Highlights
-
•
Two cases of large cervical mullerian adenosarcoma with sarcomatous overgrowth or heterologous elements and contrasting survival outcomes are reported.
-
•
When the diagnosis of mullerian adenosarcoma is uncertain or suspected, review of pathology by a national expert may be considered.
-
•
Rhabdomyoblastic differentiation of mullerian adenosarcoma may be a more aggressive histologic type.
Keywords: Mullerian adenosarcoma, Sarcomatous overgrowth, Heterologous elements, Cervical cancer
Introduction
Mullerian adenosarcomas (MAs) are malignant mixed mullerian tumors with benign epithelial and malignant stromal components that most often originate in the endometrium (71%), ovary (15%), or pelvis (12%), and less frequently develop in the cervix (2%) (Clement and Scully, 1974, 1990; Verschraegen et al., 1998). MAs behave as low-grade malignancies with a proclivity for local recurrence; distant metastases are infrequent (Clement and Scully, 1990; Verschraegen et al., 1998). Cervical MAs usually present as cervical polyps and can be confused with benign cervical polyps both clinically and pathologically; microscopic differentiation is critical for accurate diagnosis and appropriate treatment. Since cervical MAs are rare, experience with their diagnosis and treatment is limited. Due to a paucity of reports and an absence of long term follow-up data, the presentation, prognosis and management of cervical MA continue to be explored.
Two types of MA with unusual pathologic features are associated with poorer prognoses: MA with sarcomatous overgrowth and MA with heterologous elements. Sarcomatous overgrowth is diagnosed when the pure sarcomatous portion of the neoplasm constitutes more than 25% of the primary tumor. Heterologous elements are tissue types, such as skeletal muscle or cartilage, present in the neoplasm and not native to the primary site. Here, we report two cases of cervical MA, one with sarcomatous overgrowth and another with heterologous elements.
Case 1 — cervical mullerian adenosarcoma with sarcomatous overgrowth
A 54-year-old woman presented to her gynecologist with acute urinary retention. She reported irregular menses for the past four years. She was a former smoker, had a remote history of myomectomy, and had no family history of any cancer. On examination, she had a distended abdomen and suprapubic tenderness. Pelvic examination revealed a large cervical mass which appeared to originate from the endocervical canal. Tumor markers were as follows: CA 125 = 127 U/ml, CEA = 0.7 ng/ml, and CA 19-9 = 52 U/ml. Computed tomography demonstrated an 8 cm heterogeneous mass involving the cervix without extension of tumor into the bladder or rectum (Fig. 1A). No significant lymphadenopathy was noted. Exam under anesthesia found an 8 cm smooth, firm, and hemorrhagic mass with a broad base involving the cervix. The parametria were free. A prominent left ovary was palpated. Cystoscopy and proctoscopy were unremarkable. Cervical biopsies were performed and demonstrated fibrovascular tissue with severe hemorrhage.
Fig. 1.
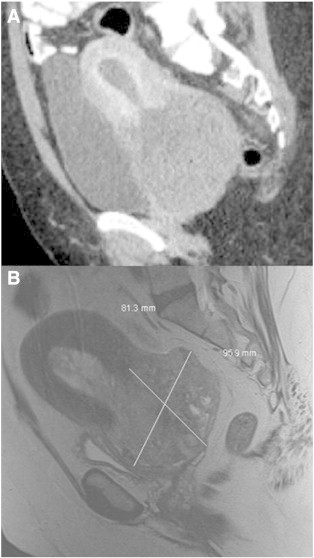
A: Representative computed tomography sagittal image of large cervical mullerian adenosarcoma with sarcomatous overgrowth (Case 1). B: Representative magnetic resonance sagittal image, T2 weighted, of large cervical mullerian adenosarcoma with heterologous elements (Case 2).
The patient was counseled extensively, and she decided to undergo radical hysterectomy and bilateral salpingo-oophorectomy with frozen section, which was of concern for cervical sarcoma. Bilateral pelvic and para-aortic lymph node dissection was performed and all 27 identified nodes were negative for malignancy. Final pathology confirmed cervical low grade MA with sarcomatous overgrowth measuring 4 × 3 × 2 cm (Fig. 2 and Supplemental Information 1). Tumor immunohistochemistry was diffusely positive for muscle markers SMA and MSA, as well as estrogen and progesterone receptors, and focally positive for desmin, CKAE1/3, and CAM 5.2, but negative for myogenin, EMA, CD10, caldesmon, HMB-45, CD31, CD34, factor VIII, inhibin, and S100. She received adjuvant vaginal cuff brachytherapy (25 Gy of iridium-192) followed by six cycles of doxorubicin (40 mg/m2). Her postoperative recovery and adjuvant treatment courses were uncomplicated. She remains without evidence of disease now 66 months after diagnosis of her stage IB2 cervical MA with sarcomatous overgrowth.
Fig. 2.
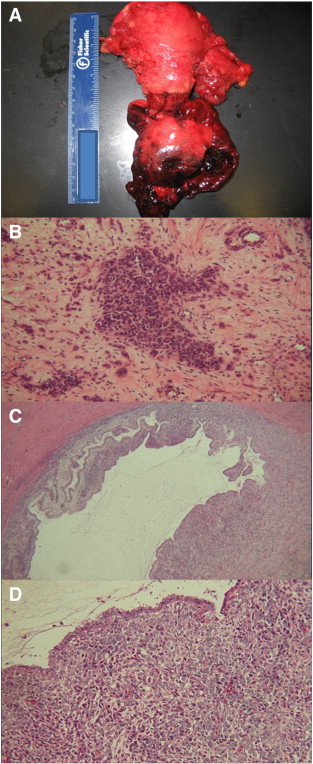
A: Gross pathologic specimen. B: Tumor glands within collagenous stroma. C: Low power field showing benign glands with stromal condensation. D: High power field showing benign glands with stromal condensation.
Case 2 — cervical mullerian adenosarcoma with heterologous elements
A 47-year-old woman with no gynecologic care for many years presented with irregular vaginal bleeding. Her past medical and surgical histories included hypertension, obesity, cholecystectomy, and a unilateral oophorectomy for an unknown benign indication. Physical examination revealed a 10 cm polypoid, partially necrotic cervical mass arising from a pedicle in the endocervical canal. Assessment of the uterus and adnexa was very limited secondary to body habitus. Exam under anesthesia further revealed bilateral shortening of the parametria. Cystoscopy and proctoscopy were negative for bladder or rectal involvement. Cervical biopsies demonstrated cervical adenosarcoma with a histologically high grade stromal component having rhabdomyoblastic differentiation (Fig. 3 and Supplemental Information 1). Magnetic resonance imaging demonstrated a 9.5 cm × 9.6 cm × 8.1 cm cervical tumor with bilateral infiltration of the parametria and right parametrial lymph nodes (Fig. 1B), without evidence of abdominal or lung metastases.
Fig. 3.
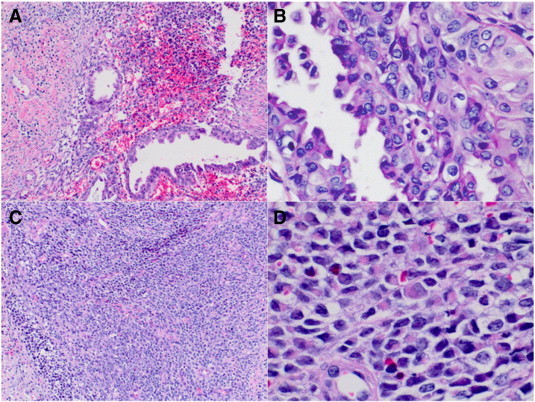
A: Biphasic cervical malignant neoplasm with atypical glands and high-grade spindle cell sarcomatous elements (H&E stain, magnification × 100). B: Area of atypical glands with mild nuclear pleomorphism and focal squamoid features (H&E stain, magnification × 400). C: Region of undifferentiated sarcoma with round cell morphology (H&E stain, magnification × 100). D: Area of high-grade sarcoma with rhabdoid morphology, including polygonal and strap-shaped tumor cells with eccentric nuclei and abundant eosinophilic cytoplasm with vague cross-striations (H&E stain, magnification × 400).
Given her stage IIB disease, initial treatment was preoperative whole pelvis external beam radiation (5040 cGy) and chemotherapy consisting of three cycles of ifosfamide (2000 mg/m2) and doxorubicin (40 mg/m2). Significant toxicity of this treatment included colitis necessitating a four-day hospitalization, during which interval computed tomography showed an ill-defined cervical mass, new enlargement of the uterus, abdominal carcinomatosis and multiple pulmonary metastases. Chemotherapy was changed to gemcitabine (675 mg/m2) and docetaxel (75 mg/m2). Repeat imaging after 3 cycles demonstrated disease progression. She began palliative docetaxel (35 mg/m2) for two additional cycles before electing for comfort care only. She subsequently died due to metastatic cervical MA 12 months after diagnosis.
Discussion
MAs are generally low grade neoplasms that may recur locally after initial surgical resection and infrequently metastasize to distal sites. Unfavorable prognostic factors for MAs are cytologic atypia, high mitotic rate, sarcomatous overgrowth, heterologous elements, deep myometrial invasion, necrosis and extrauterine spread (Jones and Lefkowitz, 1995; Kerner and Lichtig, 1993; Kaku et al., 1992). The diagnosis of MA is established by the criteria proposed by Clement and Scully (Clement and Scully, 1990) and Jones and Lefkowitz (Jones and Lefkowitz, 1995): formation of periglandular cuffs and intraglandular protrusions of cellular stroma, non-invasive glands lined by benign-appearing mullerian epithelia of various types showing mild to marked nuclear atypia, an average of ≥ 2 mitotic figures per 10 high powered fields in the stromal component, and more than mild nuclear atypia of the stromal cells. Given the rarity of this disease, we recommend a pathology review by a national expert when the diagnosis of adenosarcoma is suspected or uncertain. The clinical behavior and pathologic features of Case 2 were suggestive of carcinosarcoma. A pathology review by a leading national expert in adenosarcoma was obtained. The expert was confident in the diagnosis of a high grade adenosarcoma with rhabdomyoblastic differentiation, which was suggested to be a feature associated with relatively aggressive disease, although reported clinical experience with this entity is insufficient to provide robust prognostic information.
Approximately 2% of MAs develop in the cervix. In the largest series of cervical MA (24 cases), the mean age at presentation was 31 years (range 11–65 years), with one-third of patients presenting before age 15 (Jones and Lefkowitz, 1995). Most patients presented with abnormal vaginal bleeding and a polypoid appearing lesion protruding through the external cervical os. The differential diagnosis of cervical MA includes benign (adenofibroma, atypical endocervical polyp, and adenomyoma of the cervix) and malignant (uterine adenosarcoma with secondary involvement of the cervix, carcinosarcoma, and embryonal rhabdomyosarcoma) lesions. Nine cases of cervical MA with sarcomatous overgrowth (Table S1) and eighteen cases of MA with heterologous elements (Table S2) are reported in the literature. Four cases had both sarcomatous overgrowth and heterologous elements (Table S1). The most common heterologous elements are skeletal muscle and cartilage.
The prognosis and management of cervical MA continue to be defined as the number of reported cases increases. Much of the management of cervical MA is extrapolated from experience with uterine MAs, which, while uncommon among uterine cancers, are seen much more frequently than cervical MAs. Some authors recommend hysterectomy and bilateral salpingo-oophorectomy (Clement and Scully, 1990; Gast et al., 1989; Zaloudek and Norris, 1981). Local excision has been curative in rare cases (Table S2). Fertility sparing surgery may be an alternative in patients with pedunculated cervical tumors with uninvolved stalks (Clement and Scully, 1990; Chen, 1985). However, obtaining a negative margin is necessary, and reoperation should be considered when disease extends to the surgical margins (Michener and Simon, 2001). No standard of care exists for radiation or chemotherapy of uterine or cervical MA due to lack of evidence that any particular therapy is more beneficial than other options in reported series (Krivak et al., 2001; Tanner et al., 2013; Bernard et al., 2013). Adjuvant treatment recommendations are poorly defined. Cases 1 and 2 were managed with radiation and standard sarcoma chemotherapy regimens that are among many treatment options per National Comprehensive Cancer Network guidelines (Annon, 2014). Management options for uterine sarcomas have been recently reviewed (Reichardt, 2012; Trope et al., 2012). Trials of radiation or chemotherapy for uterine sarcomas often included multiple uterine sarcoma types, such as the early GOG trial of stage I–II uterine sarcomas that randomized patients to adjuvant doxorubicin or no further treatment and showed no statistically significant benefit but did establish a precedent for use of adjuvant doxorubicin for uterine sarcomas (Omura et al., 1985). Retrospective data suggested decreased recurrence with radiation therapy, but no benefit was found in a prospective trial (Reed et al., 2008). Adenosarcomas with sarcomatous overgrowth are often treated according to guidelines for high-grade undifferentiated endometrial sarcoma (Trope et al., 2012). In Case 1, vaginal brachytherapy and single agent doxorubicin were offered due to the presence of sarcomatous overgrowth. Case 2 was stage IIB and high grade. The patient received second opinions regarding treatment options and elected whole pelvic radiation and one of the most studied combination chemotherapies for uterine sarcomas (ifosfamide/doxorubicin), with a plan for post-treatment surgery. Due to early disease progression with systemic metastases, she was switched to combination gemcitabine/docetaxel, which is preferred for advanced or recurrent leiomyosarcoma (NCCN, 2014). Her disease continued to progress and she began palliative docetaxel before electing for comfort measures only.
Recurrences after 5 years are not infrequent for uterine MAs; therefore long-term clinical follow-up is recommended (Clement and Scully, 1990; Jones and Lefkowitz, 1995; Zaloudek and Norris, 1981). A local recurrence rate of 24% and a distant recurrence rate of 2% after hysterectomy and bilateral salpingo-oophorectomy were reported with recurrences occurring between 0.5 and 9.5 years; one-third of recurrences occurred after 5 years (Clement and Scully, 1990). In GOG 40, Kaku et al. (1992) reported a 30% recurrence rate of uterine MAs, with most recurrences occurring within 24 months after hysterectomy, bilateral salpingo-oophorectomy, and lymph node sampling. One series compared survival of patients diagnosed with uterine adenosarcoma with sarcomatous overgrowth (N = 11) to survival of patients with uterine carcinosarcoma (N = 33), reporting a trend toward worse prognosis of uterine MA with sarcomatous overgrowth (p = 0.052) with a median survival of 13 months (Krivak et al., 2001). A recent small (N = 5) series of uterine MA with sarcomatous overgrowth reported a 20% 2-year survival rate (Tanner et al., 2013). The prognosis of cervical MA with sarcomatous overgrowth or heterologous elements remains less characterized. Cases need to be reported as only continued accumulation of clinicopathologic data will lead to improved understanding of this disease. Here we report two cases of large cervical MA tumors with contrasting clinical outcomes: one case of stage IB2 low grade cervical MA with sarcomatous overgrowth and long-term disease free survival after surgical resection, vaginal brachytherapy, and single-agent chemotherapy and the second case being a patient with stage IIB high grade cervical MA with rhabdomyoblastic differentiation who experienced a short 12 month disease specific survival despite whole pelvic external beam radiation and multiple courses of chemotherapy. The disease progression of Case 2 was more rapid than is typical of MAs and may represent a more aggressive nature of high grade MA with rhabdomyoblastic features: more reports of clinical outcomes of patients with this rare histologic feature are needed to establish its prognostic significance.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgments
We wish to thank Dr. Robert Soslow of Memorial Sloan Kettering Cancer Center for expert pathology consultation on both cases. We wish to thank Dr. Steven Sieber, Director of Anatomy Pathology, Western Connecticut Health Network, for providing histologic images and captions for Fig. 3.
Disclaimer
Written informed consent was obtained from each patient or next of kin for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-In-Chief of this journal on request.
Appendix A. Supplementary data
Supplemental Information 1: Pathology reports of Cases 1 and 2.
Table S1: Clinicopathologic features of cervical mullerian adenosarcomas with sarcomatous overgrowth reported in the literature in chronological order.
Table S2: Clinicopathologic features of cervical mullerian adenosarcomas with heterologous elements reported in the literature in chronological order.
References
- Clement P.B., Scully R.E. Mullerian adenosarcoma of the uterus: a clinicopathologic analysis of ten cases of a distinctive type of mullerian mixed tumor. Cancer. 1974;34:1138–1149. doi: 10.1002/1097-0142(197410)34:4<1138::aid-cncr2820340425>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Clement P.B., Scully R.E. Mullerian adenosarcoma of the uterus: a clinicopathologic analysis of 100 cases with a review of the literature. Hum. Pathol. 1990;21:363–381. doi: 10.1016/0046-8177(90)90198-e. [DOI] [PubMed] [Google Scholar]
- Verschraegen C.F., Vasuratna A., Edwards C., Freedman R., Kudelka A.P., Tornos C. Clinicopathologic analysis of mullerian adenosarcoma: the MD Anderson Cancer Center experience. Oncol. Rep. 1998;5:939–944. doi: 10.3892/or.5.4.939. [DOI] [PubMed] [Google Scholar]
- Jones M.W., Lefkowitz M. Adenosarcoma of the uterine cervix: a clinicopathologic study of 12 cases. Int. J. Gynecol. Pathol. 1995;14:223–229. doi: 10.1097/00004347-199507000-00005. [DOI] [PubMed] [Google Scholar]
- Kerner H., Lichtig C. Mullerian adenosarcoma presenting as cervical polyps: a report of seven cases and review of the literature. Obstet. Gynecol. 1993;81:655–659. [PubMed] [Google Scholar]
- Kaku T., Silverberg S.G., Major F.J., Miller A., Fetter B., Brady M.F. Adenosarcoma of the uterus: a Gynecologic Oncology Group clinicopathologic study of 31 cases. Int. J. Gynecol. Pathol. 1992;11:75–88. [PubMed] [Google Scholar]
- Gast M.J., Radkins L.V., Jacobs A.J., Gersell D. Mullerian adenosarcoma of the cervix with heterologous elements: diagnostic and therapeutic approach. Gynecol. Oncol. 1989;32:381–384. doi: 10.1016/0090-8258(89)90646-x. [DOI] [PubMed] [Google Scholar]
- Zaloudek C.J., Norris H.J. Adenofibroma and adenosarcoma of the uterus: a clinicopathologic study of 35 cases. Cancer. 1981;48:354–366. doi: 10.1002/1097-0142(19810715)48:2<354::aid-cncr2820480222>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Chen K.T. Rhabdomyosarcomatous uterine adenosarcoma. Int. J. Gynecol. Pathol. 1985;4:146–152. doi: 10.1097/00004347-198506000-00006. [DOI] [PubMed] [Google Scholar]
- Michener C.M., Simon N.L. Ovarian conservation in a woman of reproductive age with mullerian adenosarcoma. Gynecol. Oncol. 2001;83:424–427. doi: 10.1006/gyno.2001.6398. [DOI] [PubMed] [Google Scholar]
- Krivak T.C., Seidman J.D., McBroom J.W., MacKoul P.J., Aye L.M., Rose G.S. Uterine adenosarcoma with sarcomatous overgrowth versus uterine carcinosarcoma: comparison of treatment and survival. Gynecol. Oncol. 2001;83:89–94. doi: 10.1006/gyno.2001.6334. [DOI] [PubMed] [Google Scholar]
- Tanner E.J., Toussaint T., Leitao M.M., Jr., Hensley M.L., Soslow R.A., Gardner G.J. Management of uterine adenosarcomas with and without sarcomatous overgrowth. Gynecol. Oncol. 2013;129:140–144. doi: 10.1016/j.ygyno.2012.12.036. [DOI] [PubMed] [Google Scholar]
- Bernard B., Clarke B.A., Malowany J.I., McAlpine J., Lee C.H., Atenafu E.G. Uterine adenosarcomas: a dual-institution update on staging, prognosis and survival. Gynecol. Oncol. 2013;131:634–639. doi: 10.1016/j.ygyno.2013.09.011. [DOI] [PubMed] [Google Scholar]
- NCCN clinical practice guidelines in oncology: uterine neoplasms. 2014. http://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf (Accessed April 12, 2014)
- Reichardt P. The treatment of uterine sarcomas. Ann. Oncol. 2012;23:x151–x157. doi: 10.1093/annonc/mds359. [DOI] [PubMed] [Google Scholar]
- Trope C.G., Abeler V.M., Kristensen G.B. Diagnosis and treatment of sarcoma of the uterus: a review. Acta Oncol. 2012;51:694–705. doi: 10.3109/0284186X.2012.689111. [DOI] [PubMed] [Google Scholar]
- Omura G.A., Blessing J.A., Major F., Lifshitz S., Ehrlich C.E., Mangan C. A randomized clinical trial of adjuvant adriamycin in uterine sarcomas: a Gynecologic Oncology Group study. J. Clin. Oncol. 1985;3:1240–1245. doi: 10.1200/JCO.1985.3.9.1240. [DOI] [PubMed] [Google Scholar]
- Reed N.S., Manigioni C., Malmstrom H., Scarfone G., Poveda A., Pecorelli S. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: an European Organization for Research and Treatment of Cancer Gynaecological Cancer Group study (protocol 55874) Eur. J. Cancer. 2008;44:808–818. doi: 10.1016/j.ejca.2008.01.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Information 1: Pathology reports of Cases 1 and 2.
Table S1: Clinicopathologic features of cervical mullerian adenosarcomas with sarcomatous overgrowth reported in the literature in chronological order.
Table S2: Clinicopathologic features of cervical mullerian adenosarcomas with heterologous elements reported in the literature in chronological order.


