services. Outpatient induction may be more convenient to, and preferred by, women; it may reduce hospital bed occupancy and, therefore, be associated with lower healthcare costs.
A number of papers have set out indications for outpatient cervical ripening or induction such as post-dates pregnancy in women who are otherwise well, and where there have been no signs of fetal distress. Several outpatient induction protocols have been described in observational studies suggesting that such inductions are feasible, safe and acceptable to women (Elliott 1992; McGill 2007; Neale 2002).
Why it is important to do this review
For some methods, and for selected groups of women, induction of labour is already being carried out in outpatient settings. A number of randomised controlled trials examining cervical ripening and induction of labour in outpatient settings have now been completed. This review brings together evidence from these trials to provide an overview of the feasibility of outpatient induction. If sufficient data were available we had also planned to provide information on the relative costs of different methods and their acceptability to women. Where possible, we have pooled data from trials examining the same methods to address questions of safety. In the context of this review, the issue of safety is of great importance. At the same time, it is unlikely that the safety will be adequately addressed in studies of randomised cohorts. Severe maternal and neonatal mortality and morbidity are likely to be very rare events in the low-risk population included in studies of outpatient induction. Information on adverse events and the relative safety of outpatient methods is most likely to emerge where there have been several large studies and where the same methods have been directly compared in different settings. Information on rare adverse events takes time to accumulate, but by systematically recording information on adverse events in all the studies included in the review, we may shed some light on this question.
In this review we have not included studies where the same method of cervical ripening or induction of labour was compared in outpatient versus inpatient settings: this has been addressed in a related Cochrane review (Kelly 2009b).
OBJECTIVES
To examine pharmacological and mechanical interventions to induce labour in outpatient settings in terms of feasibility, effectiveness, maternal satisfaction, healthcare costs and, where information is available, safety. The review complements existing reviews on labour induction examining effectiveness and safety.
METHODS
Criteria for considering studies for this review
Types of studies
All published and unpublished randomised trials which compared different methods of cervical ripening or induction of labour carried out in outpatient settings. All trials included random allocation to intervention and control groups. We did not include quasi-randomised trials. We included studies reported in abstracts and brief reports provided sufficient information was available to allow us to assess eligibility and risk of bias; where such information was not provided we attempted to contact trial authors. We planned to include cluster-randomised trials if they were otherwise eligible. We have not included crossover studies as we did not consider that they were appropriate in this topic area.
Types of participants
Pregnant women with a viable fetus suitable for cervical ripening or induction of labour at or near term (greater than 35 weeks) in an outpatient setting.
Types of interventions
We have included studies examining outpatient cervical ripening or induction of labour with pharmacological agents or mechanical methods. We have included studies where different methods of induction of labour in outpatient settings are compared; where a method is compared with a placebo; where a method is compared with expectant management; or where different doses of the same drug are compared. ‘Outpatient’ has been defined by the trialists, and includes any cervical ripening or induction of labour intervention (with the exception of membrane sweeping) that can be carried out at home or within community healthcare settings. It also includes a package of care initially provided in hospital (fetal monitoring, drug administration) after which the woman is allowed home until later review or until admission in labour. We have not included interventions where women remain in hospital throughout (even if they are in ‘day-care’ settings, or in other parts of the hospital, but are not formally admitted as inpatients), as a purpose of the review is to examine outcomes where women do not have immediate access to emergency care facilities.
Types of outcome measures
Clinically relevant outcomes for trials of methods of cervical ripening and labour induction have been pre-specified by two authors of labour induction reviews (Justus Hofmeyr and Zarko Alfirevic) (Hofmeyr 2000). We have used most of these outcomes (relevant to both inpatient and outpatient settings) in this review.
In addition, we have attempted to use relevant outcome measures to quantify any cost effectiveness benefits of outpatient ripening.
Primary outcomes
Failure to achieve vaginal delivery within 24 hours.
Additional induction agents required.
Length of hospital stay.
Use of emergency services.
Mother not satisfied.
Caregiver not satisfied.
Serious neonatal morbidity or perinatal death (composite outcome will include, for example, seizures, birth asphyxia defined by trialists, neonatal encephalopathy, disability in childhood).
Serious maternal morbidity or death (composite outcome will include, for example, uterine rupture, admission to intensive care unit, septicaemia).
Secondary outcomes
Outcomes related to measures of effectiveness, complications and satisfaction.
Measures of effectiveness
Vaginal delivery not achieved within 48 and 72 hours.
Randomisation to delivery interval.
Oxytocin augmentation.
Pain relief requirements (epidural, opioids).
Complications
Uterine hyperstimulation (with fetal heart rate (FHR) changes).
Uterine hyperstimulation (without FHR changes).
Instrumental vaginal delivery.
Caesarean section.
Apgar score less than seven at five minutes.
Neonatal intensive care unit admission.
Perinatal death.
Postpartum haemorrhage (as defined by the trial authors).
Serious maternal complications (considered as separate outcomes, e.g. intensive care unit admission, septicaemia, uterine rupture).
Serious neonatal complications (considered as separate outcomes).
In the absence of formal economic evaluation, we had planned to estimate potential cost savings and the impact of interventions used within an outpatient setting. These estimates could involve using some measures of effectiveness and complications in combination with estimates of healthcare provision. There were insufficient data reported in the trials to allow us to carry out this analysis.
We have also included some additional outcomes that may serve as ‘proxy’ measures of progress towards labour or delivery.
Indicators of ‘progress’ in labour such as: preterm rupture of membranes, diagnosis of active/spontaneous labour, self-referral back to hospital, Bishop scores at fixed time points post-randomisation.
‘Failed induction’ (as defined by trialists, but excluding the use of oxytocin for augmentation in women already in established labour).
Time to delivery including the interval from randomisation to delivery; interval to admission along with length of labour.
Detailed definitions for outcomes
Perinatal and maternal morbidity and mortality are composite outcomes. This is not an ideal solution because some components are clearly less severe than others. It is possible for one intervention to cause more deaths but less severe morbidity. However, in the context of labour induction at term, this is unlikely. All these events are rare, and a modest change in their incidence will be easier to detect if composite outcomes are presented. The incidence of individual components are explored as secondary outcomes (see above).
‘Uterine rupture’ includes all clinically significant ruptures of unscarred or scarred uteri. Trivial scar dehiscence noted incidentally at the time of surgery is excluded.
The terminology of uterine hyperstimulation is problematic (Curtis 1987). In the reviews, the term ‘uterine hyperstimulation’ is defined as uterine tachysystole (more than five contractions per 10 minutes for at least 20 minutes) and uterine hypersystole/hypertonus (a contraction lasting at least two minutes).
‘Uterine hyperstimulation with FHR changes’ is usually defined as uterine hyperstimulation syndrome (tachysystole or hypersystole with FHR changes such as persistent decelerations, tachycardia or decreased short-term variability). However, due to varied reporting, there is the possibility of subjective bias in the interpretation of these outcomes. Also, it is not always clear from the trials if these outcomes are reported in a mutually exclusive manner. More importantly, continuous monitoring is unlikely in an outpatient setting. Therefore, there is a high risk of biased reporting of uterine hyperstimulation (with or without FHR changes). It is possible that bias will favour the outpatient setting (i.e. by failure to recognise mild forms of hyperstimulation without continuous monitoring). On the other hand, clinicians who favour inpatient induction may, in the absence of continuous monitoring, label any maternal description of painful, frequent uterine contractions as hyperstimulation. Therefore, in the absence of blinding, hyperstimulation and other ‘soft’ outcomes should be interpreted with extreme caution.
While we sought data on all of the outcomes listed above, we have documented only those with data in the analysis tables. We have included outcomes in the analysis if reasonable measures were taken to minimise observer bias, and data were available according to original treatment allocation.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co-ordinator (December 2009).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co-ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co-ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language restrictions.
Data collection and analysis
We carried out data collection and analysis (including the selection of studies, data extraction and management, assessment of risk of bias, and data entry and analysis) using methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009).
Selection of studies
Two review authors (A Kelly, T Dowswell) independently assessed the eligibility for inclusion of all the studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third review author.
Data extraction and management
We adapted the data extraction form used in the series of Cochrane reviews on the induction of labour. All review authors were involved in data extraction, with two authors independently extracting data from each study report. We resolved discrepancies through discussion or, if required, we consulted a third author. We entered data into Review Manager software (RevMan 2008) and checked them for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). We resolved any disagreement by discussion or by involving a third author.
(1) Sequence generation (checking for possible selection bias)
We have described for each included study the method used to generate the allocation sequence.
We assessed the method as:
adequate (any truly random process, e.g. random number table; computer random number generator);
inadequate (any non random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear.
(2) Allocation concealment (checking for possible selection bias)
We have described for each included study the method used to conceal the allocation sequence and have assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed or non-opaque envelopes, alternation; date of birth); or
unclear.
(3) Blinding (checking for possible performance bias)
Interventions to ripen the cervix or to induce labour in outpatient settings include both pharmacological and mechanical methods. Although some studies may be placebo controlled, we envisaged that in many studies blinding women and care providers would not be feasible. However, we have described for each included study any methods used to blind study participants and personnel from knowledge of which intervention a participant received. We have noted where there was partial blinding (e.g. outcome assessment may be blind for some types of outcomes).
We have assessed the methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel;
adequate, inadequate or unclear for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We have described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We have stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or supplied by the trial authors, we have re-included missing data in the analyses. We assessed methods as:
adequate (where there was no or low levels of attrition (less than 20%) and where attrition was balanced across groups);
inadequate (where there were high levels of attrition or where attrition was not balanced across groups);
unclear.
(5) Selective reporting bias
We have noted if we had concerns about selective reporting bias, for example, where not all the study’s pre-specified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; or where a study failed to include results of a key outcome.
(6) Other sources of bias
We have described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
yes;
no;
unclear.
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2009). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses - see Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we have presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we have used the mean difference if outcomes were measured in the same way between trials. We have used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster-randomised trials
No cluster randomised trials were identified by the search strategy. However, if such trials are identified in the future, provided that they are otherwise eligible, we will include them in updates of the review and will analyse them along with individually randomised trials. Their sample sizes will be adjusted using the methods described in Gates 2005 and Higgins 2009 using an estimate of the intracluster correlation co-efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources are used, this will be reported and sensitivity analyses conducted to investigate the effect of variation in the ICC.
Crossover trials
We have not included crossover trials in the review; it was unlikely that such trials would be identified in this topic area.
Dealing with missing data
For included studies, we have noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we have carried out analyses, as far as possible, on an intention-to-treat basis; i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We have assessed heterogeneity by visual inspection of the forest plots for each analyses and have quantified the level of heterogeneity by examining the I2, T2 and Chi2 statistics for each analysis. Where we identified substantial heterogeneity (T2 greater than zero and either I2 greater than 30% or a significant Chi2 test for heterogeneity (P value less than 0.1)) we explored it by pre-specified subgroup analysis. In the presence of moderate or high levels of heterogeneity, we have used random-effects meta-analysis and have included along with the risk ratio and the 95% confidence interval, the values of I2, T2 and the P value of the Chi2 test for heterogeneity with the 95% prediction interval. In analyses where an intervention appears to favour a particular intervention, but where there is high heterogeneity and the prediction interval includes the null value of one, we would advise caution in the interpretation of results. In such cases we cannot rule out the possibility that the treatment effect in a single study may be different (in size and direction) from that suggested by the meta-analysis.
Assessment of reporting biases
We did not formally assess reporting bias as part of the risk of bias assessment. For most of the studies we carried out data extraction from published study reports; without access to study protocols it can be difficult to assess whether there has been any selective reporting. Where we suspected reporting bias (see ‘Selective reporting bias’ above), we planned to contact study authors asking them to provide any suspected missing outcome data.
We were not able to explore possible publication bias using funnel plots as none of the comparisons included sufficient studies.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2008). We anticipated that there would be a variety of methods for cervical ripening and induction of labour used in different trials. We planned therefore only to pool data to calculate an overall treatment effect where the same method of induction was used in different trials. We have used fixed-effect meta-analysis for combining data where trials examined the same intervention, and the trials’ populations and methods were judged sufficiently similar. Where we suspected clinical or methodological heterogeneity between studies sufficient to suggest that treatment effects differed between trials, we used random-effects meta-analysis. Where we identified substantial statistical heterogeneity we have used a random-effects method and have indicated the levels of heterogeneity as part of the presentation of results.
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analyses using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). Data permitting, we planned subgroup analysis by:
nulliparous versus multiparous women;
induction indication (e.g. postdate (41 weeks’ gestation or greater)).
We planned to use only primary outcomes in subgroup analysis. In view of the small number of studies included in each comparison we were not able to carry out the planned analyses. In all but three of the included studies (Incerpi 2001; Lelaidier 1994; Rayburn 1999) the main indication for induction of labour was ‘postdates’ pregnancy. The majority of studies recruited both primi- and multiparous women, and separate figures were not provided for subgroups. Further, very few of the included studies provided information on the review’s primary outcomes. In updates of the review, as more studies are added, and more data become available, we may be able to include planned subgroup analysis.
Sensitivity analysis
If, in the future, we identify any cluster randomised trials and use published ICC values, we will carry out sensitivity analysis examining the effect of changing the ICC.
We planned to investigate the impact of including poorer quality studies (for example, where allocation concealment was unclear) by carrying out sensitivity analysis. We intended to temporarily remove those studies assessed as being of poorer quality from the analysis to examine any changes in the size of the treatment effect, or the direction of findings. We did not carry out this additional analysis as only a small number of studies contributed outcome data for each different comparison, and very few studies reported on the review’s primary outcomes. In updates of the review as more studies become available, we plan to carry out sensitivity analysis by study quality.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
Results of the search
We identified 72 reports, representing 55 separate studies (some trials were reported in more than one published paper). We have included 28 studies in the review, excluded 25, and two studies are awaiting further assessment (Ascher-Walsh 2000; Thakur 2005; see Characteristics of studies awaiting classification tables).
Included studies
Twenty-eight studies including 2616 women.
The studies included a variety of different comparisons.
Vaginal prostaglandin (PGE2) versus expectant management or placebo (five studies) (Hage 1993; Newman 1997; O’Brien 1995; Sawai 1991; Sawai 1994).
Intracervical prostaglandin (PgE2) versus expectant management or placebo (seven studies) (Buttino 1990; Gittens 1996; Larmon 2002; Lien 1998; Magann 1998; McKenna 1999; Rayburn 1999).
Vaginal misoprostol versus placebo (four studies) (Incerpi 2001; McKenna 2004; Oboro 2005; Stitely 2000). In addition, one study compared two different doses of misoprostol (Kipikasa 2005).
Intracervical prostaglandin (PGE2) versus vaginal misoprostol (one study) (Meyer 2005).
Oral misoprostol versus placebo (one study) (Lyons 2001).
Mifepristone versus placebo (five studies) (Elliott 1998; Frydman 1992; Giacalone 1998; Lelaidier 1994; Stenlund 1999).
Oestrogen versus placebo (one study) (Larmon 2002).
Vaginal isosorbide mononitrate (IMN) versus placebo (three studies) (Bollapragada 2006; Bullarbo 2007; Habib 2008).
Acupuncture versus routine care (one study) (Harper 2006).
(The study by Larmon 2002 was a three-arm trial comparing intracervical PGE2, oestrogen and placebo and is included in more than one comparison.)
In all trials it was intended that women would spend part of the study period at home. In the majority of studies women received the initial treatment in a hospital setting (and frequently underwent a period of surveillance) before discharge home. Women were advised to seek help or return to hospital if any problems arose, if labour commenced, or after a predefined period. In some studies, women self-administered the study intervention at home, and again were advised to return either if they had concerns, if labour started, or for review after a specified period (e.g. in the study by Bollapragada 2006 women scheduled for labour induction were given vaginal IMN with instructions on self-administration 48, 32 and 16 hours before the scheduled induction time).
The studies almost invariably recruited healthy women at term. A small number of studies focused on women with particular histories. In the trials by Gittens 1996, Lelaidier 1994 and Rayburn 1999 women that had had a previous caesarean delivery were recruited; Incerpi 2001 focused on women with insulin-dependent diabetes and Newman 1997 included women with diabetes along with those requiring induction of labour for postmaturity. Two studies (Lelaidier 1994; Rayburn 1999) recruited women who had had a previous caesarean section (CS) and who were aiming to achieve a vaginal delivery. In the remaining studies the main indication for induction of labour was prolonged pregnancy, although recruitment was not always restricted to this group. Four studies included only primiparous women (Bollapragada 2006; Elliott 1998; Hage 1993; Harper 2006) and two multiparous women only (Lelaidier 1994; Rayburn 1999).
The main recruitment criterion in all of these studies was that labour had not already started (i.e. women were not having regular painful contractions). Most of the studies also specified a Bishop score indicating an unfavourable cervix as an inclusion criterion although the definition of an unfavourable cervix (low Bishop score) varied. No studies specifically recruited women where the cervix was favourable. Where it was mentioned, studies invariably recruited women with intact membranes; no studies specifically focused on women with ruptured membranes. Most of these studies specifically mentioned that multiple pregnancies were excluded, and at recruitment it was usually specified as an inclusion criterion that the fetus was in good condition with no signs of distress (e.g. normal fetal heart rate monitoring and normal amniotic fluid volume).
Further information on interventions, participants and inclusion and exclusion criteria are set out in the Characteristics of included studies tables.
Excluded studies
We excluded 25 trials. The main reason for excluding studies was their study design.
Four studies used a crossover design; we had decided to exclude crossover trials as we did not think this design was appropriate in this topic area; in all of these studies the focus was on breast stimulation. Women in the control groups initially received no intervention, while those in intervention groups were asked to stimulate their nipples for a specified time period; after this time period, women then crossed over into the control or intervention arm (Adewole 1993; Di Lieto 1989; Elliott 1984; Salmon 1986). In four studies (Damania 1988; Griffin 2003; Manidakis 1999) there was too little information on study methods to allow us to ascertain whether group allocation was truly random, or to allow us to carry out an assessment of risk of bias (the studies by Griffin 2003 and Manidakis 1999 were reported in brief abstracts; we attempted to contact the authors for more information without success). Two studies used quasi-randomisation and were at high risk of bias (Garry 2000; Kadar 1990). Evans 1983 described findings from two separate studies, one of which seemed to be carried out in a hospital setting and included a control group receiving no treatment; a second “outpatient” study did not include a control group; different doses of porcine ovarian relaxin were compared. In the study by Ohel 1996, whilst there seemed to be random allocation to treatment groups, results were not reported by randomisation group, and we were not able to include data in the review. In one study reported in a brief abstract, no original data were reported in the results section (Krammer 1995).
A number of studies focused on interventions that we had either specifically excluded (e.g. Doany 1997; Kaul 2004; Magann 1999; and Salamalekis 2000 looked at membrane sweeping), or interventions that are not used nowadays in clinical practice (extra amniotic saline infusion was examined by Moghtadaei 2007; it was not clear that women in both arms of this trial were discharged home; Spallicci 2007 examined the use of hyaluronidase injection).
In five studies it was not clear that the study was carried out in an outpatient setting or that the women were expected to spend some of the study period at home (Damania 1992; Herabutya 1992; Rayburn 1988; Voss 1996; Ziaei 2003).
Rijnders 2007 looked at the same intervention comparing home versus hospital settings and has been included in a related review (Kelly 2009b).
Finally, Dorfman 1987 looked at homeopathic preparations (caulophyllum-arnica-actea and racemosa-pulsatilla-gelsemium) used with the intention of generally preparing women for childbirth rather than for labour induction.
More information on excluded studies is set out in the Characteristics of excluded studies tables.
Risk of bias in included studies
Allocation
We assessed most of the studies included in the review as using adequate methods to generate the randomisation sequence and to conceal group allocation.
Sequence generation was either computer generated or derived from random number tables in 22 of the 28 included studies. In six trials the methods used to generate the randomisation order were not clear (Elliott 1998; Gittens 1996; Hage 1993; Lyons 2001; Newman 1997; Sawai 1991).
Sixteen studies used either external or pharmacy randomisation services, or identical coded drug packs from pharmacy to conceal group allocation (Bollapragada 2006; Buttino 1990; Frydman 1992; Giacalone 1998; Habib 2008; Incerpi 2001; Kipikasa 2005; Lelaidier 1994; Lien 1998; McKenna 1999; McKenna 2004; O’Brien 1995; Oboro 2005; Rayburn 1999; Sawai 1994; Stitely 2000). Four trials used sealed, opaque, sequentially numbered envelopes to conceal allocation (Bullarbo 2007; Harper 2006; Larmon 2002; Magann 1998); envelopes were also used in the Meyer 2005 and Stenlund 1999 trials, although in the former it was not stated that they were sealed, and in the latter that they were opaque. In six trials methods to conceal group allocation were not clear (Elliott 1998; Gittens 1996; Hage 1993; Lyons 2001; Newman 1997; Sawai 1991).
Blinding
Most (21) of the included studies were placebo controlled, and women and clinical staff were described as blind to group allocation. However, it was not always clear when the randomisation code was broken, so it was difficult to assess whether outcome assessment was carried out by blinded investigators. In two of the placebo controlled trials blinding may not have been convincing; in the Kipikasa 2005 trial women in the two groups were both given tablet fragments (either an eighth or a quarter of whole tablets) so the tablets may have not appeared identical (at least to staff). In the Larmon 2002 study women may have been blind to intra-vaginal preparations, but staff are unlikely to have been.
In six trials women in the two arms of the studies were given different interventions and therefore blinding was not feasible, or not attempted (Gittens 1996; Harper 2006; Meyer 2005; Newman 1997; Rayburn 1999; Stenlund 1999). The lack of blinding in these studies may have affected some of the outcomes examined in the review.
Incomplete outcome data
Loss of women to follow up and missing data were not serious problems in most of the included studies. Rates of attrition were less than 10% in 19 of the trials. In six studies the levels of attrition were not clear (Gittens 1996; Hage 1993; Harper 2006; Incerpi 2001; Lyons 2001; Newman 1997). In the study by Sawai 1991, attrition was approximately 12% and some of the exclusions were for non-compliance. Attrition was also high in the study by Bollapragada 2006; in this trial randomisation occurred up to nine days before the initiation of treatment, hence 80 of the 350 women did not start treatment as they had already gone into labour. To reduce risk of bias, the authors reported an intention-to-treat analysis (including all women randomised) for the trial’s primary outcomes but not for secondary outcomes. In the study by Kipikasa 2005 there were inconsistencies in figures between the text and the tables.
Other potential sources of bias
In many of these studies women were likely to receive other interventions at some stage in their treatment as well as the study allocated intervention (e.g. amniotomy, membrane sweeping, additional medication) and these in turn may have affected other outcomes (e.g. length of labour and rate of CS). Without adequate blinding, it is possible that women in intervention and control groups may have had different co-interventions, or co-interventions at different stages. For example, in the study by Harper 2006 women in the intervention group attended for treatment on three occasions, and at these visits (not available to women in the control group) women may have been exposed to a range of co-interventions, or additional tests or observations, that may have had an impact on outcomes.
Other sources of bias included baseline imbalance in parity between groups (Oboro 2005) and imbalance in numbers of randomised women between the treatment and control groups (Elliott 1998).
Effects of interventions
Induction of labour in outpatient settings: 28 studies with 2616 women
(1) Vaginal prostaglandin (PGE2) versus expectant management or placebo: five studies, 335 women
Primary outcomes
We included five studies in this comparison (Hage 1993; Newman 1997; O’Brien 1995; Sawai 1991; Sawai 1994). None of the studies collected information on most of the review’s primary outcomes. We do not have information on the numbers of women achieving vaginal delivery within 24 hours, on length of hospital stay, on the use of emergency services or on maternal satisfaction. Maternal and perinatal deaths were not reported.
O’Brien 1995 and Sawai 1991 reported the numbers of women requiring further (non-study) induction agents with fewer women in the PGE2 group needing further medication to induce labour. While 14.8% of the PGE2 group needed further induction agents this applied to 28.9% of the control group. However, as only two relatively small studies contributed data for this outcome, results were of borderline statistical significance (risk ratio (RR) 0.52, 95% confidence interval (CI) 0.27 to 0.99).
Secondary outcomes
There was only limited information on the impact of interventions on the health of mothers and babies. O’Brien 1995 and Sawai 1994 reported rates of chorioamnionitis and results favoured women in the PGE2 group (RR 0.37, 95% CI 0.15 to 0.90). There was no statistically significant differences between groups for uterine hyperstimulation (with or without fetal heart rate (FHR) changes) (Analysis 1.5). There was no information on the use of antibiotics or on rates of endometritis.
There was no statistically significant evidence of differences between groups for Apgar scores at five minutes (Analysis 1.18) or for admission to a neonatal intensive care unit (NICU) (RR 0.32, 95% CI 0.10 to 1.03). There was no information on neonatal infection or on the use of antibiotics.
Four of the studies reported rates of CS and there was no evidence of a difference between groups (Analysis 1.7). O’Brien 1995 examined the use of epidural; again, there was no strong evidence of any difference between groups (Analysis 1.10).
Additional outcomes
While none of these five studies reported the numbers of women achieving vaginal delivery within a certain specified period, other ‘proxy’ measures of progress towards labour or delivery were included. Each study reported different outcomes.
Hage 1993 reported on the rate of change in Bishop scores and, compared with women receiving PGE2, those in the control group were more likely to have score changes of less than three at follow up (RR 0.13, 95% CI 0.03 to 0.47) although it was not clear when follow up occurred.
Newman 1997 reported figures for the number of women going into “spontaneous labour” within 48 hours of treatment commencing; it was more likely for labour to start in the PGE2 group compared with women receiving routine care (RR 6.43, 95% CI 2.12 to 19.48).
O’Brien 1995 reported that the median interval from study enrolment to delivery was four days in the PGE2 group (range 0 to 28 days) versus 10 days (range 0 to 26 days) in controls (P = 0.002). The shorter interval between randomisation and delivery was reflected in a lower gestational age at delivery in the intervention group (Analysis 1.26). It was also reported that, during the five-day treatment period, compared with controls, significantly more women in the intervention group were admitted to hospital “for labour” (RR 2.70, 95% CI 1.47 to 4.97), although it was not clear whether this included women in active labour only, or women admitted after PROM or for other reasons. The numbers of women diagnosed with post-term pregnancy was small in both groups (two women in the intervention group and three in the control group).
Sawai 1991 describes Bishop scores in control and intervention groups at hospital admission, but there were differences between groups at baseline and the authors report no significant differences between groups at follow up (data not shown). Sawai 1994 reported the mean gestational age at hospital admission (although the indications for admission included pregnancy complications as well as signs of the onset of labour). The difference between groups was not significant (Analysis 1.27).
(2) Intracervical prostaglandin (PGE2) versus expectant management or placebo: seven studies, 678 women
Primary outcomes
We included seven studies in this comparison (Buttino 1990; Gittens 1996; Larmon 2002; Lien 1998; Magann 1998; McKenna 1999; Rayburn 1999).
Three studies looked at whether, compared with no treatment or placebo, women receiving intracervical PGE2 were less likely to need further (non-study) interventions to induce labour. There was no strong evidence of a difference between groups (RR 0.98, 95% CI 0.74 to 1.32). Lien 1998 also examined whether women given intracervical PGE2 were less likely to receive further doses of prostaglandin to induce labour. Again, there was no evidence to suggest a difference between groups (RR 1.61, 95% CI 0.22 to 1.67).
Buttino 1990 reported on the number of women failing to achieve vaginal delivery within 48 to 72 hours and, although results favoured the PGE2 group, they did not reach statistical significance (RR 0.83, 95% CI 0.68 to 1.02).
Rayburn 1999 reported rates of uterine rupture, and there were no events in either the PGE2 group or amongst controls (Analysis 2.6). There was no information on serious neonatal morbidity of death, or on the review’s other primary outcomes.
Secondary outcomes
The impact of interventions on maternal health were explored in five of these studies (Buttino 1990; Larmon 2002; Lien 1998; McKenna 1999; Rayburn 1999). There was no strong evidence that there were differences between women receiving PGE2 compared to controls for uterine hyperstimulation (with or without FHR changes), postpartum haemorrhage, chorioamnionitis or endometritis (Analysis 2.7; Analysis 2.9; Analysis 2.16; Analysis 2.17). Two studies looked at side effects from treatment, and there was no evidence of a difference between women in the two arms of these trials (Analysis 2.26).
There were no statistically significant differences between groups for neonatal Apgar scores at five minutes, or for the number of babies admitted to NICU (Analysis 2.20; Analysis 2.21).
All seven studies examined the numbers of women undergoing CS; numbers were similar for women receiving PGE2 compared with controls (RR 0.90, 95% CI 0.72 to 1.12). There was no strong evidence of differences between groups for other interventions in labour including the number of women having assisted vaginal delivery or oxytocin augmentation. (Analysis 2.11; Analysis 2.12). The included studies did not provide information on other review outcomes including the use of antibiotics, neonatal infection, or use of epidural.
Additional outcomes
All seven studies collected information on progress towards labour and delivery; again reported outcomes were different in each study. Buttino 1990 and Lien 1998 reported no significant differences between women in the PGE2 group and controls in the interval between the first dose of drug or placebo and delivery (Analysis 2.24).
Larmon 2002 found no differences between groups for the median number of days from recruitment to hospital admission (16.8 days for the PGE2 group versus 15.4 days for controls). For other outcomes reported in this study (Bishop score on admission, and estimated gestational age on admission) there were no significant differences between groups. However, some women were admitted for induction rather than in labour and it was not clear if these mean figures included all women.
Lien 1998 and Magann 1998 reported the estimated gestational age at delivery and found no difference between groups for this outcome (Analysis 2.27) (there was high heterogeneity for this outcome and results should be interpreted with caution). These same two studies provided information on the number of women requiring induction for ‘postdates’ pregnancy (women reaching 42 weeks’ gestation). In view of high heterogeneity and different clinical management in the two studies, we did not pool results for this outcome but have set out the data in Analysis 2.28. While in the Magann 1998 study more women in the control group required induction (22 of 35 women) compared to the PGE2 group (seven of 35 women) the results were difficult to interpret as some women had been admitted to hospital for induction at an earlier stage because of changes in Bishop score, or for other reasons.
McKenna 1999 reported the median time from recruitment to admission to hospital, and the interval was shorter in the PGE2 group compared with controls (2.5 days versus 7 days, P = 0.02) however, reasons for admission included change in Bishop score, as well as for onset of labour. This study also reported the number of women delivering within two days of treatment commencing; more women gave birth within two days if they had had the active treatment (RR 3.10, 95% CI 1.29 to 7.47).
Finally, Rayburn 1999 reported the numbers of women delivering at various gestational ages (all deliveries). There were no statistically significant differences between groups at any of the time points measured (data not shown).
(3) Vaginal misoprostol versus placebo: four studies, 274 women
Primary outcomes
Four studies compared vaginal misoprostol with placebo (Incerpi 2001; McKenna 2004; Oboro 2005; Stitely 2000). In all four studies the initial dose of misoprostol was 25 mcg; in the study by Incerpi 2001 women received a second dose after three to four days if labour had not commenced, and in the study by Stitely 2000 a second dose was administered after one day.
For this comparison, only Oboro 2005 reported on the rate of perinatal death with no significant differences between groups; there were no deaths in the active treatment group (n = 38) compared with one stillbirth (reason not reported) in the control group (n = 39) (RR 0.34, 95% CI 0.01 to 8.14).
None of the studies provided information on the number of women achieving vaginal delivery within 24 hours.
There was no other information on serious maternal or neonatal morbidity.
Secondary outcomes
There was little information from these studies on the impact of interventions on mothers’ and babies’ health. There was no strong evidence of differences between intervention and control groups for uterine hyperstimulation (with or without FHR changes), Apgar scores at five minutes, neonatal infection, or admission to NICU (Analysis 3.5; Analysis 3.6; Analysis 3.18; Analysis 3.19; Analysis 3.21).
There were no statistically significant differences between groups for interventions in labour including the number of women undergoing CS, assisted vaginal delivery, or epidural (Analysis 3.7; Analysis 3.8; Analysis 3.10).
No information was provided in these studies on other review outcomes including maternal or neonatal infection, use of antibiotics or other maternal or neonatal complications.
Additional outcomes
Incerpi 2001 reported the mean dose of oxytocin used for each group; there was no significant evidence of any difference between the two groups (Analysis 3.24).
McKenna 2004 provides data on the interval from treatment to vaginal delivery; the difference between groups was not significant (Analysis 3.32). This author also reported the mean interval from recruitment to delivery, which was less for the misoprostol group compared with women receiving placebo (Analysis 3.33); information was provided separately for nulliparous and multiparous women (Analysis 3.34). It was not clear whether the figures included those women who had had CS, or other interventions in labour.
Stitely 2000 gave information about the number doses of medication given to the women (Analysis 3.25) and the number of women requiring subsequent doses on study days two and three; fewer women received further doses in the intravaginal misoprostol group (P < 0.01 for both time points, Analysis 3.26; Analysis 3.27).
Oboro 2005 reports that the interval from the commencement of treatment to hospital admission was significantly shorter for the misoprostol group both for nulliparous and parous women (Analysis 3.28; Analysis 3.29; Analysis 3.30). There was also evidence from this trial that the gestational age at labour was reduced in the misoprostol group compared with controls, with labour approximately a week earlier in the misoprostol group (mean difference (MD) −0.80, 95% CI −1.05 to −0.55). There was also evidence that the time to preterm rupture of membranes was shorter in the misoprostol group (Analysis 3.31), although it was not clear whether this was the interval from commencement of treatment or from hospital admission.
(4) Vaginal misoprostol 25 mcg versus 50 mcg: one study with 52 women
Primary and secondary outcomes
Kipikasa 2005 looked at two different doses of vaginal misoprostol. There was no information on any of the review’s primary outcomes or most secondary outcomes. There were no differences between groups in the number of women requiring further induction agents, undergoing CS or in the number of babies admitted to NICU (Analysis 4.1; Analysis 4.3; Analysis 4.4). There were no cases of uterine hyperstimulation in either group. One baby in the higher dose group had a low Apgar score (less than six) at five minutes.
Additional outcomes
The interval to delivery was reported to be shorter in the group receiving the higher dose of misoprostol; with women receiving 50 mcg delivering, on average, one and a half days earlier than those receiving 25 mcg (MD 1.5, 95% CI 1.19 to 1.81).
(5) Intracervical PGE2 versus vaginal misoprostol: one study, 84 women
Primary outcomes
One study is included in this comparison between intracervical PGE2 and vaginal misoprostol (Meyer 2005). None of the review’s primary outcomes were considered in this study.
Secondary outcomes
There was no strong evidence of differences between intervention and control groups for uterine hyperstimulation, rate of CS, Apgar scores at five minutes and admission to NICU (Analysis 5.1; Analysis 5.2; Analysis 5.3; Analysis 5.4).
Additional outcomes
It was reported that the proportion of women not requiring oxytocin was 22% in the misoprostol group versus 2% in for those in the PGE2 group (P = 0.006). The dose of oxytocin used was also reported to be significantly decreased in those women receiving misoprostol (P = 0.008 for cumulative dose of oxytocin) (data not shown).
The interval from the administration of the cervical ripening agent to admission was shorter for women who received misoprostol (Analysis 5.6), and misoprostol was also reported to increase by 32% the number of women starting labour or with SROM during the ripening period (Analysis 5.7).
Misoprostol was reported to increase the number of deliveries within 24 and 48 hours, but the differences between groups were not statistically significant (Analysis 5.8; Analysis 5.9).
(6) Oral misoprostol versus placebo: one study, 40 women
Primary outcomes and secondary outcomes
Lyons 2001 was the only study included in this comparison and it provided information on only three of the review’s pre-specified outcomes. There was no evidence of differences between women in the misoprostol and placebo groups in the need for further induction agents, uterine hyperstimulation or chorioamnionitis (Analysis 6.3; Analysis 6.4; Analysis 6.2).
Additional outcomes
Management of post-term pregnancies with oral misoprostol was associated with a reported significant decrease in the need for postdates inductions: 27% versus 59% with placebo (P < 0.05). Women in the misoprostol group had a significantly shorter interval from the first dose to active labor (Analysis 6.5), and this is reflected in the longer duration of treatment in the placebo group (Analysis 6.6).
(7) Mifepristone versus placebo: five studies, 393 women
Primary outcomes
We included five studies in this comparison (Elliott 1998; Frydman 1992; Giacalone 1998; Lelaidier 1994; Stenlund 1999). Women in the mifepristone group were less likely to require further medication to induce labour compared with controls ((random-effects) RR 0.59, 95% CI 0.37 to 0.95). However, there was considerable heterogeneity for this outcome (heterogeneity: I2 = 74%, T2 = 0.16, Chi2 test for heterogeneity P = 0.009). The wide 95% prediction interval (0.08 to 4.39) indicates that this result should be interpreted cautiously as some further study might yield a ‘negative’ result.
Stenlund 1999 examined serious neonatal morbidity (the number of babies requiring antI-convulsive therapy); there was no statistically significant difference between groups (Analysis 7.2). Lelaidier 1994 reported on perinatal mortality and there were no deaths in either group (Analysis 7.17).
Secondary outcomes
There was only limited evidence on the impact of mifepristone on maternal and neonatal health. There were no significant differences between intervention and control groups for uterine rupture, chorioamnionitis, infant Apgar scores at five minutes or admission to NICU (Analysis 7.12; Analysis 7.14; Analysis 7.18; Analysis 7.19).
There was no strong evidence that the intervention had an impact on interventions in labour including CS, assisted vaginal delivery, oxytocin augmentation or use of epidural (Analysis 7.7; Analysis 7.8; Analysis 7.9; Analysis 7.10).
Two of the five studies looked at failure to achieve changes in the cervix after 24 to 48 hours and here results favoured the mifepristone group (RR 0.36, 95% CI 0.20 to 0.63) (Analysis 7.23). Information was not reported on the other secondary review outcomes.
Additional outcomes
None of the studies reported on the number of women achieving vaginal delivery within 24 hours but Elliott 1998 described the number of women in spontaneous labour within 72 hours. There was no evidence of a difference between groups receiving mifepristone versus placebo (Analysis 7.24). The time to onset of labour was similar in all three study groups, with amedian of 81 hours 15 minutes for placebo, 80 hours 20 minutes for 50 mg mifepristone, and 75 hours 50 minutes for 200 mg mifepristone.
Giacalone 1998 reported on “spontaneous labour” within 48 hours and results favoured the mifepristone group (RR 2.05, 95% CI 1.27 to 3.30). There was a shorter interval between the beginning of treatment and onset of labour, and between treatment and vaginal delivery for the mifepristone group (the median interval to labour onset was 31.7 hours for mifepristone group versus 53.9 hours for placebo, and 31.3 hours versus 58.5 hours between treatment and delivery; with a reported P value = 0.02 for both outcomes).
Stenlund 1999 reported that during the first 48 hours after treatment started, 83.3% of women with mifepristone and 41.7% with placebo went into labour or had a ripe cervix (Analysis 7.22). The median time to onset of labour from commencing treatment was 24 hours 10 minutes for women who had mifepristone and 52 hours for women with placebo.
Mifepristone significantly reduced the total dose of oxytocin for women having both vaginal and caesarean deliveries in the study by Frydman 1992 (Analysis 7.26; Analysis 7.27) and also by Lelaidier 1994 (Analysis 7.28). Lelaidier 1994 reported the interval between the start of treatment and the onset of labour; the interval was significantly shorter in the mifepristone group (Analysis 7.29).
(8) Oestrogen versus placebo: one study, analysis for 77 women
Primary and secondary outcomes
We included one study in this comparison and there was no information reported on any of the review’s primary outcomes (Larmon 2002).
There were no statistically significant differences between intervention and control groups for outcomes relating to maternal and infant wellbeing, or interventions in labour including the numbers of women having CS, assisted delivery, oxytocin augmentation or infection, or in infant admission to NICU (Analysis 8.7; Analysis 8.8; Analysis 8.9; Analysis 8.14; Analysis 8.15; Analysis 8.19).
(9) Vaginal isosorbide mononitrate (IMN) versus placebo: three studies, 652 women
Primary outcomes
Bollapragada 2006 examined the number of women failing to achieve vaginal delivery within 24 hours of the intervention, and found no statistically significant difference between groups (RR 0.97, 95% CI 0.83 to 1.15). Three studies presented information on the need for further induction agents and the results favoured the IMN group (RR 0.83, 95% CI 0.74 to 0.92).
Bollapragada 2006 provided information on perinatal death, but with one reported perinatal death in this study the confidence interval is wide and estimates unreliable (Analysis 9.18).
Two of these studies reported on maternal satisfaction with the induction process. Bullarbo 2007 found no difference in levels of satisfaction between women in the two arms of the trial. In the study by Bollapragada 2006 et al, women were asked to rate their satisfaction with the induction process at home. On five of the six measures of satisfaction, women in the placebo group were slightly more satisfied with their care compared with those in the IMN group, although the differences between groups were not large, and the mean scores in both groups suggested general satisfaction (Analysis 9.5).
Secondary outcomes
There was no evidence that the intervention had any significant impact on maternal health including uterine hyperstimulation and postpartum haemorrhage (Analysis 9.6; Analysis 9.15). IMN seemed to be associated with increased side effects, including nausea and particularly headaches (Analysis 9.11; Analysis 9.12). In one study 22/112 women in the IMN group reported severe headaches compared with only 1/108 in the placebo group (Analysis 9.16).
The intervention did not seem to be associated with any differences between groups for neonatal infection, Apgar scores at five minutes or admission to NICU (Analysis 9.19; Analysis 9.20; Analysis 9.22).
Women in the two arms of these trials had similar rates of interventions in labour and there were no statistically significant differences for CS, assisted vaginal delivery, oxytocin augmentation or use of epidural (Analysis 9.7; Analysis 9.8; Analysis 9.9; Analysis 9.10). Bollapragada 2006 reported on the number of women who had no change to the cervix 24 to 48 hours following treatment, and women in the placebo group were more likely to have no change in Bishop score (RR 0.83, 95% CI 0.70 to 0.97). Bollapragada 2006 collected information on the cost of providing care; the mean overall cost of the care package was very similar for women in the two groups (MD 11.98, 95% CI −105.34 to 129.30).
Non pre-specified outcomes
Bollapragada 2006 reported the mean interval from hospital admission to delivery for all women, and for those women having vaginal deliveries, along with the mean change in Bishop scores at 48 hours after baseline; there were no significant differences between groups for any of these outcomes (data not shown).
Bullarbo 2007 reported that women in the IMN group were more likely to start labour within 24 hours compared with controls (RR 2.75, 95% CI 1.29 to 5.88) while Habib 2008 reported no significant difference between groups for women admitted either in labour or with a ripe cervix (Bishop score six or more) (RR 3.80, 95% CI 1.54 to 9.40). Habib 2008 reported a result favouring IMN for the interval between admission and delivery, although it was not clear that those women undergoing CS or receiving other interventions were excluded from this analysis (MD −6.67 hours, 95% CI −9.56 to −3.78).
(10) Acupuncture versus routine care: one study 56 women
Primary and secondary outcomes
Harper 2006 presented limited information relevant to this review. There was no strong evidence that the intervention had any impact on the number of women having CS or on the use of medication to induce labour (Analysis 10.1; Analysis 10.2). There were no significant differences between groups for women starting labour spontaneously, cervical dilatation at hospital admission, or the mean time from study enrolment to delivery (data not shown).
DISCUSSION
Summary of main results
The studies included in the review examined 10 different types of interventions in outpatient settings. Overall, the results demonstrate that outpatient induction of labour is feasible and that important adverse events are rare (Table 1; Table 2; Table 3). However, the safety data should be treated with considerable caution. First, very few of the studies provided information on maternal and neonatal death or serious morbidity. It may not be safe to assume that because adverse outcomes were not reported, they did not occur. Further, even where outcomes such as perinatal mortality, maternal complications or serious neonatal morbidity were reported, the finding that there was no apparent difference between groups was not surprising as none of these studies had the statistical power to detect differences for such rare outcomes in relatively low-risk populations.
Table 1. Uterine hyperstimulation with outpatient inductions.
| UTERINE HYPERSTIMULATION | |
|---|---|
| PGE2 (vaginal) | |
| Hage 1993 | 1/18 PGE group (FHR status unknown), 0/18 in placebo group |
| Newman 1997 | 2/28 PGE group (FHR status unknown), 0/30 in controls (no treatment) |
| O’Brien 1995 | 1/50 PGE group (normal FHR), 0/50 in placebo group |
| TOTAL | 4/96 PGE, 0/98 in controls |
| PGE2 (intracervical) | |
| Buttino 1990 | 1/23 PGE group (with FHR decelerations), 0/20 in placebo group |
| Lien 1998 | 2/43 PGE group, 1/47 placebo group with FHR deceleration in both |
| McKenna 1999 | 1/30 PGE group (fetal bradycardia), 0/31 placebo group |
| Rayburn 1999 | 1/143 PGE group, 0/151 control (no treatment) with hyperstimulation 11/143 FHR decelerations in PGE group, 12/151 in control |
| TOTAL | 5/239 PGE, 1/249 control with hyperstimulation |
| Intravaginal misoprostol | |
| Stitely 2000 | 2/27 misoprostol group with FHR deceleration, 2/33 placebo group 1/27 misoprostol with tachysystole without FHR changes, 0/33 placebo group |
| Incerpi 2001 | 3/57 misoprostol with hyperstimulation (FHR unknown), 2/63 placebo group 2/57 misoprostol with hypertonus, 5/57 misoprostol with tachysystole, none control |
| McKenna 2004 | 1/33 misoprostol (FHR deceleration), 0/35 placebo group |
| Oral misoprostol | |
| Lyons 2001 | 1/18 misoprostol, 2/22 placebo group (FHR unknown) with hyperstimulation |
| Mifepristone | |
| Giacalone 1998 | 4/41 mifepristone group, 0/42 placebo group with hypertonia (FHR unknown) |
| Lelaidier 1994 | 0/16 in both groups |
| TOTAL | 4/57 mifepristone, 0/58 placebo with hypertonia |
| IMN | |
| Habib 2008 |
0/51 IMN group, 2/51 placebo group with hyperstimulation (abnormal FHR)
1/51 IMN, 8/51 placebo group with tachysystolia (FHR normal) |
FHR: fetal heart rate
Table 2. Neonatal complications following induction in outpatient setting.
| NEONATAL COMPLICATIONS | |
|---|---|
| PGE2 vaginal | |
| Sawai 1991 | 0/24 in PGE2 group; 2/26 in placebo group to NICU |
| Sawai 1994 | 2/38 in PGE2; 4/42 in placebo group to NICU |
| O’Brien 1995 | 1/50 in PGE2; 5/50 in placebo group to NICU |
| TOTAL | 3/112 PGE, 11/118 control to NICU |
| PGE2 intracervical | |
| Larmon 2002 | 6/41 PGE group, 8/43 placebo group with complication such as tachypnea, meconium aspiration, meconium or admission to NICU |
| Magann 1998 | 3/35 PGE2 vs 0/35 control NICU admission |
| McKenna 1999 | 1/30 PGE, 2/31 placebo group with complication |
| TOTAL | 10/106 PGE, 10/109 controls with neonatal complications/admitted to NICU |
| Vaginal misoprostol | |
| Stitely 2000 | 1/27 misoprostol, 3/33 placebo group to NICU |
| Incerpi 2001 | 18/57 misoprostol, 20/63 placebo group to NICU |
| McKenna 2004 | 0/33 misoprostol, 1/35 placebo group to NICU |
| Oboro 2005 | 1/38 misoprostol, 1/39 control (no treatment) to NICU |
| TOTAL | 20/155 misoprostol, 25/170 control to NICU |
| Misoprostol 25mcg versus 50mcg | |
| Kipikasa 2005 | 1/23 25 mcg, 2/26 50 mcg misoprostol to NICU |
| Intracervical PGE2 versus intravaginal misoprostol | |
| Meyer 2005 | 5/42 PGE, 4/42 misoprostol to NICU |
| Mifepristone | |
| Elliott 1998 | 0/50 mifepristone, 1/30 placebo group to NICU |
| Giacalone 1998 | 5/41 mifepristone, 4/42 control to NICU |
| TOTAL | 5/91 mifepristone, 5/72 control to NICU |
| IMN | |
| Bollapragada 2006 | 18/177 IMN, 16/173 placebo group to NICU |
| Bullarbo 2007 | 13/100 IMN, 9/100 placebo group to NICU |
| Habib 2008 | 0/51 IMN, 1/51 placebo group to NICU |
| TOTAL | 31/328 IMN, 26/324 placebo group to NICU |
NICU: neonatal intensive-care unit
Table 3. Maternal complications following induction of labour in outpatient setting.
| MATERNAL COMPLICATIONS | |
|---|---|
| Intracervical PGE2 | |
| Larmon 2002 | 4/41 PGE, 10/43 placebo group with complication such as endometritis, chorioamnionitis and pre-eclampsia |
| Lien 1998 | 6/43 PGE, 3/47 placebo group with complication such as endometritis and chorioamnionitis |
| McKenna 1999 | 1/30 PGE with PPH, 0/31 placebo group 2/30 PGE, 2/31 placebo group with infection |
| Rayburn 1999 | 8/143 PGE, 7/151 control (no treatment) with endometritis |
| TOTAL | 21/257 PGE2, 22/272 control with maternal complications |
| IMN | |
| Bollapragada 2006 | blood loss > 500 ml: 59/177 IMN, 47/173 placebo group |
| Bullarbo 2007 | blood loss > 1000 ml: 14/100 IMN, 12/100 placebo group |
| Habib 2008 | PPH: 2/51 IMN, 3/51 placebo group |
| TOTAL | 75/328 IMN, 62/324 placebo group with maternal complications |
PPH: postpartum haemorrhage
There was some evidence that, compared with placebo or no treatment, induction agents reduced the need for further intervention to induce labour, and shortened the interval from intervention to delivery. However, we were not able to pool results on outcomes relating to progress in labour, as studies tended to measure a very broad range of outcomes.
There was no evidence that induction agents increased interventions in labour such as operative deliveries. Only two studies (Bollapragada 2006; Bullarbo 2007) collected information on women’s views about the induction process, and overall there was very little information on the costs to health services of different methods of induction of labour in outpatient settings.
There seemed to be general satisfaction with induction in outpatient settings, although the Bollapragada 2006 trial suggested that women receiving isosorbide mononitrate were less satisfied than controls. This finding may have been associated with the relatively high number of women in the intervention group experiencing unpleasant side effects (particularly headaches) during the treatment period.
Overall completeness and applicability of evidence
It is debatable what would constitute definitive evidence on the effectiveness and safety of various induction protocols in the outpatient (home) environment. The issues that are likely to be important to women and healthcare providers were not adequately addressed in the included trials here, or in a related Cochrane review comparing home and hospital inductions (Kelly 2009b).
Safety
Adverse events in the pregnant population that is likely to be eligible for outpatient induction are rare (Table 1; Table 2). There is no consensus on what would be an unacceptable risk of an outpatient induction; views may vary between different healthcare systems and between women, doctors and healthcare commissioners in the same system. Assuming that one additional serious adverse event (e.g. perinatal death/serious morbidity) for every 500 outpatient inductions is considered unacceptable irrespective of the cost savings made, a very large randomised trial or meta-analysis including thousands of women would be needed to be able to exclude a possibility of such an excess risk. A trial of this size (or meta-analysis) designed to exclude such an excess risk (equivalence trial) is unlikely to be funded, irrespective of the method used.
In the absence of adequate safety data from randomised trials, the only pragmatic solution is to rely on observational data from large cohorts with relatively robust surrogate outcomes like emergency caesarean section for presumed fetal distress or emergency transfer to hospital. A recent paper from Canada (Salvador 2009) reports on 567 outpatient inductions with no serious complications, but it is not entirely clear what is included in this composite outcome. Other surrogate outcomes such as uterine hyperstimulation or fetal heart rate abnormalities (which have been reported in some studies (e.g. Ramsey 2005)) may be difficult to interpret unless there are clear definitions of what these outcomes mean. The use of common outcomes with agreed definitions applicable to all healthcare settings would be welcome; see Implications for research.
Experience of women and staff
Outpatient induction may be more convenient to women, they may feel more comfortable at home, and prefer being there rather than in hospital. On the other hand, women may feel worried about the induction process (especially if they live at some distance from emergency facilities) and the induction agent may cause side effects that are distressing, so some women may prefer the reassurance offered by hospital care. We have very limited information on what women would prefer, and no evidence on whether any women were forced to make arrangements for rapid transfer to hospital.
Outcomes such as average time to ‘admission in labour’ may be difficult to understand if there is no clear definition of what this means. The time may be partly determined by women’s decisions about when to attend hospital, which may depend on a broad range of physiological, psychological, social and practical factors. For example, a woman experiencing unpleasant side effects, living at a distance from emergency facilities may seek early admission; under these circumstances the outcome does not serve as a good proxy for progress in labour. Criteria for admission to hospital in the trials were frequently not specified and included active labour (variously defined), ruptured membranes and a range of other indications. Further, a short interval to admission is not necessarily a good thing; a very short interval means that sending women home may not be worthwhile, a long interval may not be harmful provided women are reasonably comfortable and there is no urgent need for delivery. A short interval to admission is also meaningless if it is offset by prolonged and painful labour. Reporting these two outcomes separately may not, therefore, be helpful.
Measures of cervical change (Bishop score) may also be problematic, for example, mean increases in Bishop scores on hospital admission, or Bishop scores reaching a certain level at given time points, are not straightforward to interpret. Such outcomes may not give any clear idea of when delivery will occur, whether more rapid cervical dilatation is predictive of a more rapid labour, or whether the delivery will be more or less likely to be normal.
Cost
Health service providers may also assume that transferring care to community or outpatient settings may reduce the total costs of care; we have no evidence to support this assumption. In the absence of formal economic evaluation, descriptive information on the total length of hospital stay for mothers and babies receiving active or placebo interventions may have been helpful in understanding the impact of outpatient procedures on health service utilisation. Such information was generally not provided. Instead, studies tended to focus on proxy measures for progress in labour, but we would advise caution in the way such information is collected and interpreted.
It is possible that different induction agents perform quite differently at different stages of cervical dilatation or at different gestational ages. The majority of studies included in this review recruited women requiring induction for ‘postdates pregnancy’. In different studies ‘postdates’ was defined differently, and may have been any gestational age between approximately 39 to 44 weeks; in some studies women were recruited from 37 weeks. The cervical status at recruitment also varied considerably with Bishop scores at recruitment being any value less than nine. One of the included studies recruited women with diabetes; there is insufficient evidence to know whether outpatient induction is safe and acceptable for women in high-risk groups.
With one or two exceptions, information on costs to women was generally not reported in the trials included in this review. In the absence of such data the assumption must be that women were not asked for their views on care, or about costs or inconvenience associated with hospital or outpatient care. The potential importance of such outcomes (patient related outcome measures) is increasingly being recognised by commissioners of healthcare services.
Quality of the evidence
Most of the studies included in the review were assessed as being at relatively low risk of bias; most of the trials were placebo controlled with adequate methods of randomisation and low levels of attrition. There was no blinding in seven trials where interventions were compared with no intervention or routine care. Lack of blinding may be a particular problem in these studies as many of the outcomes reported may have depended on clinical judgements by staff (e.g. need for hospital admission, prescription of additional drugs to induce or augment labour, and other interventions in labour). In other words, clinical decisions may have been affected by knowledge of treatment allocation.
Potential biases in the review process
We acknowledge that there was a possibility of introducing bias at every stage of the reviewing process. We attempted to minimise bias in a number of ways; two review authors assessed eligibility for inclusion, carried out data extraction and assessed risk of bias. Each worked independently. Nevertheless, the process of assessing risk of bias, for example, is not an exact science and includes many personal judgements. Further, the process of reviewing research studies is known to be affected by prior beliefs and attitudes. It is difficult to control for this type of bias.
While we attempted to be as inclusive as possible in the search strategy, the literature identified was predominantly written in English and published in North American and European journals. We did not attempt to formally assess reporting bias, constraints of time meant that assessment of risk of bias largely relied on information available in the published trial reports and thus, reporting bias was not usually apparent. Too few studies were included in each comparison in the review to allow us to explore possible publication bias.
Agreements and disagreements with other studies or reviews
A number of related Cochrane reviews have examined the same methods of induction of labour considered in this review, namely: vaginal PGE2 (Kelly 2009), intracervical PGE2 (Boulvain 2008), vaginal misoprostol (Hofmeyr 2003), oral misoprostol (Alfirevic 2006), mifepristone (Hapangama 2009), oestrogens (Thomas 2001), and acupuncture (Smith 2004), and a review is currently being prepared on isosorbide mononitrate (IMN) and other nitric oxide donors (Kelly 2008). Compared with these other reviews (which included both hospital (inpatient) and home (outpatient) inductions), the current review contains relatively few studies, and therefore, has insufficient statistical power to demonstrate differences between groups. This is particularly the case for relatively rare outcomes such as uterine rupture, but is also true for more common complications such as uterine hyperstimulation.
Evidence from the related Cochrane reviews is mainly in agreement with the findings of this review. Findings from these reviews indicate that compared with placebo, PGE2 (vaginal and intracervical) and vaginal and oral misoprostol are effective induction agents in that vaginal delivery within 24 hours was more likely for women receiving these agents. There is less evidence regarding the effectiveness of mifepristone, oestrogens, nitric oxide donors (including IMN) and acupuncture. Findings regarding safety suggest that some methods of induction (PGE2 and vaginal misoprostol) may be associated with an increased risk of uterine hyperstimulation. However, despite the relatively large number of studies included in some of these reviews, even pooled results from studies do not provide strong evidence regarding serious maternal and neonatal morbidity and death; as we have discussed above, with such rare outcomes very large trials are needed to exclude excess risk, or risk must be imputed by examining surrogate outcomes. None of these reviews specifically considered the issue of outpatient induction and we must remain cautious about assuming that methods that appear safe in hospital will achieve the same levels of safety (and indeed effectiveness) in outpatient settings. As we have indicated in this review, related reviews also illustrate that very little attention has been paid to consumer views or the costs of care.
Most of the related Cochrane reviews have examined the effectiveness of induction agents compared with placebo. Relatively few studies have examined different methods of induction directly. Where different agents have been compared (e.g. IMN with vaginal PGE2 (Osman 2006)) some agents may have advantages over others, and the safety profile of different agents (and doses) may differ. This may mean that they are more or less suitable for outpatient use.
A recent Cochrane review has compared the same method of induction in home and hospital settings directly, but this review contained only three trials and was unable to shed much light on issues of either the relative effectiveness, safety or costs associated with outpatient induction (Kelly 2009b).
AUTHORS’ CONCLUSIONS
Implications for practice
Induction of labour in outpatient settings appears feasible. We do not have sufficient evidence to know which methods are most safe or effective in outpatient settings.
Implications for research
For most methods of induction there have been no direct comparisons between outpatient versus inpatient management; we cannot address the question of the relative safety of settings unless such research is conducted. As part of such work it is important to ask women what sort of management they would prefer. There needs to be more careful consideration of outcomes purporting to measure progress in labour and more consistency in what is measured in trials.
It would be helpful to have information from trials on the use of emergency services. Data on the utilisation of out of hours community health services and emergency ambulance services might allow those providing health services to decide the best types of induction agents to use, to set out criteria for selecting women for outpatient induction, and would allow women to make more informed choices about their care.
PLAIN LANGUAGE SUMMARY.
Inducing labour for pregnant women at term in outpatient settings
Women may be more comfortable waiting for labour to start at home, and outpatient care may be less costly for providers of health services. Induction of labour (where labour is started artificially) is carried out for a variety of reasons including when women have passed their due dates or on an individual basis, such as having diabetes. A range of different drugs (including vaginal and cervical prostaglandin E2, vaginal and oral misoprostol and isosorbide mononitrate) and other methods (including acupuncture) have been used to induce labour. Induction of labour has been carried out in hospital, but some methods may be suitable for use in outpatient settings. Women may be able to administer treatment themselves at home, or to be discharged home after treatment in hospital. This review examined the feasibility, effectiveness, maternal satisfaction, healthcare costs and, where information was available, safety of outpatient induction of labour.
We have included 28 controlled studies with 2616 women randomised to induction or who received placebo or no treatment. In all studies women received treatment at home or were discharged home after initial treatment and monitoring in hospital. There was some evidence that the induction agents used in outpatient settings reduced the need for further drugs such as oxytocin to induce labour, and shortened the time from the beginning of treatment to the birth of the baby. Induction agents used in this way did not appear to increase the likelihood of the need for caesarean section or other interventions in labour. Only two studies provided information on women’s views about the induction process. Overall there was very little information on the costs to health services of different methods. Induction of labour in outpatient settings appears feasible and safe to use. We do not know which methods are preferred by women, or the interventions that are most effective and safe to use in outpatient settings.
ACKNOWLEDGEMENTS
As part of the pre-publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group’s international panel of consumers and the Group’s Statistical Adviser.
SOURCES OF SUPPORT
Internal sources
The University of Liverpool, UK.
External sources
TD is supported by a grant from the National Institute for Health Research (NIHR), UK.
NIHR NHS Cochrane Collaboration Programme Grant Scheme award for NHS prioritised, centrally managed, pregnancy and childbirth systematic reviews: CPGS02
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | RCT. | |
| Participants | Setting: large teaching hospital in Glasgow, Scotland, UK. 350 women randomised. Inclusion criteria: primiparous women at term (gestational age > 37 weeks) with singleton pregnancy and Bishop score < 7. Women were scheduled for induction (97% for prolonged pregnancy: 40 weeks + 10 days gestation). Women recruited were willing to self-administer vaginal tablets Exclusion criteria: women with ruptured membranes, less than 16 years age, who needed delivery within the next 48 hrs, or with fetal compromise requiring daily fetal monitoring |
|
| Interventions | Intervention group: self-administered vaginal IMN 40 mg every 16 hrs to max of 3 doses (48 hrs, 32 hrs and 16 hrs prescheduled admission for induction) Comparison group: self-administered placebo, same regimen as intervention group |
|
| Outcomes | Time from hospital admission to delivery, women’s views on induction process, pain, mode of delivery, cost to NHS, neonatal outcomes | |
| Notes | One of the review authors, Jane Norman (JN) was an investigator on this trial. JN was not involved in assessing the eligibility of the study for inclusion, data extraction or assessment of risk of bias | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer generated sequence. |
| Allocation concealment? | Yes | Central randomisation with automated telephone service. Women were given information and consented after the decision to induce labour had been made. Randomisation in the antenatal clinic up to 9 days before treatment commenced |
| Blinding? Women |
Yes | Treatment packs for intervention and control groups were described as identical, prepared by pharmacy |
| Blinding? Clinical staff |
Yes | |
| Blinding? Outcome assessor |
Yes | |
| Incomplete outcome data addressed? All outcomes |
Yes | 350 randomised. 80 women did not initiate treatment as they went in to labour before the scheduled time for taking medication, a further 11 women withdrew (including 2 with breech presentation). All women randomised were included in an ITT analysis for primary outcomes (but not in secondary analysis) |
| Free of other bias? | Yes | No baseline imbalance apparent. |
| Methods | RCT. | |
| Participants | Setting: two hospitals in Gothenburg, Sweden. 200 women randomised. Inclusion criteria: women with uncomplicated pregnancies, singleton, cephalic presentation, intact membranes, > 42 weeks’ gestation (confirmed by ultrasound before 20 weeks) normal AFI, reactive NST Exclusion criteria: serious medical or obstetric complication (daily use of medication), history of headache, regular contractions, alcohol abuse, intolerance of IMN |
|
| Interventions | Intervention group: 40 mg IMN intravaginal. Comparison: placebo. Review arranged for the next day, if labour had not started then IOL was carried out according to local protocol |
|
| Outcomes | Additional induction agents required, maternal satisfaction, CS PPH | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Random number tables. |
| Allocation concealment? | Yes | Sealed sequentially numbered envelopes. |
| Blinding? Women |
Yes | Described as double blind. |
| Blinding? Clinical staff |
Yes | |
| Incomplete outcome data addressed? All outcomes |
Yes | Report that all women completed the study. |
| Methods | RCT, 2-arm trial. | |
| Participants | Setting: 43 women attending antenatal clinics in California, USA Inclusion criteria: women with “post-dates” pregnancies (gestational age > 41 weeks and 6 days based on reliable menstrual history and early ultrasound confirmation) with reactive NST Exclusion criteria: contraindications to prostaglandins. |
|
| Interventions | Intervention group: intracervical PGE2 0.5 mg. Comparison group: visually identical placebo gel. Women in both groups were observed for 1 hr with external fetal monitoring and then discharged home |
|
| Outcomes | Bishop score on admission, mode of delivery, interval to delivery, length of labour, infant birthweight and Apgar score | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | External sequence generation by hospital pharmacy. |
| Allocation concealment? | Yes | Coded syringes of identical appearance were dispensed from pharmacy |
| Blinding? Women |
Yes | Placebo controlled trial. |
| Blinding? Clinical staff |
Yes | |
| Blinding? Outcome assessor |
Unclear | Not clear when code was revealed. |
| Incomplete outcome data addressed? All outcomes |
Yes | All women randomised appeared to be included in the analyses |
| Free of other bias? | Yes | No other bias apparent. |
| Methods | RCT. 4-arm trial. | |
| Participants | Setting: Edinburgh, UK. 80 women recruited with induction of labour scheduled 72 hrs after recruitment Inclusion criteria: primiparous women aged 18-40, normal viable fetus, 37-41 weeks (confirmed by 1st trimester ultrasound scan), cephalic presentation, Bishop score < 5 Exclusion criteria: women who showed signs of labour onset, placental insufficiency or contraindication to mifepristone, |
|
| Interventions | Intervention: group 1: (25 women) oral mifepristone 50 mg. Group 2: (25 women) oral mifepristone 200 mg. (In this review we have combined both groups in the analysis although it was not clear how randomisation was achieved in the higher dose study.) Comparison groups: placebo (2 groups of women 25 compared with the lower dose and 5 with the higher dose. We have combined placebo groups in the analysis in this review as data were reported together in the results in the study reports; group size was very unbalanced for the second part of the study.) |
|
| Outcomes | Additional induction agents required, labour within 72 hrs, CS, oxytocin augmentation. NICU admission | |
| Notes | It was not clear why the placebo group for the higher dose comparison was so small (5 women) or how randomisation was performed to achieve the unbalanced intervention and control groups | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | “Pre-determined randomisation code.” |
| Allocation concealment? | Unclear | “Treatment in predetermined numeric order”. It was not clear why the group allocation in the placebo arms of the trial were very unbalanced |
| Blinding? Women |
Yes | “Neither the patient nor the physician had knowledge of whether a simple oral dose of mifepristone or placebo was given.” |
| Blinding? Clinical staff |
Yes | |
| Blinding? Outcome assessor |
Yes | |
| Incomplete outcome data addressed? All outcomes |
Unclear | All women randomised seemed to be accounted for in the analysis, although there was serious imbalance in group size |
| Free of other bias? | Unclear | In the second part of the study (higher dose) the treatment to placebo ratio was 1:5. It was not clear how randomisation was performed, or why the control group was so small |
| Methods | RCT 2-arm parallel group design. | |
| Participants | 120 women attending an antenatal clinic in a hospital in France, 1990-91 Inclusion criteria: termpregnancy scheduled for induction (range of indications), Bishop score < 4 Exclusion criteria: malpresentation, ruptured membranes, multiple pregnancy, > 1 previous CS or known medical condition |
|
| Interventions | Intervention group: active tablets mifepristone 200 mg. All women received a box with 2 tablets, the first to be taken on the morning of day 1 and the second on the morning of day 2 Comparison group: placebo tablets. Same regimen as intervention group IOL scheduled for 4 days after intervention, women reported to the hospital each day over the 4 day study period and were asked to report drug reactions, pain, bleeding or contractions |
|
| Outcomes | Labour within 4-day study period, other induction agents required, duration of labour, mode of birth, Apgar score < 7 at 5 min | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Tablets were supplied by pharmacy according to a “balanced randomisation list”. Block size 4 |
| Allocation concealment? | Yes | Small block size might mean that allocation order could potentially be anticipated in advance but the drug packs were described as being of similar appearance |
| Blinding? Women |
Yes | Described as a double-blind study. |
| Blinding? Clinical staff |
Yes | Placebo described as being of similar appearance. |
| Blinding? Outcome assessor |
Yes | |
| Incomplete outcome data addressed? All outcomes |
Unclear | 120 women were randomised but 8 were excluded from the results because of a deterioration in their condition within 12 hours of the first pill (3 in the mifepristone group and 5 in the placebo group) |
| Free of other bias? | Unclear | Additional induction agents were used for some women so labour and other outcomes may be affected by co-interventions |
| Methods | RCT, 2-arm trial. | |
| Participants | Setting: study carried out in 2 hospitals in France, 1991-2. 84 women randomised. Inclusion criteria: women with gestational age 41 weeks and 3 days or more and scheduled for induction for “post-dates” pregnancy, Bishop score < 6, induction could be postponed for 48 hrs Exclusion criteria: women with multiple pregnancies, ruptured membranes, contraindication to vaginal delivery, no uterine scarring, parity < 4, no FHR abnormalities, serious medical disease or obstetric complication |
|
| Interventions | Intervention group: mifepristone 400 mg, single oral dose. Comparison group: placebo tablets of identical appearance. Women in both groups returned after 1 day for review. If Bishop score > 6 then women had labour induction or returned the next day for labour induction |
|
| Outcomes | Change in Bishop score after 48 hrs, treatment to delivery interval, mode of birth, oxytocin augmentation, neonatal condition at delivery | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Balanced randomisation list in permuted blocks (block size not stated) |
| Allocation concealment? | Yes | Coded drug bottles. The “code for each subject was to be kept sealed in an opaque envelope to be opened in case of an emergency” |
| Blinding? Women |
Yes | Described as double blind study. Placebo described as being of identical appearance |
| Blinding? Clinical staff |
Yes | |
| Blinding? Outcome assessor |
Yes | |
| Incomplete outcome data addressed? All outcomes |
Yes | 84 women were recruited, 1 woman (from the mifepristone group) was lost to follow up |
| Free of other bias? | Yes | |
| Methods | RCT (little information on study methods). | |
| Participants | 32 women. Inclusion criteria: women with previous CS, gestational age 39 weeks with Bishop score < 6 |
|
| Interventions | Intervention group: Intracervical PGE2 repeated weekly. Comparison group: expectant management. |
|
| Outcomes | CS. | |
| Notes | Brief abstract, little information provided. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | No information. |
| Allocation concealment? | Unclear | “prospectively randomised.” |
| Blinding? Women |
No | Not feasible. |
| Blinding? Clinical staff |
No | |
| Methods | RCT. | |
| Participants | Setting: 102 women in a Cairo hospital, Egypt. Inclusion criteria: women at term (> 37 weeks’ gestation) scheduled for induction, singleton viable fetus, intact membranes, no uterine contractions Exclusion criteria: malpresentation, placenta previa, previous uterine surgery, contraindications to induction |
|
| Interventions | Intervention group: self-administered IMN, 40 mg, 3 doses 12 hrs apart (scheduled for 36, 24 and 12 hrs before induction Comparison group: placebo same regiment as intervention group |
|
| Outcomes | CS, further induction agents required, PPH, Apgar score >7 at 5mins, NICU admission, side effects | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer generated sequence. |
| Allocation concealment? | Yes | Coded treatment packs prepared by pharmacy. |
| Blinding? Women |
Yes | Placebo controlled trial. |
| Blinding? Clinical staff |
Yes | |
| Incomplete outcome data addressed? All outcomes |
Yes | All women randomised appear to be accounted for in the analysis |
| Methods | RCT, placebo controlled trial. | |
| Participants | Setting: not clear. 36 women. Inclusion criteria: healthy, nulliparous women, 41 weeks’ gestation and Bishop score < 9 |
|
| Interventions | Intervention group: 2.5 mg intravaginal PGE2, with 2nd dose if labour not established 24 hrs later Comparison group: placebo gel, with 2nd dose after 24 hrs if labour was not established |
|
| Outcomes | Change in cervix after 48hrs. | |
| Notes | Information from brief abstract. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Described as “randomized”. |
| Allocation concealment? | Unclear | No information. |
| Blinding? Women |
Yes | Described as double-blind trial with placebo gel. |
| Blinding? Clinical staff |
Yes | |
| Blinding? Outcome assessor |
Unclear | No information. |
| Incomplete outcome data addressed? All outcomes |
Unclear | Little information on methods. It appeared that all women were available at follow up |
| Methods | RCT with block randomisation. | |
| Participants | Setting: outpatient clinic in North Carolina USA. 56 women randomised Inclusion criteria: primiparous women at term (39 weeks and 4 days to 41 weeks) with singleton, cephalic, pregnancy and Bishop score < 7 Exclusion criteria: cannot tolerate acupuncture, uncertain dates, breech presentation, placenta praevia, contraindication to vaginal delivery |
|
| Interventions | Cervical examination and ultrasound at recruitment. Intervention group: acupuncture + routine care on 3 of 4 consecutive days, visits also included fetal monitoring, treatment by trained acupuncturist to hands, legs and lower back and low voltage stimulation Comparison group: routine care with follow up after 3 or 4 days |
|
| Outcomes | Vaginal delivery not achieved in 24 hrs, additional induction agents required. CS, mean time to delivery | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer generated sequence in balanced blocks of 2 or 4. |
| Allocation concealment? | Yes | Sealed, opaque, sequentially numbered envelopes. |
| Blinding? Women |
No | Blinding not feasible. |
| Blinding? Clinical staff |
No | |
| Blinding? Outcome assessor |
No | |
| Incomplete outcome data addressed? All outcomes |
Unclear | Data were available for all women randomised but denominators were not clear for some outcomes |
| Free of other bias? | Unclear | Women receiving acupuncture attended for 3 additional visits where other interventions occurred as well as acupuncture that may have affected outcomes |
| Methods | RCT, 2-arm trial. | |
| Participants | Setting: Los Angeles hospital, USA, 1996-2000 120 women with diabetes. Inclusion criteria: women with insulin dependent or other diabetes, gestational age 38 weeks (confirmed by ultrasound), not in labour, normal AFI (> 5 cm), normal FHR. Women compliant with hospital appointments and home glucose monitoring Exclusion criteria: women with multiple pregnancies, ruptured membranes, vaginal bleeding, prior uterine surgery, active genital herpes, glaucoma, serious medical disease, parity > 5, fetal weight > 4500 g or < 2000 g |
|
| Interventions | Study over 7 days. Intervention group: single dose of vaginal misoprostol 25 mcg Comparison group: placebo of similar appearance. Both groups were observed for 4 hrs and if there were no signs of fetal distress of painful contractions women were sent home. Reviewed after 3-4 days. If labour had not started then intervention/placebo was repeated. At 7 days women not in labour were induced |
|
| Outcomes | Additional induction agents required (oxytocin), mode of delivery, uterine hyperstimulation, neonatal condition at delivery | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer generated sequence. |
| Allocation concealment? | Yes | Coded drug boxes. Pharmacy prepared and distributed medication according to the randomisation schedule |
| Blinding? Women |
Yes | Placebo controlled trial. |
| Blinding? Clinical staff |
Yes | |
| Blinding? Outcome assessor |
Unclear | Not clear when code revealed. |
| Incomplete outcome data addressed? All outcomes |
Unclear | 120 women randomised and no loss to follow up was apparent but denominators in the data tables were not always clear |
| Free of other bias? | Yes | |
| Methods | RCT 2-arm parallel group design (dose comparison study). | |
| Participants | 52 women attending a large teaching hospital and scheduled for induction of labour Inclusion criteria: singleton, cephalic presentation, not in active labour, gestational age > 40 weeks (confirmed by menstrual dates and ultrasound before 20 weeks) Exclusion criteria: previous CS, FHR abnormalities, contraindication to prostaglandin or vaginal delivery |
|
| Interventions | Intervention group: 50 mcg oral misoprostol. Comparison group: 25 mcg misoprostol. Prior to randomisation women received an ultrasound to assess fetal growth and AFV and a fetal NST was carried out. In both groups medication was administered by a nurse and in the absence of labour or contraindications the dose was repeated after 3 days to a max of 3 doses over 9 days. Women returned to hospital every 3 days unless labour started or there was any reduction in fetal kicks |
|
| Outcomes | Days to delivery, uterine hyperstimulation, further induction agents required, CS, Apgar score < 6 at 5 min, NICU admission, meconium staining, perinatal death | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer generated sequence. |
| Allocation concealment? | Yes | Coded drug boxes. |
| Blinding? Women |
Unclear | Tablets were described as indistinguishable but they were cut from larger tablets (1/4 or 1/8) |
| Blinding? Outcome assessor |
Yes | Described as blind. |
| Incomplete outcome data addressed? All outcomes |
Unclear | There were some inconsistencies in the figures; while 49 women seem to have been randomised there were 52 in the results tables |
| Methods | RCT, 3-arm trial. | |
| Participants | Setting: not clear (outpatient setting). 136 women randomised. Inclusion criteria: women at term (37 weeks’ gestation), Bishop score < 6, candidates for vaginal delivery with uncomplicated pregnancy Exclusion criteria: women with diabetes or serious pregnancy complications including hypertension, or chronic medical conditions |
|
| Interventions | Intervention group (1): PGE2 0.5 mg intracervical. Intervention group (2): vaginal oestrogen cream (estradiol) 4 mg Comparison group: inert lubricant vaginal jelly. Women were assessed weekly until an indication for delivery arose. Medication was repeated weekly |
|
| Outcomes | Mode of delivery, use of oxytocin, condition of newborn. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Random number tables. |
| Allocation concealment? | Yes | Opaque, sequentially numbered envelopes. |
| Blinding? Women |
Yes | Placebo controlled. |
| Blinding? Clinical staff |
Unclear | Interventions not identical. |
| Incomplete outcome data addressed? All outcomes |
Yes | 136 women were randomised, 8 were excluded after randomisation |
| Methods | RCT. | |
| Participants | Setting: not clear. 32 women. Inclusion criteria: women that had had a previous CS with gestational age > 38 and < 42 weeks confirmed by ultrasound. All women were scheduled for induction (21 for “postdates”, 7 for hypertension and 4 for fetal growth retardation); Bishop score < 4 |
|
| Interventions | The study was carried out over a 4 day observation period, induction was planned for the 4th day (PGE2 and amniotomy or oxytocin induction if Bishop score > 3). Women attended the outpatient’s department for NST daily Intervention group: 200 mg oral mifepristone on days 1 and 2 Comparison group: placebo, same regime as intervention group |
|
| Outcomes | CS, assisted delivery, uterine scar separation, fetal distress | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Described as “randomisation list” using block design (block size 4) |
| Allocation concealment? | Yes | Coded drug boxes. |
| Blinding? Women |
Unclear | Described as double-blind placebo controlled study. “External appearance of the tablets was similar.” |
| Blinding? Clinical staff |
Yes | |
| Incomplete outcome data addressed? All outcomes |
Yes | All women appeared to be accounted for in the analysis. |
| Methods | RCT 2-arm parallel group design. | |
| Participants | 90 women attending 4 USA hospitals. Inclusion criteria: women with post-dates pregnancy (gestational age > 40 weeks + 3 days) attending for FHR testing. Gestation confirmed by ultrasound before 24 weeks, AFI > 5 cm, reactive NST Exclusion criteria: malpresentation, multiple pregnancy, previous CS, evidence of hyperstimulation or suspicious FHR patterns, grand multiparity (> 4 previous deliveries), placenta praevia or other contraindications to vaginal delivery |
|
| Interventions | Intervention group: intracervical PGE2 gel (Prepidil) 0.5 mg Comparison group: placebo gel. Gel was inserted by doctor or midwife in an antenatal testing centre or in the labour unit within rapid transport distance of delivery facilities. After insertion there was 40 min of continuous monitoring. Women returned to hospital after 3-4 days for fetal testing and further gel up to a max of 4 doses |
|
| Outcomes | Further induction agents required, CS rates, uterine hyperstimulation, FHR changes and side effects | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer generated sequence (permuted block design, but block size not stated) |
| Allocation concealment? | Yes | Central randomisation with coded drug boxes. |
| Blinding? Women |
Yes | Unblinding was reported to occur only after completion of all the data collection |
| Blinding? Clinical staff |
Yes | |
| Blinding? Outcome assessor |
Yes | |
| Incomplete outcome data addressed? All outcomes |
Yes | 2 women that were randomised were not included in the analysis as they did not meet the inclusion criteria (the study was described as ITT) |
| Free of other bias? | Yes | |
| Methods | RCT, 2-arm trial. | |
| Participants | Setting: 40 women, setting not clear. Inclusion criteria: women with gestational age 40-42 weeks, singleton, cephalic presentation, intact membranes, Bishop score < 6, reassuring FHR and < 3 contractions in 10 minutes |
|
| Interventions | Intervention group: 100 mg oral misoprostol, dose repeated every 24 hrs with max of 3 doses. 2 hrs continual fetal monitoring after each dose Comparison group: placebo, with same regime and monitoring as the intervention group |
|
| Outcomes | Chorioamnionitis, meconium aspiration, uterine hyperstimulation, mean time to active labour | |
| Notes | Study reported in brief abstract. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Described as “randomized”. |
| Allocation concealment? | Unclear | Placebo controlled, no information on randomisation procedure |
| Blinding? Women |
Yes | Described as double-blind, placebo controlled study. |
| Blinding? Clinical staff |
Yes | |
| Blinding? Outcome assessor |
Unclear | Not described. |
| Incomplete outcome data addressed? All outcomes |
Unclear | All women appeared to have been followed up, but little information |
| Methods | RCT. 3-arm trial. | |
| Participants | Setting: California, USA, women attending a naval medical centre 70 women included in the analysis (2 of 3 treatment arms included, total recruited 105 women) Inclusion criteria: women with “post dates” pregnancy - gestational age 41 weeks confirmed by menstrual dates and pre-20 weeks ultrasound. Uncomplicated pregnancy. Bishop score < 5 Exclusion criteria: women with any contraindication to vaginal delivery |
|
| Interventions | (1 intervention group had daily membrane stripping; this group has not been included in the analysis in this review.) Intervention group: intracervical PGE2 0.5 mg, daily for 3 days Comparison group: gentle cervical examination, daily for 3 days Women were instructed to return to hospital is they had bleeding, membrane rupture, regular contractions of reduction in fetal movements. Once Bishop score = 8 or women reached 42 weeks they were admitted to hospital for induction |
|
| Outcomes | Induced at 42 weeks, CS, instrumental delivery. Apgar score < 7 at 5 min, admission to NICU | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Random number tables. |
| Allocation concealment? | Yes | Sealed, opaque, sequentially numbered envelopes. |
| Blinding? Women |
No | Not feasible. |
| Blinding? Clinical staff |
No | |
| Blinding? Outcome assessor |
Unclear | Outcome assessment of cervical changes were reported to be blind |
| Incomplete outcome data addressed? All outcomes |
Yes | No apparent loss to follow up. |
| Methods | RCT. | |
| Participants | Setting: Ohio hospital USA (65 women). Inclusion criteria: women at term (gestational age > 37 weeks), age > 17 years, Bishop score < 7. “Well dated pregnancy” with no indication for immediate induction Exclusion criteria: multiple pregnancy, insulin dependent diabetes, ruptured membranes, non-reassuring NST, contraindications to a trial of labour, chronic hypertension |
|
| Interventions | Intervention group: intracervical PGE2 0.5 mg. Comparison group: placebo. Both groups had continuous monitoring for 1 hr, if labour started women were admitted to hospital, otherwise they were discharged home |
|
| Outcomes | Uterine hyperstimulation, further induction agents required, uterine hyperstimulation, CS | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Random number tables. |
| Allocation concealment? | Yes | Placebo controlled trial. |
| Blinding? Women |
Yes | Placebo was described as identical to active PGE2. |
| Blinding? Clinical staff |
Yes | |
| Blinding? Outcome assessor |
Yes | |
| Incomplete outcome data addressed? All outcomes |
Yes | 65 women were randomised, there were 4 post randomisation exclusions |
| Free of other bias? | Yes | No baseline imbalance apparent. |
| Methods | RCT, 2-arm trial. | |
| Participants | Setting: not clear. 68 women included, Inclusion criteria: women with “well-dated” pregnancies with gestational age > 40 weeks and Bishop score < 9 Exclusion criteria: current indication for IOL, malpresentation, multiple pregnancy, previous CS, oligohydramnios (AFI < 5 cm). any contraindication to a trial of labour, current regular contractions |
|
| Interventions | All women were assessed prior to randomisation. Intervention group: vaginal misoprostol 25 mcg. Comparison group: placebo gel. Fetal and uterine monitoring for 1 hr after treatment then women were discharged home. Labour was induced if BIshop score > 8 after 41 weeks or all women after 42 weeks |
|
| Outcomes | Uterine hyperstimulation, mode of delivery, epidural, Apgar score, NICU admission. (Women with PROM were given oxytocin to “stimulate labour” but were not included as inductions in the analyses.) | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer generated sequence performed in hospital pharmacy. |
| Allocation concealment? | Yes | Placebo controlled trial. |
| Blinding? Women |
Yes | Placebo of similar appearance. |
| Blinding? Clinical staff |
Yes | |
| Incomplete outcome data addressed? All outcomes |
Yes | 68 women were randomised, 4 were excluded after randomisation and did not receive the study medication, but were included in an ITT analysis |
| Free of other bias? | Unclear | No baseline imbalance apparent. |
| Methods | RCT 2-arm parallel group design. | |
| Participants | 84 women attending a USA hospital between 199 to 2001. Inclusion criteria: singleton, cephalic presentation, intact membranes, Bishop score of 6 or less, reactive NST Exclusion criteria: ruptured membranes, Bishop score > 6, contraindication to induction, > 3 contractions in 10 min, uterine scar |
|
| Interventions | Intervention group: vaginal misoprostol 25 mcg. Comparison group: intracervical PGE2 gel (dinoprostone) 0.5 mg Women in both groups were randomised after a reactive NST. After drug administration women had continuous fetal heart rate monitoring for 3hrs with discharge home if clinically stable. Women were asked to return the next day (after 18 hrs) for oxytocin induction if labour was not established |
|
| Outcomes | Vaginal delivery within 24 or 48 hrs, uterine hyperstimulation, CS, oxytocin required, Apgar score < 7 at 5 min, NICU admission | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Random number table. |
| Allocation concealment? | Yes | Opaque sequentially numbered envelopes (not stated whether sealed) |
| Blinding? Women |
No | Blinding women would be feasible but the study was not blinded |
| Blinding? Clinical staff |
No | |
| Blinding? Outcome assessor |
No | |
| Incomplete outcome data addressed? All outcomes |
Yes | 84 women were randomised (42 in each group), 2 women were lost to follow up in the misoprostol group but were included in the denominators |
| Free of other bias? | Yes | None apparent. |
| Methods | RCT, 2-arm trial. | |
| Participants | 58 women. Inclusion criteria: women with diabetes at term or women with prolonged pregnancy (> 42 weeks) requiring induction, Bishop score < 7 |
|
| Interventions | Intervention group: 2 mg intravaginal PGE2 after reassuring NST, then continuous fetal monitoring for 3 hrs. Women were admitted if labour started or cervix favourable. Treatment repeated after 24 and 48 hrs and admitted after 3rd dose Comparison group: expectant management with weekly assessment of AFI and NST. Admission in labour or if signs of fetal distress. IOL at 44 weeks |
|
| Outcomes | Spontaneous labour within 48 hrs, uterine hyperstimulation, CS | |
| Notes | Results reported in brief abstract. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Described as “prospectively randomised”. |
| Allocation concealment? | Unclear | Not described. |
| Blinding? Women |
No | Not feasible. |
| Blinding? Clinical staff |
No | |
| Blinding? Outcome assessor |
No | |
| Incomplete outcome data addressed? All outcomes |
Unclear | Little information. No loss to follow up apparent. |
| Methods | Placebo controlled RCT. | |
| Participants | Setting: outpatient clinic in Memphis, USA. 100 women recruited. Inclusion criteria: gestation 38-40 weeks with Bishop score < 7 Exclusion criteria: non-reactive NST, oligohydramnios (AFI < 5.0 cm) macrosomia (> 4000 g or 10th centile), medical indication for delivery, more than 1 previous CS |
|
| Interventions | All women underwent NST, AFV and ultrasound assessment. Intervention group: 2 mg intravaginal PGE2 for 5 consecutive days Comparison group: identical placebo for 5 consecutive days. After each dose women were monitored for 30 min to rule out labour or fetal distress. Women were reviewed twice weekly (NST and AFV) |
|
| Outcomes | Other induction agents required, uterine hyperstimulation, CS, epidural, chorioamnionitis, Apgar score, NICU admission, gestational age at delivery | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Random number table. Permuted blocks with variable block size. The randomisation schedule was kept in pharmacy |
| Allocation concealment? | Yes | Coded drug boxes prepared by pharmacy. |
| Blinding? Women |
Yes | Placebo controlled trial. |
| Blinding? Clinical staff |
Yes | |
| Incomplete outcome data addressed? All outcomes |
Yes | State that “no post randomisation exclusions were allowed”. All women included in the analysis |
| Free of other bias? | Yes | No baseline imbalance apparent. |
| Methods | RCT, 2-arm trial. | |
| Participants | Setting: 36 women attending hospital in Stockholm, Sweden. Inclusion criteria: women 14 days post-term scheduled for induction but where IOL could be postponed for 48 hrs, Bishop score < 7 Exclusion criteria: parity > 4, contraindications to vaginal delivery, oligohydramnios, prior uterine surgery, obstetric or medical complications |
|
| Interventions | Intervention group: mifepristone. Comparison group: placebo. Women returned for review after 24 and 48 hrs if labour did not start. If Bishop score > 6 then ARM and oxytocin induction, if < 6 then PGE2 0.5 mg intracervical up to 2 treatments |
|
| Outcomes | Use of additional induction agents, mode of delivery, cervical change after 48 hrs, Apgar score at 5 min | |
| Notes | Unbalanced randomisation 24 in intervention group vs 12 in control group | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Random number tables. |
| Allocation concealment? | Yes | Coded drug boxes, “sealed pre-numbered boxes containing either mifepristone or placebo tablets” |
| Blinding? Women |
Yes | “the type of treatment the women were given was not known until the entire study was finished” |
| Blinding? Clinical staff |
Yes | |
| Blinding? Outcome assessor |
Yes | |
| Incomplete outcome data addressed? All outcomes |
Yes | No loss to follow up apparent. |
| Free of other bias? | Unclear | Some baseline imbalance, intervention group 79% primiparous vs 58% in the control group |
| Methods | RCT, 2-arm trial. | |
| Participants | Setting: USA. FHR tracings and uterine activity monitored for 20 minutes before randomisation Inclusion criteria: 294 women who had had one previous CS and were candidates for vaginal delivery with accurate gestational age dating (39 - 41 weeks) by ultrasound before 20 weeks, with no signs of labour, no fetal growth abnormalities and reassuring FHR tracing. Bishop score < 6 Exclusion criteria: malpresentation, multiple pregnancies, diabetes, hypertension, vaginal bleeding, ruptured membranes, cephalopelvic disproportion, contraindication to oxytocic drugs or hypersensitivity to PGE2, more than 1 previous CS |
|
| Interventions | Intervention group: intracervical PGE2 0.5 mg. Women were monitored for 2hrs after insertion Comparison group: expectant management. Women in both groups were reviewed at 40 and 41 weeks for routine assessments |
|
| Outcomes | Further induction agents required, uterine hyperstimulation, CS, instrumental vaginal delivery, maternal infection, Apgar score at 5 min, side effects, birthweight | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer generated sequence provided by pharmaceutical company |
| Allocation concealment? | Unclear | “Blocks of the list were sent with the drugs to the study centres where new subjects were assigned to the next number on the list to determine treatment group.” |
| Blinding? Women |
No | Study described as “open-label”. |
| Blinding? Clinical staff |
No | Not feasible. |
| Blinding? Outcome assessor |
No | |
| Incomplete outcome data addressed? All outcomes |
Yes | 300 were enrolled but 6 were not included in analysis “because of improper entry or non compliance with clinic visits” |
| Free of other bias? | Unclear | Groups appeared similar at baseline. Research was supported by the manufacturers of the study intervention (Prepidil) |
| Methods | RCT. | |
| Participants | Setting: post-dates clinic in Florida hospital USA. 50 women with prolonged pregnancy (> 41 weeks, 287 days). Inclusion criteria: reactive NST and normal ultrasound, EDD confirmed by menstrual dates, clinical exam and early ultrasound. Bishop score < 9 Exclusion criteria: malpresentations, multiple pregnancy, diabetes, hypertension, vaginal bleeding, abnormal FHR, established contractions, macrosomia (> 4500 g), FGR, fetal abnormalities or oligohydramnios |
|
| Interventions | Intervention group: Intravaginal PGE2 gel 2 mg. Repeated twice weekly Comparison group: placebo gel. Repeated twice weekly. Uterine activity and FHR was monitored for 1-2 hrs after gel insertion, if no regular contractions or side effects, women were discharged home returning for weekly sonograms and AFV assessment, and returning twice weekly for NST and repeat interventions |
|
| Outcomes | Further induction agents required, uterine hyperstimulation, CS, NICU admission | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | “randomly generated assignments”. |
| Allocation concealment? | Unclear | “drawing of envelopes”. |
| Blinding? Women |
Yes | Placebo controlled study. |
| Blinding? Clinical staff |
Yes | |
| Blinding? Outcome assessor |
Unclear | Not reported. |
| Incomplete outcome data addressed? All outcomes |
Yes | All women randomised accounted for in the analysis. |
| Methods | RCT. | |
| Participants | Setting: 91 women with prolonged pregnancy (gestational age > 41 weeks) attending a Florida, USA hospital Inclusion criteria: uncomplicated pregnancy, reliable dating, Bishop score < 9, reactive NST and ultrasound Exclusion criteria: vaginal bleeding, ruptured membranes, macrosomia (estimated fetal weight > 4500 g) previous uterine surgery or stillbirth, abnormal FHR or ultrasound, regular contractions |
|
| Interventions | Intervention group: daily self-administered vaginal PGE2 2mg before bed (women were given instructions re placement and storage of suppositories) Comparison group: self-administered placebo. Telephone contact available 24 hrs per day for both groups. Twice weekly clinic attendance for post-dates surveillance (NST and AFV); induction if indicated or at 44 weeks |
|
| Outcomes | CS rates, chorioamnionitis, Apgar score at 5 min, NICU admission | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer generated. |
| Allocation concealment? | Yes | Coded drug boxes. |
| Blinding? Women |
Yes | Described as “double blind” placebo controlled. |
| Blinding? Clinical staff |
Yes | |
| Incomplete outcome data addressed? All outcomes |
Unclear | 91 were enrolled but 11 were lost to follow up (3 were excluded as they were non compliant) |
| Methods | RCT, 2-arm trial. | |
| Participants | Setting: district hospital in southern Nigeria, 2000-2001. 77 women randomised. Inclusion criteria: women with gestational age > 40 weeks, Bishop score < 9, uncomplicated pregnancy, candidates for vaginal delivery Exclusion criteria: women with previous CS, vaginal bleeding, ruptured membranes of indication for immediate IOL, uncertain dates, non reactive stress test or estimated fetal weight > 4500 g |
|
| Interventions | Intervention group: vaginal misoprostol 25 mcg. Comparison group: expectant management with gentle vaginal examinations only Women were monitored for 1 hr after treatment. If regular contractions started women were admitted otherwise they were discharged home |
|
| Outcomes | Mode of delivery, uterine hyperstimulation, perinatal; death, NICU admission, PPH, interval to admission in labour | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer generated. |
| Allocation concealment? | Yes | Sealed, sequentially numbered envelopes (not stated that envelopes opaque) |
| Blinding? Women |
No | Described as an “open” randomised controlled trial. |
| Blinding? Clinical staff |
No | |
| Blinding? Outcome assessor |
No | |
| Incomplete outcome data addressed? All outcomes |
Yes | Outcome data were available for all women randomised. |
| Free of other bias? | Yes | Groups appeared similar at baseline. |
| Methods | RCT. | |
| Participants | Setting: USA, naval medical centre. 50 women. Inclusion criteria: women with prolonged pregnancy (41-42 weeks’ gestation) confirmed by ultrasound, clinical examination and menstrual dates. Singleton, cephalic presentation, intact membranes, Bishop score < 5, < 8 contractions per hour, AFI > 5 cm, reactive NST, maternal age > 18, < 50 Exclusion criteria: malpresentations, multiple pregnancy, previous CS, vaginal bleeding, ruptured membranes, non reactive NST, estimated fetal weight > 4500 g or < 2000 g, placenta previa, active herpes, hypersensitivity to prostaglandin, signs of infection, asthma or serious disease |
|
| Interventions | Intervention group: vaginal misoprostol 25 mcg. (with 2nd dose after 24 hrs.) Comparison group: placebo, packaged and labelled to appear indistinguishable Both groups were observed for 4 hrs with FHR and uterine activity monitoring. If women showed no sign of labour of fetal distress they were discharged and asked to return after 24 hrs for a second dose, then review after a further 24 hrs for inpatient management |
|
| Outcomes | Uterine hyperstimulation, CS, Apgar score < 7 at 5 min, meconium staining | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer generated sequence by pharmacy (permuted block design) |
| Allocation concealment? | Yes | The list was maintained by inpatient pharmacy and drugs were dispensed to appear identical |
| Blinding? Women |
Yes | Placebo controlled trial. |
| Blinding? Clinical staff |
Yes | |
| Blinding? Outcome assessor |
Yes | |
| Incomplete outcome data addressed? All outcomes |
Yes | No apparent loss to follow up. |
AFI: amniotic fluid index
AFV: amniotic fluid volume
CS: caesarean section
EDD: expected date of delivery
FGR: fetal growth retardation
FHR: fetal heart rate
hrs: hours
IMN: isosorbide mononitrate
IOL: induction of labour
ITT: intention to treat
max: maximum
min: minutes
NHS: National Health Service (UK)
NICU: neonatal intensive care unit
NST: non-stress test
PPH: postpartum haemorrhage
PROM: premature rupture of the membranes
RCT: randomised controlled trial
vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Adewole 1993 | This study examined breast stimulation and used a crossover design. Women were allocated to either breast stimulation versus no stimulation; after 3 days, if labour had not started women crossed over into the other study group |
| Damania 1988 | Very little information was provided on study methods. It was not clear that this was a RCT |
| Damania 1992 | In this study breast stimulation was compared with an oxytocin infusion. It was not clear that women in the oxytocin group were discharged home |
| Di Lieto 1989 | This study used a crossover design. |
| Doany 1997 | In this study intravaginal PGE2 with or without membrane sweeping was compared with placebo with or without membrane sweeping. Complex interventions or interventions involving membrane sweeping are not included in this review |
| Dorfman 1987 | In this study women received a range of homeopathic herbal preparations versus placebo. The intervention was to prepare women for childbirth generally rather than to induce labour |
| Elliott 1984 | This study focused on breast stimulation and used a crossover design |
| Evans 1983 | It was not clear that this was a RCT: “the assignment [of medication] to patients was by consecutive entry into either of the studies”. The paper described findings for two separate studies both examining the use of intracervical porcine ovarian relaxin. The first study appeared to be conducted in hospital and women receiving medication were compared with a control group. In the “outpatient study” there was no control group; women received either 2 mg or 4 mg of relaxin 5-7 days before scheduled induction; no outcomes were reported relevant for inclusion in the review |
| Garry 2000 | This study compared castor oil with no treatment, women were alternately allocated to groups; otherwise there was little information on methods |
| Griffin 2003 | This study was reported in a brief abstract and insufficient information was available on methods and results to include the study. We contacted the study author and further data are not available |
| Herabutya 1992 | This study examined intracervical prostaglandin. Little information was provided on study methods. Women “randomized” to the intervention group received intracervical PGE2 and then monitored for 4-6 hrs, some had a repeat dose after 6hrs, some had a repeat dose the next day and if labour did not start on the third day these women were admitted to hospital for amniotomy and oxytocin infusion. It was not clear what happened to women in the control group other than that they had weekly fetal monitoring; these women were not admitted unless there were signs of abnormality or until they reached 44 weeks’ gestation. The management of women in the two groups was so different that results are difficult to interpret |
| Kadar 1990 | This study focused on nipple stimulation. Group allocation was by a quasi-randomised method; there were serious protocol violations and analysis was not by randomisation group making results very difficult to interpret |
| Kaul 2004 | This study focused on membrane sweeping. This intervention is not included in this review |
| Krammer 1995 | This study was reported in a very brief abstract. No original data were presented in the results |
| Magann 1999 | This study compared PGE2 and membrane sweeping. Membrane sweeping is not included in this review |
| Manidakis 1999 | This study was reported in a brief abstract. It was not clear that it was a RCT. We were unable to find contact details for the author to obtain further information |
| Moghtadaei 2007 | This study focused on extra-amniotic saline infusion, an intervention rarely used nowadays. It was not clear that this intervention was carried out in an outpatient setting |
| Ohel 1996 | This quasi randomised trial compared women receiving vaginal PGE2 with expectant management. Analysis was not by randomisation group. Of 96 cases randomised to PGE2 26 preferred expectant management and were therefore omitted from the analysis. As there was no intention to treat analysis results of this study were very difficult to interpret |
| Rayburn 1988 | In this study some of the women included in the study were admitted to hospital rather that being treated as outpatients. No separate results were available for women in the outpatient group |
| Rijnders 2007 | In this study the same method of inducing labour was used in both groups, 1 group was treated in hospital and 1 at home |
| Salamalekis 2000 | In this study membrane sweeping was compared with oxytocin for labour induction. It was not clear that women were discharged home after interventions and membrane sweeping is not included in this review |
| Salmon 1986 | This study focused on breast stimulation and used a crossover design. Women were allocated to either breast stimulation versus no stimulation; after three days, if labour had not started women crossed over into the other study group |
| Spallicci 2007 | The intervention in this trial was an intracervical injection of hyalurodinase. This intervention is no longer used in clinical practice |
| Voss 1996 | It was not clear that this intervention was carried out in an outpatient setting or that women were discharged home after treatment |
| Ziaei 2003 | This study compared dexamethasone with oxytocin. it was not clear that the intervention was carried out in an outpatient setting |
hrs: hours
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Double blind RCT. |
| Participants | 30 women at term (40-41 weeks) with a Bishop score < 7. |
| Interventions | Intervention: (2 groups) 200 mcg or 100 mcg of oral misoprostol Comparison group: placebo. FHR and uterine monitoring for 2 hrs after medication. Procedure was repeated after three days if labour did not start until 42 weeks |
| Outcomes | Interval to delivery, CS, Induction at 42 weeks, hyperstimulation, Apgar scores |
| Notes | This study was reported in a brief abstract and the data was described as “preliminary”. We attempted to contact authors for further information (8th September 2009) |
| Methods | Double blind RCT. |
| Participants | 50 primiparous women with unfavourable cervix with gestational age > 41 weeks |
| Interventions | Intervention group: 2 tablets (400 mg) mifepristone 48 hrs before scheduled induction of labour Comparison group: 2 tablets placebo. |
| Outcomes | Interval to delivery, CS, onset of spontaneous labour. |
| Notes | This study was reported in a brief abstract. The setting was not clear. We attempted to contact the authors for further information (11th September 2009) |
CS: caesarean section
FHR: fetal heart rate
hrs: hours
RCT: randomised controlled trial
DATA AND ANALYSES
Comparison 1.
Intravaginal PGE2 gel versus placebo or expectant management
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Additional induction agents required | 2 | 150 | Risk Ratio (M-H, Fixed, 95% CI) | 0.52 [0.27, 0.99] |
| 2 Serious neonatal morbidity or death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Serious maternal morbidity or death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4 Vaginal delivery not achieved in 24/48 hrs | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Uterine hyperstimulation (FHR changes unclear) | 4 | 244 | Risk Ratio (M-H, Fixed, 95% CI) | 3.76 [0.64, 22.24] |
| 6 Uterine hyperstimulation (without FHR changes) | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Caesarean section | 4 | 288 | Risk Ratio (M-H, Fixed, 95% CI) | 0.80 [0.49, 1.31] |
| 8 Assisted (instrumental) vaginal delivery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9 Oxytocin augmentation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10 Epidural | 1 | 100 | Risk Ratio (M-H, Fixed, 95% CI) | 0.83 [0.62, 1.12] |
| 11 Maternal death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Uterine rupture | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13 Serious maternal complication | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Chorioamnionitis | 2 | 180 | Risk Ratio (M-H, Fixed, 95% CI) | 0.37 [0.15, 0.90] |
| 15 Endometritis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16 Maternal antibiotics | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Perinatal death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18 Apgar score < 7 at 5 minutes | 2 | 180 | Risk Ratio (M-H, Fixed, 95% CI) | 0.45 [0.07, 2.93] |
| 19 NICU admission | 3 | 230 | Risk Ratio (M-H, Fixed, 95% CI) | 0.32 [0.10, 1.03] |
| 20 Neonatal antibiotics | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21 Neonatal infection | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22 Interval from intervention to delivery | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 23 Cervix unchanged at follow up | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 0.13 [0.03, 0.47] |
| 24 “Spontaneous labour” within 48 hours | 1 | 58 | Risk Ratio (M-H, Fixed, 95% CI) | 6.43 [2.12, 19.48] |
| 25 Admitted to hospital for labour | 1 | 100 | Risk Ratio (M-H, Fixed, 95% CI) | 2.7 [1.47, 4.97] |
| 26 Gestational age at delivery (weeks) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | −0.60 [−0.99, −0.21] |
| 27 Gestational age on admission (days) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | −2.0 [−4.17, 0.17] |
Comparison 2.
Intracervical PGE2 versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Additional induction agent required (induction with oxytocin or other means) | 3 | 445 | Risk Ratio (M-H, Fixed, 95% CI) | 0.98 [0.74, 1.32] |
| 2 Additional induction agents required (further prostaglandin required) | 1 | 90 | Risk Ratio (M-H, Fixed, 95% CI) | 0.61 [0.22, 1.67] |
| 3 Serious neonatal morbidity or death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4 Serious maternal morbidity or death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Delivery not achieved in 48-72 hrs | 1 | 43 | Risk Ratio (M-H, Fixed, 95% CI) | 0.83 [0.68, 1.02] |
| 6 Uterine rupture | 1 | 294 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Uterine hyperstimulation (with FHR changes) | 4 | 488 | Risk Ratio (M-H, Fixed, 95% CI) | 2.66 [0.63, 11.25] |
| 8 Uterine hyperstimulation (without FHR changes) | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9 Postpartum haemorrhage (> 500 ml) | 1 | 61 | Risk Ratio (M-H, Fixed, 95% CI) | 3.10 [0.13, 73.16] |
| 10 Caesarean section | 7 | 674 | Risk Ratio (M-H, Fixed, 95% CI) | 0.90 [0.72, 1.12] |
| 11 Assisted (instrumental) vaginal delivery | 4 | 538 | Risk Ratio (M-H, Fixed, 95% CI) | 1.29 [0.85, 1.96] |
| 12 Oxytocin augmentation | 1 | 84 | Risk Ratio (M-H, Fixed, 95% CI) | 0.67 [0.40, 1.12] |
| 13 Epidural | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Maternal death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 15 Serious maternal complication | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16 Chorioamnionitis | 3 | 468 | Risk Ratio (M-H, Fixed, 95% CI) | 2.03 [0.66, 6.18] |
| 17 Endometritis | 2 | 174 | Risk Ratio (M-H, Fixed, 95% CI) | 1.60 [0.27, 9.37] |
| 18 Maternal antibiotics | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19 Perinatal death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20 Apgar score < 7 at 5 minutes | 4 | 515 | Risk Ratio (M-H, Fixed, 95% CI) | 0.82 [0.42, 1.60] |
| 21 NICU admission | 3 | 215 | Risk Ratio (M-H, Fixed, 95% CI) | 1.61 [0.43, 6.05] |
| 22 Neonatal antibiotics | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23 Neonatal infection | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 24 Interval from intervention to delivery (days) | 2 | 133 | Std. Mean Difference (IV, Fixed, 95% CI) | −0.20 [−0.55, 0.14] |
| 25 Cervix unchanged after 24/48 hrs | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 26 Maternal side effects | 2 | 384 | Risk Ratio (M-H, Fixed, 95% CI) | 0.59 [0.13, 2.77] |
| 27 Gestational age at delivery (weeks) | 2 | 156 | Mean Difference (IV, Random, 95% CI) | −0.06 [−0.35, 0.23] |
| 28 Induction for gestational age > 42 weeks | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 29 Delivery within 48 hours of treatment (all deliveries) | 1 | 61 | Risk Ratio (M-H, Fixed, 95% CI) | 3.1 [1.29, 7.47] |
Comparison 3.
Vaginal misoprostol versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Additional induction agents required | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2 Serious neonatal morbidity or death | 1 | 77 | Risk Ratio (M-H, Fixed, 95% CI) | 0.34 [0.01, 8.14] |
| 3 Serious maternal morbidity or death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4 Vaginal delivery not achieved in 24/48 hrs | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Uterine hyperstimulation (with FHR changes) | 3 | 265 | Risk Ratio (M-H, Fixed, 95% CI) | 1.97 [0.43, 9.00] |
| 6 Uterine hyperstimulation (without FHR changes) | 2 | 137 | Risk Ratio (M-H, Fixed, 95% CI) | 3.64 [0.15, 85.97] |
| 7 Caesarean section | 4 | 325 | Risk Ratio (M-H, Fixed, 95% CI) | 0.94 [0.61, 1.46] |
| 8 Assisted (instrumental) vaginal delivery | 2 | 145 | Risk Ratio (M-H, Fixed, 95% CI) | 0.91 [0.50, 1.67] |
| 9 Oxytocin augmentation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10 Epidural | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | 0.98 [0.77, 1.26] |
| 11 Maternal death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Uterine rupture | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13 Serious maternal complication | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Chorioamnionitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 15 Endometritis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16 Maternal antibiotics | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Perinatal death | 1 | 77 | Risk Ratio (M-H, Fixed, 95% CI) | 0.34 [0.01, 8.14] |
| 18 Apgar score < 7 at 5 minutes | 3 | 248 | Risk Ratio (M-H, Fixed, 95% CI) | 0.21 [0.01, 4.25] |
| 19 NICU admission | 4 | 325 | Risk Ratio (M-H, Fixed, 95% CI) | 0.89 [0.54, 1.47] |
| 20 Neonatal antibiotics | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21 Neonatal infection | 1 | 68 | Risk Ratio (M-H, Fixed, 95% CI) | 0.30 [0.07, 1.36] |
| 22 Cervix unchanged after 24/48 hrs | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23 Gestational age at labour | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | −0.80 [−1.05, −0.55] |
| 24 Oxytocin dose used (mU) | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 1508.70 [−2357.55, 5374.95] |
| 25 Number of medication dose | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | −0.44 [−0.49, −0.39] |
| 26 Number of patients requiring dosing on day 2 | 1 | 60 | Risk Ratio (M-H, Fixed, 95% CI) | 0.61 [0.43, 0.87] |
| 27 Number of patients requiring induction on day 3 | 1 | 60 | Risk Ratio (M-H, Fixed, 95% CI) | 0.13 [0.04, 0.38] |
| 28 Days to admission (all) | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | −2.90 [−4.99, −0.81] |
| 29 Days to admission (nulliparous) | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | −3.20 [−6.44, 0.04] |
| 30 Days to admission (parous) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | −3.10 [−6.24, 0.04] |
| 31 Days to PROM | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | −2.5 [−4.14, −0.86] |
| 32 Interval from intervention to vaginal delivery | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | −1.4 [−3.51, 0.71] |
| 33 Days to delivery (all) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | −1.90 [−3.74, −0.06] |
| 34 Days to delivery (subgroups by parity) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 34.1 Nulliparous women | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | −3.0 [−5.42, −0.58] |
| 34.2 Parous women | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | −0.60 [−3.51, 2.31] |
Comparison 4.
Vaginal misoprostol 25 mcg versus 50 mcg
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Additional induction agents required (oxytocin) | 1 | 49 | Risk Ratio (M-H, Fixed, 95% CI) | 2.26 [0.22, 23.33] |
| 2 Uterine hyperstimulation | 1 | 49 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 NICU admission | 1 | 49 | Risk Ratio (M-H, Fixed, 95% CI) | 0.57 [0.05, 5.83] |
| 4 Caesarean section | 1 | 49 | Risk Ratio (M-H, Fixed, 95% CI) | 0.94 [0.33, 2.68] |
| 5 Interval from treatment to delivery (in days, all deliveries) | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [1.19, 1.81] |
Comparison 5.
Intracervical PGE2 versus vaginal misoprostol
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation (with or without FHR changes) | 1 | 64 | Risk Ratio (M-H, Fixed, 95% CI) | 0.26 [0.03, 2.73] |
| 2 Caesarean section | 1 | 84 | Risk Ratio (M-H, Fixed, 95% CI) | 0.89 [0.38, 2.08] |
| 3 Apgar score < 7 after 5 minutes | 1 | 84 | Risk Ratio (M-H, Fixed, 95% CI) | 0.33 [0.01, 7.96] |
| 4 Admission to NICU | 1 | 84 | Risk Ratio (M-H, Fixed, 95% CI) | 1.25 [0.36, 4.33] |
| 5 Vaginal delivery not achieved within 48 hours | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Interval from administration to admission (hours) | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [2.22, 2.78] |
| 7 Labour or SROM during ripening | 1 | 83 | Risk Ratio (M-H, Fixed, 95% CI) | 0.31 [0.14, 0.69] |
| 8 Delivery within 24 hours | 1 | 83 | Risk Ratio (M-H, Fixed, 95% CI) | 0.90 [0.75, 1.07] |
| 9 Delivery within 48 hours (cumulative) | 1 | 83 | Risk Ratio (M-H, Fixed, 95% CI) | 0.93 [0.81, 1.06] |
Comparison 6.
Oral misoprostol versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2 Chorioamnionitis | 1 | 38 | Risk Ratio (M-H, Fixed, 95% CI) | 1.65 [0.43, 6.38] |
| 3 Additional induction agents required | 1 | 40 | Risk Ratio (M-H, Fixed, 95% CI) | 0.47 [0.21, 1.07] |
| 4 Uterine hyperstimulation (FHR changes unclear) | 1 | 40 | Risk Ratio (M-H, Fixed, 95% CI) | 0.61 [0.06, 6.21] |
| 5 Time from first dose to active labor (hours) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | −34.98 [−61.23, −8.73] |
| 6 Total doses of medication | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | −0.51 [−0.92, −0.10] |
Comparison 7.
Mifepristone versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Additional induction agents required | 4 | 311 | Risk Ratio (M-H, Random, 95% CI) | 0.59 [0.37, 0.95] |
| 2 Serious neonatal morbidity or death | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 1.56 [0.07, 35.67] |
| 3 Serious maternal morbidity or death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4 Vaginal delivery not achieved in 24/48 hrs | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Uterine hyperstimulation (with FHR changes) | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Uterine hyperstimulation (without FHR changes) | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Caesarean section | 5 | 343 | Risk Ratio (M-H, Fixed, 95% CI) | 0.88 [0.62, 1.25] |
| 8 Assisted (instrumental) vaginal delivery | 5 | 343 | Risk Ratio (M-H, Fixed, 95% CI) | 1.35 [0.93, 1.97] |
| 9 Oxytocin augmentation | 2 | 116 | Risk Ratio (M-H, Fixed, 95% CI) | 0.89 [0.63, 1.26] |
| 10 Epidural | 1 | 112 | Risk Ratio (M-H, Fixed, 95% CI) | 0.87 [0.73, 1.03] |
| 11 Maternal death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Uterine scar separation | 1 | 32 | Risk Ratio (M-H, Fixed, 95% CI) | 1.0 [0.07, 14.64] |
| 13 Serious maternal complication | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Chorioamnionitis | 1 | 32 | Risk Ratio (M-H, Fixed, 95% CI) | 2.0 [0.20, 19.91] |
| 15 Endometritis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16 Maternal antibiotics | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Perinatal death | 1 | 32 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18 Apgar score < 7 at 5 minutes | 2 | 119 | Risk Ratio (M-H, Fixed, 95% CI) | 1.56 [0.07, 35.67] |
| 19 NICU admission | 2 | 163 | Risk Ratio (M-H, Fixed, 95% CI) | 0.93 [0.31, 2.79] |
| 20 Neonatal antibiotics | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21 Neonatal infection | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22 Labour or ripe cervix in 48 hours | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 2.0 [1.00, 4.00] |
| 23 Cervix unchanged after 24/48 hrs | 2 | 119 | Risk Ratio (M-H, Fixed, 95% CI) | 0.36 [0.20, 0.63] |
| 24 Spontaneous labour within 72 hours | 1 | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 1.46 [0.68, 3.10] |
| 25 Spontaneous labour within 48 hrs | 1 | 83 | Risk Ratio (M-H, Fixed, 95% CI) | 2.05 [1.27, 3.30] |
| 26 Total dose of oxytocin in vaginal delivery (IM) | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | −2.07 [−3.21, −0.93] |
| 27 Total dose of oxytocin in CS (IM) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | −1.97 [−3.37, −0.57] |
| 28 Oxytocin requirements (IU) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | −2.56 [−4.01, −1.11] |
| 29 Interval between day 1 and start of labour (hours) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | −22.15 [−35.96, −8.34] |
Comparison 8.
Oestrogens versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Additional induction agents required | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2 Serious neonatal morbidity or death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Serious maternal morbidity or death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4 Vaginal delivery not achieved in 24/48 hrs | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Uterine hyperstimulation (with FHR changes) | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Uterine hyperstimulation (without FHR changes) | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Caesarean section | 1 | 87 | Risk Ratio (M-H, Fixed, 95% CI) | 1.27 [0.63, 2.58] |
| 8 Assisted (instrumental) vaginal delivery | 1 | 87 | Risk Ratio (M-H, Fixed, 95% CI) | 0.84 [0.44, 1.60] |
| 9 Oxytocin augmentation | 1 | 87 | Risk Ratio (M-H, Fixed, 95% CI) | 0.93 [0.61, 1.43] |
| 10 Epidural | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11 Maternal death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Uterine rupture | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13 Serious maternal complication | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Chorioamnionitis | 1 | 87 | Risk Ratio (M-H, Fixed, 95% CI) | 1.95 [0.38, 10.12] |
| 15 Endometritis | 1 | 87 | Risk Ratio (M-H, Fixed, 95% CI) | 2.93 [0.32, 27.10] |
| 16 Maternal antibiotics | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Perinatal death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18 Apgar score < 7 at 5 minutes | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19 NICU admission | 1 | 87 | Risk Ratio (M-H, Fixed, 95% CI) | 0.98 [0.06, 15.13] |
| 20 Neonatal antibiotics | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21 Neonatal infection | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22 Interval from intervention to delivery | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 23 Cervix unchanged after 24/48 hrs | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
Comparison 9.
Vaginal isosorbide mononitrate (IMN) versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24/48 hrs | 1 | 238 | Risk Ratio (M-H, Fixed, 95% CI) | 0.97 [0.83, 1.15] |
| 2 Additional induction agents required | 3 | 559 | Risk Ratio (M-H, Fixed, 95% CI) | 0.83 [0.74, 0.92] |
| 3 Serious maternal morbidity or death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4 Maternal satisfaction (women would recommend procedure) | 1 | 193 | Risk Ratio (M-H, Fixed, 95% CI) | 1.01 [0.94, 1.08] |
| 5 Maternal satisfaction (various questions, low score= satisfied, high score = dissatisfied, score out of 10) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 How do you think your labour went? (easy/very difficult) | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | −0.34 [−0.94, 0.26] |
| 5.2 What do you think about home treatment? (extremely good/not at all good) | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.61 [0.03, 1.19] |
| 5.3 How painful was the treatment at home? (not at all/very) | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.58 [−0.00, 1.16] |
| 5.4 How anxious were you being at home taking the treatment? (not at all/very) | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [−0.39, 0.61] |
| 5.5 Would you have the same treatment at home again? (definitely/definitely not) | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.62 [−0.02, 1.26] |
| 5.6 Would you advise a friend to have the same treatment at home? (definitely/definitely not) | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [−0.17, 0.99] |
| 6 Postpartum haemorrhage (> 500 ml) | 3 | 256 | Risk Ratio (M-H, Fixed, 95% CI) | 1.19 [0.89, 1.59] |
| 7 Caesarean section | 3 | 652 | Risk Ratio (M-H, Fixed, 95% CI) | 1.03 [0.81, 1.31] |
| 8 Assisted (instrumental) vaginal delivery | 1 | 350 | Risk Ratio (M-H, Fixed, 95% CI) | 0.85 [0.61, 1.18] |
| 9 Oxytocin augmentation | 1 | 254 | Risk Ratio (M-H, Fixed, 95% CI) | 1.07 [0.88, 1.30] |
| 10 Epidural | 1 | 350 | Risk Ratio (M-H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| 11 Cervix unchanged after 48 hrs | 1 | 257 | Risk Ratio (M-H, Fixed, 95% CI) | 0.83 [0.70, 0.97] |
| 12 Maternal side effect - headache | 3 | 522 | Risk Ratio (M-H, Fixed, 95% CI) | 5.41 [3.85, 7.60] |
| 13 Maternal side effect - nausea | 2 | 420 | Risk Ratio (M-H, Fixed, 95% CI) | 2.06 [1.22, 3.50] |
| 14 Admitted in established labour within 24 hours | 1 | 200 | Risk Ratio (M-H, Fixed, 95% CI) | 2.75 [1.29, 5.88] |
| 15 Uterine hyperstimulation (with FHR changes) | 1 | 102 | Risk Ratio (M-H, Fixed, 95% CI) | 0.2 [0.01, 4.07] |
| 16 Side effects: severe headache | 1 | 220 | Risk Ratio (M-H, Fixed, 95% CI) | 21.21 [2.91, 154.65] |
| 17 Total cost of care package (GB £) | 1 | 350 | Mean Difference (IV, Fixed, 95% CI) | 11.98 [−105.34, 129.30] |
| 18 Perinatal death | 1 | 350 | Risk Ratio (M-H, Fixed, 95% CI) | 0.33 [0.01, 7.94] |
| 19 Apgar score < 7 at 5 minutes 3 | 3 | 256 | Risk Ratio (M-H, Fixed, 95% CI) | 1.21 [0.35, 4.17] |
| 20 NICU admission | 3 | 652 | Risk Ratio (M-H, Fixed, 95% CI) | 1.17 [0.72, 1.92] |
| 21 Interval from admission to vaginal delivery (hours) | 1 | 128 | Mean Difference (IV, Fixed, 95% CI) | −0.70 [−6.11, 4.71] |
| 22 Neonatal infection | 1 | 200 | Risk Ratio (M-H, Fixed, 95% CI) | 1.0 [0.26, 3.89] |
| 23 Bishop score > 6 or active labour at 36 hours | 1 | 102 | Risk Ratio (M-H, Fixed, 95% CI) | 3.8 [1.54, 9.40] |
| 24 Time in hours from admission to delivery (all women) | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | −6.67 [−9.56, −3.78] |
| 25 Onset of labour within 24 hours | 1 | 200 | Risk Ratio (M-H, Fixed, 95% CI) | 2.75 [1.29, 5.88] |
Comparison 10.
Acupuncture versus routine care
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Additional induction agents required | 1 | 56 | Risk Ratio (M-H, Fixed, 95% CI) | 0.6 [0.31, 1.17] |
| 2 Caesarean section | 1 | 56 | Risk Ratio (M-H, Fixed, 95% CI) | 0.43 [0.17, 1.11] |
Analysis 1.1. Comparison 1 Intravaginal PGE2 gel versus placebo or expectant management, Outcome 1 Additional induction agents required
Review: Different methods for the induction of labour in outpatient settings
Comparison: 1 Intravaginal PGE2 gel versus placebo or expectant management
Outcome: 1 Additional induction agents required
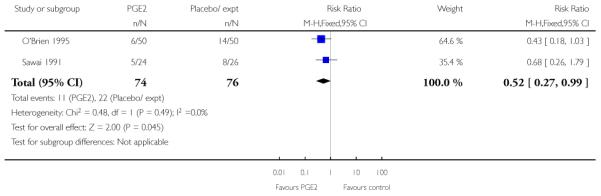
|
Analysis 1.5. Comparison 1 Intravaginal PGE2 gel versus placebo or expectant management, Outcome 5 Uterine hyperstimulation (FHR changes unclear)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 1 Intravaginal PGE2 gel versus placebo or expectant management
Outcome: 5 Uterine hyperstimulation (FHR changes unclear)
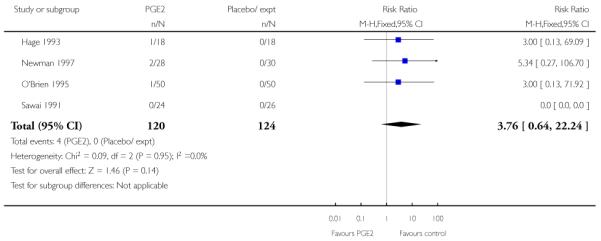
|
Analysis 1.7. Comparison 1 Intravaginal PGE2 gel versus placebo or expectant management, Outcome 7 Caesarean section
Review: Different methods for the induction of labour in outpatient settings
Comparison: 1 Intravaginal PGE2 gel versus placebo or expectant management
Outcome: 7 Caesarean section
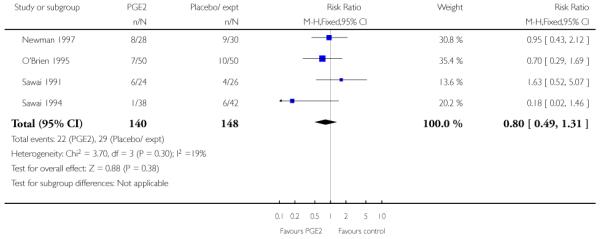
|
Analysis 1.10. Comparison 1 Intravaginal PGE2 gel versus placebo or expectant management, Outcome 10 Epidural
Review: Different methods for the induction of labour in outpatient settings
Comparison: 1 Intravaginal PGE2 gel versus placebo or expectant management
Outcome: 10 Epidural
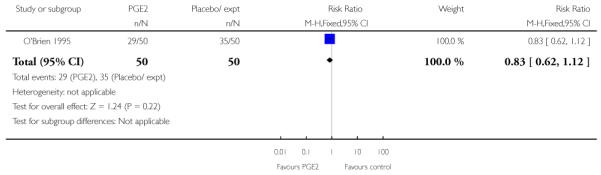
|
Analysis 1.14. Comparison 1 Intravaginal PGE2 gel versus placebo or expectant management, Outcome 14 Chorioamnionitis
Review: Different methods for the induction of labour in outpatient settings
Comparison: 1 Intravaginal PGE2 gel versus placebo or expectant management
Outcome: 14 Chorioamnionitis
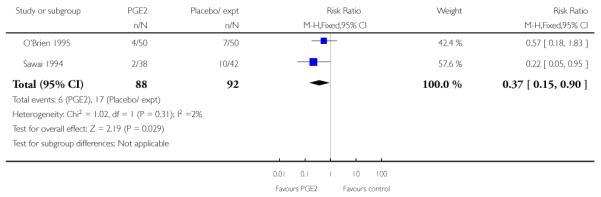
|
Analysis 1.18. Comparison 1 Intravaginal PGE2 gel versus placebo or expectant management, Outcome 18 Apgar score < 7 at 5 minutes
Review: Different methods for the induction of labour in outpatient settings
Comparison: 1 Intravaginal PGE2 gel versus placebo or expectant management
Outcome: 18 Apgar score < 7 at 5 minutes
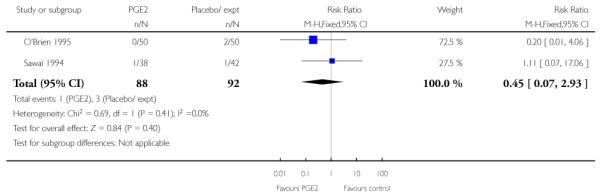
|
Analysis 1.19. Comparison 1 Intravaginal PGE2 gel versus placebo or expectant management, Outcome 19 NICU admission
Review: Different methods for the induction of labour in outpatient settings
Comparison: 1 Intravaginal PGE2 gel versus placebo or expectant management
Outcome: 19 NICU admission
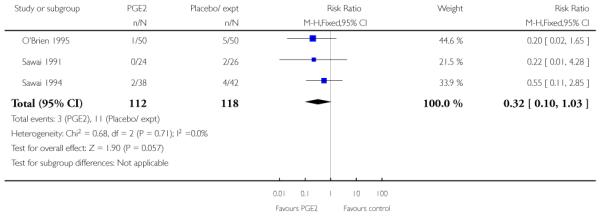
|
Analysis 1.23. Comparison 1 Intravaginal PGE2 gel versus placebo or expectant management, Outcome 23 Cervix unchanged at follow up
Review: Different methods for the induction of labour in outpatient settings
Comparison: 1 Intravaginal PGE2 gel versus placebo or expectant management
Outcome: 23 Cervix unchanged at follow up
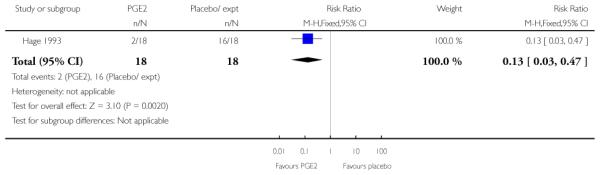
|
Analysis 1.24. Comparison 1 Intravaginal PGE2 gel versus placebo or expectant management, Outcome 24 “Spontaneous labour” within 48 hours
Review: Different methods for the induction of labour in outpatient settings
Comparison: 1 Intravaginal PGE2 gel versus placebo or expectant management
Outcome: 24 “Spontaneous labour” within 48 hours
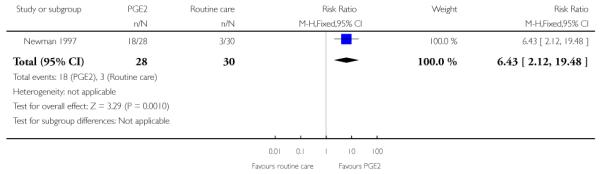
|
Analysis 1.25. Comparison 1 Intravaginal PGE2 gel versus placebo or expectant management, Outcome 25 Admitted to hospital for labour
Review: Different methods for the induction of labour in outpatient settings
Comparison: 1 Intravaginal PGE2 gel versus placebo or expectant management
Outcome: 25 Admitted to hospital for labour
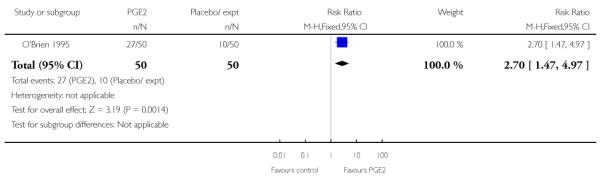
|
Analysis 1.26. Comparison 1 Intravaginal PGE2 gel versus placebo or expectant management, Outcome 26 Gestational age at delivery (weeks)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 1 Intravaginal PGE2 gel versus placebo or expectant management
Outcome: 26 Gestational age at delivery (weeks)
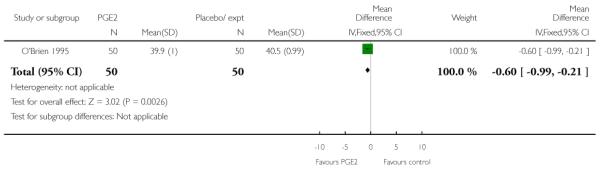
|
Analysis 1.27. Comparison 1 Intravaginal PGE2 gel versus placebo or expectant management, Outcome 27 Gestational age on admission (days)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 1 Intravaginal PGE2 gel versus placebo or expectant management
Outcome: 27 Gestational age on admission (days)
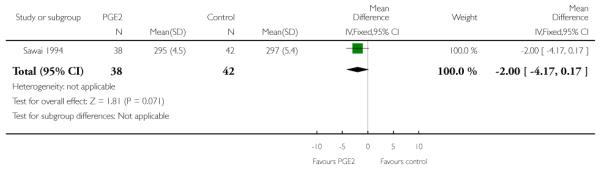
|
Analysis 2.1. Comparison 2 Intracervical PGE2 versus placebo, Outcome 1 Additional induction agent required (induction with oxytocin or other means)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 2 Intracervical PGE2 versus placebo
Outcome: 1 Additional induction agent required (induction with oxytocin or other means)
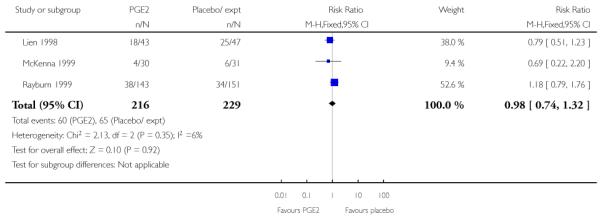
|
Analysis 2.2. Comparison 2 Intracervical PGE2 versus placebo, Outcome 2 Additional induction agents required (further prostaglandin required)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 2 Intracervical PGE2 versus placebo
Outcome: 2 Additional induction agents required (further prostaglandin required)
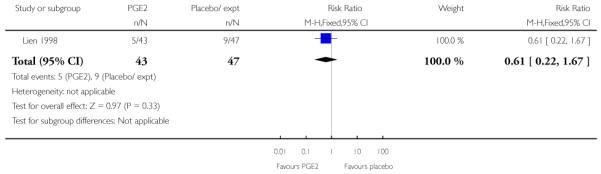
|
Analysis 2.5. Comparison 2 Intracervical PGE2 versus placebo, Outcome 5 Delivery not achieved in 48-72 hrs
Review: Different methods for the induction of labour in outpatient settings
Comparison: 2 Intracervical PGE2 versus placebo
Outcome: 5 Delivery not achieved in 48-72 hrs
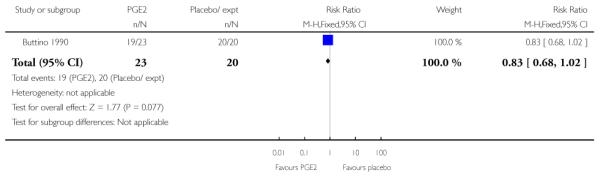
|
Analysis 2.6. Comparison 2 Intracervical PGE2 versus placebo, Outcome 6 Uterine rupture
Review: Different methods for the induction of labour in outpatient settings
Comparison: 2 Intracervical PGE2 versus placebo
Outcome: 6 Uterine rupture
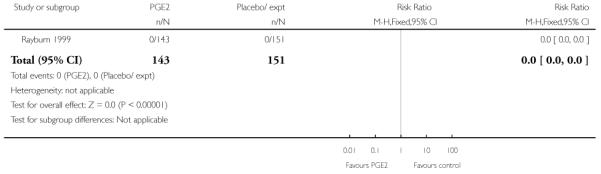
|
Analysis 2.7. Comparison 2 Intracervical PGE2 versus placebo, Outcome 7 Uterine hyperstimulation (with FHR changes)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 2 Intracervical PGE2 versus placebo
Outcome: 7 Uterine hyperstimulation (with FHR changes) (1) Not clear if FHR changes
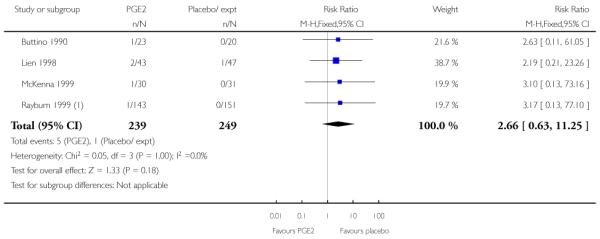
|
|
|
Analysis 2.9. Comparison 2 Intracervical PGE2 versus placebo, Outcome 9 Postpartum haemorrhage (> 500 ml)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 2 Intracervical PGE2 versus placebo
Outcome: 9 Postpartum haemorrhage (> 500 ml)
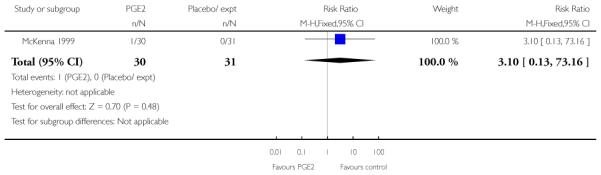
|
Analysis 2.10. Comparison 2 Intracervical PGE2 versus placebo, Outcome 10 Caesarean section
Review: Different methods for the induction of labour in outpatient settings
Comparison: 2 Intracervical PGE2 versus placebo
Outcome: 10 Caesarean section
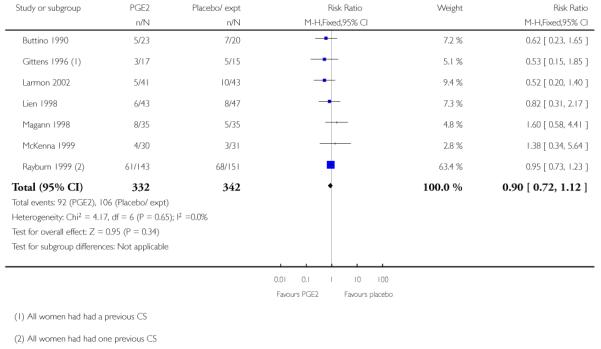
|
Analysis 2.11. Comparison 2 Intracervical PGE2 versus placebo, Outcome 11 Assisted (instrumental) vaginal delivery
Review: Different methods for the induction of labour in outpatient settings
Comparison: 2 Intracervical PGE2 versus placebo
Outcome: 11 Assisted (instrumental) vaginal delivery
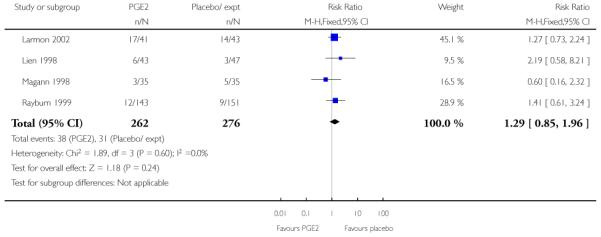
|
Analysis 2.12. Comparison 2 Intracervical PGE2 versus placebo, Outcome 12 Oxytocin augmentation
Review: Different methods for the induction of labour in outpatient settings
Comparison: 2 Intracervical PGE2 versus placebo
Outcome: 12 Oxytocin augmentation
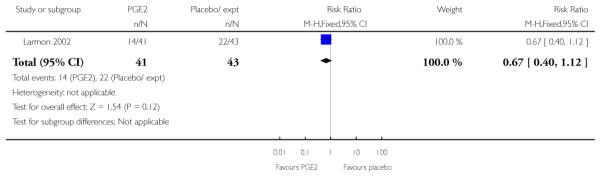
|
Analysis 2.16. Comparison 2 Intracervical PGE2 versus placebo, Outcome 16 Chorioamnionitis
Review: Different methods for the induction of labour in outpatient settings
Comparison: 2 Intracervical PGE2 versus placebo
Outcome: 16 Chorioamnionitis
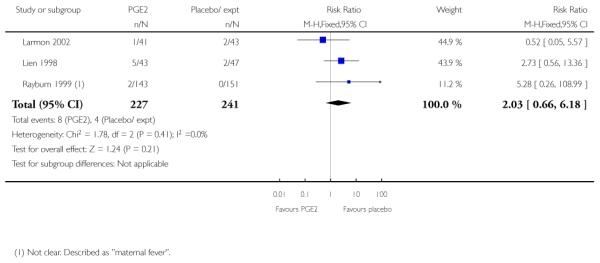
|
Analysis 2.17. Comparison 2 Intracervical PGE2 versus placebo, Outcome 17 Endometritis
Review: Different methods for the induction of labour in outpatient settings
Comparison: 2 Intracervical PGE2 versus placebo
Outcome: 17 Endometritis
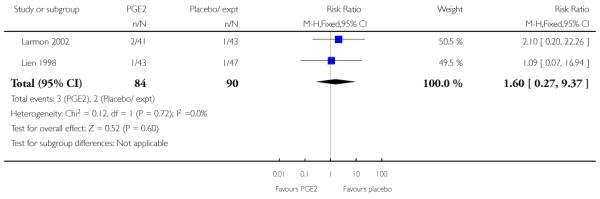
|
Analysis 2.20. Comparison 2 Intracervical PGE2 versus placebo, Outcome 20 Apgar score < 7 at 5 minutes
Review: Different methods for the induction of labour in outpatient settings
Comparison: 2 Intracervical PGE2 versus placebo
Outcome: 20 Apgar score < 7 at 5 minutes
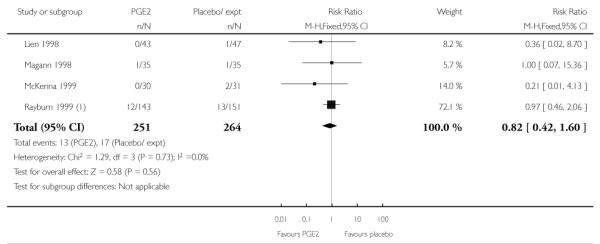
|
|
|
Analysis 2.21. Comparison 2 Intracervical PGE2 versus placebo, Outcome 21 NICU admission
Review: Different methods for the induction of labour in outpatient settings
Comparison: 2 Intracervical PGE2 versus placebo
Outcome: 21 NICU admission
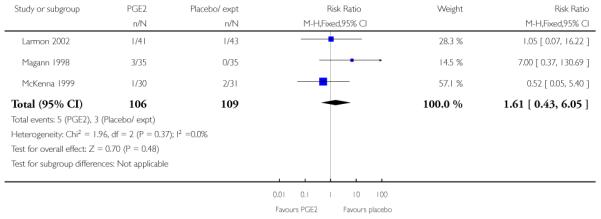
|
Analysis 2.24. Comparison 2 Intracervical PGE2 versus placebo, Outcome 24 Interval from intervention to delivery (days)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 2 Intracervical PGE2 versus placebo
Outcome: 24 Interval from intervention to delivery (days)
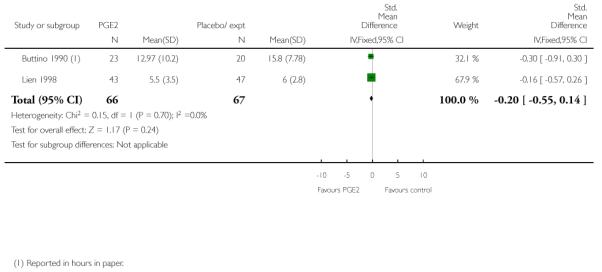
|
Analysis 2.26. Comparison 2 Intracervical PGE2 versus placebo, Outcome 26 Maternal side effects
Review: Different methods for the induction of labour in outpatient settings
Comparison: 2 Intracervical PGE2 versus placebo
Outcome: 26 Maternal side effects
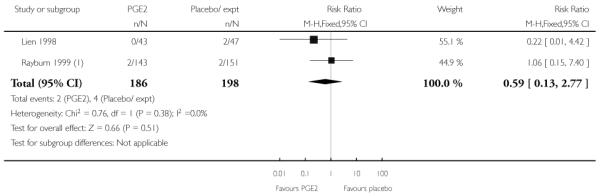
|
|
|
Analysis 2.27. Comparison 2 Intracervical PGE2 versus placebo, Outcome 27 Gestational age at delivery (weeks)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 2 Intracervical PGE2 versus placebo
Outcome: 27 Gestational age at delivery (weeks)
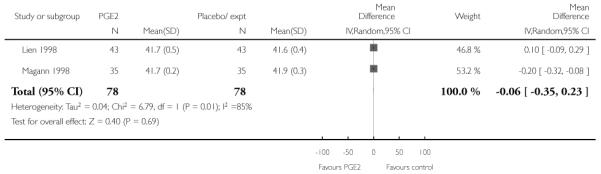
|
Analysis 2.28. Comparison 2 Intracervical PGE2 versus placebo, Outcome 28 Induction for gestational age > 42 weeks
Review: Different methods for the induction of labour in outpatient settings
Comparison: 2 Intracervical PGE2 versus placebo
Outcome: 28 Induction for gestational age > 42 weeks

|
Analysis 2.29. Comparison 2 Intracervical PGE2 versus placebo, Outcome 29 Delivery within 48 hours of treatment (all deliveries)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 2 Intracervical PGE2 versus placebo
Outcome: 29 Delivery within 48 hours of treatment (all deliveries)
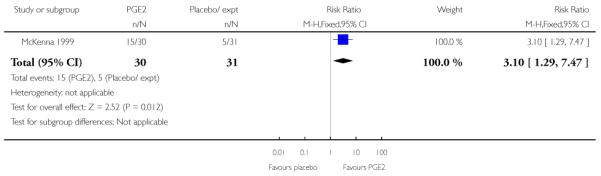
|
Analysis 3.2. Comparison 3 Vaginal misoprostol versus placebo, Outcome 2 Serious neonatal morbidity or death
Review: Different methods for the induction of labour in outpatient settings
Comparison: 3 Vaginal misoprostol versus placebo
Outcome: 2 Serious neonatal morbidity or death
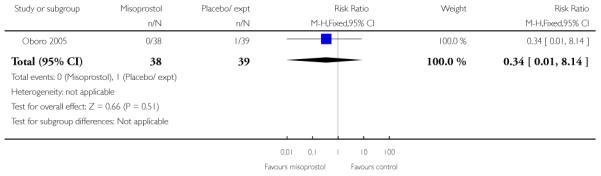
|
Analysis 3.5. Comparison 3 Vaginal misoprostol versus placebo, Outcome 5 Uterine hyperstimulation (with FHR changes)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 3 Vaginal misoprostol versus placebo
Outcome: 5 Uterine hyperstimulation (with FHR changes)
|
|
|
Analysis 3.6. Comparison 3 Vaginal misoprostol versus placebo, Outcome 6 Uterine hyperstimulation (without FHR changes)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 3 Vaginal misoprostol versus placebo
Outcome: 6 Uterine hyperstimulation (without FHR changes)
|
Analysis 3.7. Comparison 3 Vaginal misoprostol versus placebo, Outcome 7 Caesarean section
Review: Different methods for the induction of labour in outpatient settings
Comparison: 3 Vaginal misoprostol versus placebo
Outcome: 7 Caesarean section
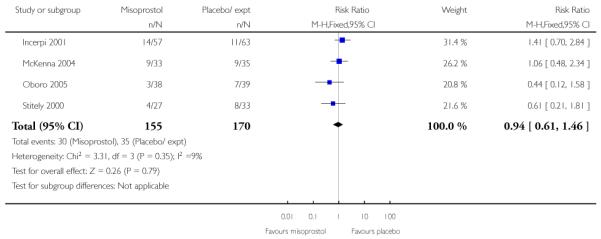
|
Analysis 3.8. Comparison 3 Vaginal misoprostol versus placebo, Outcome 8 Assisted (instrumental) vaginal delivery
Review: Different methods for the induction of labour in outpatient settings
Comparison: 3 Vaginal misoprostol versus placebo
Outcome: 8 Assisted (instrumental) vaginal delivery
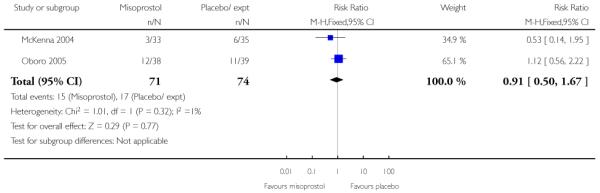
|
Analysis 3.10. Comparison 3 Vaginal misoprostol versus placebo, Outcome 10 Epidural
Review: Different methods for the induction of labour in outpatient settings
Comparison: 3 Vaginal misoprostol versus placebo
Outcome: 10 Epidural
|
Analysis 3.17. Comparison 3 Vaginal misoprostol versus placebo, Outcome 17 Perinatal death
Review: Different methods for the induction of labour in outpatient settings
Comparison: 3 Vaginal misoprostol versus placebo
Outcome: 17 Perinatal death
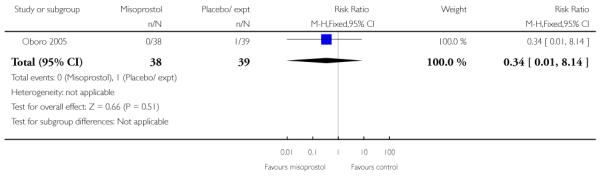
|
Analysis 3.18. Comparison 3 Vaginal misoprostol versus placebo, Outcome 18 Apgar score < 7 at 5 minutes
Review: Different methods for the induction of labour in outpatient settings
Comparison: 3 Vaginal misoprostol versus placebo
Outcome: 18 Apgar score < 7 at 5 minutes
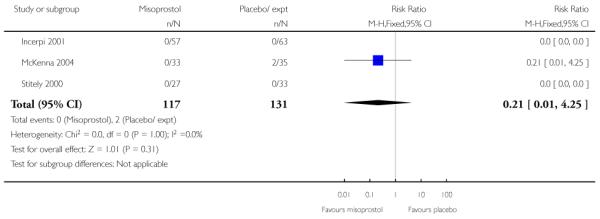
|
Analysis 3.19. Comparison 3 Vaginal misoprostol versus placebo, Outcome 19 NICU admission
Review: Different methods for the induction of labour in outpatient settings
Comparison: 3 Vaginal misoprostol versus placebo
Outcome: 19 NICU admission
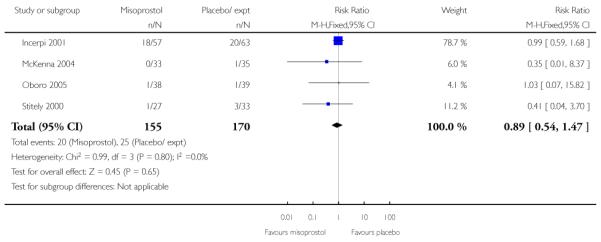
|
Analysis 3.21. Comparison 3 Vaginal misoprostol versus placebo, Outcome 21 Neonatal infection
Review: Different methods for the induction of labour in outpatient settings
Comparison: 3 Vaginal misoprostol versus placebo
Outcome: 21 Neonatal infection
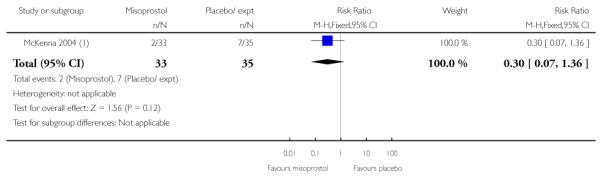
|

|
Analysis 3.23. Comparison 3 Vaginal misoprostol versus placebo, Outcome 23 Gestational age at labour
Review: Different methods for the induction of labour in outpatient settings
Comparison: 3 Vaginal misoprostol versus placebo
Outcome: 23 Gestational age at labour
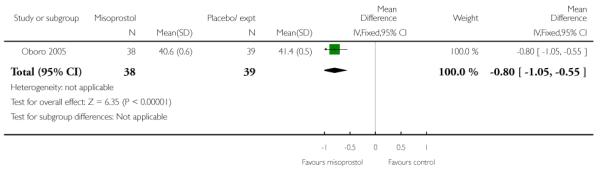
|
Analysis 3.24. Comparison 3 Vaginal misoprostol versus placebo, Outcome 24 Oxytocin dose used (mU)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 3 Vaginal misoprostol versus placebo
Outcome: 24 Oxytocin dose used (mU)
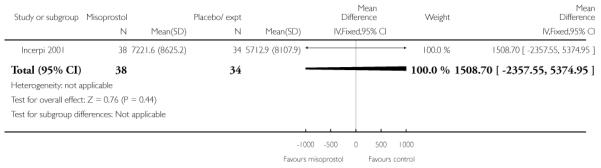
|
Analysis 3.25. Comparison 3 Vaginal misoprostol versus placebo, Outcome 25 Number of medication dose
Review: Different methods for the induction of labour in outpatient settings
Comparison: 3 Vaginal misoprostol versus placebo
Outcome: 25 Number of medication dose
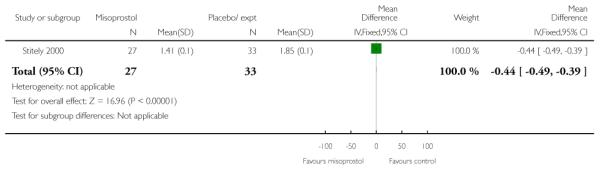
|
Analysis 3.26. Comparison 3 Vaginal misoprostol versus placebo, Outcome 26 Number of patients requiring dosing on day 2
Review: Different methods for the induction of labour in outpatient settings
Comparison: 3 Vaginal misoprostol versus placebo
Outcome: 26 Number of patients requiring dosing on day 2
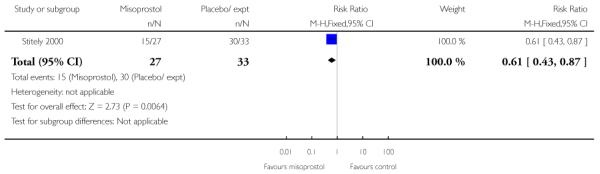
|
Analysis 3.27. Comparison 3 Vaginal misoprostol versus placebo, Outcome 27 Number of patients requiring induction on day 3
Review: Different methods for the induction of labour in outpatient settings
Comparison: 3 Vaginal misoprostol versus placebo
Outcome: 27 Number of patients requiring induction on day 3
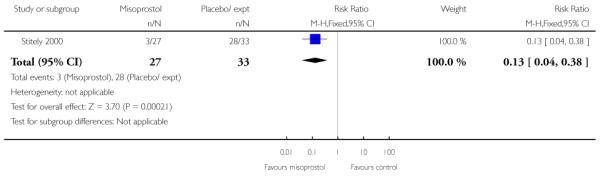
|
Analysis 3.28. Comparison 3 Vaginal misoprostol versus placebo, Outcome 28 Days to admission (all)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 3 Vaginal misoprostol versus placebo
Outcome: 28 Days to admission (all)
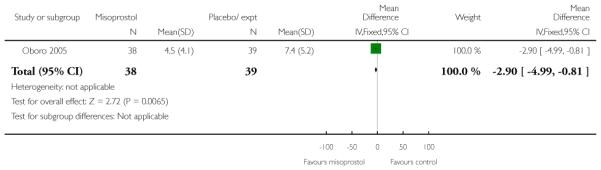
|
Analysis 3.29. Comparison 3 Vaginal misoprostol versus placebo, Outcome 29 Days to admission (nulliparous)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 3 Vaginal misoprostol versus placebo
Outcome: 29 Days to admission (nulliparous)
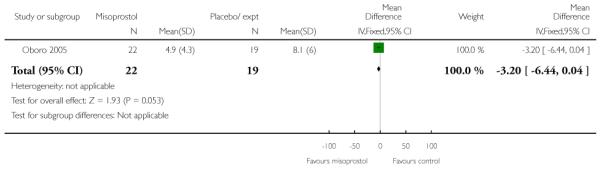
|
Analysis 3.30. Comparison 3 Vaginal misoprostol versus placebo, Outcome 30 Days to admission (parous)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 3 Vaginal misoprostol versus placebo
Outcome: 30 Days to admission (parous)
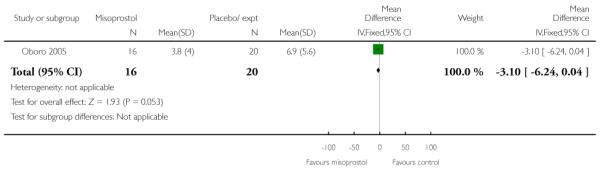
|
Analysis 3.31. Comparison 3 Vaginal misoprostol versus placebo, Outcome 31 Days to PROM
Review: Different methods for the induction of labour in outpatient settings
Comparison: 3 Vaginal misoprostol versus placebo
Outcome: 31 Days to PROM
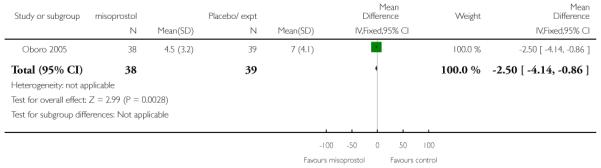
|
Analysis 3.32. Comparison 3 Vaginal misoprostol versus placebo, Outcome 32 Interval from intervention to vaginal delivery
Review: Different methods for the induction of labour in outpatient settings
Comparison: 3 Vaginal misoprostol versus placebo
Outcome: 32 Interval from intervention to vaginal delivery
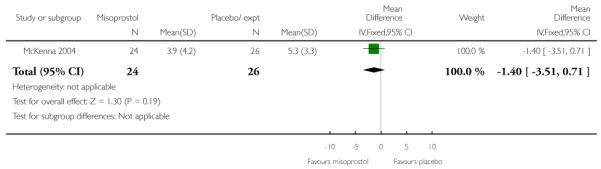
|
Analysis 3.33. Comparison 3 Vaginal misoprostol versus placebo, Outcome 33 Days to delivery (all)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 3 Vaginal misoprostol versus placebo
Outcome: 33 Days to delivery (all)
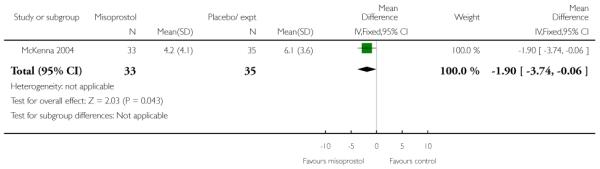
|
Analysis 3.34. Comparison 3 Vaginal misoprostol versus placebo, Outcome 34 Days to delivery (subgroups by parity)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 3 Vaginal misoprostol versus placebo
Outcome: 34 Days to delivery (subgroups by parity)
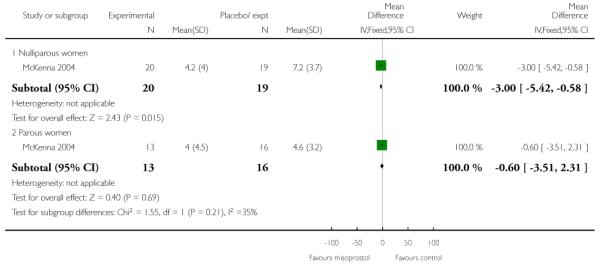
|
Analysis 4.1. Comparison 4 Vaginal misoprostol 25 mcg versus 50 mcg, Outcome 1 Additional induction agents required (oxytocin)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 4 Vaginal misoprostol 25 mcg versus 50 mcg
Outcome: 1 Additional induction agents required (oxytocin)
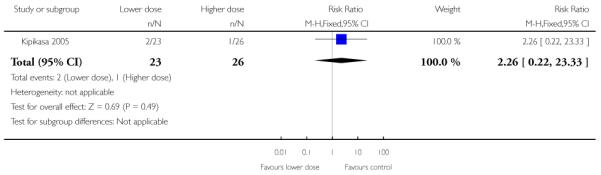
|
Analysis 4.2. Comparison 4 Vaginal misoprostol 25 mcg versus 50 mcg, Outcome 2 Uterine hyperstimulation
Review: Different methods for the induction of labour in outpatient settings
Comparison: 4 Vaginal misoprostol 25 mcg versus 50 mcg
Outcome: 2 Uterine hyperstimulation
|
Analysis 4.3. Comparison 4 Vaginal misoprostol 25 mcg versus 50 mcg, Outcome 3 NICU admission
Review: Different methods for the induction of labour in outpatient settings
Comparison: 4 Vaginal misoprostol 25 mcg versus 50 mcg
Outcome: 3 NICU admission
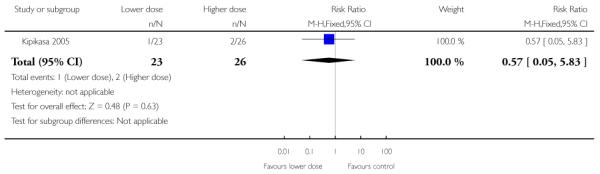
|
Analysis 4.4. Comparison 4 Vaginal misoprostol 25 mcg versus 50 mcg, Outcome 4 Caesarean section
Review: Different methods for the induction of labour in outpatient settings
Comparison: 4 Vaginal misoprostol 25 mcg versus 50 mcg
Outcome: 4 Caesarean section
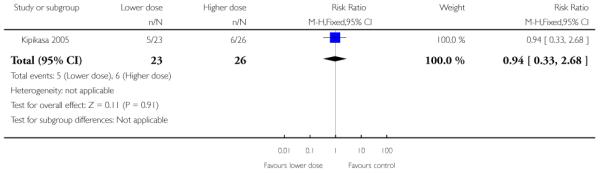
|
Analysis 4.5. Comparison 4 Vaginal misoprostol 25 mcg versus 50 mcg, Outcome 5 Interval from treatment to delivery (in days, all deliveries)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 4 Vaginal misoprostol 25 mcg versus 50 mcg
Outcome: 5 Interval from treatment to delivery (in days, all deliveries)
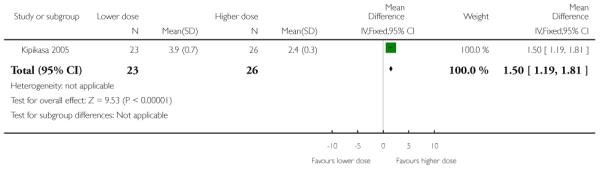
|
Analysis 5.1. Comparison 5 Intracervical PGE2 versus vaginal misoprostol, Outcome 1 Uterine hyperstimulation (with or without FHR changes)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 5 Intracervical PGE2 versus vaginal misoprostol
Outcome: 1 Uterine hyperstimulation (with or without FHR changes)
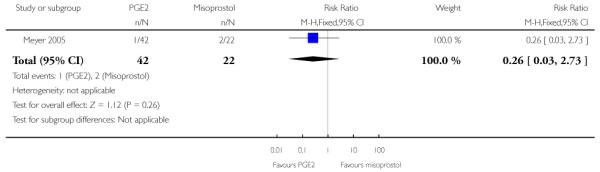
|
Analysis 5.2. Comparison 5 Intracervical PGE2 versus vaginal misoprostol, Outcome 2 Caesarean section
Review: Different methods for the induction of labour in outpatient settings
Comparison: 5 Intracervical PGE2 versus vaginal misoprostol
Outcome: 2 Caesarean section
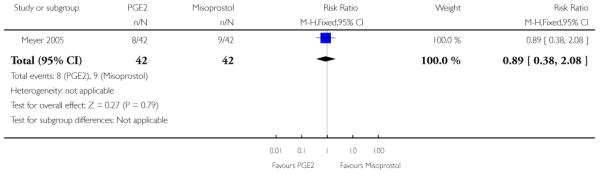
|
Analysis 5.3. Comparison 5 Intracervical PGE2 versus vaginal misoprostol, Outcome 3 Apgar score < 7 after 5 minutes
Review: Different methods for the induction of labour in outpatient settings
Comparison: 5 Intracervical PGE2 versus vaginal misoprostol
Outcome: 3 Apgar score < 7 after 5 minutes
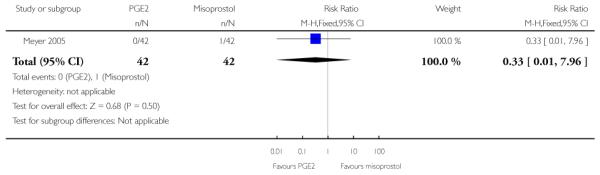
|
Analysis 5.4. Comparison 5 Intracervical PGE2 versus vaginal misoprostol, Outcome 4 Admission to NICU
Review: Different methods for the induction of labour in outpatient settings
Comparison: 5 Intracervical PGE2 versus vaginal misoprostol
Outcome: 4 Admission to NICU
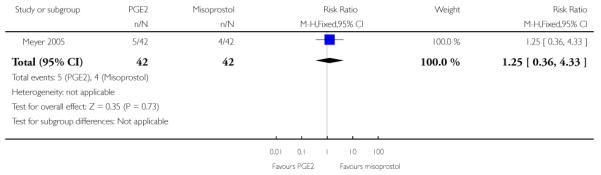
|
Analysis 5.6. Comparison 5 Intracervical PGE2 versus vaginal misoprostol, Outcome 6 Interval from administration to admission (hours)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 5 Intracervical PGE2 versus vaginal misoprostol
Outcome: 6 Interval from administration to admission (hours)
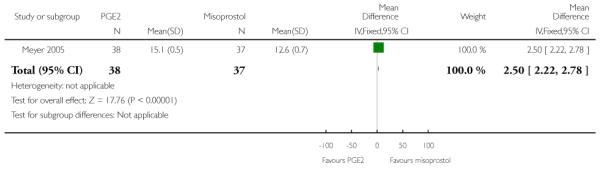
|
Analysis 5.7. Comparison 5 Intracervical PGE2 versus vaginal misoprostol, Outcome 7 Labour or SROM during ripening
Review: Different methods for the induction of labour in outpatient settings
Comparison: 5 Intracervical PGE2 versus vaginal misoprostol
Outcome: 7 Labour or SROM during ripening
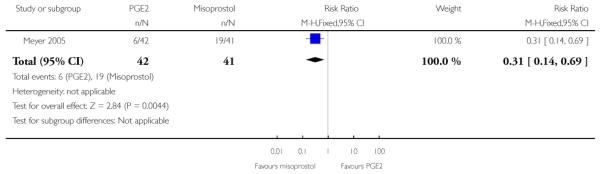
|
Analysis 5.8. Comparison 5 Intracervical PGE2 versus vaginal misoprostol, Outcome 8 Delivery within 24 hours
Review: Different methods for the induction of labour in outpatient settings
Comparison: 5 Intracervical PGE2 versus vaginal misoprostol
Outcome: 8 Delivery within 24 hours
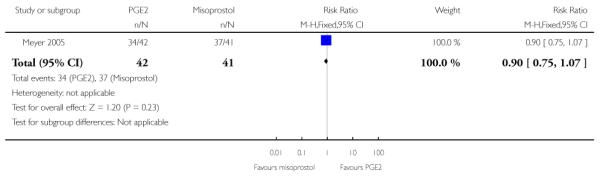
|
Analysis 5.9. Comparison 5 Intracervical PGE2 versus vaginal misoprostol, Outcome 9 Delivery within 48 hours (cumulative)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 5 Intracervical PGE2 versus vaginal misoprostol
Outcome: 9 Delivery within 48 hours (cumulative)
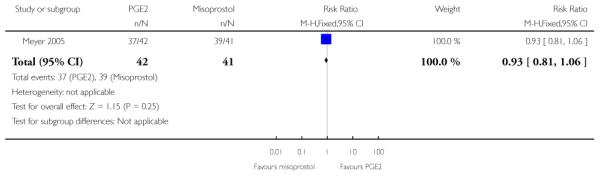
|
Analysis 6.2. Comparison 6 Oral misoprostol versus placebo, Outcome 2 Chorioamnionitis
Review: Different methods for the induction of labour in outpatient settings
Comparison: 6 Oral misoprostol versus placebo
Outcome: 2 Chorioamnionitis
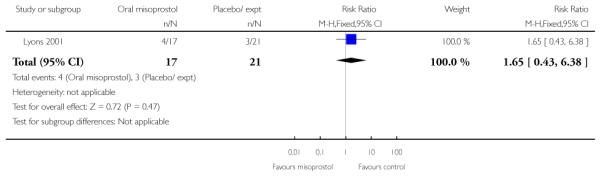
|
Analysis 6.3. Comparison 6 Oral misoprostol versus placebo, Outcome 3 Additional induction agents required
Review: Different methods for the induction of labour in outpatient settings
Comparison: 6 Oral misoprostol versus placebo
Outcome: 3 Additional induction agents required
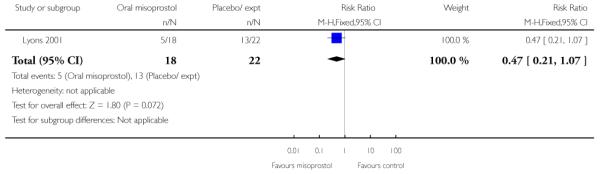
|
Analysis 6.4. Comparison 6 Oral misoprostol versus placebo, Outcome 4 Uterine hyperstimulation (FHR changes unclear)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 6 Oral misoprostol versus placebo
Outcome: 4 Uterine hyperstimulation (FHR changes unclear)
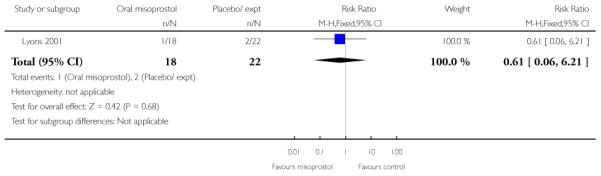
|
Analysis 6.5. Comparison 6 Oral misoprostol versus placebo, Outcome 5 Time from first dose to active labor (hours)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 6 Oral misoprostol versus placebo
Outcome: 5 Time from first dose to active labor (hours)
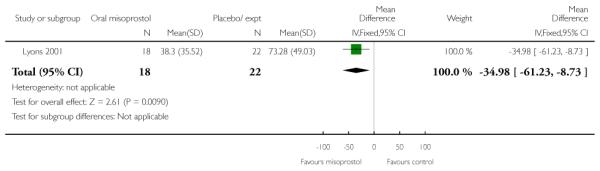
|
Analysis 6.6. Comparison 6 Oral misoprostol versus placebo, Outcome 6 Total doses of medication
Review: Different methods for the induction of labour in outpatient settings
Comparison: 6 Oral misoprostol versus placebo
Outcome: 6 Total doses of medication
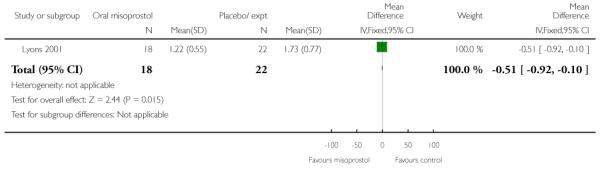
|
Analysis 7.1. Comparison 7 Mifepristone versus placebo, Outcome 1 Additional induction agents required
Review: Different methods for the induction of labour in outpatient settings
Comparison: 7 Mifepristone versus placebo
Outcome: 1 Additional induction agents required
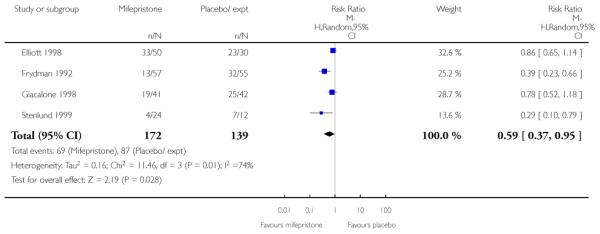
|
Analysis 7.2. Comparison 7 Mifepristone versus placebo, Outcome 2 Serious neonatal morbidity or death
Review: Different methods for the induction of labour in outpatient settings
Comparison: 7 Mifepristone versus placebo
Outcome: 2 Serious neonatal morbidity or death
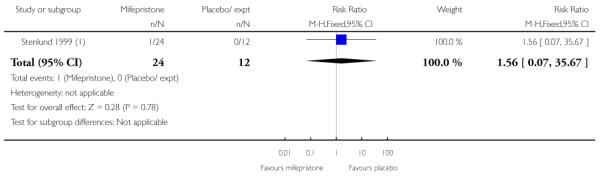
|
|
|
Analysis 7.7. Comparison 7 Mifepristone versus placebo, Outcome 7 Caesarean section
Review: Different methods for the induction of labour in outpatient settings
Comparison: 7 Mifepristone versus placebo
Outcome: 7 Caesarean section
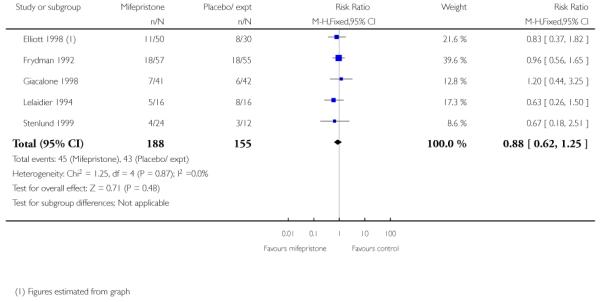
|
Analysis 7.8. Comparison 7 Mifepristone versus placebo, Outcome 8 Assisted (instrumental) vaginal delivery
Review: Different methods for the induction of labour in outpatient settings
Comparison: 7 Mifepristone versus placebo
Outcome: 8 Assisted (instrumental) vaginal delivery
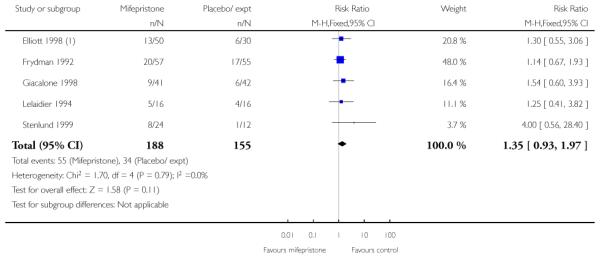
|
Analysis 7.9. Comparison 7 Mifepristone versus placebo, Outcome 9 Oxytocin augmentation
Review: Different methods for the induction of labour in outpatient settings
Comparison: 7 Mifepristone versus placebo
Outcome: 9 Oxytocin augmentation
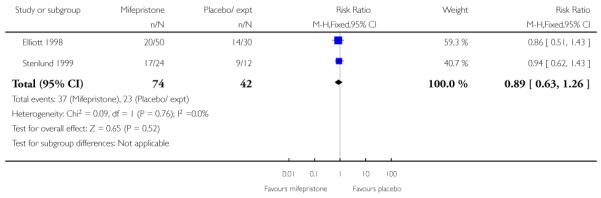
|
Analysis 7.10. Comparison 7 Mifepristone versus placebo, Outcome 10 Epidural
Review: Different methods for the induction of labour in outpatient settings
Comparison: 7 Mifepristone versus placebo
Outcome: 10 Epidural
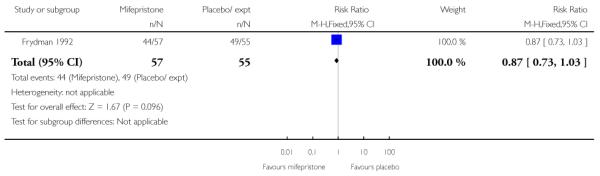
|
Analysis 7.12. Comparison 7 Mifepristone versus placebo, Outcome 12 Uterine scar separation
Review: Different methods for the induction of labour in outpatient settings
Comparison: 7 Mifepristone versus placebo
Outcome: 12 Uterine scar separation
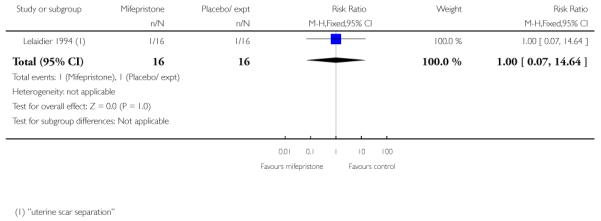
|
Analysis 7.14. Comparison 7 Mifepristone versus placebo, Outcome 14 Chorioamnionitis
Review: Different methods for the induction of labour in outpatient settings
Comparison: 7 Mifepristone versus placebo
Outcome: 14 Chorioamnionitis
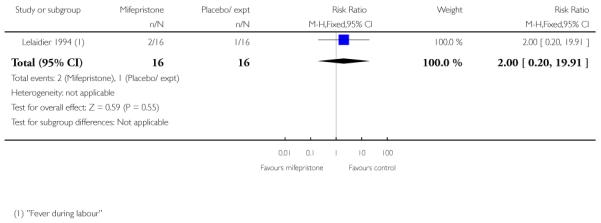
|
Analysis 7.17. Comparison 7 Mifepristone versus placebo, Outcome 17 Perinatal death
Review: Different methods for the induction of labour in outpatient settings
Comparison: 7 Mifepristone versus placebo
Outcome: 17 Perinatal death
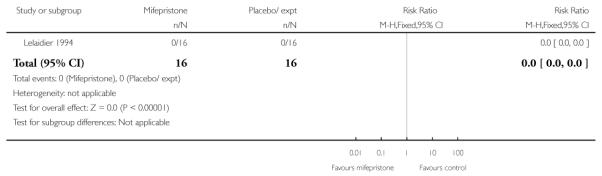
|
Analysis 7.18. Comparison 7 Mifepristone versus placebo, Outcome 18 Apgar score < 7 at 5 minutes
Review: Different methods for the induction of labour in outpatient settings
Comparison: 7 Mifepristone versus placebo
Outcome: 18 Apgar score < 7 at 5 minutes
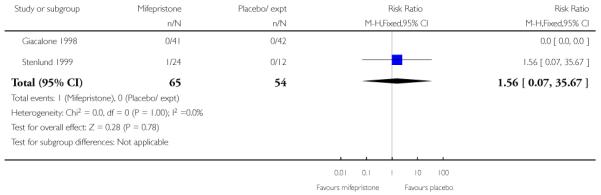
|
Analysis 7.19. Comparison 7 Mifepristone versus placebo, Outcome 19 NICU admission
Review: Different methods for the induction of labour in outpatient settings
Comparison: 7 Mifepristone versus placebo
Outcome: 19 NICU admission
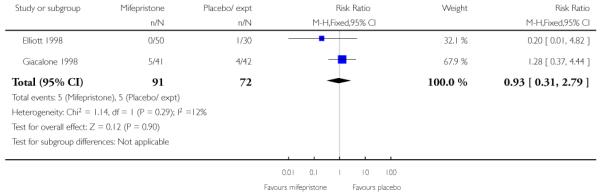
|
Analysis 7.22. Comparison 7 Mifepristone versus placebo, Outcome 22 Labour or ripe cervix in 48 hours
Review: Different methods for the induction of labour in outpatient settings
Comparison: 7 Mifepristone versus placebo
Outcome: 22 Labour or ripe cervix in 48 hours
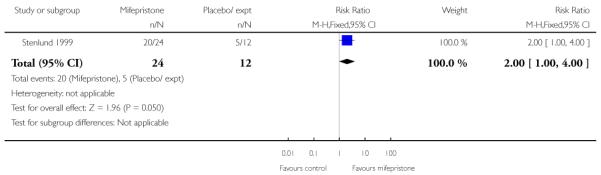
|
Analysis 7.23. Comparison 7 Mifepristone versus placebo, Outcome 23 Cervix unchanged after 24/48 hrs
Review: Different methods for the induction of labour in outpatient settings
Comparison: 7 Mifepristone versus placebo
Outcome: 23 Cervix unchanged after 24/48 hrs
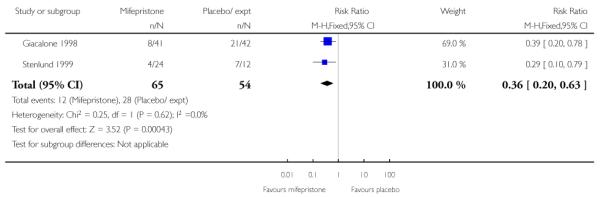
|
Analysis 7.24. Comparison 7 Mifepristone versus placebo, Outcome 24 Spontaneous labour within 72 hours
Review: Different methods for the induction of labour in outpatient settings
Comparison: 7 Mifepristone versus placebo
Outcome: 24 Spontaneous labour within 72 hours
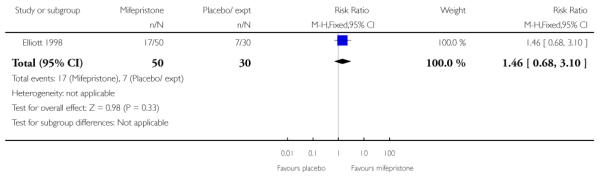
|
Analysis 7.25. Comparison 7 Mifepristone versus placebo, Outcome 25 Spontaneous labour within 48 hrs
Review: Different methods for the induction of labour in outpatient settings
Comparison: 7 Mifepristone versus placebo
Outcome: 25 Spontaneous labour within 48 hrs
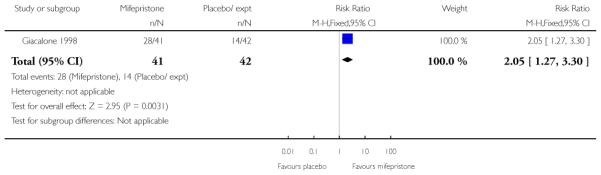
|
Analysis 7.26. Comparison 7 Mifepristone versus placebo, Outcome 26 Total dose of oxytocin in vaginal delivery (IM)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 7 Mifepristone versus placebo
Outcome: 26 Total dose of oxytocin in vaginal delivery (IM)
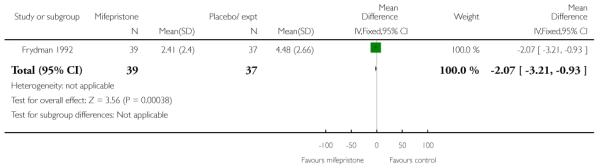
|
Analysis 7.27. Comparison 7 Mifepristone versus placebo, Outcome 27 Total dose of oxytocin in CS (IM)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 7 Mifepristone versus placebo
Outcome: 27 Total dose of oxytocin in CS (IM)
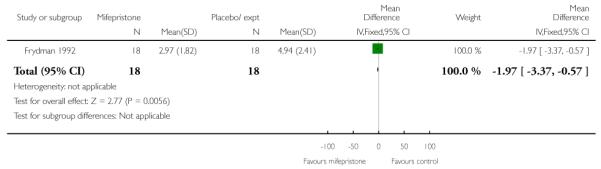
|
Analysis 7.28. Comparison 7 Mifepristone versus placebo, Outcome 28 Oxytocin requirements (IU)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 7 Mifepristone versus placebo
Outcome: 28 Oxytocin requirements (IU)
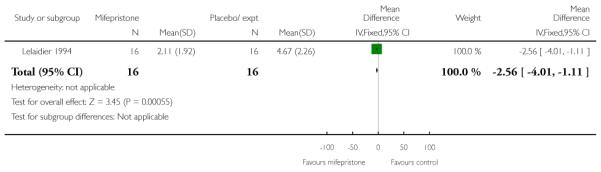
|
Analysis 7.29. Comparison 7 Mifepristone versus placebo, Outcome 29 Interval between day 1 and start of labour (hours)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 7 Mifepristone versus placebo
Outcome: 29 Interval between day 1 and start of labour (hours)
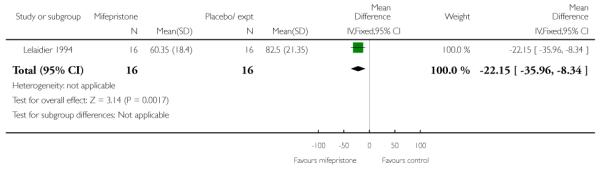
|
Analysis 8.7. Comparison 8 Oestrogens versus placebo, Outcome 7 Caesarean section
Review: Different methods for the induction of labour in outpatient settings
Comparison: 8 Oestrogens versus placebo
Outcome: 7 Caesarean section
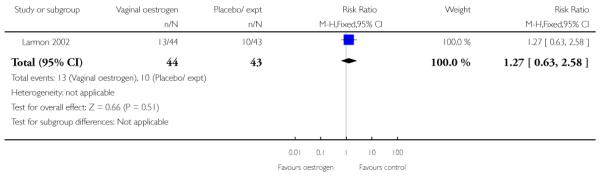
|
Analysis 8.8. Comparison 8 Oestrogens versus placebo, Outcome 8 Assisted (instrumental) vaginal delivery
Review: Different methods for the induction of labour in outpatient settings
Comparison: 8 Oestrogens versus placebo
Outcome: 8 Assisted (instrumental) vaginal delivery
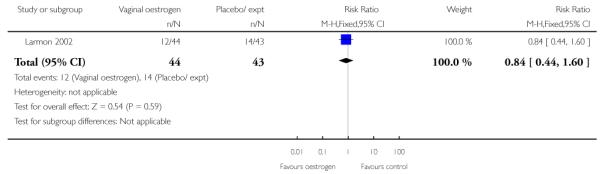
|
Analysis 8.9. Comparison 8 Oestrogens versus placebo, Outcome 9 Oxytocin augmentation
Review: Different methods for the induction of labour in outpatient settings
Comparison: 8 Oestrogens versus placebo
Outcome: 9 Oxytocin augmentation
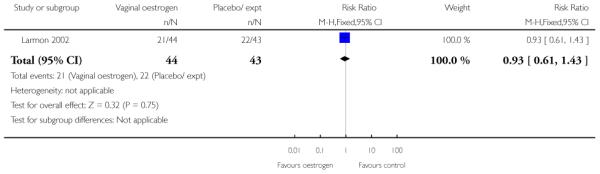
|
Analysis 8.14. Comparison 8 Oestrogens versus placebo, Outcome 14 Chorioamnionitis
Review: Different methods for the induction of labour in outpatient settings
Comparison: 8 Oestrogens versus placebo
Outcome: 14 Chorioamnionitis
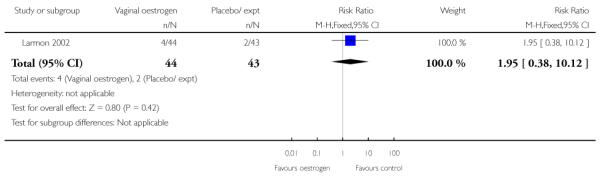
|
Analysis 8.15. Comparison 8 Oestrogens versus placebo, Outcome 15 Endometritis
Review: Different methods for the induction of labour in outpatient settings
Comparison: 8 Oestrogens versus placebo
Outcome: 15 Endometritis
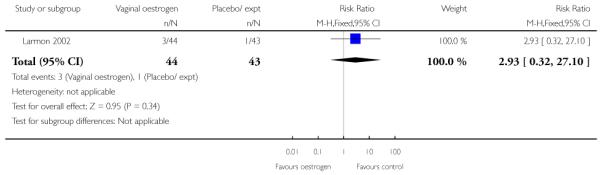
|
Analysis 8.19. Comparison 8 Oestrogens versus placebo, Outcome 19 NICU admission
Review: Different methods for the induction of labour in outpatient settings
Comparison: 8 Oestrogens versus placebo
Outcome: 19 NICU admission
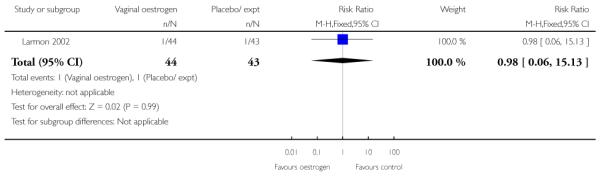
|
Analysis 9.1. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 1 Vaginal delivery not achieved in 24/48 hrs
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 1 Vaginal delivery not achieved in 24/48 hrs
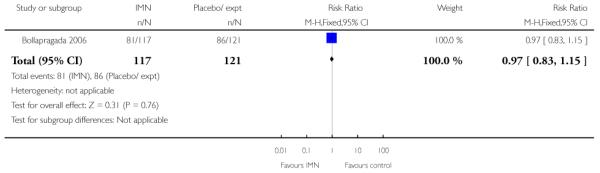
|
Analysis 9.2. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 2 Additional induction agents required
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 2 Additional induction agents required
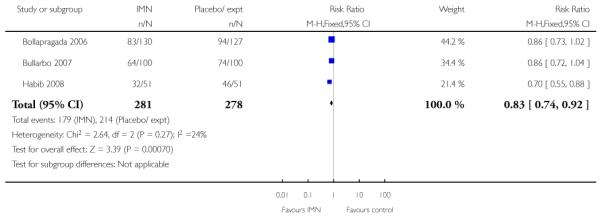
|
Analysis 9.4. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 4 Maternal satisfaction (women would recommend procedure)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 4 Maternal satisfaction (women would recommend procedure)
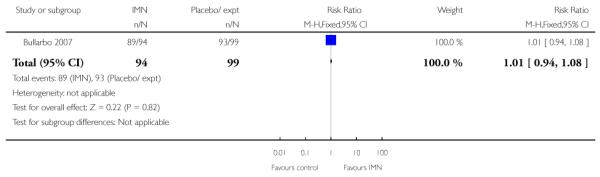
|
Analysis 9.5. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 5 Maternal satisfaction (various questions, low score= satisfied, high score = dissatisfied, score out of 10)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 5 Maternal satisfaction (various questions, low score= satisfied, high score = dissatisfied, score out of 10)
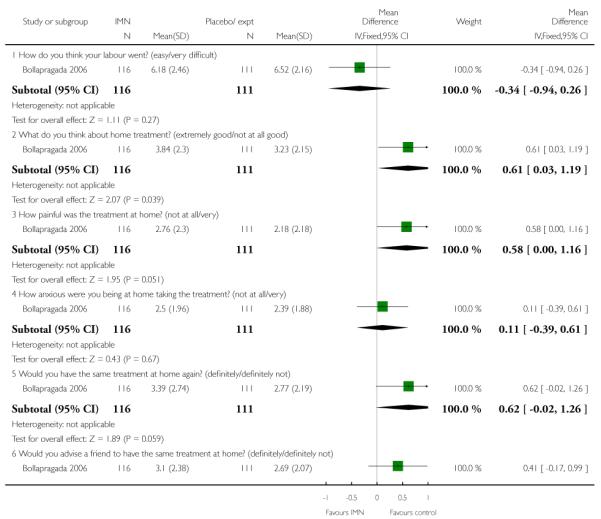
|
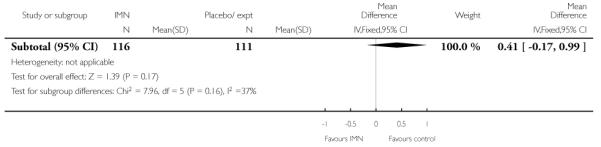
|
Analysis 9.6. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 6 Postpartum haemorrhage (> 500 ml)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 6 Postpartum haemorrhage (> 500 ml)
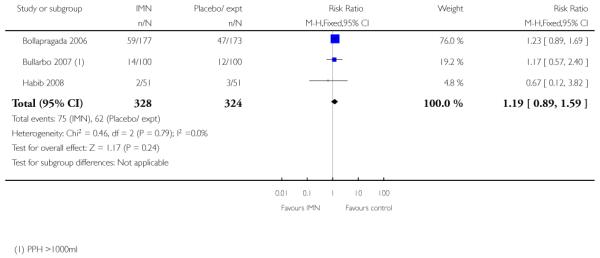
|
Analysis 9.7. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 7 Caesarean section
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 7 Caesarean section
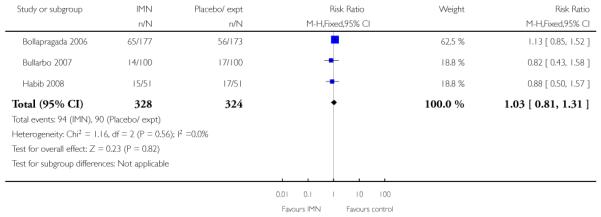
|
Analysis 9.8. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 8 Assisted (instrumental) vaginal delivery
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 8 Assisted (instrumental) vaginal delivery
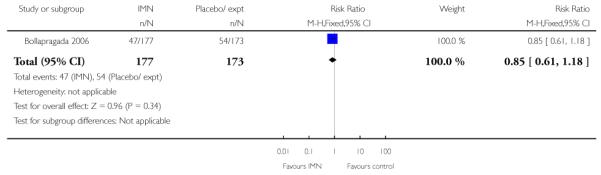
|
Analysis 9.9. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 9 Oxytocin augmentation
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 9 Oxytocin augmentation
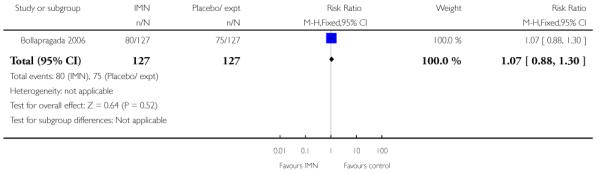
|
Analysis 9.10. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 10 Epidural
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 10 Epidural
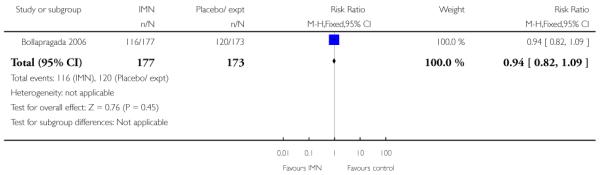
|
Analysis 9.11. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 11 Cervix unchanged after 48 hrs
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 11 Cervix unchanged after 48 hrs
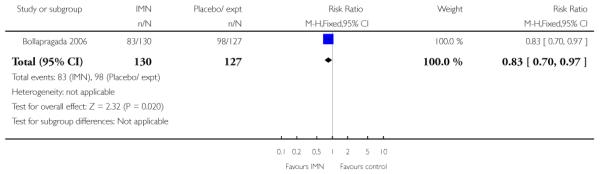
|
Analysis 9.12. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 12 Maternal side effect - headache
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 12 Maternal side effect - headache
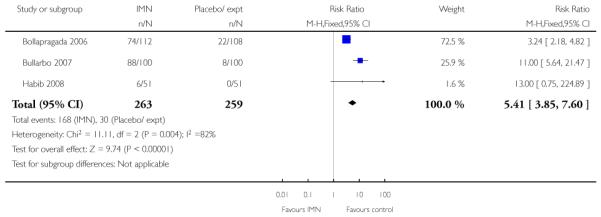
|
Analysis 9.13. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 13 Maternal side effect - nausea
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 13 Maternal side effect - nausea
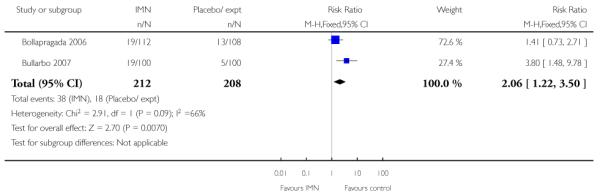
|
Analysis 9.14. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 14 Admitted in established labour within 24 hours
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 14 Admitted in established labour within 24 hours
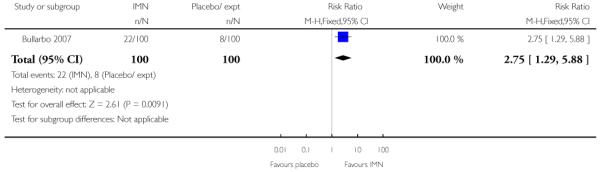
|
Analysis 9.15. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 15 Uterine hyperstimulation (with FHR changes)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 15 Uterine hyperstimulation (with FHR changes)
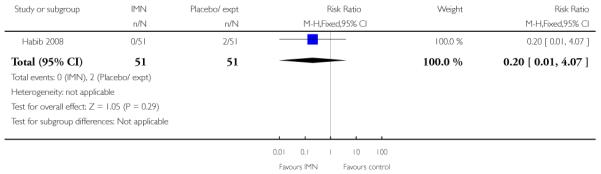
|
Analysis 9.16. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 16 Side effects: severe headache
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 16 Side effects: severe headache
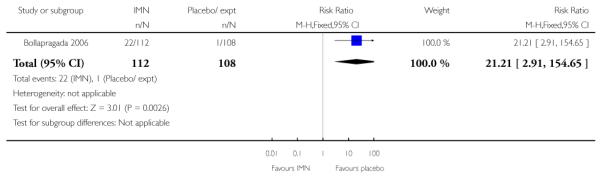
|
Analysis 9.17. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 17 Total cost of care package (GB £)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 17 Total cost of care package (GB )
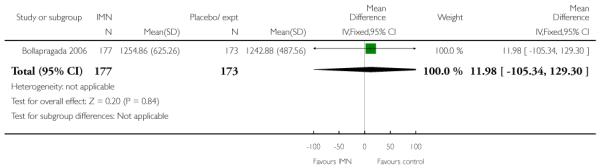
|
Analysis 9.18. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 18 Perinatal death
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 18 Perinatal death
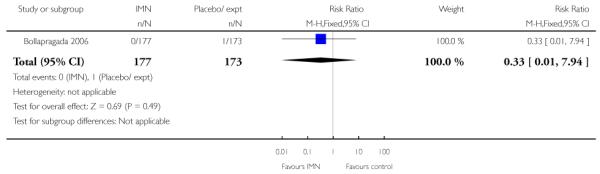
|
Analysis 9.19. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 19 Apgar score < 7 at 5 minutes
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 19 Apgar score < 7 at 5 minutes
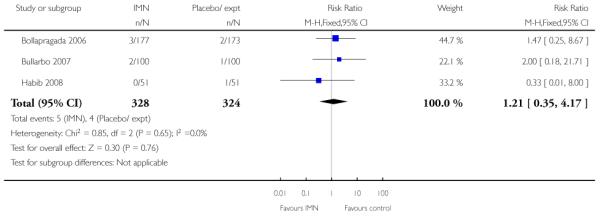
|
Analysis 9.20. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 20 NICU admission
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 20 NICU admission
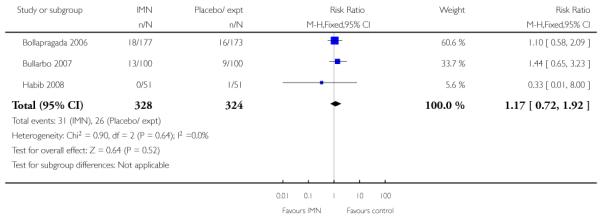
|
Analysis 9.21. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 21 Interval from admission to vaginal delivery (hours)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 21 Interval from admission to vaginal delivery (hours)
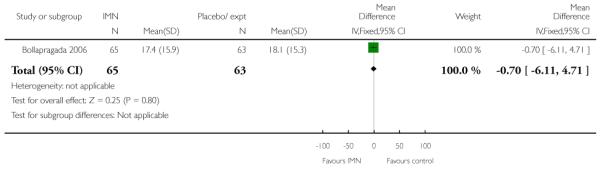
|
Analysis 9.22. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 22 Neonatal infection
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 22 Neonatal infection
|
Analysis 9.23. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 23 Bishop score > 6 or active labour at 36 hours
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 23 Bishop score > 6 or active labour at 36 hours
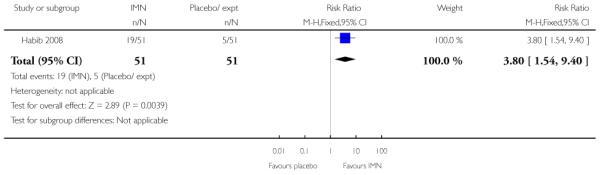
|
Analysis 9.24. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 24 Time in hours from admission to delivery (all women)
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 24 Time in hours from admission to delivery (all women)
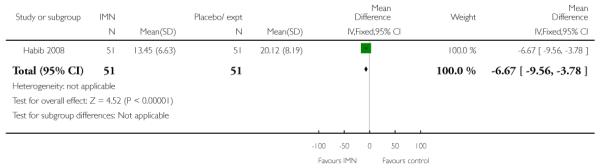
|
Analysis 9.25. Comparison 9 Vaginal isosorbide mononitrate (IMN) versus placebo, Outcome 25 Onset of labour within 24 hours
Review: Different methods for the induction of labour in outpatient settings
Comparison: 9 Vaginal isosorbide mononitrate (IMN) versus placebo
Outcome: 25 Onset of labour within 24 hours
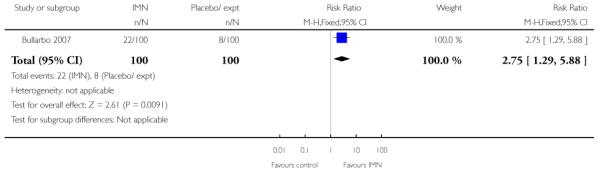
|
Analysis 10.1. Comparison 10 Acupuncture versus routine care, Outcome 1 Additional induction agents required
Review: Different methods for the induction of labour in outpatient settings
Comparison: 10 Acupuncture versus routine care
Outcome: 1 Additional induction agents required
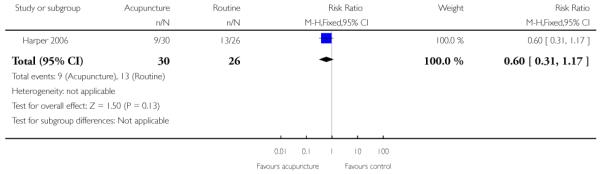
|
Analysis 10.2. Comparison 10 Acupuncture versus routine care, Outcome 2 Caesarean section
Review: Different methods for the induction of labour in outpatient settings
Comparison: 10 Acupuncture versus routine care
Outcome: 2 Caesarean section
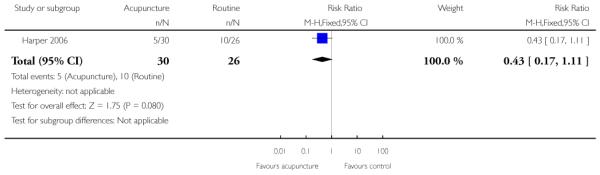
|
HISTORY
Protocol first published: Issue 2, 2009
Review first published: Issue 8, 2010
Footnotes
DECLARATIONS OF INTEREST: Jane Norman was an investigator in a trial examining cervical ripening in an outpatient setting included in this review (Bollapragada 2006); the reports from this trial were independently assessed by two other review authors. Additionally, she has received research grants from pharmaceutical companies developing labour induction agents.
Zarko Alfirevic has received a grant from Ferring and Monica Healthcare Ltd administered by his employers, University of Liverpool and Liverpool Women’s NHS Foundation Trust, to support research related to non-invasive fetal ECG monitoring and outpatient induction of labour.
DIFFERENCES BETWEEN PROTOCOL AND REVIEW: We have added a number of additional (non-prespecified) outcomes focusing on proxy measures of progress towards delivery.
References to studies included in this review
- Bollapragada 2006 [published data only] .Bollapragada S, Mackenzie F, Norrie J, Petrou S, Reid M, Greer I, et al. IMOP: randomised placebo controlled trial of outpatient cervical ripening with isosorbide mononitrate (IMN) prior to induction of labour - clinical trial with analyses of efficacy, cost effectiveness and acceptability. BMC Pregnancy and Childbirth. 2006;6:25. doi: 10.1186/1471-2393-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bollapragada SS, MacKenzie F, Norrie J, Petrou S, Reid M, Greer IA, et al. Randomized placebo controlled trial of outpatient cervical ripening with isosorbide mononitrate (IMN) prior to induction of labour - clinical trial with analyses of efficacy, cost effectiveness and acceptability. The IMOP study [abstract] Journal of Obstetrics and Gynaecology. 2007;27(Suppl 1):S22. doi: 10.1111/j.1471-0528.2009.02216.x. [DOI] [PubMed] [Google Scholar]; * Bollapragada SS, MacKenzie F, Norrie JD, Eddama O, Petrou S, Reid M, et al. Randomised placebo-controlled trial of outpatient (at home) cervical ripening with isosorbide mononitrate (IMN) prior to induction of labour-clinical trial with analyses of efficacy and acceptability. The IMOP study. BJOG: an international journal of obstetrics and gynaecology. 2009;116(9):1185–95. doi: 10.1111/j.1471-0528.2009.02216.x. [DOI] [PubMed] [Google Scholar]; Eddama O, Petrou S, Schroeder L, Bollapragada SS, Mackenzie F, Norrie J, et al. The cost-effectiveness of outpatient (at home) cervical ripening with isosorbide mononitrate prior to induction of labour. BJOG: an international journal of obstetrics and gynaecology. 2009;116(9):1196–203. doi: 10.1111/j.1471-0528.2009.02236.x. [DOI] [PubMed] [Google Scholar]
- Bullarbo 2007 [published data only] .Bullarbo M, Orrskog ME, Andersch B, Granstrom L, Norstrom A, Ekerhovd E. Outpatient vaginal administration of the nitric oxide donor isosorbide mononitrate for cervical ripening and labor induction postterm: a randomized controlled study. American Journal of Obstetrics & Gynecology. 2007;196(1):50.e1–50.e5. doi: 10.1016/j.ajog.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Buttino 1990 [published data only] .Buttino LTCL, Garite TJ. Intracervical prostaglandin in postdate pregnancy. A randomized trial. Journal of Reproductive Medicine. 1990;35:155–8. [PubMed] [Google Scholar]
- Elliott 1998 [published data only] .Elliot CL, Brennand JE, Calder AA. The effect of mifepristone (RU486) on cervical ripening and induction of labour in human pregnancy. 27th British Congress of Obstetrics and Gynaecology; Dublin, Ireland. 1995 July 4-7.1995. p. 207. [Google Scholar]; * Elliott CL, Brennand JE, Calder AA. The effects of mifepristone on cervical ripening and labor induction in primigravidae. Obstetrics & Gynecology. 1998;92(5):804–9. doi: 10.1016/s0029-7844(98)00284-1. [DOI] [PubMed] [Google Scholar]
- Frydman 1992 [published data only] .Frydman R, Baton C, Lelaidier C, Vial M, Bourget P, Fernandez H. Mifepristone for induction of labour. Lancet. 1991;337:488–9. doi: 10.1016/0140-6736(91)93421-5. [DOI] [PubMed] [Google Scholar]; * Frydman R, Lelaidier C, Baton-Saint-Mleux C, Fernandez H, Vial M, Bourget P. Labor induction in women at term with mifepristone (RU 486): a double blind, randomized, placebo-controlled study. Obstetrics & Gynecology. 1992;80:972–5. [PubMed] [Google Scholar]; Frydman R, Lelaidier C, Baton-Saint-Mleux C, Fernandez H, Vial M, Bourget P. Labor induction in women at term with mifepristone (RU 486): a double-blind, randomized, placebo-controlled study. International Journal of Gynecology & Obstetrics. 1993;42(2):220. [PubMed] [Google Scholar]; Frydman R, Taylor S, Paoli C, Pourade A. Ru 486 (mifepristone): A new tool for labour induction in term women with fetus alive [Le RU 486 (mifepristone) un novel outil pour le declenchment du travail a terme] Contraception, Fertilite, Sexualite. 1992;20(12):1133–6. [PubMed] [Google Scholar]; Lelaidier C, Benifla JL, Fernandez H, Baton C, Bourget P, Bourrier MC, et al. RU 486 (mifepristone) in medical indications for labour induction in pregnancies at term: results of a randomized, double-blind study of RU 486 vs placebo. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction. 1993;22:92–100. [PubMed] [Google Scholar]
- Giacalone 1998 [published data only] .Giacalone PL, Targosz V, Laffargue F, Boog G, Faure JM. Cervical ripening with mifepristone before labor induction: a randomized study. Obstetrics & Gynecology. 1998;92(4 Pt 1):487–92. doi: 10.1016/s0029-7844(98)00225-7. [DOI] [PubMed] [Google Scholar]
- Gittens 1996 [published data only] .Gittens L, Schenkel C, Strassberg S, Apuzzio J. Vaginal birth after cesarean section: comparison of outpatient use of prostaglandin gel to expectant management. American Journal of Obstetrics and Gynecology. 1996;174(1 Pt 2):354. [Google Scholar]
- Habib 2008 [published data only] .Habib SM, Emam SS, Saber AS. Outpatient cervical ripening with nitric oxide donor isosorbide mononitrate prior to induction of labor. International Journal of Gynecology & Obstetrics. 2008;101(1):57–61. doi: 10.1016/j.ijgo.2007.09.027. [DOI] [PubMed] [Google Scholar]
- Hage 1993 [published data only] .Hage P, Shaw J, Zarou D, Fleisher J, Wehbeh H. Double blind randomized trial to evaluate the role of outpatient use of PGE 2 in cervical ripening. American Journal of Obstetrics and Gynecology. 1993;168:430. [Google Scholar]
- Harper 2006 [published data only] .Harper TC, Coeytaux RR, Chen W, Campbell K, Kaufman JS, Moise KJ, et al. A randomized controlled trial of acupuncture for initiation of labor in nulliparous women. Journal of Maternal-Fetal & Neonatal Medicine. 2006;19(8):465–70. doi: 10.1080/14767050600730740. [DOI] [PubMed] [Google Scholar]
- Incerpi 2001 [published data only] .Incerpi M, Fassett M, Kjos S, Tran S, Wing D. Vaginally administered misoprostol for outpatient labor induction in pregnancies with diabetes mellitus [abstract] American Journal of Obstetrics and Gynecology. 2001;184(1):S120. doi: 10.1067/mob.2001.117306. [DOI] [PubMed] [Google Scholar]; * Incerpi MH, Fassett MJ, Kjos SL, Tran SH, Wing DA. Vaginally administered misoprostol for outpatient cervical ripening in pregnancies complicated by diabetes mellitus. American Journal of Obstetrics and Gynecology. 2001;185(4):916–9. doi: 10.1067/mob.2001.117306. [DOI] [PubMed] [Google Scholar]
- Kipikasa 2005 [published data only] .Kipikasa JH, Adair CD, Williamson J, Breen JM, Medford LK, Sanchez-Ramos L. Use of misoprostol on an outpatient basis for postdate pregnancy. International Journal of Gynecology & Obstetrics. 2005;88:108–11. doi: 10.1016/j.ijgo.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Larmon 2002 [published data only] .Larmon JE, Magann EF, Dickerson GA, Morrison JC. Outpatient cervical ripening with prostaglandin E2 and estradiol. Journal of Maternal-Fetal & Neonatal Medicine. 2002;11(2):113–7. doi: 10.1080/jmf.11.2.113.117. [DOI] [PubMed] [Google Scholar]
- Lelaidier 1994 [published data only] .Lelaidier C, Baton C, Benifla JL, Fernandez H, Bourget P, Frydman R. Mifepristone for labour induction after previous caesarean section. British Journal of Obstetrics and Gynaecology. 1994;101:501–3. doi: 10.1111/j.1471-0528.1994.tb13150.x. [DOI] [PubMed] [Google Scholar]
- Lien 1998 [published data only] .Lien JM, Morgan MA, Garite TJ, Kennedy KA, Sassoon DA, Freeman RK. Antepartum cervical ripening: applying prostaglandin e2 gel in conjunction with scheduled nonstress tests in postdate pregnancies. American Journal of Obstetrics and Gynecology. 1998;179(2):453–8. doi: 10.1016/s0002-9378(98)70378-3. [DOI] [PubMed] [Google Scholar]
- Lyons 2001 [published data only] .Lyons C, Rumney P, Huang W, Morrison E, Thomas S, Nageotte M, et al. Outpatient cervical ripening with oral misoprostol post-term: induction rates decreased. American Journal of Obstetrics and Gynecology. 2001;184(1):S116. [Google Scholar]
- Magann 1998 [published data only] .Magann E, Chauhan SP, Nevils BG, McNamara MF, Kinsella MJ, Morrison JC. Management of pregnancies beyond forty-one weeks’ gestation with an unfavorable cervix. American Journal of Obstetrics and Gynecology. 1998;178:1279–87. doi: 10.1016/s0002-9378(98)70334-5. [DOI] [PubMed] [Google Scholar]
- McKenna 1999 [published data only] .McKenna DS, Costa SW, Samuels P. Prostaglandin E2 cervical ripening without subsequent induction of labor. Obstetrics & Gynecology. 1999;94(1):11–4. doi: 10.1016/s0029-7844(99)00244-6. [DOI] [PubMed] [Google Scholar]
- McKenna 2004 [published data only] .McKenna DS, Ester JB, Proffitt M, Waddell KR. Misoprostol outpatient cervical ripening without subsequent induction of labor: a randomized trial. Obstetrics & Gynecology. 2004;104(3):579–84. doi: 10.1097/01.AOG.0000136479.72777.56. [DOI] [PubMed] [Google Scholar]
- Meyer 2005 [published data only] .Meyer M, Pflum J. Outpatient administration of misoprostol decreases induction time. American Journal of Obstetrics and Gynecology. 2002;187(6 Pt 2):S167. [Google Scholar]; * Meyer M, Pflum J, Howard D. Outpatient misoprostol compared with dinoprostone gel for preinduction cervical ripening: a randomized controlled trial. Obstetrics & Gynecology. 2005;105(3):466–72. doi: 10.1097/01.AOG.0000152341.31873.d9. [DOI] [PubMed] [Google Scholar]
- Newman 1997 [published data only] .Newman M, Newman R. Multiple-dose PGE2 cervical ripening on an outpatient basis: safety and efficacy. American Journal of Obstetrics and Gynecology. 1997;176(1 Pt 2):S112. [Google Scholar]
- O’Brien 1995 [published data only] .O’Brien JM, Mercer B, Cleary N, Sibai BM. Efficacy of outpatient induction with low dose intravaginal prostaglandin E2: A randomized, double-blind, placebo-controlled trial. American Journal of Obstetrics and Gynecology. 1995;172:424. doi: 10.1016/0002-9378(95)90440-9. [DOI] [PubMed] [Google Scholar]; O’Brien JM, Mercer BM, Cleary NT, Sibai BM. Efficacy of outpatient induction with low-dose intravaginal prostaglandin E2: A randomized, double blind, placebo-controlled trial. American Journal of Obstetrics and Gynecology. 1995;173:1855–9. doi: 10.1016/0002-9378(95)90440-9. [DOI] [PubMed] [Google Scholar]
- Oboro 2005 [published data only] .Oboro VO, Tabowei TO. Outpatient misoprostol cervical ripening without subsequent induction of labor to prevent post-term pregnancy. Acta Obstetricia et Gynecologica Scandinavica. 2005;84(7):628–31. doi: 10.1111/j.0001-6349.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- Rayburn 1999 [published data only] .Rayburn W, Lucas M, Gittens L, Goodwin TM, Baxi L, Gall S, et al. Attempted vaginal birth after cesarean section: a multicenter comparison of outpatient prostaglandin E2 gel with expectant management. Primary Care Update for Ob/Gyns. 1998;5(4):182–3. doi: 10.1016/s1068-607x(98)00096-1. [DOI] [PubMed] [Google Scholar]; Rayburn WF, Gittens LN, Lucas MJ, Gall SA, Martin ME. Weekly administration of prostaglandin e2 gel compared with expectant management in women with previous cesareans Prepidil gel study group. Obstetrics & Gynecology. 1999;94(2):250–4. doi: 10.1016/s0029-7844(99)00300-2. [DOI] [PubMed] [Google Scholar]
- Sawai 1991 [published data only] .Sawai SK, Williams MC, O’Brien WF, Angel JL, Mastrogiannis DS, Johnson L. Sequential outpatient application of intravaginal prostaglandin E2 gel in the management of postdates pregnancies. Obstetrics & Gynecology. 1991;78(1):19–23. [PubMed] [Google Scholar]; Williams MG, O’Brien WF, Sawai SK, Knuppel RA. Outpatient cervical ripening in the postdates pregnancy. Proceedings of 10th Annual Meeting of Society of Perinatal Obstetricians; Houston, Texas, USA. 1990 Jan 23-27.1990. p. 533. [Google Scholar]
- Sawai 1994 [published data only] .* Sawai SK, O’Brien WF, Mastrogiannis DS, Krammer J, Mastry MG, Porter GW. Patient-administered outpatient intravaginal prostaglandin E2 suppositories in post-date pregnancies: a double-blind, randomized, placebo-controlled study. Obstetrics & Gynecology. 1994;84(5):807–10. [PubMed] [Google Scholar]; Sawai SK, O’Brien WF, Mastrogiannis MS, Mastry MG, Porter GW, Johnson L. Outpatient prostaglandin E2 suppositories in postdates pregnancies. American Journal of Obstetrics and Gynecology. 1992;166(1 Pt 2):400. [Google Scholar]
- Stenlund 1999 [published data only] .Stenlund PM, Bygdeman M, Ekman G. Induction of labor with mifepristone (RU 486). A randomized double-blind study in post-term pregnant women with unripe cervices. Acta Obstetricia et Gynecologica Scandinavica Supplement. 1994;73(161):FP50. [PubMed] [Google Scholar]; Stenlund PM, Ekman G, Aedo AR, Bygdeman M. Induction of labour with mifepristone - a randomized double-blind study versus placebo. Acta Obstetricia et Gynecologica Scandinavica. 1999;78:793–8. [PubMed] [Google Scholar]
- Stitely 2000 [published data only] .Stitely ML, Browning J, Fowler M, Gendron RT, Gherman RB. Outpatient cervical ripening with intravaginal misoprostol. Obstetrics & Gynecology. 2000;96:684–8. doi: 10.1016/s0029-7844(00)01034-6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Adewole 1993 [published data only] .Adewole IF, Franklin O, Matiluko AA. Cervical ripening and induction of labour by breast stimulation. African Journal of Medicine and Medical Sciences. 1993;22:81–6. [PubMed] [Google Scholar]
- Damania 1988 [published data only] .Damania KR, Nanavati MS, Dastur NA, Daftary SN. Breast stimulation for cervical ripening. Journal of Obstetrics and Gynaecology of India. 1988;58:663–5. [Google Scholar]
- Damania 1992 [published data only] .Damania KK, Natu U, Mhatre PN, Mataliya M, Mehta AC, Daftary SN. Evaluation of two methods employed for cervical ripening. Journal of Postgraduate Medicine. 1992;38(2):58–9. [PubMed] [Google Scholar]
- Di Lieto 1989 [published data only] .Di Lieto A, Miranda L, Ardito P, Favale P, Albano G. Changes in the bishop score induced by manual nipple stimulation. A cross-over randomized study. Clinical and Experimental Obstetrics and Gynecology. 1989;16:26–9. [PubMed] [Google Scholar]
- Doany 1997 [published data only] .Doany W. Outpatient management of postdate pregnancy with intravaginal prostaglandin E2 and membrane stripping. American Journal of Obstetrics and Gynecology. 1996;174(1Pt 2):351. [Google Scholar]; * Doany W, McCarty J. Outpatient management of the uncomplicated postdate pregnancy with intravaginal prostaglandin E2 gel and membrane stripping. Journal of Maternal-Fetal Medicine. 1997;6(2):71–8. doi: 10.1002/(SICI)1520-6661(199703/04)6:2<71::AID-MFM2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Dorfman 1987 [published data only] .Dorfman P, Lasserre MN, Tetau M. Preparation for childbirth by homeopathy [Preparation a l’accouchement par homeopathie – experimentation en double insu versus placebo] Cahiers de BiothÄrapie. 1987;94:77–81. [Google Scholar]
- Elliott 1984 [published data only] .Elliott JP, Flaherty JF. The use of breast stimulation to prevent postdate pregnancy. American Journal of Obstetrics and Gynecology. 1984;149:628–32. doi: 10.1016/0002-9378(84)90247-3. [DOI] [PubMed] [Google Scholar]; Elliott JP, Flaherty JF. The use of breast stimulation to ripen the cervix in term pregnancies. American Journal of Obstetrics and Gynecology. 1983;145:553–6. doi: 10.1016/0002-9378(83)91194-8. [DOI] [PubMed] [Google Scholar]
- Evans 1983 [published data only] .Evans MI, Dougan MB, Moawad AH, Evans WJ, Bryant-Greenwood GD, Greenwood FC. Ripening of the human cervix with porcine ovarian relaxin. American Journal of Obstetrics and Gynecology. 1983;147(4):410–4. doi: 10.1016/s0002-9378(16)32236-0. [DOI] [PubMed] [Google Scholar]
- Garry 2000 [published data only] .Garry D, Figueroa R, Guillaume J, Cucco V. Use of castor oil in pregnancies at term. Alternative Therapies in Health and Medicine. 2000;6(1):77–9. [PubMed] [Google Scholar]
- Griffin 2003 [published data only] .Griffin C. Outpatient cervical ripening using sequential oestrogen - a randomised controlled pilot study. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2003;43:183. [Google Scholar]
- Herabutya 1992 [published data only] .Herabutya Y, Prasertsawat PO, Tongyai T, Isarangura N, Ayudthya N. Prolonged pregnancy: the management dilemma. International Journal of Gynecology & Obstetrics. 1992;37:253–8. doi: 10.1016/0020-7292(92)90325-d. [DOI] [PubMed] [Google Scholar]
- Kadar 1990 [published data only] .Kadar N, Tapp A, Wong A. The influence of nipple stimulation at term on the duration of pregnancy. Journal of Perinatology. 1990;10:164–6. [PubMed] [Google Scholar]
- Kaul 2004 [published data only] .Kaul V, Aggarwal N, Ray P. Membrane stripping versus single dose intracervical prostaglandin gel administration for cervical ripening. International Journal of Gynecology & Obstetrics. 2004;86:388–9. doi: 10.1016/j.ijgo.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Krammer 1995 [published data only] .Krammer J, O’Brien W, Williams M. Outpatient cervical ripening does not affect gestational age at delivery. American Journal of Obstetrics and Gynecology. 1995;172:425. [Google Scholar]
- Magann 1999 [published data only] .Magann EF, Chauhan SP, McNamara MF, Bass JD, Estes CM, Morrison JC. Membrane stripping vs dinoprostone vaginal insert in the management of pregnancies beyond 41 weeks with unfavorable cervix. American Journal of Obstetrics and Gynecology. 1998;178(1 Pt 2):S30. [Google Scholar]; * Magann EF, Chauhan SP, McNamara MF, Bass JD, Estes CM, Morrison JC. Membrane sweeping versus dinoprostone vaginal insert in the management of pregnancies beyond 41 weeks with an unfavorable cervix. Journal of Perinatology. 1999;19(2):88–91. doi: 10.1038/sj.jp.7200133. [DOI] [PubMed] [Google Scholar]
- Manidakis 1999 [published data only] .Manidakis G, Sifakis S, Orfanoudaki E, Mikelakis G, Prokopakis P, Magou M, et al. Prostaglandin versus stripping of membranes in management of pregnancy beyond 40-41 weeks [abstract] European Journal Obstetrics, Gynecology and Reproductive Biology. 1999;86:S79–S80. [Google Scholar]
- Moghtadaei 2007 [published data only] .Moghtadaei P. A randomized trial comparing outpatient vaginal isosorbide-mononitrate versus extra-amnion saline infusion with concurrent oxytocin for cervical ripening and labor induction in nulliparous women. American Journal of Obstetrics and Gynecology. 2007;197(6 Suppl 1):S103. [Google Scholar]
- Ohel 1996 [published data only] .Ohel G, Rahav D, Rothbart H, Ruach M. Randomised trial of outpatient induction of labor with vaginal PGE2 at 40-41 weeks of gestation versus expectant management. Archives of Gynecology & Obstetrics. 1996;258(3):109–12. doi: 10.1007/s004040050110. [DOI] [PubMed] [Google Scholar]
- Rayburn 1988 [published data only] .Rayburn W, Gosen R, Ramadei C, Woods R, Scott J., Jr Outpatient cervical ripening with prostaglandin E2 gel in uncomplicated postdate pregnancies. American Journal of Obstetrics and Gynecology. 1988;158(6 Pt 1):1417–23. doi: 10.1016/0002-9378(88)90376-6. [DOI] [PubMed] [Google Scholar]
- Rijnders 2007 [published data only] .Rijnders MEB. [accessed 15 February 2007];Costs and effects of amniotomy at home for induction of post term pregnancy. Current Controlled Trials. http://controlled-trials.com. [Google Scholar]
- Salamalekis 2000 [published data only] .Salamalekis E, Vitoratos N, Kassanos D, Loghis C, Batalias L, Panayotopoulos N, et al. Sweeping of the membranes versus uterine stimulation by oxytocin in nulliparous women: a randomized controlled trial. Gynecologic and Obstetric Investigation. 2000;49:240–3. doi: 10.1159/000010267. [DOI] [PubMed] [Google Scholar]
- Salmon 1986 [published data only] .Salmon YM, Kee WH, Tan SL, Jen SW. Cervical ripening by breast stimulation. Obstetrics & Gynecology. 1986;67:21–4. [PubMed] [Google Scholar]
- Spallicci 2007 [published data only] .* Spallicci MD, Chiea MA, Singer JM, Albuquerque PB, Bittar RE, Zugaib M. Use of hyaluronidase for cervical ripening: a randomized trial. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2007;130(1):46–50. doi: 10.1016/j.ejogrb.2005.10.028. [DOI] [PubMed] [Google Scholar]; Spallicci MDB, Bittar RE. Randomized double blind study of ripening the cervix with hyaluronidase in term gestations [abstract] [Estudo clinico aleatorizado com grupo controle e mascaramento duplo da maturacao do colo uterino pela hialuronidase em gestacoes a termo] Revista Brasileira de Ginecologia e Obstetricia. 2003;25(1):67. [Google Scholar]
- Voss 1996 [published data only] .Voss DH, Cumminsky KC, Cook VD, Nethers MS, Spinnato JA, Gall SA. Effect of three concentrations of intracervical prostaglandin E2 gel for cervical ripening. Journal of Maternal-Fetal Medicine. 1996;5(4):186–93. doi: 10.1002/(SICI)1520-6661(199607/08)5:4<186::AID-MFM5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Ziaei 2003 [published data only] .Ziaei S, Rosebehani N, Kazeminejad A, Zafarghandi S. The effects of intramuscular administration of corticosteroids on the induction of parturition. Journal of Perinatal Medicine. 2003;31(2):134–9. doi: 10.1515/JPM.2003.018. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Ascher-Walsh 2000 [published data only] .Ascher-Walsh C, Burke B, Baxi L. Outpatient management of prolonged pregnancy with misoprostol (mp): a randomized double-blind placebo controlled study, prelim data. American Journal of Obstetrics and Gynecology. 2000;182(1 Pt 2):S20. [Google Scholar]
- Thakur 2005 [published data only] .Thakur V, Dorman E, Sanu L, Harrington K. Mifepristone is an effective ripening agent in postdates primips with cervical length >= 2.5cm, but mode of delivery correlates with birthweight: a randomised, placebo controlled double blind study. Ultrasound in Obstetrics and Gynecology. 2005;26:452. [Google Scholar]
Additional references
- Alfirevic 2006 .Alfirevic Z, Weeks A. Oral misoprostol for induction of labour. Cochrane Database of Systematic Reviews. 2006;(Issue 2) doi: 10.1002/14651858.CD001338.pub2. [DOI: 10.1002/14651858.CD001338.pub2] [DOI] [PubMed] [Google Scholar]
- Bollapragada 2006 .Bollapragada S, Mackenzie F, Norrie J, Petrou S, Reid M, Greer I, et al. IMOP: randomised placebo controlled trial of outpatient cervical ripening with isosorbide mononitrate (IMN) prior to induction of labour - clinical trial with analyses of efficacy, cost effectiveness and acceptability. BMC Pregnancy and Childbirth. 2006;6:25. doi: 10.1186/1471-2393-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulvain 2008 .Boulvain M, Kelly AJ, Irion O. Intracervical prostaglandins for induction of labour. Cochrane Database of Systematic Reviews. 2008;(Issue 1) doi: 10.1002/14651858.CD006971. [DOI: 10.1002/14651858.CD006971] [DOI] [PubMed] [Google Scholar]
- Curtis 1987 .Curtis P, Evans S, Resnick J. Uterine hyperstimulation. The need for standard terminology. Journal of Reproductive Medicine. 1987;32:91–5. [PubMed] [Google Scholar]
- Elliott 1992 .Elliott JP, Clewell WH, Radin TG. Intracervical prostaglandin E2 gel. Safety for outpatient cervical ripening before induction of labor. Journal of Reproductive Medicine. 1992;37(8):713–6. [PubMed] [Google Scholar]
- Gates 2005 .Gates S. Methodological Guidelines. In: The Editorial Team. Pregnancy and Childbirth Group. About the Cochrane Collaboration (Collaborative Review Groups (CRGs)) 2005;(Issue 2) [Google Scholar]
- Glantz 2003 .Glantz JC. Labor induction rate variation in upstate New York: what is the difference? Birth. 2003;30(3):168–74. doi: 10.1046/j.1523-536x.2003.00241.x. [DOI] [PubMed] [Google Scholar]
- Gülmezoglu 2006 .Gülmezoglu AM, Crowther CA, Middleton P. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database of Systematic Reviews. 2006;(Issue 4) doi: 10.1002/14651858.CD004945.pub2. [DOI: 10.1002/14651858.CD004945.pub2] [DOI] [PubMed] [Google Scholar]
- Hapangama 2009 .Hapangama D, Neilson JP. Mifepristone for induction of labour. Cochrane Database of Systematic Reviews. 2009;(Issue 3) doi: 10.1002/14651858.CD002865.pub2. [DOI: 10.1002/14651858.CD002865.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2009 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.2 [updated September 2009] The Cochrane Collaboration; 2009. Available from www.cochrane-handbook.org. [Google Scholar]
- Hofmeyr 2000 .Hofmeyr GJ, Alfirevic Z, Kelly T, Kavanagh J, Thomas J, Brocklehurst P, et al. Methods for cervical ripening and labour induction in late pregnancy: generic protocol. Cochrane Database of Systematic Reviews. 2000;(Issue 2) doi: 10.1002/14651858.CD000941. [DOI: 10.1002/14651858.CD002074] [DOI] [PubMed] [Google Scholar]
- Hofmeyr 2003 .Hofmeyr GJ, Gülmezoglu AM. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews. 2003;(Issue 1) doi: 10.1002/14651858.CD000941. [DOI: 10.1002/14651858.CD000941] [DOI] [PubMed] [Google Scholar]
- Kelly 2001 .Kelly A, Alfirevic Z, Hofmeyr GJ, Kavanagh J, Neilson JP, Thomas J. Induction of labour in specific clinical situations: generic protocol. Cochrane Database of Systematic Reviews. 2001;(Issue 4) [DOI: 10.1002/14651858.CD003398] [Google Scholar]
- Kelly 2008 .Kelly AJ, Kavanagh J. Nitric oxide donors for cervical ripening and induction of labour (Protocol) Cochrane Database of Systematic Reviews. 2008;(Issue 1) [DOI: 10.1002/14651858.CD006901] [Google Scholar]
- Kelly 2009 .Kelly AJ, Malik S, Smith L, Kavanagh J, Thomas J. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database of Systematic Reviews. 2009;(Issue 4) doi: 10.1002/14651858.CD003101.pub2. [DOI: 10.1002/14651858.CD003101.pub2] [DOI] [PubMed] [Google Scholar]
- Kelly 2009b .Kelly AJ, Alfirevic Z, Dowswell T. Outpatient versus inpatient induction of labour for improving birth outcomes. Cochrane Database of Systematic Reviews. 2009;(Issue 2) doi: 10.1002/14651858.CD007372.pub2. [DOI: 10.1002/14651858.CD007372.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby 2004 .Kirby RS. Trends in labor induction in the United States: is it true that what goes up must come down? Birth. 2004;31(2):148–51. doi: 10.1111/j.0730-7659.2004.00294.x. [DOI] [PubMed] [Google Scholar]
- McGill 2007 .McGill J, Shetty A. Mifepristone and misoprostol in the induction of labor at term. International Journal of Gynecology & Obstetrics. 2007;96(2):80–4. doi: 10.1016/j.ijgo.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Neale 2002 .Neale E, Pachulski A, Whiterod S, McGuinness E, Gallagher N, Wallace R. Outpatient cervical ripening prior to induction of labour. Journal of Obstetrics and Gynaecology. 2002;22(6):634–5. doi: 10.1080/0144361021000020411. [DOI] [PubMed] [Google Scholar]
- NHS 2007 .Richardson A, Mmata C. NHS Maternity Statistics, England: 2005-06. National Statistics, The Information Centre; London: 2007. [Google Scholar]
- Osman 2006 .Osman I, MacKenzie F, Norrie J, Murray HM, Greer IA, Norman JE. The “PRIM” study: a randomized comparison of prostaglandin E2 gel with the nitric oxide donor isosorbide mononitrate for cervical ripening before the induction of labor at term. American Journal of Obstetrics and Gynecology. 2006;194(4):1012–21. doi: 10.1016/j.ajog.2005.10.812. [DOI] [PubMed] [Google Scholar]
- Ramsey 2005 .Ramsey PS, Meyer L, Walkes BA, Harris D, Ogburn PL, Jr, Heise RH, et al. Cardiotocographic abnormalities associated with dinoprostone and misoprostol cervical ripening. Obstetrics & Gynecology. 2005;105(1):85–90. doi: 10.1097/01.AOG.0000146638.51536.09. [DOI] [PubMed] [Google Scholar]
- Rayburn 2002 .Rayburn WF, Zhang J. Rising rates of labor induction: present concerns and future strategies. Obstetrics & Gynecology. 2002;100(1):164–7. doi: 10.1016/s0029-7844(02)02047-1. [DOI] [PubMed] [Google Scholar]
- RevMan 2008 .The Nordic Cochrane Centre, The Cochrane Collaboration . Review Manager (RevMan). 5.0. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2008. [Google Scholar]
- Salvador 2009 .Salvador SC, Simpson ML, Cundiff GW. Dinoprostone vaginal insert for labour induction: a comparison of outpatient and inpatient settings. Journal of Obstetrics & Gynaecology Canada: JOGC. 2009;31:1028–34. doi: 10.1016/S1701-2163(16)34347-X. [DOI] [PubMed] [Google Scholar]
- Sawai 1995 .Sawai SK, O’Brien WF. Outpatient cervical ripening. Clinical Obstetrics & Gynecology. 1995;38(2):301–9. doi: 10.1097/00003081-199506000-00013. [DOI] [PubMed] [Google Scholar]
- Shetty 2005 .Shetty A, Burt R, Rice P, Templeton A. Women’s perceptions, expectations and satisfaction with induced labour--a questionnaire-based study. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2005;123(1):56–61. doi: 10.1016/j.ejogrb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Smith 2004 .Smith CA, Crowther CA. Acupuncture for induction of labour. Cochrane Database of Systematic Reviews. 2004;(Issue 1) doi: 10.1002/14651858.CD002962.pub2. [DOI: 10.1002/14651858.CD002962.pub2] [DOI] [PubMed] [Google Scholar]
- Thomas 2001 .Thomas J, Kelly AJ, Kavanagh J. Oestrogens alone or with amniotomy for cervical ripening or induction of labour. Cochrane Database of Systematic Reviews. 2001;(Issue 4) doi: 10.1002/14651858.CD003393. [DOI: 10.1002/14651858.CD003393] [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Indicates the major publication for the study


