Abstract
Background
Volasertib (BI 6727) is a potent inhibitor of Polo-like kinase 1 (Plk1), that is overexpressed in several childhood cancers and cell lines. Because of its novel mechanism of action, volasertib was evaluated through the PPTP.
Procedures
Volasertib was tested against the PPTP in vitro cell line panel at concentrations from 0.1 nM to 1.0 μM and against the PPTP in vivo xenograft panels administered I.V at a dose of 30 mg/kg (solid tumors) or 15 mg/kg (ALL models) using a q7dx3 schedule.
Results
In vitro volasertib demonstrated cytotoxic activity, with a median relative IC50 value of 14.1 nM, (range 6.0 nM to 135 nM). Volasertib induced significant differences in EFS in 19 of 32 (59%) of the evaluable solid tumor xenografts and in 2 of 4 (50%) of the evaluable ALL xenografts. Volasertib induced tumor growth inhibition meeting criteria for intermediate EFS T/C (>2) activity in 11 of 30 (37%) evaluable solid tumor xenografts, including neuroblastoma (4 of 6) and glioblastoma (2 of 3) panels, and 2 of 4 ALL models. Objective responses (CR’s) were observed for 4 of 32 solid tumor (2 neuroblastoma, 1 glioblastoma, and 1 rhabdomyosarcoma) and 1 of 4 ALL xenografts.
Conclusions
Volasertib shows potent in vitro activity against the PPTP cell lines with no histotype selectivity. In vivo, volasertib induced regressions in several xenograft models. However, pharmacokinetic data suggest that mice tolerate higher systemic exposure to volasertib than humans, suggesting that the current results may over-estimate potential clinical efficacy against the childhood cancers studied.
Keywords: Preclinical Testing, Developmental Therapeutics, Plk inhibitor
INTRODUCTION
Volasertib (Bl 6727) is a dihydropteridinone that targets the Polo-like Kinase (Plk) family of proteins in an ATP-competitive manner at low nanomolar concentrations and thereby induces mitotic arrest and apoptosis [1]. Polo-Like Kinase 1 (Plk1) is a serine/threonine specific kinase that regulates multiple steps in mitosis and that is essential for progression through mitosis [2]. Numerous lines of evidence suggest that Plk1 is oncogenic through driving cell cycle progression, and overexpression of the gene transforms NIH 3T3 cells [3]. Plk1 is highly expressed in multiple cancers [2,4,5], and in some malignancies expression of Plk1 may be prognostic [4]. Inhibition of the Plk1 blocks proliferation of cancer cells [6,7]. Blocking of Plk1 activity with small molecule inhibitors such as Bl 2536 and volasertib produced regressions in multiple preclinical adult cancer models [1,8]. Volasertib has entered clinical evaluation and has advanced to phase 2 testing [9–11].
Plk1 is overexpressed in several childhood cancers and cell lines. RNA interference and small molecule inhibitor screens suggest that Plk1 may be a relevant therapeutic target in a variety of pediatric malignancies including neuroblastoma, rhabdomyosarcoma and osteosarcoma [12–14]. The Pediatric Preclinical Testing Program (PPTP) therefore evaluated volasertib to gain further insight into the role of Plk1 inhibition as a therapeutic strategy for childhood cancers.
MATERIALS AND METHODS
In vitro testing
Testing was performed using DIMSCAN [20], as previously described in a characterized panel of 24 cell lines [15]. Cells were incubated in the presence of volasertib for 96 hours at concentrations from 0.1 nM to 1 μM and analyzed as previously described [16].
In vivo tumor growth inhibition studies
CB17SC scid−/− female mice (Taconic Farms, Germantown NY), were used to propagate subcutaneously implanted kidney/rhabdoid tumors, sarcomas (Ewing, osteosarcoma, rhabdomyosarcoma), neuroblastoma, and non-glioblastoma brain tumors, while BALB/c nu/nu mice were used for glioma models, as previously described [17]. Human leukemia cells were propagated by intravenous inoculation in female non-obese diabetic (NOD)/scid−/− mice as described previously [18]. Female mice were used irrespective of the patient gender from which the original tumor was derived. All mice were maintained under barrier conditions and experiments were conducted using protocols and conditions approved by the institutional animal care and use committee of the appropriate consortium member. Eight to ten mice were used in each control or treatment group. Tumor volumes (cm3) [solid tumor xenografts] or percentages of human CD45-positive [%hCD45+] cells [ALL xenografts] were determined and responses were determined using three activity measures as previously described [17]. An in-depth description of the analysis methods is included in the Supplemental Response Definitions section.
Statistical Methods
The exact log-rank test, as implemented using Proc StatXact for SAS®, was used to compare event-free survival distributions between treatment and control groups. P-values were two-sided and were not adjusted for multiple comparisons given the exploratory nature of the studies.
Drugs and Formulation
Volasertib was provided to the Pediatric Preclinical Testing Program by Boehringer Ingleheim AG, through the Cancer Therapy Evaluation Program (NCI). Volasertib was formulated in sterile saline and stored for up to 7 days at 4°C, protected from light. Volasertib was administered IV at 30 mg/kg (solid tumors) and 15 mg/kg (ALL models) to mice using a q 7 days x 3 schedule with an additional 3 weeks of observation. Volasertib was provided to each consortium investigator in coded vials for blinded testing.
RESULTS
In vitro testing
Volasertib was tested against the PPTP’s in vitro cell line panel at concentrations ranging from 0.1 nM to 1.0 μM using the PPTP’s standard 96 hour exposure period. The median relative IC50 (rIC50) value for the PPTP cell lines was 14.1 nM, with a range from 6.0 nM (CHLA-136) to 135 nM (Rh18). Observed Ymin values ranged from 0% to 20.2%, with 10 of 23 models having non-zero model-based Ymin values. Each of the ALL cell lines and NHL cell lines had model based Ymin values of 0% (indicative of a complete cytotoxic response), as did 3 of 4 Ewing tumor cell lines. The Relative In/Out (I/O)% values represent the percentage difference between the observed Ymin value and the estimated starting cell number and either the control cell number (for agents with Ymin > starting cell number) or 0 (for agents with Ymin < estimated starting cell number). Relative I/O% values range between 100% (no treatment effect) to −100% (complete cytotoxic effect), Table I. For most of the PPTP cell lines the Relative I/O% values were between −80% and −100% indicating a potent cytotoxic effect. Several cell lines had Relative I/O% values closer to 0%, consistent with a cytostatic effect for these lines, including the rhabdomyosarcoma cell line Rh30 and the rhabdoid tumor cell line BT-12.
Table I.
In vitro activity of Volasertib against PPTP cell lines.
| Cell Line | Histotype | rIC50 (nM) | Panel rIC50/Line rIC50 | Ymin (%) (Observed) | Ymin (%) (Model Based) | Relative In/Out (Observed Ymin) |
|---|---|---|---|---|---|---|
| RD | Rhabdomyosarcoma | 16.5 | 0.85 | 1.7 | 0.0 | −69% |
| Rh41 | Rhabdomyosarcoma | 6.9 | 2.04 | 7.7 | 11.4 | −65% |
| Rh18 | Rhabdomyosarcoma | 135.2 | 0.10 | 20.2 | 0.0 | −55% |
| Rh30 | Rhabdomyosarcoma | 8.2 | 1.71 | 11.0 | 12.8 | −34% |
| BT-12 | Rhabdoid | 55.7 | 0.25 | 13.1 | 15.2 | 5% |
| CHLA-266 | Rhabdoid | 47.5 | 0.30 | 9.2 | 10.1 | −65% |
| TC-71 | Ewing sarcoma | 13.8 | 1.02 | 0.1 | 0.0 | −94% |
| CHLA-9 | Ewing sarcoma | 17.7 | 0.80 | 0.5 | 0.0 | −85% |
| CHLA-10 | Ewing sarcoma | 17.3 | 0.82 | 1.9 | 0.0 | −70% |
| CHLA-258 | Ewing sarcoma | 37.4 | 0.38 | 6.1 | 5.8 | −84% |
| SJ-GBM2 | Glioblastoma | 11.8 | 1.19 | 1.9 | 0.0 | −81% |
| NB-1643 | Neuroblastoma | 15.5 | 0.91 | 4.4 | 5.5 | −79% |
| NB-EBc1 | Neuroblastoma | 34.5 | 0.41 | 0.7 | 0.0 | −97% |
| CHLA-90 | Neuroblastoma | 24.6 | 0.58 | 14.9 | 15.4 | −47% |
| CHLA-136 | Neuroblastoma | 6.0 | 2.35 | 1.9 | 3.3 | −93% |
| NALM-6 | ALL | 12.7 | 1.11 | 0.0 | 0.0 | −99% |
| COG-LL-317 | ALL | 11.3 | 1.25 | 0.0 | 0.0 | −100% |
| RS4;11 | ALL | 7.8 | 1.82 | 0.8 | 0.0 | −95% |
| MOLT-4 | ALL | 12.2 | 1.16 | 0.0 | 0.0 | −100% |
| CCRF-CEM (1) | ALL | 11.5 | 1.23 | 0.0 | 0.0 | −100% |
| CCRF-CEM (2) | ALL | 14.2 | 1.00 | 0.0 | 0.0 | −99% |
| Kasumi-1 | AML | 14.1 | 1.00 | 1.2 | 3.5 | −96% |
| Karpas-299 | ALCL | 14.9 | 0.95 | 0.5 | 0.0 | −93% |
| Ramos-RA1 | NHL | 13.0 | 1.08 | 0.0 | 0.0 | −100% |
| Median | 14.1 | 1.00 | 1.5 | 0.00 | −89% | |
| Minimum | 6.0 | 0.10 | 0.0 | 0.00 | −100% | |
| Maximum | 135.2 | 2.35 | 20.2 | 15.38 | 5% |
A metric used to compare the relative responsiveness of the PPTP cell lines to volasertib is the ratio of the median rIC50 of the entire panel to that of each cell line. Higher ratios indicate greater sensitivity to volasertib and are shown in Figure 1 by bars to the right of the midpoint line. The median rIC50 values were lowest for the ALL cell line panel compared to the remaining cell lines (11.9 versus 16.0 nM, respectively), but this difference was not significant, and overall the rIC50 values did not show histotype dependency.
Figure 1.
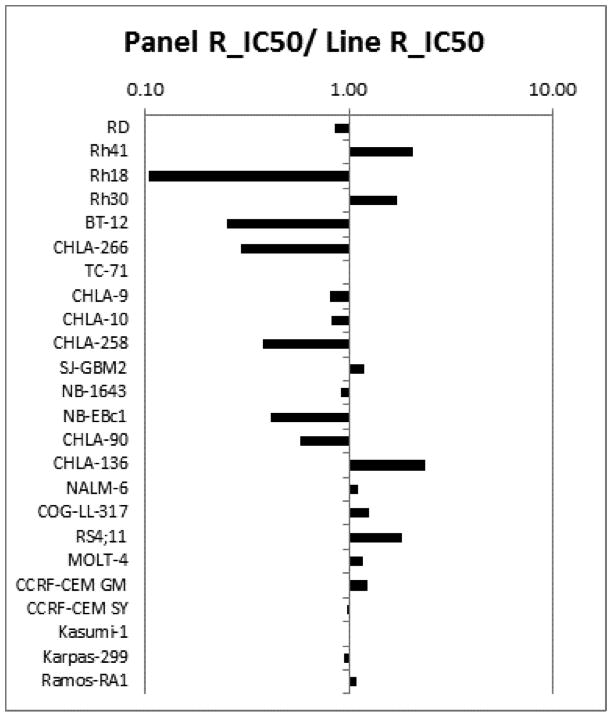
Volasertib in vitro activity: The median rIC50 ratio graph shows the relative rIC50 values for the cell lines of the PPTP panel. Each bar represents the ratio of the panel rIC50 to the rIC50 value of the indicated cell line. Bars to the right represent cell lines with higher sensitivity, while bars to the left indicate cell lines with lesser sensitivity.
In vivo testing
Volasertib was tested against the PPTP solid tumor xenografts using a dose of 30 mg/kg (solid tumors) and 15 mg/kg (ALL) administered intravenously weekly x 3. The total planned treatment period was 3 weeks with an additional 3 weeks observation. Volasertib induced a 7.1% toxicity rate (vs 0.8% in controls) in the treated groups, with toxicity being greater for the ALL panel versus the solid tumor panels (16.4% versus 5.2%, respectively) despite the reduced volasertib dose used for the ALL panel.
Thirty-four of 40 tested xenograft models were considered evaluable for efficacy. Lines inevaluable because of toxicity included four ALL xenografts (ALL-4, ALL-7, ALL-31, and MLL7) and two rhabdomyosarcoma xenografts (Rh30 and Rh30R). Testing was repeated for the latter two xenografts and volasertib was tolerated in the repeat testing. Complete details of testing are provided in Supplemental Table I including total numbers of mice, number of mice that died (or were otherwise excluded), numbers of mice with events and average times to event, tumor growth delay, as well as numbers of responses and T/C values.
Volasertib induced significant differences in EFS distribution compared to control in 19 of 32 (59%) of the evaluable solid tumor xenografts and in 2 of 4 (50%) of the evaluable ALL xenografts, Table II. Significant differences in EFS distribution were most consistently noted in the osteosarcoma (5 of 6), neuroblastoma (5 of 6), and Wilms tumor (2 of 2) panels. For those xenografts with a significant difference in EFS distribution between treated and control groups, the EFS T/C activity measure additionally requires an EFS T/C value of > 2.0 for intermediate activity and indicates a substantial agent effect in slowing tumor growth. High activity further requires a reduction in final tumor volume compared to the starting tumor volume. Volasertib induced tumor growth inhibition meeting criteria for intermediate EFS T/C activity in 11 of 30 (37%) evaluable solid tumor xenografts. Intermediate activity for the EFS T/C metric was most consistently observed in the neuroblastoma (4 of 6) and glioblastoma (2 of 3) panels. For the ALL panel, 2 of 4 (50%) xenografts met criteria for intermediate activity.
Table II.
Summary of in vivo activity of Volasertib
| Line Description | Tumor Type | Estimate of Median Time to Event | P-value | EFS T/C | Median RTV/CD45 at End of Study | Tumor Volume T/C1 | Median Group Response2 | EFS Activity3 |
|---|---|---|---|---|---|---|---|---|
| BT-29 | Rhabdoid | 31.2 | 0.091 | 1.3 | >4 | 0.91 | PD1 | Low |
| KT-14 | Rhabdoid | 25.6 | <0.0014 | 1.2 | >4 | 0.70 | PD1 | Low |
| KT-12 | Rhabdoid | > EP | <0.001 | > 2.3 | 3.1 | 0.36 | PD2 | Int |
| KT-10 | Wilms | 19.2 | 0.049 | 1.2 | >4 | 0.58 | PD1 | Low |
| KT-13 | Wilms | 30.3 | <0.001 | 2.1 | >4 | 0.26 | PD2 | Int |
| SK-NEP-1 | Ewing | 23.8 | <0.001 | 2.8 | >4 | 0.29 | PD2 | Int |
| EW5 | Ewing | 8.0 | 0.198 | 1.2 | >4 | 0.86 | PD1 | Low |
| TC-71 | Ewing | 11.5 | 0.300 | 1.2 | >4 | 0.84 | PD1 | Low |
| CHLA258 | Ewing | 20.1 | 0.168 | 1.2 | >4 | 0.79 | PD1 | Low |
| Rh10 | ALV RMS | 20.3 | 0.066 | 1.1 | >4 | 0.76 | PD1 | Low |
| Rh28 | ALV RMS | 20.1 | 0.006 | 1.5 | >4 | 0.63 | PD1 | Low |
| Rh30 | ALV RMS | 19.4 | 0.052 | 1.7 | >4 | 0.73 | PD2 | Low |
| Rh30R | ALV RMS | 36.9 | <0.001 | 3.1 | >4 | 0.31 | CR | Int |
| Rh18 | EMB RMS | 14.9 | 0.652 | 1.3 | >4 | 0.79 | PD1 | Low |
| BT-28 | Medulloblastoma | 5.9 | 0.513 | 0.9 | >4 | 1.09 | PD1 | Low |
| BT-45 | Medulloblastoma | 6.2 | 0.157 | 0.9 | >4 | 1.10 | PD1 | Low |
| BT-50 | Medulloblastoma | > EP | 0.474 | . | 1.5 | 0.38 | SD | NE |
| GBM2 | Glioblastoma | 33.7 | <0.001 | 3.5 | >4 | 0.17 | CR | Int |
| BT-39 | Glioblastoma | 12.6 | 0.843 | 1.1 | >4 | 0.59 | PD1 | Low |
| D645 | Glioblastoma | 13.0 | <0.001 | 2.1 | >4 | 0.26 | PD2 | Int |
| NB-SD | Neuroblastoma | > EP | <0.001 | > 3.8 | 1.9 | 0.08 | CR | Int |
| NB-1771 | Neuroblastoma | 16.3 | 0.006 | 2.4 | >4 | 0.47 | PD2 | Int |
| NB-1691 | Neuroblastoma | 25.4 | 0.048 | 1.7 | >4 | 0.40 | CR | Low |
| NB-EBc1 | Neuroblastoma | 13.1 | <0.001 | 2.6 | >4 | 0.22 | PD2 | Int |
| CHLA-79 | Neuroblastoma | 10.0 | 0.155 | 0.8 | >4 | 1.74 | PD1 | Low |
| NB-1643 | Neuroblastoma | 27.3 | <0.001 | 2.9 | >4 | 0.30 | SD | Int |
| OS-1 | Osteosarcoma | > EP | 0.001 | > 1.1 | 2.8 | 0.52 | PD2 | NE |
| OS-2 | Osteosarcoma | 22.2 | 0.026 | 1.2 | >4 | 0.84 | PD1 | Low |
| OS-17 | Osteosarcoma | 21.5 | 0.277 | 1.0 | >4 | 0.93 | PD1 | Low |
| OS-9 | Osteosarcoma | > EP | <0.001 | > 2.2 | 3.9 | 0.53 | PD2 | Int |
| OS-33 | Osteosarcoma | 30.6 | 0.014 | 1.4 | >4 | 0.75 | PD1 | Low |
| OS-31 | Osteosarcoma | 27.7 | <0.001 | 1.6 | >4 | 0.63 | PD2 | Low |
| ALL-2 | ALL B-precursor | 22.5 | 0.082 | 1.4 | >25 | . | PD1 | Low |
| ALL-8 | ALL T-cell | 34.8 | <0.001 | 4.9 | >25 | . | CR | Int |
| ALL-17 | ALL B-precursor | 17.1 | <0.001 | 2.4 | >25 | . | PD2 | Int |
| ALL-19 | ALL B-precursor | 20.8 | 0.379 | 2.1 | >25 | . | PD2 | Low |
Tumor Volume T/C value: Relative tumor volumes (RTV) for control (C) and treatment (T) mice were calculated at day 21 or when all mice in the control and treated groups still had measurable tumor volumes (if less than 21 days). The T/C value is the mean RTV for the treatment group divided by the mean RTV for the control group. High activity = T/C ≤ 0.15; Intermediate activity = T/C ≤ 0.45 but > 0.15; and Low activity = T/C > 0.45.
Objective response measures are described in detail in the Supplemental Response Definitions. PD1 = progressive disease with EFS T/C ≤ 1.5, and PD2 = progressive disease with EFS T/C > 1.5.
EFS T/C values = the ratio of the median time to event of the treatment group and the median time to event of the respective control group. High activity requires: a) an EFS T/C > 2; b) a significant difference in EFS distributions, and c) a net reduction in median tumor volume for animals in the treated group at the end of treatment as compared to at treatment initiation. Intermediate activity = criteria a) and b) above, but not having a net reduction in median tumor volume for treated animals at the end of the study. Low activity = EFS T/C < 2.
P< 0.05.
Objective responses were observed for 4 of 32 solid tumor and 1 of 4 ALL xenografts. Two of 6 neuroblastoma xenografts demonstrated CRs, as did a single glioblastoma and a single rhabdomyosarcoma xenograft, Figure 2. The in vivo testing results for the objective response measure of activity are presented in Figure 3 in a ‘heat-map’ format as well as a ‘COMPARE’-like format, based on the scoring criteria described in the Supplemental Response Definitions section. The latter analysis demonstrates relative tumor sensitivities around the midpoint score of 5 (stable disease).
Figure 2.
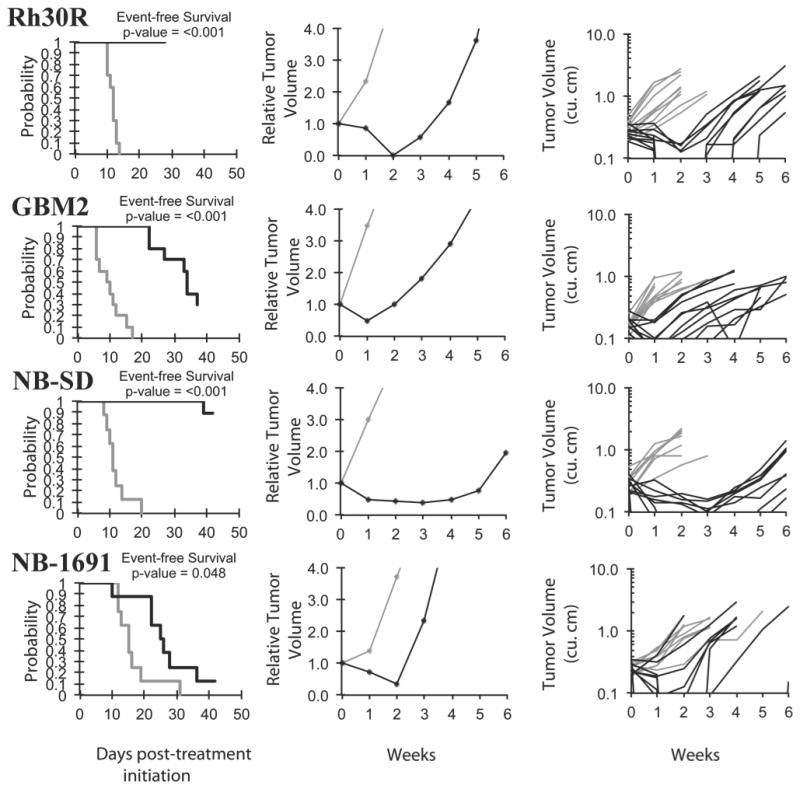
Volasertib in vivo objective response activity for solid tumor and brain tumor models. Rh30R (rhabdomyosarcoma), GBM2 (glioblastoma), and neuroblastoma lines (NB-EBc1 and NB-1691): Kaplan-Meier curves for EFS (left), median relative tumor volume graphs (center), and individual tumor volume graphs (right) are shown for selected lines. Treated (black lines), statistical significance (p values) of the difference between treated and control groups are included.
DISCUSSION
The potential relevance of Plk1 as a therapeutic target for various childhood cancers has been described in prior reports. For example, a genome-wide small-interfering RNA (siRNA) library screen identified Plk1 as one of the most important survival kinases for rhabdomyosarcoma [12]. Ackermann, et al., found that Plk1 was highly expressed in unfavorable neuroblastoma clinical specimens and in neuroblastoma cell lines and that the Plk1 inhibitor BI 2536 showed low nanomolar IC50 values against neuroblastoma cell lines [2]. Furthermore, BI 2536 abrogated growth of neuroblastoma xenografts in nude mice. Grinshtein, et al., conducted a small molecule kinase inhibitor library screen against neuroblastoma tumor initiating cells (TICs) and identified Plk1 as a promising neuroblastoma target [13]. Low nanomolar sensitivity of neuroblastoma TICs to BI 2536 was demonstrated as was inhibition of growth of a neuroblastoma xenograft treated with BI 2536. RNA interference screens also demonstrated that knocking down Plk1 expression resulted in mitotic cell cycle arrest and subsequent apoptotic cell death in osteosarcoma cell lines [14,19]. Morales, et al., demonstrated the in vitro antiproliferative effects of BI 2536 on osteosarcoma cell lines at low nanomolar concentrations and documented mitotic arrest and aneuploidy in treated cell lines [20]. Liu, et al., showed that BI 2536 inhibited proliferation and induced apoptosis in two-dimensional and three-dimensional cultures of osteosarcoma cell lines (KHOS and U-2OS) and that BI 2536 inhibited the growth of KHOS grown as a xenograft [21].
The PPTP in vitro results are in general agreement with prior results describing the antitumor activity associated with Plk1 inhibition in childhood cancer cell lines. The PPTP cell lines demonstrated a median rIC50 of 14 nM, with almost all cell lines showing rIC50 values between 5 and 40 nM. These results are similar to those previously reported for adult cancer cell lines treated with volasertib [1]. There was no histotype selectivity for volasertib, as all cell line panels showed median rIC50 values in the 12 nM to 18 nM range. This observation is consistent with the reports described above in which Plk1 inhibition was shown to be effective in vitro against a range of childhood cancer cell lines.
Volasertib demonstrated significant in vivo tumor growth inhibition against most of the PPTP xenografts, and 11 of 30 (37%) evaluable solid tumor xenografts showed a two-fold or greater increase in time to event for volasertib-treated animals compared to control. Four of 6 xenografts in the neuroblastoma panel showed this degree of delay in time to progression. Objective responses (PR or CR) were noted in 4 xenografts, with only the neuroblastoma panel having more than 1 tumor with an objective response. Two other small molecule anticancer agents have shown preferential activity against the neuroblastoma xenograft panel, topotecan and the aurora kinase inhibitor MLN8237 [22,23]. It is tempting to speculate that the strong cell cycle progression drive provided by MYCN plays a role in sensitizing neuroblastoma cells to selected agents that block mitotic progression. Of some interest is that all of the solid tumors demonstrating regressions were derived from patients that had failed prior therapies.
For the ALL panel that utilized NOD-SCID mice, toxicity limited the evaluation of volasertib activity even though a lower dose was used compared to that employed for the solid tumor panels (15 mg/kg versus 30 mg/kg). Volasertib showed clear anti-leukemia activity against the ALL panel, with a CR noted in one evaluable model and with several inevaluable models that would have been coded PR or CR if less toxicity had been observed. However, the depth of remissions induced by the three weeks of volasertib was not deep, as low levels of leukemia cells were commonly observed in the peripheral blood and as regrowth occurred shortly after treatment was stopped in most cases.
Volasertib has entered clinical testing in adults. A phase 1 trial in adults with progressive advanced or metastatic solid tumors evaluated volasertib as a single 1-hour infusion administered every 3 weeks [24]. Reversible hematological toxicity was the main side-effect, with thrombocytopenia, neutropenia, and febrile neutropenia constituting the main dose-limiting events. The recommended phase 2 dose was 300 mg, and three patients from among 46 treated with volasertib at doses of 300 mg or higher achieved confirmed partial response. A phase 2 trial for patients with advanced or metastatic urothelial cancer evaluated volasertib at 300 mg using the every three week schedule with the option for dose escalation to 350 mg if the initial course was well tolerated [10]. Partial response was observed in 14% of patients (7 of 50), with a median duration of response of 30 weeks. Volasertib has also been studied in adults with AML, both as a single agent and in combination with low-dose cytarabine [11,25]. Seven out of 32 patients treated with volasertib plus low-dose cytarabine achieved a complete remission in the phase 1 trial of this combination [25]. Preliminary phase 2 data demonstrate a significantly improved CR/CRi rate and a trend for EFS benefit for volasertib plus low-dose cytarabine compared with low-dose cytarabine alone in patients with newly diagnosed AML ineligible for intensive treatment [11].
A central question in evaluating the clinical relevance of in vivo xenograft testing results is how the drug exposures for treated animals relate to the drug exposures that humans can tolerate [26]. Volasertib pharmacokinetics have been determined in nude mice and in humans in phase 1 clinical trials. The systemic exposure (AUC) for a single 35 mg/kg intravenous dose in nude mice was 100 μmol h/L [1], whereas the AUC at the recommended phase 2 dose in humans was approximately 10 μmol h/L [24]. Together with differences in the schedules of administration between rodents and in phase 2 trials (weekly x 3 versus every 3 weeks, respectively), exposures in rodents that lead to regressions may be greater than can be achieved clinically. For responding tumor models, future experiments could address whether responsiveness is maintained with doses/schedules more closely related to the human experience. The observation of higher volasertib exposure in the mouse compared to the human makes it unlikely that the PPTP results represent an under-prediction of the activity of volasertib against the selected childhood cancers evaluated, and the possibility of over-prediction needs to be considered. A caveat that applies to this statement is that pharmacokinetic parameters other than systemic exposure may be more relevant to volasertib activity than AUC and these parameters may be less different between mouse and man than AUC.
In summary, volasertib shows potent in vitro activity against the PPTP cell lines with no evidence for histotype selectivity. In vivo, volasertib induced regressions in a minority of the PPTP xenograft models, with the neuroblastoma panel showing the most consistent pattern of responsiveness to volasertib. Given available pharmacokinetic data showing that mice tolerate higher systemic exposure to volasertib compared to humans, the possibility that these results represent an over-estimation of the antitumor activity of volasertib against the childhood cancers studied needs to be considered.
Supplementary Material
Figure 3.
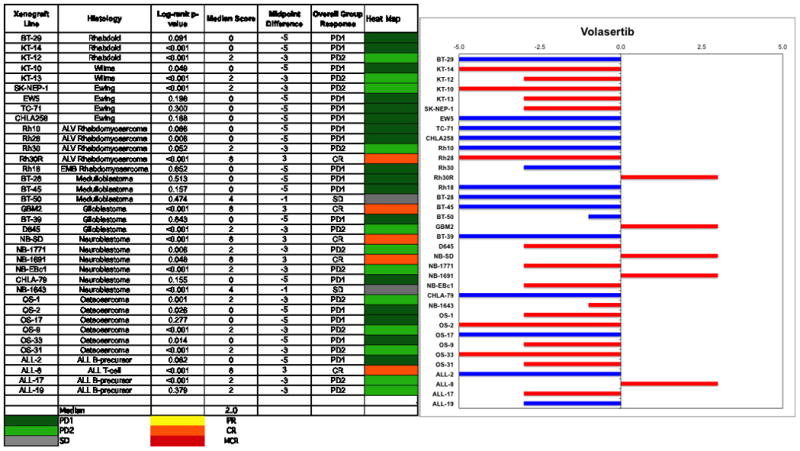
Left: The colored heat map depicts group response scores. A high level of activity is indicated by a score of 6 or more, intermediate activity by a score of ≥2 but <6, and low activity by a score of <2. Right: representation of tumor sensitivity based on the difference of individual tumor lines from the midpoint response (stable disease). Bars to the right of the median represent lines that are more sensitive, and to the left are tumor models that are less sensitive. Red bars indicate lines with a significant difference in EFS distribution between treatment and control groups, while blue bars indicate lines for which the EFS distributions were not significantly different.
Acknowledgments
This work was supported by NO1-CM-42216, CA21765, and CA108786 from the National Cancer Institute and used volasertib supplied by Boehringer Ingleheim, AG. In addition to the authors this paper represents work contributed by the following: Sherry Ansher, Ingrid Boehm, Joshua Courtright, Kathryn Evans, Edward Favours, Henry S. Friedman, Danuta Gasinski, Nicholas Pettit, Melissa Sammons, Joe Zeidner, Jianrong Wu, Ellen Zhang, and Jian Zhang. Children’s Cancer Institute Australia for Medical Research is affiliated with the University of New South Wales and the Sydney Children’s Hospitals Network.
Footnotes
Conflict of interest statement: The authors consider that there are no actual or perceived conflicts of interest.
References
- 1.Rudolph D, Steegmaier M, Hoffmann M, et al. BI 6727, a Polo-like kinase inhibitor with improved pharmacokinetic profile and broad antitumor activity. Clin Cancer Res. 2009;15(9):3094–3102. doi: 10.1158/1078-0432.CCR-08-2445. [DOI] [PubMed] [Google Scholar]
- 2.Ackermann S, Goeser F, Schulte JH, et al. Polo-like kinase 1 is a therapeutic target in high-risk neuroblastoma. Clin Cancer Res. 2011;17(4):731–741. doi: 10.1158/1078-0432.CCR-10-1129. [DOI] [PubMed] [Google Scholar]
- 3.Smith MR, Wilson ML, Hamanaka R, et al. Malignant transformation of mammalian cells initiated by constitutive expression of the polo-like kinase. Biochem Biophys Res Commun. 1997;234(2):397–405. doi: 10.1006/bbrc.1997.6633. [DOI] [PubMed] [Google Scholar]
- 4.Strebhardt K, Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer. 2006;6(4):321–330. doi: 10.1038/nrc1841. [DOI] [PubMed] [Google Scholar]
- 5.Weichert W, Ullrich A, Schmidt M, et al. Expression patterns of polo-like kinase 1 in human gastric cancer. Cancer Sci. 2006;97(4):271–276. doi: 10.1111/j.1349-7006.2006.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renner AG, Dos Santos C, Recher C, et al. Polo-like kinase 1 is overexpressed in acute myeloid leukemia and its inhibition preferentially targets the proliferation of leukemic cells. Blood. 2009;114(3):659–662. doi: 10.1182/blood-2008-12-195867. [DOI] [PubMed] [Google Scholar]
- 7.Guan R, Tapang P, Leverson JD, et al. Small interfering RNA-mediated Polo-like kinase 1 depletion preferentially reduces the survival of p53-defective, oncogenic transformed cells and inhibits tumor growth in animals. Cancer Res. 2005;65(7):2698–2704. doi: 10.1158/0008-5472.CAN-04-2131. [DOI] [PubMed] [Google Scholar]
- 8.Steegmaier M, Hoffmann M, Baum A, et al. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr Biol. 2007;17(4):316–322. doi: 10.1016/j.cub.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 9.Schoffski P, Awada A, Dumez H, et al. A phase I, dose-escalation study of the novel Polo-like kinase inhibitor volasertib (BI 6727) in patients with advanced solid tumours. Eur J Cancer. 2012;48(2):179–186. doi: 10.1016/j.ejca.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Stadler WM, Vaughn DJ, Sonpavde G, et al. Phase II study of single-agent volasertib (BI 6727) for second-line treatment of urothelial cancer (UC) J Clin Oncol. 2011:29. [Google Scholar]
- 11.Maertens J, Lubbert M, Fiedler W, et al. Phase I/II Study of Volasertib (BI 6727), an Intravenous Polo-Like Kinase (Plk) Inhibitor, in Patients with Acute Myeloid Leukemia (AML): Results From the Randomized Phase II Part for Volasertib in Combination with Low-Dose Cytarabine (LDAC) Versus LDAC Monotherapy in Patients with Previously Untreated AML Ineligible for Intensive Treatment. Blood (ASH Annual Meeting Abstracts) 2012;120(21):Abstr #411. [Google Scholar]
- 12.Hu K, Lee C, Qiu D, et al. Small interfering RNA library screen of human kinases and phosphatases identifies polo-like kinase 1 as a promising new target for the treatment of pediatric rhabdomyosarcomas. Molecular cancer therapeutics. 2009;8(11):3024–3035. doi: 10.1158/1535-7163.MCT-09-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grinshtein N, Datti A, Fujitani M, et al. Small Molecule Kinase Inhibitor Screen Identifies Polo-Like Kinase 1 as a Target for Neuroblastoma Tumor-Initiating Cells. Cancer research. 2011;71(4):1385–1395. doi: 10.1158/0008-5472.CAN-10-2484. [DOI] [PubMed] [Google Scholar]
- 14.Duan Z, Ji D, Weinstein EJ, et al. Lentiviral shRNA screen of human kinases identifies PLK1 as a potential therapeutic target for osteosarcoma. Cancer letters. 2010;293(2):220–229. doi: 10.1016/j.canlet.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Frgala T, Kalous O, Proffitt RT, et al. A fluorescence microplate cytotoxicity assay with a 4-log dynamic range that identifies synergistic drug combinations. Mol Cancer Ther. 2007;6(3):886–897. doi: 10.1158/1535-7163.MCT-04-0331. [DOI] [PubMed] [Google Scholar]
- 16.Kang MH, Smith MA, Morton CL, et al. National Cancer Institute Pediatric Preclinical Testing Program: Model description for in vitro cytotoxicity testing. Pediatr Blood Cancer. 2011;56(2):239–249. doi: 10.1002/pbc.22801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houghton PJ, Morton CL, Tucker C, et al. The pediatric preclinical testing program: Description of models and early testing results. Pediatr Blood Cancer. 2006 doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 18.Liem NL, Papa RA, Milross CG, et al. Characterization of childhood acute lymphoblastic leukemia xenograft models for the preclinical evaluation of new therapies. Blood. 2004;103(10):3905–3914. doi: 10.1182/blood-2003-08-2911. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi U, Honda K, Satow R, et al. Functional genome screen for therapeutic targets of osteosarcoma. Cancer science. 2009;100(12):2268–2274. doi: 10.1111/j.1349-7006.2009.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morales AG, Brassesco MS, Pezuk JA, et al. BI 2536-mediated PLK1 inhibition suppresses HOS and MG-63 osteosarcoma cell line growth and clonogenicity. Anti-cancer drugs. 2011;22(10):995–1001. doi: 10.1097/CAD.0b013e32834a16d4. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Choy E, Harmon D, et al. Inhibition of polo-like kinase 1 leads to the suppression of osteosarcoma cell growth in vitro and in vivo. Anti-cancer drugs. 2011;22(5):444–453. doi: 10.1097/CAD.0b013e32834513f4. [DOI] [PubMed] [Google Scholar]
- 22.Carol H, Houghton PJ, Morton CL, et al. Initial testing of topotecan by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2010;54(5):707–715. doi: 10.1002/pbc.22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maris JM, Morton CL, Gorlick R, et al. Initial testing of the aurora kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP) Pediatr Blood Cancer. 2010;55(1):26–34. doi: 10.1002/pbc.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schoffski P, Awada A, Dumez H, et al. A phase I, dose-escalation study of the novel Polo-like kinase inhibitor volasertib (BI 6727) in patients with advanced solid tumours. Eur J Cancer. 2011 doi: 10.1016/j.ejca.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Bug G, Muller-Tidow C, Schlenk RF, et al. Phase I/II Study of Volasertib (BI 6727), An Intravenous Polo-Like Kinase (Plk) Inhibitor, in Patients with Acute Myeloid Leukemia (AML): Updated Results of the Dose Finding Phase I Part for Volasertib in Combination with Low-Dose Cytarabine (LD-Ara-C) and As Monotherapy in Relapsed/Refractory AML. Blood (ASH Annual Meeting Abstracts) 2011;118(21):Abstr #1549. [Google Scholar]
- 26.Wong H, Choo EF, Alicke B, et al. Antitumor activity of targeted and cytotoxic agents in murine subcutaneous tumor models correlates with clinical response. Clin Cancer Res. 2012;18(14):3846–3855. doi: 10.1158/1078-0432.CCR-12-0738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


