Abstract
From historical studies of developing chick hearts to recent advances in regenerative injury models, the epicardium has arisen as a key player in heart genesis and repair. The epicardium provides paracrine signals to nurture growth of the developing heart from mid-gestation, and epicardium-derived cells act as progenitors of numerous cardiac cell types. Interference with either process is terminal for heart development and embryogenesis. In adulthood, the dormant epicardium reinstates an embryonic gene programme in response to injury. Furthermore, injury-induced epicardial signalling is essential for heart regeneration in zebrafish. Given these critical roles in development, injury response and heart regeneration, the application of epicardial signals following adult heart injury could offer therapeutic strategies for the treatment of ischaemic heart disease and heart failure.
Graphical abstract
Highlights
-
•
The epicardium is a dynamic signalling centre during heart development and injury.
-
•
Heart repair in lower vertebrates highlights the importance of epicardial signalling.
-
•
Epicardial signals may be targeted to regenerate adult mammalian hearts.
Introduction
In 1909, Kurkiewicz observed the developing chick embryo and declared the epicardium a secondary covering, originating from the ‘pericardial villi’ and distinct from the looping muscular tube that would form the heart (Kurkiewicz, 1909). More than half a century passed before electron microscopy confirmed this observation; until which, the prevailing dogma of epicardium origin was from the myocardium itself (Manasek, 1968, 1969). Following numerous descriptive studies in multiple systems (Ho and Shimada, 1978; Viragh and Challice, 1981; Komiyama et al., 1987; Kuhn and Liebherr, 1988; Fransen and Lemanski, 1990) including humans (Hirakow, 1992), the first experimental demonstration of the ‘Kurkiewicz hypothesis’ came in 1992, when Männer obstructed the migration of the pericardial villi (now termed the proepicardial organ (PEO)) to the surface of the developing chick heart resulting in defective epicardial formation and impaired heart development (Manner, 1993). This early seminal work established the basis for the transcendence of the epicardium from a dormant mesothelium to a critical cell and signalling source for the developing heart.
It is now established that, in all vertebrates studied, cells from the PEO encapsulate the looping heart at mid-gestation, forming the critical outer layer. The epicardium then enters a complex ‘dialogue’ with the underlying myocardium; secretes trophic factors essential for myocardial maturation, and directly contributes precursors of numerous cardiac cell types. The sequence and mechanisms of this complex interplay are a major research focus, whilst the relative contributions of epicardium-derived cells (EPDCs) to cardiac lineages remain a source of debate. The relevance of understanding epicardial potential in development is paramount given its response to injury. Post-development, embryonic epicardial gene programmes are shut down, at least in mammals, and in the healthy adult heart, the epicardium is said to become quiescent. Following heart injury, however, such ‘quiescence’ is rapidly lost as epicardial cells revert to an embryonic-like phenotype, proliferating at the site of injury and secreting factors to modulate wound healing. In adult mammals, this response is characterised by mass fibrosis and scar formation, which, whilst necessary to prevent exsanguination of the compromised ventricle and retain contractile force, ultimately leads to pathological remodelling and heart failure. Conversely, organ-wide epicardial activation in the zebrafish heart in response to injury is central to the regenerative capacity of this species (Kikuchi et al., 2011; Lepilina et al., 2006).
It has long been known that the mammalian heart is very limited in its regenerative capacity. Shortly after birth, a majority of cardiomyocytes (CMs) exit the cell cycle, and whilst there is evidence of limited turnover (of between 0.5 and 1% per year in adult humans during normal homeostasis (Bergmann et al., 2009)), muscle regeneration is insufficient to restore the billions of CMs lost post-infarction. That is, unless the infarction occurs during the first few days of life. Recently, Porrello and colleagues demonstrated the remarkable regenerative capacity of the neonatal mouse heart. Following amputation of 15–20% of the apex (Porrello et al., 2011) or ischemia induced by chronic ligation of the lateral anterior descending coronary artery (LAD) (Porrello et al., 2013; Haubner et al., 2012), the neonate (one day old) was shown to regenerate lost myocardium in a timescale that exceeds the regenerative capacity of zebrafish. However, this capacity was lost within the first week of life, such that if the same injury was sustained on or after postnatal day (P) 7, default scar formation and adult-like wound healing was observed. This first demonstration of effective mammalian heart regeneration was again associated with organ-wide epicardial activation, and was lost coincident with the loss of epicardial potential (Chen et al., 2002).
Thus, the epicardium represents a critical developmental source of cells and signals which, whilst quiescent under normal conditions, can revert to act as a multipotent cell source and trophic signalling centre to modulate both pathological and regenerative wound healing. Recent advances in models of heart injury and repair have highlighted the potential of the epicardium in promoting regeneration. Such advances are discussed here, with a focus on myogenic epicardial signalling events conserved in development and the injury response, to highlight the therapeutic potential of modulating epicardial signals to instruct heart repair in adult mammals including humans.
The epicardium in heart development
At around Embryonic day (E) 9.75 in the mouse, Hamburger Hamilton (HH) stage 18 in the chick, and 72 h post fertilisation (hpf) in the zebrafish; the mature PEO migrates to the myocardial surface to encapsulate the heart. After forming a uniform epithelium (at around E11 or equivalent) a proportion of epicardial cells undergo epithelial-to-mesenchymal transition (EMT) and populate the subepicardial space. EPDCs then migrate into the underlying myocardium and give rise to numerous cardiac lineages.
Cellular contributions to the developing heart
EPDCs can be detected through the expression of the transcription factors Wt1, Tbx18, Tcf21 (also known as capsulin/pod1/epicardin), as well as the retinoic acid (RA) synthesising enzyme, Raldh2. There is consensus regarding EPDC contribution to coronary vascular smooth muscle cells and interstitial and perivascular fibroblasts: in standard culture medium, vascular smooth muscle cells are the default EPDC fate and studies in chick and mouse have consistently identified EPDCs as the major source of embryonic cardiac fibroblasts (Mikawa and Fischman, 1992; Manner, 1999; Cai et al., 2008; Zhou et al., 2008; Mikawa and Gourdie, 1996). Contributions to coronary endothelium and CMs, however, remain contentious, but highly relevant given the importance of these lineages for both neovascularization and myogenesis. In the past, the controversy surrounding the epicardial fate map has been fuelled by divergent findings from chick and mouse studies, but more so in recent years due to (mis-) interpretation of lineage trace data arising from cre-loxP-based reporter lines in the mouse.
In the chick, Perez-Pomarez and colleagues described a direct Wt1 positive contribution to endothelial cells supporting an epicardial origin (Perez-Pomares et al., 2002). This conflicted with earlier findings from Poelmann and colleagues, who described no such differentiation following the formation of the subepicardial mesenchyme, and instead reported that the coronary endothelium was derived from subsequent sproutings of the sinus venosus at the liver primordium (Poelmann et al., 1993). However, the argument for an EPDC origin persisted based on evidence from Dil labelling experiments, chimaera studies and retroviral analyses (Mikawa and Fischman, 1992; Manner, 1999; Guadix et al., 2006; Gourdie et al., 2000). Of note, none of the aforementioned studies demonstrated an EPDC contribution to CMs in the chick, although, two independent explant studies demonstrated that BMP2 stimulation could drive chick PE cells towards a CM fate in vitro (van Wijk et al., 2009; Kruithof et al., 2006).
In the mouse, little or no EPDC contribution to the endothelial lineage was found in fate mapping studies utilising GATA5 (Merki et al., 2005), Tbx18 (Cai et al., 2008) and Wt1 (Zhou et al., 2008) reporter lines. In contrast, Red-Horse and colleagues described a sinus venosus origin of coronary endothelial cells analogous to that described in chicks (Red-Horse et al., 2010) and that these cells subsequently populate the subepicardium and migrate to form the ventricular capillary plexus (Tian et al., 2013). Wu and co-workers further proposed an endocardial origin for coronary endothelium (Wu et al., 2012). Interestingly, sub-populations of PEO cells with endothelial potential have since been defined using Semaphorin 3D (Sema3D) and Scleraxis (Scx) as cre-drivers in lineage trace experiments. Distinct domains of Wt1, Tbx18, Sema3D and Scx expression were observed in the PEO, and whilst Sema3D and Scx expression sometimes overlapped, both were mutually exclusive from the canonical markers Tbx18 and Wt1. In these studies, whilst Tbx18 and Wt1 were reported to give rise to vascular smooth muscle cells and fibroblasts but not coronary endothelium, Sema3D and Scx progenitors contributed to all three lineages. A modest contribution to CMs was also reported (Katz et al., 2012). Further, these populations were observed to contribute to the sinus venosus and the endocardium, offering an explanation of a potential common endothelial cell progenitor source to reconcile the previous studies (Poelmann et al., 1993; Red-Horse et al., 2010; Tian et al., 2013; Wu et al., 2012).
Though an epicardial contribution to the myocardial lineage is minimal in the chick, fate mapping using Wt1 and Tbx18 cre lines described significant epicardial contributions to the developing myocardium in the mouse (Cai et al., 2008; Zhou et al., 2008). However, this finding is controversial: Christoffels and co-workers since demonstrated Tbx18 expression in the inter-ventricular sulcus (IVS) and left ventricular myocardium, even in epicardium-deficient embryos (Christoffels et al., 2009). Rudat and Kispert further observed ‘leaky’ and inefficient recombination in the Wt1 reporter lines used for the developmental myocardial lineage trace studies, coupled to endogenous endocardial Wt1 expression (Rudat and Kispert, 2012). Arising from these findings, therefore, is the need to ensure the specificity of cre-Lox based fate mapping, coupled to a requirement for rigorous characterisation of the native expression pattern of the gene driving the cre recombinase, alongside validation through alternate cre drivers and parallel approaches such as DiI-labelling and clonal analyses.
Epicardial signalling in heart development
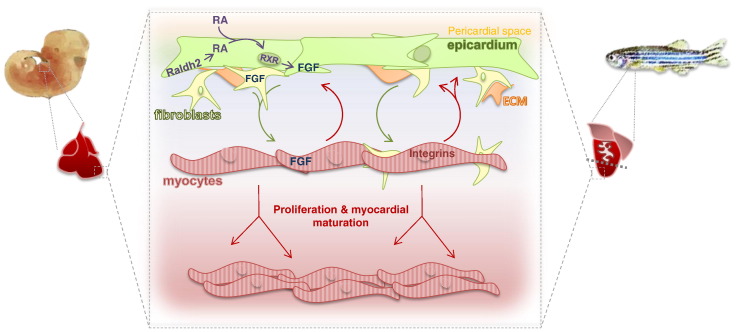
In addition to cellular contributions to the developing heart, the epicardium and its derivatives provide paracrine signals which influence myocardial maturation and development of the coronary vasculature; the latter of which is extensively reviewed (Olivey and Svensson, 2010; Perez-Pomares and de la Pompa, 2011). A number of key epicardial signals have arisen as important functional mitogens during development, injury and regeneration, which include most prominently retinoic acid (RA), fibroblast growth factors (FGFs) and signals arising from the extracellular matrix (ECM). These will be discussed in more detail throughout the remainder of this review.
Retinoic acid signalling is critical for early heart morphogenesis. Mice carrying the null mutation for Raldh2 fail to undergo axial rotation and the heart comprises a single dilated chamber (Niederreither et al., 1999). Paradoxically, exposing embryos to a teratogenic dose of RA also leads to heart defects (D'Aniello et al., 2013), highlighting the necessity for tight regulation of RA signalling in heart development. Interestingly, mice lacking the retinoid X receptor α (RXRα), a critical receptor for RA signalling, die mid-gestation due to a detached epicardium and hypoplastic myocardium (Sucov et al., 1994). This phenotype was shown to be epicardium specific: deletion of myocardial RXRα led to a normally formed heart (Chen et al., 1998), whilst Gata5-cre mediated RXRα deletion recapitulated the RXRα null phenotype (Chen et al., 2002; Merki et al., 2005). This suggested an epicardial specific response to RA that is necessary for heart development, irrespective of a direct myocardial RA signalling component. In this instance, FGF2 was identified as the downstream mitogenic factor (Merki et al., 2005). An epicardium-dependent RA effect was also demonstrated in chick heart slice cultures: RA induced CM proliferation in slices incorporating the epicardium, but not in those without (Stuckmann et al., 2003). Cultured mouse embryonic epicardial cells and stable epicardial cell lines further secreted trophic factors in response to RA treatment, and this conditioned medium stimulated proliferation of cultured CMs. However, by postnatal day 4, both the potential of cultured epicardial cells to secrete factors in response to RA, and the ability of CMs to respond to secreted factors, were lost. Intriguingly, this coincides with the loss of regenerative capacity of the mouse heart in early neonatal life (Porrello et al., 2011, 2013). Also of note, recent studies by Brade and colleagues demonstrated parallel properties of hepatic RA-erythropoietin (Epo) signalling in mice (Brade et al., 2011). In response to RA, the liver secreted Epo, which then initiated IGF2 production in the epicardium. IGF2 secretion induces CM proliferation (Li et al., 2011). Interestingly, blocking Epo signalling in slice culture blocked CM proliferation; which could then be rescued by RA administration. Equally, inhibition of RA-induced CM proliferation was rescued by addition of Epo, suggesting that RA and Epo act synergistically on the epicardium. Together, these epicardial signalling pathways represent key mitogens for heart development.
Fibroblast growth factors constitute another important class of epicardial signalling molecules which are known to exert a number of biological effects including the regulation of cell-survival effects (Ornitz and Itoh, 2001), and may act downstream of RA signalling (Merki et al., 2005). Numerous FGF family members (FGF1, 2, 4, 9, 16 and 20) are expressed in the mammalian epicardium (Lavine et al., 2005; Pennisi and Mikawa, 2005). Concomitantly, CMs express FGF receptors, and in some instances, ligand and receptor cellular expression is reversed between the two lineages, suggesting a reciprocal interaction which functions to exert a proliferative effect on the myocardium (Lavine et al., 2005). This is underscored by loss of function studies: mice lacking FGF9 die at birth due to a poorly formed compact myocardium associated with reduced CM cycling. An equivalent phenotype is observed following deletion of FGFR1 and FGFR2 (Lavine et al., 2005). Epicardial–myocardial interaction of FGF10 and FGFR1 and FGFR2b was also shown to be critical for EPDC migration into the myocardium, with a specific impact on fibroblast differentiation, linked to decreased heart size (Vega-Hernandez et al., 2011). Also, in zebrafish, signalling between myocardial FGF17b and epicardial FGFR2 and FGFR4 is thought to underlie constant myocardial homeostasis (Wills et al., 2008) and is further implicated in the response to injury (Lepilina et al., 2006).
A major source of FGF signalling is the cardiac fibroblast population, which constitute upwards of two thirds of the cells of the heart (Nag, 1980; Jugdutt, 2003). EPDCs are the main source of cardiac fibroblasts and their emergence during development coincides with CM proliferation and myocardial expansion (Ieda et al., 2009). In culture, embryonic fibroblasts exert proliferative signals by paracrine secretions of extracellular matrix (ECM) components, fibronectin, collagen, and heparin-binding EGF-like growth factor 1 (Ieda et al., 2009). Ieda and colleagues observed a positive correlation between CM proliferation and fibroblast density, which was dependent upon myocardial β1-integrin expression. Conversely, adult cardiac fibroblast cultures stimulated myocyte hypertrophy but not proliferation (Ieda et al., 2009). Epicardial–myocardial FGF signalling thus represents another important mitogenic pathway during heart development.
Another molecule that has attracted attention in recent years due to its potential roles in both the developing epicardium and adult counterpart following injury, is the actin monomer binding protein, thymosin beta-4 (Tβ4) (Smart et al., 2007; Bock-Marquette et al., 2009). During development, Tβ4 is expressed in the developing myocardium. Smart and colleagues utilised short hairpin RNA (shRNA) to knockdown Tβ4 expression in CMs and demonstrated defective epicardial development and disrupted vasculogenesis, associated with diminished EPDC coronary endothelial cell and vascular smooth muscle cell differentiation and migration. These observations preceded embryonic lethality at E15.5 in the most effected embryos, suggesting that Tβ4 signals to the developing epicardium in a paracrine manner to promote EPDC differentiation towards vascular lineages. This was supported by the enhanced differentiation of EPDCs to coronary endothelium and vascular smooth muscle cell fates following addition of Tβ4 to epicardial explant cultures; an effect that was optimised by parallel treatment with VEGF and FGF7 (Smart et al., 2007). In contrast to these findings, Banerjee and colleagues observed no phenotype in global and cardiac specific Tβ4 knockout mice, suggesting that Tβ4 is dispensable for heart development (Banerjee et al., 2012, 2013) although this has since been refuted (Smart and Riley, 2013).
The ‘quiescent’ epicardium
Following development, the epicardium becomes relatively dormant. Chen and colleagues described the loss of epicardial potential by P4 in the mouse; simultaneous with a loss of myocardial responsiveness to epicardial paracrine secretions (Chen et al., 2002). Early embryonic marker genes, including Raldh2, were switched off, with low levels persisting only in epicardium surrounding the atria and atrioventricular sulcus (AVS) in the mouse (van Wijk et al., 2012; Zhou et al., 2011). The role of the epicardium in adult mammalian heart homeostasis is not well characterised, but in the adult zebrafish the epicardium is suggested to regularly contribute to myocardial homeostasis via an FGF dependent dialogue with CMs throughout life (Wills et al., 2008), and may underpin continual CM turnover in the zebrafish heart.
The ‘reactive’ epicardium in the non-regenerative injury response
Following myocardial infarction (MI) in adult mammals, billions of CMs are lost and replaced by proliferation and activation of fibroblasts which deposit ECM (most notably collagen) which results in scar formation. Whilst required as an immediate and early response to prevent organ rupture, the resulting loss in contractile function results in changes to ventricular geometry (enlargement and dilation) which in turn causes haemodynamic uncoupling and progressive deterioration of pump function (Jugdutt, 2003; Jessup and Brozena, 2003). For over a century, animal models of heart injury have facilitated understanding of the pathophysiological consequences of MI and heart failure. Traditionally, this has been induced by ligation of the LAD to induce temporary or permanent occlusion of blood flow to the left ventricle (with or without reperfusion, respectively) and consequent MI facilitating studies on cardiovascular wound healing and repair.
Epicardium-derived cellular contributions to the injured adult mammalian heart
Following MI in the adult mouse heart, a rapid and robust epicardial response has been well documented and is characterised by an initial reactivation of an embryonic gene programme (van Wijk et al., 2012; Zhou et al., 2011; Huang et al., 2012; Mercer et al., 2013; Wang et al., 2013; Limana et al., 2010). Van Wijk and colleagues describe an initial loss of the epicardium over the site of injury, followed by organ-wide activation of Wt1, Tbx18 and Raldh2 and subsequent epicardial expansion from the adjacent epicardium to ensheath the exposed myocardium (van Wijk et al., 2012). Embryonic epicardial gene expression was also recently reported in hypertensive and ischemic patients in a disease specific manner (Braitsch et al., 2013). Further lineage analyses in mouse revealed the formation of a Wt1 positive sub-epicardial mesenchyme, from which predominantly fibroblasts were derived, followed by a contribution of coronary endothelium and at later stages, a modest number of CMs (van Wijk et al., 2012).
As during development, the contribution of EPDCs to endothelial cells and CMs post-injury remains contentious. Using an inducible Wt1-CreERT2 based reporter mouse, Pu and colleagues report no epicardial CM or endothelial cell contribution post-MI, and instead focused on the secretion of pro-angiogenic paracrine signals by Wt1 + EPDCs. FACS isolation and culturing of this population generated conditioned medium containing relatively high concentrations of VEGF and FGF2 that were further shown to improve cardiac remodelling when administered following MI (Zhou et al., 2011). Smart and colleagues subsequently revealed that Tβ4 ‘priming’ prior to MI induction could induce a small sub-population of Wt1 + EPDCs towards a CM fate (Smart et al., 2011). Tβ4 administration had no effect on EPDC fate when administered after MI, with EPDCs contributing almost exclusively fibroblasts (Zhou et al., 2012; Kispert, 2012). How and why the timing of administration should affect EPDC fate requires further study, but may reflect the need to reactivate a larger cohort of Wt1 positive progenitors prior to injury to capture vascular and myocardial cell fates. However, regardless of the potential of re-activated EPDCs to contribute de novo vasculature and CMs to the injured adult mammalian heart, the contribution, as it stands, is insufficient for effective myocardial regeneration.
Epicardial signalling in the injured adult mammalian heart
Reactivation of RA signalling following adult injury is suggested by re-expression of epicardial Raldh2. A recent study aligned the upregulation or Raldh2 with a downregulation of RA degrading Cytochrome P450 26B1 (CYP45026B1), and further utilised a retinoic acid response-element (RARE)-Luciferase reporter mouse to investigate RA signalling post-MI (Bilbija et al., 2012). Fluorescent luciferase activity was detectable through the thorax, and ex vivo analysis identified the heart as the major source of this signal. Furthermore, when isolated post-MI, CMs and fibroblasts displayed robust luciferase activity compared to sham treated controls. Interestingly, when all-trans retinoic acid (ATRA) was added to cardiac fibroblast cultures, proliferation was inhibited. This suggests that RA signalling may influence cardiac remodelling through modulating fibrosis at the level of activation and collagen deposition, as opposed to influencing fibroblast number in the injured heart.
Injury-induced epicardial signalling was also recently implicated in the robust immune and inflammatory response associated with cardiac injury. Huang and colleagues analysed enhancer regions of epicardial genes activated during development and in response to injury and identified exclusively conserved CCAAT/enhancer binding protein (C/EBP) binding sites in Wt1 and Raldh2 promoter regions. Interestingly, inhibition of epicardial C/EBP signalling resulted in significantly decreased fibrosis and improved contractile function after ischemia–reperfusion injury, associated with reduced neutrophil influx to the infarct zone (Huang et al., 2012). Thus it is possible that injury-induced epicardial RA, through Raldh2, acts as a cue for immune cell infiltration and modulation of the inflammatory response.
An altered response to MI has also been reported in adult mice with loss and gain of function for FGF2. In the FGF2-null animals, Virag and colleagues reported decreased CM hypertrophy and reduced fibroblast proliferation, which was coupled to decreased collagen density, leading to infarct expansion and ultimately chamber dilation. Conversely, overexpression led to increased fibroblast proliferation, collagen deposition, and preserved ventricular wall integrity (Virag et al., 2007). Whilst further demonstrating the importance of epicardial signalling in the mammalian injury response, these findings also highlight the importance of scar formation in mammals, reflecting the mechanical stress under which wound healing must take place.
The ‘reactive’ epicardium in heart regeneration
In contrast to the insufficient endogenous repair mechanisms of the adult mammalian heart, many lower vertebrates, and recently the neonatal mouse, have been shown to retain remarkable regenerative capacities following substantial cardiac injury. Early seminal work revealed the first demonstrations of vertebrate heart regeneration in the newt: following amputation of 10% of the ventricle, the newt heart showed signs of regeneration within 30 days (Oberpriller and Oberpriller, 1974). More recently, regenerative responses to heart injury have been extrapolated to the adult zebrafish and neonatal mouse (Porrello et al., 2011, 2013; Haubner et al., 2012; Poss et al., 2002; Xin et al., 2013a; Gonzalez-Rosa and Mercader, 2012). The amenability of these species to both forward and reverse genetics has facilitated investigation into the underlying cellular and molecular basis for heart regeneration across evolution. There are, however, important distinctions between the animal model organisms, as outlined in Table 1, which all likely contribute to the responses to cardiac injury.
Table 1.
Comparison of animal models during heart injury and regeneration.
Zebrafish
|
Neonatal (P1–P2) mouse
|
Adult mouse
|
||
|---|---|---|---|---|
| Chambers | 2 | 4 | 4 | |
| Pulmonary circulation | No | Yes | Yes | |
| Heart rate (bpm) | ≈ 130 | ≈ 400 | ≈ 600 | |
| Systolic blood pressure (mm Hg) | ≈ 2.5 | ≈ 30 | ≈ 120 | |
| Cardiomyocyte | Size | Small | Intermediate | Large |
| Density | Low | High | High | |
| Nuclei | Mononuclear | Mostly mononuclear | Mostly binuclear | |
| Cardiac fibroblast density | Low | High | High | |
| Hypoxia resistant | Yes | Likely | No | |
| Myocardial growth | Hyperplasia | Hyperplasia | Hypertrophy | |
| Injury response | Epicardial signalling | Yes | Yes | Yes |
| CM proliferation | Yes | Yes | Negligible | |
| Regeneration | Yes | Yes | No | |
≈: approximate parameters dependant on strain. Information sourced from references cited in The adult zebrafish as a model of heart regeneration and The neonatal mouse as a model of heart regeneration.
The adult zebrafish as a model of heart regeneration
The adult zebrafish heart is able to regenerate following substantial injury throughout life and comprises a two chambered, single circulation, hypoxia-resistant organ (Marques et al., 2008), which operates at a pressure that is 50 times lower than that of the human circulation (Hu et al., 2001; Le et al., 2012; Mattson, 2001; Laboratory, S.o.t.J., 2007) (Table 1). The ventricular wall is thin and highly trabeculated, with a much smaller fibroblast population than the mammalian heart (Ausoni and Sartore, 2009). Also, zebrafish CMs are smaller, mononuclear and retain the capacity to proliferate indefinitely (Wills et al., 2008; Poss, 2007). Following amputation of up to 20% of the ventricle, complete myocardial regeneration is observed after 60 days (Poss et al., 2002; Raya et al., 2003). Apical resection constitutes tissue removal, resulting in blood clot/blastema formation but not extensive scarring which is in contrast to ischemic damage (Gonzalez-Rosa et al., 2011). An alternative model is cryoinjury, which involves the application of a liquid nitrogen-cooled cryoprobe to induce ‘ischemia-like’ necrotic and apoptotic cell death across 25–30% of the ventricle. In this model, the regenerative process is prolonged to more than 130 days (Gonzalez-Rosa and Mercader, 2012; Gonzalez-Rosa et al., 2011, 2012; Chablais et al., 2011; Schnabel et al., 2011) during which an initial scar is formed at the site of injury and is gradually replaced as the myocardium regenerates (Gonzalez-Rosa et al., 2011). A further model for dissecting the mechanisms of myocardial repair in zebrafish utilised inducible CM expression of the cytotoxic diphtheria toxin-A chain to selectively ablate up to 60% of CMs, which resulted in complete regeneration after just 30 days (Wang et al., 2011).
The neonatal mouse as a model of heart regeneration
Analogous to the zebrafish, the neonatal mouse heart contains a high proportion of proliferative mononuclear CMs, which undergo karyokinesis without cytokinesis between P4 and P6, rendering more than 50% of CMs binuclear by P7. This increases to more than 80% by P14 (Ikenishi et al., 2012) at which point, most CMs have exited the cell cycle and heart growth continues by hypertrophy. It is also suggested that the mammalian heart retains hypoxia resistance transiently after birth as the newborn adapts from a reliance on glycolytic to oxidative metabolism — a ‘switch’ that further coincides with the loss of CM hyperplasia (Lopaschuk and Jaswal, 2010). Coincidentally, in contrast to the life-long regenerative capacities of the zebrafish, the neonatal mouse is capable of effective heart regeneration for just the first few days of life. Following resection of 20% of the apex at P1, the neonatal mouse heart mounts a robust regenerative response which is complete after just 21 days (Porrello et al., 2011). This regenerative capacity was also observed following MI induced by LAD ligation in the P1 and P2 mouse (Porrello et al., 2013; Xin et al., 2013a; Mahmoud et al., 2013). This is in contrast to the response of the P7 heart which, when injured by either resection or MI, invariably underwent fibrosis and scarring with adult-like wound healing. This key period immediately after birth has, therefore, been termed the ‘neonatal regenerative window’. Investigation of the ‘switch’ from regeneration to scarring and fibrosis in the neonatal mouse represents a powerful approach for elucidating the mechanisms underpinning pathological and reparative wound healing in mammals.
The relevance of this model is further emphasised by anecdotal evidence of a similar phenomenon in humans. Longitudinal studies of patients who underwent surgical correction of congenital coronary artery defects suggested that infants, despite having the worst preoperative heart function, held the best potential for functional recovery (Michielon et al., 2003). Moreover, akin to the neonatal mouse, the newborn human heart was recently shown to have the greatest proportion of proliferating CMs, with reported cytokinesis rates dropping from 0.016% at birth to 0.005% by adolescence; proportionate to the 3.4-fold CM increase in the left ventricle over the same time period (Mollova et al., 2013). These findings support a developmental loss of regenerative capacity in humans; inversely related to increased terminally differentiated CMs with ageing, elevating the translational relevance of the neonatal mouse as a means to identify regenerative pathways lost in development.
Cellular contributions of the epicardium during heart regeneration
Despite the differences in duration of regeneration and the nature of the specific injury insult, reactivation of embryonic epicardial potential is conserved in zebrafish and neonatal mouse heart regeneration (Kikuchi et al., 2010, 2011; Lepilina et al., 2006; Porrello et al., 2011; Mercer et al., 2013; Gonzalez-Rosa and Mercader, 2012; Gonzalez-Rosa et al., 2011, 2012; Chablais et al., 2011; Schnabel et al., 2011; Wang et al., 2011; Jopling et al., 2010). Analogous to the adult mammalian response, this is characterised by immediate global re-expression of embryonic epicardial genes, and subsequent epicardial proliferation at the site of injury (Kikuchi et al., 2010, 2011; Lepilina et al., 2006; Porrello et al., 2011; Wang et al., 2011, 2013; Gonzalez-Rosa et al., 2011, 2012; Schnabel et al., 2011; Jesty et al., 2012). Early studies of resection injury in the zebrafish suggested that epicardial activation and subsequent EPDC mobilisation preceded stimulation of resident cardiac progenitor cells; the proliferation and differentiation of which generated de novo myocardium (Lepilina et al., 2006). An epicardial origin for CMs was subsequently excluded by Zhou and colleagues, who fate mapped tcf21+ cells exclusively to nonmyocardial lineages (Zhou et al., 2011). However, since the epicardium in zebrafish is a mixed population it remains to be determined whether tcf21-negative EPDCs might contribute CMs to the myocardial regenerate. Subsequent lineage tracing studies identified pre-existing CMs as the apparent source of regenerated myocardium in the adult zebrafish (Jopling et al., 2010; Kikuchi et al., 2010). Fate mapping also identified resident CMs as the principle source of regenerated myocardium in the neonatal mouse heart following resection injury (Porrello et al., 2011). However, whilst Porrello and colleagues demonstrated that most CMs in the regenerating mouse heart derived from pre-existing CMs, not all were labelled, suggesting the existence of an unidentified, alternate progenitor source. This was supported by the finding that ckit+ resident progenitors can contribute de novo CMs to the neonatal mouse heart after MI (Jesty et al., 2012). More recently, Ellison and colleagues reported that ckit + progenitors are essential for regeneration in adult rodents following isoproterenol induced myocardial damage (Ellison et al., 2013).
Epicardial signalling during heart regeneration
As discussed, epicardial RA signalling acts as a potent and indispensable mitogen during development. In zebrafish, epicardial RA was further shown to be critical for heart regeneration (Kikuchi et al., 2011) as expression of a dominant-negative RA receptor α (RARα), or RA degrading enzymes blocked regenerative CM proliferation. An endocardial RA signalling origin was also implicated in this response. In Polypterus senegalis (another fish species capable of heart regeneration) a robust epicardial and endocardial signalling following resection injury was also described (Kikuchi et al., 2011). A global cardiac RA response is thus a critical signalling pathway for lower vertebrate heart regeneration. A role of RA signalling in neonatal mouse heart regeneration remains unexplored, and in the adult mouse heart no equivalent endocardial RA source has been described, suggesting the relevant RA signal might be restricted to the epicardium via Raldh2 up-regulation.
FGF signalling has also been implicated in zebrafish heart regeneration. FGF17b expression is elevated in CMs following resection injury, principally in the border zone of the wound; whilst FGFR2 and FGFR4 are induced in activated EPDCs. Abrogation of the FGF signalling via dominant-negative FGFR expression blocked EMT of Tbx18+ epicardial cells, ablating the regenerative response (Lepilina et al., 2006). FGF17 is not, however, expressed in the developing mouse heart and knockout mice exhibit no functional abnormalities (Xu et al., 1999, 2000). Further investigation of FGF dependent epicardial–myocardial signalling in the neonatal mouse heart may uncover important downstream mediators of mammalian heart regeneration.
Following resection injury, zebrafish heart regeneration was shown to be dependent on organ-wide epicardial fibronectin deposition, which later localised to the site of injury. This was associated with upregulation of integrin-β3 in CMs. Although it has been reported that fibronectin influences CM proliferation in development (Ieda et al., 2009) no direct proliferative influence on CM was observed in this setting, and instead it appeared that epicardial–myocardial fibronectin–integrin-β3 precluded CM migration potential to the regenerating ventricle (Wang et al., 2013). CM migration was recently shown to be a secondary requirement of zebrafish heart regeneration, as inhibition of epicardial–myocardial chemokine signalling prevented migration of newly formed CMs to the site of injury, impeding regeneration (Itou et al., 2012). Interestingly, following resection injury in the newt, Mercer and colleagues also report organ wide epicardial ECM deposition, associated with global CM proliferation and organ regeneration. Initial global epicardial ECM deposition again became restricted to the apex as regeneration proceeded, which led the authors to postulate that this epicardial ECM signals to CMs, guiding their migration firstly towards the epicardium, from/through which they migrate through the ECM to the amputation site (Mercer et al., 2013). Supporting this, EdU+ cells were observed to migrate to the ECM rich epicardial reservoir, and potentially from there to the regenerating apex, where fibronectin deposits persist until gradually replaced by regenerated myocardium. The epicardium and EPDC-derivatives may thus facilitate lower vertebrate regeneration by depositing a tissue matrix which instructs CM migration and myogenesis. These reports indicate a previously unappreciated role for the epicardial ECM signalling in directing vertebrate heart regeneration and highlight the exciting possibility that epicardial ECM depositions could be manipulated to promote wound healing in mammals.
Concluding remarks
Heart regeneration in lower vertebrates and neonatal mice is characterised by two conserved events: organ-wide epicardial activation and proliferation of pre-existing CMs. These events parallel the epicardial–myocardial signalling that is crucial for heart formation during embryonic development, as outlined in Fig. 1. Organ-wide epicardial activation is also a hallmark of the injury response of adult mammals; but in this setting, cardiac fibroblasts represent the proliferative population, and fibrotic wound healing ensues. Myogenic cues are thus an important factor for heart regeneration. It is widely assumed that the transient and persistent mononuclear status of CMs in zebrafish and neonatal mouse hearts, respectively, underpins their ability to proliferate; and thereby the capacity of these animals to regenerate following cardiac injury (Xin et al., 2013b; Muralidhar et al., 2013). Indeed, recent studies demonstrated that manipulation of factors which influence cell-cycle arrest hold potential in extending the regenerative capacity of the neonatal mouse heart beyond the first week of life (Porrello et al., 2013; Xin et al., 2013a; Mahmoud et al., 2013). Here we highlight epicardial signals as critical myogenic cues instructing myocardial formation and growth during both heart development and regeneration. In particular, epicardial–RA signalling is a potent mitogen for CMs; the loss of which impedes myogenesis. Following MI in the adult mouse, epicardial RA synthesis is reactivated, and activation of RA responsive genes is observed in both CMs and cardiac fibroblasts; though significantly more so in the latter, which is associated with fibrosis. The divergent RA influence in these settings likely reflects inherent differences in the CMs of the respective model systems. The ability of mammalian CMs to respond to epicardial–RA signalling is reportedly lost in the first week of life in mice, whilst the proliferative capacity of zebrafish CMs is retained throughout adulthood. The extent and source of RA signalling in these different animal models may be significant, given the endocardial supplement observed in the lower vertebrate injury response. The coincident timing of a loss of epicardial RA with the ‘switch’ in mammalian regenerative to non-regenerative injury response, however, is intriguing. Although a role for RA signalling during the ‘regenerative window’ in the neonatal mouse is unexplored, it is tempting to speculate that restoration of RA–epicardial–myocardial signalling may positively influence CM proliferation and thereby extend regeneration of the post-natal mouse heart beyond the first week of life. Similarly, FGFs, as potential downstream RA targets with mitogenic roles in development and both zebrafish heart homeostasis and regeneration, may also offer targets for promoting mammalian heart regeneration. Improved understanding of the relative contributions of epicardial–myocardial FGF signalling pathways across species is required to appreciate any potential therapeutic relevance in the regenerative setting.
Figure 1.
Key epicardial signals conserved in mammalian heart muscle development and lower vertebrate heart muscle regeneration: epicardial–myocardial retinoic acid (RA), fibroblast growth factor (FGF) and extracellular matrix (ECM) signalling are key mitogenic events during the formation and growth of mammalian heart muscle (left) and the restoration of lost muscle during zebrafish heart regeneration (right). Curved arrows indicate reciprocal epicardial (derived cell) signals (green) and myocardium-derived signals (red).
The epicardium is thus a dynamic tissue central to both heart development and the injury response. Improved understanding and modulation of epicardial signals to restrict scarring/fibrosis and promote restoration of lost muscle and coronary vasculature hold genuine potential for translating the regenerative capacities of lower vertebrates and immature mammals to human patients with heart failure.
Contributor Information
Megan Masters, Email: megan.masters@dpag.ox.ac.uk.
Paul R. Riley, Email: paul.riley@dpag.ox.ac.uk.
References
- Ausoni S., Sartore S. From fish to amphibians to mammals: in search of novel strategies to optimize cardiac regeneration. J. Cell Biol. 2009;184(3):357–364. doi: 10.1083/jcb.200810094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee I. Thymosin beta 4 is dispensable for murine cardiac development and function. Circ. Res. 2012;110(3):456–464. doi: 10.1161/CIRCRESAHA.111.258616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee I. Thymosin beta4 is not required for embryonic viability or vascular development. Circ. Res. 2013;112(3):e25–e28. doi: 10.1161/CIRCRESAHA.111.300197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann O. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbija D. Retinoic acid signalling is activated in the postischemic heart and may influence remodelling. PLoS One. 2012;7(9):e44740. doi: 10.1371/journal.pone.0044740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock-Marquette I. Thymosin beta4 mediated PKC activation is essential to initiate the embryonic coronary developmental program and epicardial progenitor cell activation in adult mice in vivo. J. Mol. Cell. Cardiol. 2009;46(5):728–738. doi: 10.1016/j.yjmcc.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade T. Retinoic acid stimulates myocardial expansion by induction of hepatic erythropoietin which activates epicardial Igf2. Development. 2011;138(1):139–148. doi: 10.1242/dev.054239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braitsch C.M. Differential expression of embryonic epicardial progenitor markers and localization of cardiac fibrosis in adult ischemic injury and hypertensive heart disease. J. Mol. Cell. Cardiol. 2013;65:108–119. doi: 10.1016/j.yjmcc.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C.L. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454(7200):104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chablais F. The zebrafish heart regenerates after cryoinjury-induced myocardial infarction. BMC Dev. Biol. 2011;11:21. doi: 10.1186/1471-213X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Kubalak S.W., Chien K.R. Ventricular muscle-restricted targeting of the RXRalpha gene reveals a non-cell-autonomous requirement in cardiac chamber morphogenesis. Development. 1998;125(10):1943–1949. doi: 10.1242/dev.125.10.1943. [DOI] [PubMed] [Google Scholar]
- Chen T. Epicardial induction of fetal cardiomyocyte proliferation via a retinoic acid-inducible trophic factor. Dev. Biol. 2002;250(1):198–207. doi: 10.1006/dbio.2002.0796. [DOI] [PubMed] [Google Scholar]
- Christoffels V.M. Tbx18 and the fate of epicardial progenitors. Nature. 2009;458(7240):E8–E9. doi: 10.1038/nature07916. (discussion E9-10) [DOI] [PubMed] [Google Scholar]
- D'Aniello E. Depletion of retinoic acid receptors initiates a novel positive feedback mechanism that promotes teratogenic increases in retinoic acid. PLoS Genet. 2013;9(8):e1003689. doi: 10.1371/journal.pgen.1003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison G.M. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154(4):827–842. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]
- Fransen M.E., Lemanski L.F. Epicardial development in the axolotl, Ambystoma mexicanum. Anat. Rec. 1990;226(2):228–236. doi: 10.1002/ar.1092260212. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rosa J.M., Mercader N. Cryoinjury as a myocardial infarction model for the study of cardiac regeneration in the zebrafish. Nat. Protoc. 2012;7(4):782–788. doi: 10.1038/nprot.2012.025. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rosa J.M. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development. 2011;138(9):1663–1674. doi: 10.1242/dev.060897. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rosa J.M., Peralta M., Mercader N. Pan-epicardial lineage tracing reveals that epicardium derived cells give rise to myofibroblasts and perivascular cells during zebrafish heart regeneration. Dev. Biol. 2012;370(2):173–186. doi: 10.1016/j.ydbio.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Gourdie R.G. Retroviral cell lineage analysis in the developing chick heart. Methods Mol. Biol. 2000;135:297–304. doi: 10.1385/1-59259-685-1:297. [DOI] [PubMed] [Google Scholar]
- Guadix J.A. In vivo and in vitro analysis of the vasculogenic potential of avian proepicardial and epicardial cells. Dev. Dyn. 2006;235(4):1014–1026. doi: 10.1002/dvdy.20685. [DOI] [PubMed] [Google Scholar]
- Haubner B.J. Complete cardiac regeneration in a mouse model of myocardial infarction. Aging (Albany NY) 2012;4(12):966–977. doi: 10.18632/aging.100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakow R. Epicardial formation in staged human embryos. Kaibogaku Zasshi. 1992;67(5):616–622. [PubMed] [Google Scholar]
- Ho E., Shimada Y. Formation of the epicardium studied with the scanning electron microscope. Dev. Biol. 1978;66(2):579–585. doi: 10.1016/0012-1606(78)90263-4. [DOI] [PubMed] [Google Scholar]
- Hu N., Yost H.J., Clark E.B. Cardiac morphology and blood pressure in the adult zebrafish. Anat. Rec. 2001;264(1):1–12. doi: 10.1002/ar.1111. [DOI] [PubMed] [Google Scholar]
- Huang G.N. C/EBP transcription factors mediate epicardial activation during heart development and injury. Science. 2012;338(6114):1599–1603. doi: 10.1126/science.1229765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieda M. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev. Cell. 2009;16(2):233–244. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenishi A. Cell cycle regulation in mouse heart during embryonic and postnatal stages. Develop. Growth Differ. 2012;54(8):731–738. doi: 10.1111/j.1440-169X.2012.01373.x. [DOI] [PubMed] [Google Scholar]
- Itou J. Migration of cardiomyocytes is essential for heart regeneration in zebrafish. Development. 2012;139:4133–4142. doi: 10.1242/dev.079756. [DOI] [PubMed] [Google Scholar]
- Jessup M., Brozena S. Heart failure. N. Engl. J. Med. 2003;348(20):2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- Jesty S.A. c-kit + precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc. Natl. Acad. Sci. U. S. A. 2012;109(33):13380–13385. doi: 10.1073/pnas.1208114109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jopling C. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464(7288):606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jugdutt B.I. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough? Circulation. 2003;108(11):1395–1403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- Katz T.C. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev. Cell. 2012;22(3):639–650. doi: 10.1016/j.devcel.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464(7288):601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev. Cell. 2011;20(3):397–404. doi: 10.1016/j.devcel.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispert A. No muscle for a damaged heart: thymosin beta 4 treatment after myocardial infarction does not induce myocardial differentiation of epicardial cells. J. Mol. Cell. Cardiol. 2012;52(1):10–12. doi: 10.1016/j.yjmcc.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Komiyama M., Ito K., Shimada Y. Origin and development of the epicardium in the mouse embryo. Anat. Embryol. (Berl) 1987;176(2):183–189. doi: 10.1007/BF00310051. [DOI] [PubMed] [Google Scholar]
- Kruithof B.P. BMP and FGF regulate the differentiation of multipotential pericardial mesoderm into the myocardial or epicardial lineage. Dev. Biol. 2006;295(2):507–522. doi: 10.1016/j.ydbio.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Kuhn H.J., Liebherr G. The early development of the epicardium in Tupaia belangeri. Anat. Embryol. (Berl) 1988;177(3):225–234. doi: 10.1007/BF00321133. [DOI] [PubMed] [Google Scholar]
- Kurkiewicz T. O Histogenezie miesna sur cowego zwierzat kregowych — Zur Histogenese des Herzmuskels der Wilbertiere. Bull. Int. Acad. Sci. Cracov. 1909:148–191. [Google Scholar]
- Laboratory, S.o.t.J. Vol. 2007. Dover Publications, Inc.; New York: 2007. Biology of the laboratory mouse. (In: Mouse Genome Informatics). [Google Scholar]
- Lavine K.J. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev. Cell. 2005;8(1):85–95. doi: 10.1016/j.devcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Le V.P., Kovacs A., Wagenseil J.E. Measuring left ventricular pressure in late embryonic and neonatal mice. J. Vis. Exp. 2012;60 doi: 10.3791/3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepilina A. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127(3):607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Li P. IGF signaling directs ventricular cardiomyocyte proliferation during embryonic heart development. Development. 2011;138(9):1795–1805. doi: 10.1242/dev.054338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limana F. Myocardial infarction induces embryonic reprogramming of epicardial c-kit(+) cells: role of the pericardial fluid. J. Mol. Cell. Cardiol. 2010;48(4):609–618. doi: 10.1016/j.yjmcc.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Lopaschuk G.D., Jaswal J.S. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J. Cardiovasc. Pharmacol. 2010;56(2):130–140. doi: 10.1097/FJC.0b013e3181e74a14. [DOI] [PubMed] [Google Scholar]
- Mahmoud A.I. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature. 2013;497(7448):249–253. doi: 10.1038/nature12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manasek F.J. Embryonic development of the heart I. A light and electron microscopic study of myocardial development in the early chick embryo. J. Morphol. 1968;125:329–366. doi: 10.1002/jmor.1051250306. [DOI] [PubMed] [Google Scholar]
- Manasek F.J. Embryonic development of the heart II. Formation of the epicardium. J. Embryol. Exp. Morpholog. 1969;22:333–348. [PubMed] [Google Scholar]
- Manner J. Experimental study on the formation of the epicardium in chick embryos. Anat. Embryol. (Berl) 1993;187(3):281–289. doi: 10.1007/BF00195766. [DOI] [PubMed] [Google Scholar]
- Manner J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat. Rec. 1999;255(2):212–226. doi: 10.1002/(sici)1097-0185(19990601)255:2<212::aid-ar11>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- Marques I.J. Transcriptome analysis of the response to chronic constant hypoxia in zebrafish hearts. J. Comp. Physiol. B. 2008;178(1):77–92. doi: 10.1007/s00360-007-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson D.L. Comparison of arterial blood pressure in different strains of mice. Am. J. Hypertens. 2001;14(5 Pt 1):405–408. doi: 10.1016/s0895-7061(00)01285-1. [DOI] [PubMed] [Google Scholar]
- Mercer S., Odelberg S.J., Simon H.G. A dynamic spatiotemporal extracellular matrix facilitates epicardial-mediated vertebrate heart regeneration. Dev. Biol. 2013;382:457–469. doi: 10.1016/j.ydbio.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merki E. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc. Natl. Acad. Sci. U. S. A. 2005;102(51):18455–18460. doi: 10.1073/pnas.0504343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michielon G. Anomalous coronary artery origin from the pulmonary artery: correlation between surgical timing and left ventricular function recovery. Ann. Thorac. Surg. 2003;76(2):581–588. doi: 10.1016/s0003-4975(03)00344-8. (discussion 588) [DOI] [PubMed] [Google Scholar]
- Mikawa T., Fischman D.A. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc. Natl. Acad. Sci. U. S. A. 1992;89(20):9504–9508. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa T., Gourdie R.G. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev. Biol. 1996;174(2):221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- Mollova M. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc. Natl. Acad. Sci. U. S. A. 2013;110(4):1446–1451. doi: 10.1073/pnas.1214608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidhar S.A. Harnessing the power of dividing cardiomyocytes. Glob. Cardiol. Sci. Pract. 2013;3(29) doi: 10.5339/gcsp.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag A.C. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1980;28(109):41–61. [PubMed] [Google Scholar]
- Niederreither K. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 1999;21(4):444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- Oberpriller J.O., Oberpriller J.C. Response of the adult newt ventricle to injury. J. Exp. Zool. 1974;187(2):249–253. doi: 10.1002/jez.1401870208. [DOI] [PubMed] [Google Scholar]
- Olivey H.E., Svensson E.C. Epicardial–myocardial signaling directing coronary vasculogenesis. Circ. Res. 2010;106(5):818–832. doi: 10.1161/CIRCRESAHA.109.209197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz D.M., Itoh N. Fibroblast growth factors. Genome Biol. 2001;2(3) doi: 10.1186/gb-2001-2-3-reviews3005. (p. REVIEWS3005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi D.J., Mikawa T. Normal patterning of the coronary capillary plexus is dependent on the correct transmural gradient of FGF expression in the myocardium. Dev. Biol. 2005;279(2):378–390. doi: 10.1016/j.ydbio.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Perez-Pomares J.M., de la Pompa J.L. Signaling during epicardium and coronary vessel development. Circ. Res. 2011;109(12):1429–1442. doi: 10.1161/CIRCRESAHA.111.245589. [DOI] [PubMed] [Google Scholar]
- Perez-Pomares J.M. Experimental studies on the spatiotemporal expression of WT1 and RALDH2 in the embryonic avian heart: a model for the regulation of myocardial and valvuloseptal development by epicardially derived cells (EPDCs) Dev. Biol. 2002;247(2):307–326. doi: 10.1006/dbio.2002.0706. [DOI] [PubMed] [Google Scholar]
- Poelmann R.E. Development of the cardiac coronary vascular endothelium, studied with antiendothelial antibodies, in chicken–quail chimeras. Circ. Res. 1993;73(3):559–568. doi: 10.1161/01.res.73.3.559. [DOI] [PubMed] [Google Scholar]
- Porrello E.R. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331(6020):1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello E.R. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc. Natl. Acad. Sci. U. S. A. 2013;110(1):187–192. doi: 10.1073/pnas.1208863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss K.D. Getting to the heart of regeneration in zebrafish. Semin. Cell Dev. Biol. 2007;18(1):36–45. doi: 10.1016/j.semcdb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Poss K.D., Wilson L.G., Keating M.T. Heart regeneration in zebrafish. Science. 2002;298(5601):2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- Raya A. Activation of Notch signaling pathway precedes heart regeneration in zebrafish. Proc. Natl. Acad. Sci. U. S. A. 2003;100(Suppl. 1):11889–11895. doi: 10.1073/pnas.1834204100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Red-Horse K. Coronary arteries form by developmental reprogramming of venous cells. Nature. 2010;464(7288):549–553. doi: 10.1038/nature08873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudat C., Kispert A. Wt1 and epicardial fate mapping. Circ. Res. 2012;111(2):165–169. doi: 10.1161/CIRCRESAHA.112.273946. [DOI] [PubMed] [Google Scholar]
- Schnabel K. Regeneration of cryoinjury induced necrotic heart lesions in zebrafish is associated with epicardial activation and cardiomyocyte proliferation. PLoS One. 2011;6(4):e18503. doi: 10.1371/journal.pone.0018503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart N., Riley P.R. Thymosin beta4 in vascular development response to research commentary. Circ. Res. 2013;112(3):e29–e30. doi: 10.1161/CIRCRESAHA.112.300555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart N. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445(7124):177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- Smart N. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474(7353):640–644. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuckmann I., Evans S., Lassar A.B. Erythropoietin and retinoic acid, secreted from the epicardium, are required for cardiac myocyte proliferation. Dev. Biol. 2003;255(2):334–349. doi: 10.1016/s0012-1606(02)00078-7. [DOI] [PubMed] [Google Scholar]
- Sucov H.M. RXR alpha mutant mice establish a genetic basis for vitamin A signaling in heart morphogenesis. Genes Dev. 1994;8(9):1007–1018. doi: 10.1101/gad.8.9.1007. [DOI] [PubMed] [Google Scholar]
- Tian X. Subepicardial endothelial cells invade the embryonic ventricle wall to form coronary arteries. Cell Res. 2013;23(9):1075–1090. doi: 10.1038/cr.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk B. Epicardium and myocardium separate from a common precursor pool by crosstalk between bone morphogenetic protein- and fibroblast growth factor-signaling pathways. Circ. Res. 2009;105(5):431–441. doi: 10.1161/CIRCRESAHA.109.203083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk B. Cardiac regeneration from activated epicardium. PLoS One. 2012;7(9):e44692. doi: 10.1371/journal.pone.0044692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Hernandez M. FGF10/FGFR2b signaling is essential for cardiac fibroblast development and growth of the myocardium. Development. 2011;138(15):3331–3340. doi: 10.1242/dev.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virag J.A. Fibroblast growth factor-2 regulates myocardial infarct repair: effects on cell proliferation, scar contraction, and ventricular function. Am. J. Pathol. 2007;171(5):1431–1440. doi: 10.2353/ajpath.2007.070003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viragh S., Challice C.E. The origin of the epicardium and the embryonic myocardial circulation in the mouse. Anat. Rec. 1981;201(1):157–168. doi: 10.1002/ar.1092010117. [DOI] [PubMed] [Google Scholar]
- Wang J. The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development. 2011;138(16):3421–3430. doi: 10.1242/dev.068601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Fibronectin is deposited by injury-activated epicardial cells and is necessary for zebrafish heart regeneration. Dev. Biol. 2013;382:427–435. doi: 10.1016/j.ydbio.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills A.A. Regulated addition of new myocardial and epicardial cells fosters homeostatic cardiac growth and maintenance in adult zebrafish. Development. 2008;135(1):183–192. doi: 10.1242/dev.010363. [DOI] [PubMed] [Google Scholar]
- Wu B. Endocardial cells form the coronary arteries by angiogenesis through myocardial–endocardial VEGF signaling. Cell. 2012;151(5):1083–1096. doi: 10.1016/j.cell.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M. Hippo pathway effector Yap promotes cardiac regeneration. Proc. Natl. Acad. Sci. U. S. A. 2013;110(34):13839–13844. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M., Olson E.N., Bassel-Duby R. Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair. Nat. Rev. Mol. Cell Biol. 2013;14(8):529–541. doi: 10.1038/nrm3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. Genomic structure, mapping, activity and expression of fibroblast growth factor 17. Mech. Dev. 1999;83(1–2):165–178. doi: 10.1016/s0925-4773(99)00034-9. [DOI] [PubMed] [Google Scholar]
- Xu J., Liu Z., Ornitz D.M. Temporal and spatial gradients of Fgf8 and Fgf17 regulate proliferation and differentiation of midline cerebellar structures. Development. 2000;127(9):1833–1843. doi: 10.1242/dev.127.9.1833. [DOI] [PubMed] [Google Scholar]
- Zhou B. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454(7200):109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J. Clin. Invest. 2011;121(5):1894–1904. doi: 10.1172/JCI45529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B. Thymosin beta 4 treatment after myocardial infarction does not reprogram epicardial cells into cardiomyocytes. J. Mol. Cell. Cardiol. 2012;52(1):43–47. doi: 10.1016/j.yjmcc.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]