Abstract
BACKGROUND
Solid organ transplantation recipients have elevated cancer incidence. Estimates of absolute cancer risk after transplantation can inform prevention and screening.
METHODS
The Transplant Cancer Match Study links the US transplantation registry with 14 state/regional cancer registries. The authors used nonparametric competing risk methods to estimate the cumulative incidence of cancer after transplantation for 2 periods (1987–1999 and 2000–2008). For recipients from 2000 to 2008, the 5-year cumulative incidence, stratified by organ, sex, and age at transplantation, was estimated for 6 preventable or screen-detectable cancers. For comparison, the 5-year cumulative incidence was calculated for the same cancers in the general population at representative ages using Surveillance, Epidemiology, and End Results data.
RESULTS
Among 164,156 recipients, 8520 incident cancers were identified. The absolute cancer risk was slightly higher for recipients during the period from 2000 to 2008 than during the period from 1987 to 1999 (5-year cumulative incidence: 4.4% vs 4.2%; P =.006); this difference arose from the decreasing risk of competing events (5-year cumulative incidence of death, graft failure, or retransplantation: 26.6% vs 31.9%; P <.001). From 2000 to 2008, the 5-year cumulative incidence of non-Hodgkin lymphoma was highest at extremes of age, especially in thoracic organ recipients (ages 0–34 years: range, 1.74%–3.28%; aged >50 years; range, 0.36%–2.22%). For recipients aged >50 years, the 5-year cumulative incidence was higher for colorectal cancer (range, 0.33%–1.94%) than for the general population at the recommended screening age (aged 50 years: range, 0.25%–0.33%). For recipients aged >50 years, the 5-year cumulative incidence was high for lung cancer among thoracic organ recipients (range, 1.16%–3.87%) and for kidney cancer among kidney recipients (range, 0.53%–0.84%). The 5-year cumulative incidence for prostate cancer and breast cancer was similar or lower in transplantation recipients than at the recommended ages of screening in the general population.
CONCLUSIONS
Subgroups of transplantation recipients have a high absolute risk of some cancers and may benefit from targeted prevention or screening.
Keywords: transplantation, screening, non-Hodgkin lymphoma, absolute risk
INTRODUCTION
From the beginning of successful solid-organ transplantation, an increased risk of cancer after transplantation has been noted.1 This elevated risk arises from several factors, including immunosuppression resulting from medications that prevent rejection, decreased control of oncogenic viral infections, and underlying medical conditions that are common in transplantation recipients. The importance of cancer after transplantation has increased as the life expectancy of transplantation recipients has improved. Among kidney, liver, and lung recipients, respectively, cancer is now the third, second, and fourth most common cause of death 5 years after transplantation.2
Although prior studies have demonstrated an elevated risk for cancer among transplantation recipients, most of these studies have not described cancer risk in absolute terms. Absolute risk, also called cumulative incidence, is the probability of a transplantation recipient developing a given cancer over a specified time interval and depends on both the risk of cancer and the risk of competing events (eg death, graft failure).3,4 Absolute risk can be used to estimate how many cancers are expected to develop in a population, which can inform evidence-based treatment guidelines.5 For example, information about the absolute risk of cancer among transplantation recipients could help frame the benefits and costs of cancer screening relative to other populations for whom screening is recommended or relative to other health needs.
The current study was designed to quantify the absolute risk of cancer after transplantation for those cancers that are potentially preventable or detectable by screening. We focused on cancers that were common malignancies in US transplantation recipients6 and where there are established or proposed approaches for prevention or screening. The 6 cancers that met these criteria were non-Hodgkin lymphoma (NHL), lung cancer, colorectal cancer, kidney cancer, prostate cancer, and breast cancer. Quantifying the absolute risk of these malignancies and identifying subgroups at highest risk may inform screening and treatment protocols for solid-organ recipients.
MATERIALS AND METHODS
The Transplant Cancer Match Study links data from the Scientific Registry of Transplant Recipients (1987–2008) with 14 population-based US cancer registries (information available at: http://transplantmatch.cancer.gov/ [accessed March 7, 2013]).6 Participating cancer registries, which together cover approximately 43% of the US transplantation population, ascertained the occurrence of malignancies based on mandatory reporting from hospitals, medical providers, and pathology laboratories. The study was approved by human subjects committees at the National Cancer Institute and, as required, at participating cancer registries.
In the current study, we included kidney, liver, heart, and lung recipients for whom cancer registry coverage was present beginning on the transplantation date. We used the linked cancer registry data to identify incident cancers after transplantation. Only first cancers after transplantation were counted. Although basal cell and squamous cell skin cancers are common in transplantation recipients, cancer registries do not capture these cancers, so we could not evaluate them in this study.
We used cumulative incidence estimates to assess the absolute risk of cancer after transplantation in 2 eras (1987–1999 and 2000–2008) defined by year of transplantation. Follow-up time for cumulative incidence computation started at transplantation and ended at the earliest of any first cancer, a competing event (death, graft failure, or retransplantation), or censoring because of loss to follow-up or end of cancer registry coverage. To avoid including prevalent cancers that were present before transplantation, liver and lung cancers that were recorded in cancer registries within 6 months of liver or lung transplantation, respectively, were not considered as events, and recipients were censored at the time of these diagnoses.
To estimate cumulative incidence for cancer overall and for the combined competing events, we used the non-parametric methods described by Coviello and Boggess stratified by era.7 A change across eras in the cumulative incidence of cancer could have 2 possible explanations: 1) the hazard (instantaneous risk) of cancer could have changed, or 2) the hazard of the competing events could have changed. In other words, the cumulative incidence of cancer depends directly on the hazard of cancer (ie, the rate of developing cancer among individuals at risk of cancer) and indirectly on the hazard of competing events (because this hazard determines who remains at risk of cancer); a decrease in the hazard of competing events translates to an increase in the time at risk available for recipients to develop cancer. To distinguish between these 2 scenarios, we calculated the hazard ratio (HR) (1987–1999 vs 2000–2008) for cancer and the HR (1987–1999 vs 2000–2008) for the competing events using Cox proportional hazards models.
Subsequent analyses were restricted to organ transplantations after January 1, 2000, to focus on the most recent period and derive results that would be directly applicable to recent recipients. For this recent period, we first assessed factors associated with the cumulative incidence of cancer overall using multivariate subdistribution hazards models as described by Fine and Gray.8 Adjusted subdistribution-HRs (aSHRs) were calculated from this model, which included terms for sex, age, race/ethnicity, and transplanted organ.
Finally, we estimated the 5-year cumulative incidence of each of the 6 cancers of interest (NHL, lung cancer, colorectal cancer, kidney cancer, prostate cancer, and breast cancer) using nonparametric competing risk methods, stratified by transplanted organ, sex, and age at transplantation. For strata with no cancer events, upper 95% confidence limits were calculated assuming a Poisson distribution for the cancer events and taking into account person-time at risk and the risk of competing events. For comparison, we also estimated the 5-year cumulative incidence of these cancers in the general US population starting at ages 25 years, 50 years, and 75 years for men and women using data on cancer incidence and all-cause mortality provided by the 17 cancer registries in the US Surveil-lance, Epidemiology, and End Results Program (available at: www.seer.cancer.gov [accessed March 7, 2013]).
All P values were 2-sided, and a P value of .05 was considered significant. All analyses were performed using Stata 11.0/MP for Linux (StataCorp, College Station, Tex). Nonparametric cumulative incidence estimates were produced using the stcompet command, and multivariate comparisons of cumulative incidence functions were completed using stcrreg.
RESULTS
There were 164,156 recipients of interest in the Transplant Cancer Match Study between 1987 and 2008 (Table 1). Demographic characteristics of recipients were similar between the 2 eras (1987–1999 and 2000–2008). Most recipients were male (range, 60.2%–61.7%) and non-Hispanic white, although the percentage of non-Hispanic white recipients decreased between the eras (64.5% vs 56.3%). Transplantation recipients were older in the most recent era (median age, 50 years vs 45 years), and the percentage of recipients aged >60 years was higher in the most recent era (19.3% vs 12.5%). Kidney was the most commonly transplanted organ in both eras (range, 61.1%–63.2%).
TABLE 1.
Demographic Characteristics of Transplantation Recipients in the Transplant Cancer Match Study, by Era of Transplantation (1987–2008)
| No. of Patients (%) |
|||
|---|---|---|---|
| Transplantation Period |
|||
| All Recipients | 1987–1999 | 2000–2008 | |
| Sex | |||
| Male | 72,985 (61) | 43,959 (60.2) | 56,250 (61.7) |
| Female | 91,171 (39) | 29,026 (39.8) | 34,921 (38.3) |
| Age at transplantation, y | |||
| 0–35 | 42,121 (25.7) | 21,759 (29.8) | 20,362 (22.3) |
| 36–50 | 51,953 (31.6) | 24,984 (34.2) | 26,969 (29.6) |
| 51–60 | 43,411 (26.4) | 17,162 (23.5) | 26,249 (28.8) |
| >60 | 26,671 (16.3) | 9080 (12.5) | 17,591 (19.3) |
| Race/ethnicity | |||
| Non-Hispanic white | 98,363 (59.9) | 47,081 (64.5) | 51,282 (56.3) |
| Non-Hispanic black | 27,842 (17) | 11,488 (15.7) | 16,354 (17.9) |
| Hispanic/other | 36,845 (22.4) | 13,988 (19.2) | 22,857 (25.1) |
| Missing | 1106 (0.67) | 428 (0.59) | 678 (0.74) |
| Transplanted organ | |||
| Kidney | 102,106 (62.2) | 44,534 (61.1) | 57,572 (63.2) |
| Liver | 37,944 (23.1) | 16,574 (22.7) | 21,370 (23.4) |
| Heart | 17,134 (10.4) | 9076 (12.4) | 8058 (8.8) |
| Lung | 6972 (4.3) | 2801 (3.8) | 4171 (4.6) |
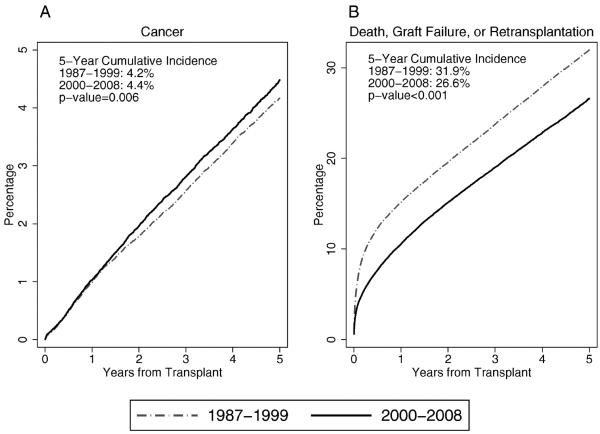
In total, 8520 incident cancers arose among the transplantation recipients between 1987 and 2008. Between the 2 eras (1987–1999 and 2000–2008), the cumulative incidence of cancer after transplantation increased slightly (5-year cumulative cancer incidence: 4.2% vs 4.4%, respectively; P = .006) (Fig. 1A). Over the same period, there was a larger decline in the cumulative incidence of the competing events (5-year cumulative incidence for death, graft failure, or retransplantation: 31.9% vs 26.6%, respectively; P < .001) (Fig. 1B). The small increase in the cumulative incidence of cancer was not a result of an increase in the hazard (instantaneous risk) of cancer (HR, 0.99; 95% confidence interval [CI], 0.94–1.04). Instead, the increasing cumulative incidence of cancer was related to a declining hazard of death, graft failure, or retransplantation between eras (HR, 0.78; 95% CI, 0.77–0.79).
Figure 1.
The cumulative incidence of cancer and of death, graft failure, or retransplantation among US solid organ transplantation recipients is illustrated. Curves indicate the cumulative incidence of (A) all incident cancers and (B) the competing events of death, graft failure, or retransplantation after kidney, liver, heart, or lung transplantation. Results are provided separately for 2 eras defined by calendar year of transplantation. The vertical axis indicates the percentage of recipients with the specified outcome; the scales differ in A and B.
Remaining analyses focused on the most recent transplantation era (2000–2008). Among the 91,171 recipients in this era, the cumulative incidence of cancer was lower for females than for males (aSHR, 0.77; 95% CI, 0.72–0.83) and increased with age at transplantation compared with recipients ages birth to 35 years (ages 36–50 years: aSHR, 1.79 [95% CI, 1.54–2.08]; ages 51–60 years: aSHR, 3.26 [95% CI, 2.83–3.76]; aged >60 years: aSHR, 4.61 [95% CI, 4.00–5.31]; Ptrend < .001). Compared with kidney recipients, lung recipients had the highest cumulative incidence of cancer (aSHR, 1.50; 95% CI, 1.30–1.73), heart recipients had intermediate cumulative incidence (aSHR, 1.19; 95% CI, 1.06–1.34), and liver recipients were similar in cumulative incidence (aSHR, 1.01; 95% CI, 0.93–1.11).
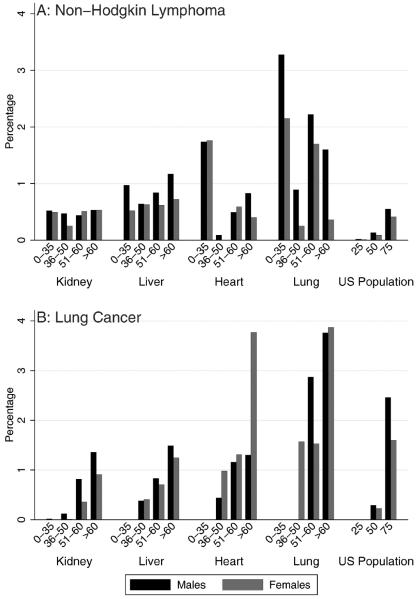
Cumulative incidence estimates for individual cancers are presented in the figures. For NHL (N = 425 cases), the 5-year cumulative incidence ranged from 0.09% to 3.28% across categories of sex, age, and transplanted organ (Fig. 2A). Five-year cumulative incidence was especially high among heart and lung recipients ages birth to 35 years (male and female heart recipients, 1.75%; male lung recipients, 3.28%; female lung recipients, 2.15%). It was also high among older lung recipients (men ages 51–60 years, 2.22%; women ages 51–60 years, 1.70%; men aged >60 years, 1.60%). Almost all subgroups of recipients had higher cumulative incidence of NHL than the US general population at ages 25 and 50 years, and most other than kidney recipients had higher cumulative incidence of NHL than the US general population at age 75 years.
Figure 2.
The 5-year cumulative incidence of (A) non-Hodgkin lymphoma and (B) lung cancer are illustrated after transplantation and for the US population from 2000 to 2008. Results correspond to the probability that individuals will develop the specified cancer over a 5-year period. Estimates for recipients are limited to patients who underwent transplantation during 2000 to 2008 and are stratified by transplanted organ, sex, and age at transplantation. Estimates of cumulative incidence for the US population were derived from Surveillance, Epidemiology, and End Results (SEER) Program data.
For lung cancer (N = 372 cases) (Fig. 2B), the 5-year cumulative incidence was 0% (or near 0%) for recipients of all organs ages birth to 35 years, but it increased with age. Five-year cumulative incidence was highest among lung recipients aged >60 years (males, 3.76%; females, 3.87%) and female heart recipients aged >60 years (3.77%). All liver, heart, and lung recipients aged >35 years had 5-year cumulative incidence of lung cancer greater than the US general population at age 50 years (males, 0.29%; females, 0.23%), and lung recipients aged >50 years had 5-year cumulative incidence that was similar to or greater than that of those aged 75 years in the US population (males, 2.46%; females, 1.60%).
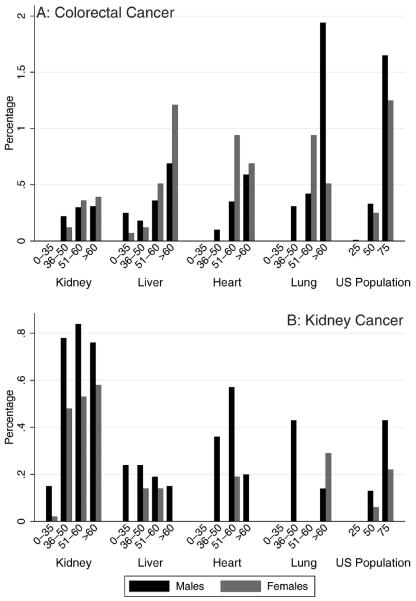
For colorectal cancer (N = 158 cases) (Fig. 3A), the 5-year cumulative incidence in transplantation recipients ranged from 0% in most recipients ages birth to 35 years to 1.94% in male lung recipients aged >60 years. Liver recipients were the only group to develop colorectal cancer at an age <35 years. For recipients of each type of organ, cumulative incidence largely increased with age. All transplantation recipients aged >50 years had 5-year cumulative incidence at or greater than that of the US general population at the recommended age of screening (US males aged 50 years, 0.33%; US females aged 50 years, 0.25%).
Figure 3.
The 5-year cumulative incidence of (A) colorectal cancer and (B) kidney cancer is illustrated after transplantation and for the US population from 2000 to 2008. Estimates for recipients are limited to patients who underwent transplantation during 2000 to 2008 and are stratified by transplanted organ, sex, and age at transplantation. Results correspond to the probability that individuals will develop the specified cancer over a 5-year period. Estimates of cumulative incidence for the US population were derived from Surveillance, Epidemiology, and End Results (SEER) Program data. Scales differ in A and B.
For kidney cancer (N = 269 cases) (Fig. 3B), 5-year cumulative incidence was highest in kidney recipients aged >35 years (range, 0.48%–0.84%). Five-year cumulative incidence was also high among some subgroups of older heart and lung recipients (range, 0.14%–0.57%). Kidney recipients, especially those aged >35 years, had greater 5-year cumulative incidence of kidney cancer than observed in the US general population at any age.
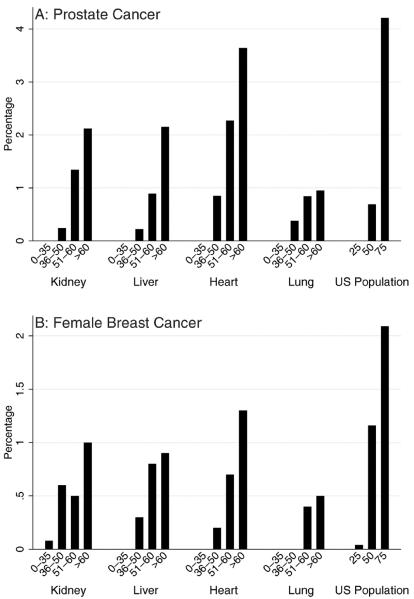
For prostate cancer (N = 350 cases) (Fig. 4A), 5-year cumulative incidence was highest in heart recipients aged >60 years (3.65%). Recipient groups who had 5-year cumulative incidence similar to or greater than the general population at age 50 years (2.34%) included kidney, liver, and lung recipients ages 51 to 60 years or older and heart recipients ages 36 to 50 years or older.
Figure 4.
The 5-year cumulative incidence of (A) prostate cancer and (B) breast cancer is illustrated after transplantation and for the US population from 2000 to 2008. Estimates for recipients are limited to patients who underwent transplantation during 2000 to 2008 and are stratified by transplanted organ and age at transplantation. Results correspond to the probability that individuals will develop the specified cancer over a 5-year period. Estimates of cumulative incidence for the US population were derived from Surveillance, Epidemiology, and End Results (SEER) Program data. Scales differ in A and B.
Results for breast cancer in females are illustrated in Figure 4B (N = 116 cases). Only heart recipients aged >60 years had the same or higher 5-year cumulative incidence of breast cancer (1.30%) as the general population at age 50 years (1.16%), which is the age cutoff used under 1 set of guidelines for mammography screening. Alternative recommendations are to start mammography screening at age 40 years in the general population. The 5-year cumulative incidence for women in the general population at age 40 years is 0.60%; kidney recipients aged >60 years (1%), and liver and heart recipients aged >50 years (range, 0.70%–1.3%) had 5-year cumulative incidence as high or higher than this benchmark.
DISCUSSION
We observed a high cumulative incidence of cancer among US solid organ recipients, with greater than 4% developing cancer over a 5-year period. This estimate of absolute cancer risk accounts for the substantial competing risks of death, graft failure, and retransplantation, and it corresponds to the probability that a transplantation recipient will develop cancer. Our results are consistent with previous Canadian and British studies, which likewise reported an approximately 4% to 4.8% cumulative incidence of cancer at 5 years after solid organ transplantation.9,10 In addition, we identified subgroups of recipients with an elevated cumulative incidence for preventable or screen-detectable cancers, which may help frame transplantation management or targeted screening protocols.
Cumulative incidence measures reflect the risk of both cancer and the competing events that may preclude the development of cancer. Among US recipients, we observed that the cumulative incidence of cancer increased slightly across 2 transplantation eras. We could hypothesize that this increase in cumulative incidence may be caused by changes in cancer risk factors or screening. If so, then we would expect those changes to translate into changes in the instantaneous risk for cancer (captured by the cancer-specific hazard function). However, the increase in cumulative incidence actually was not caused by an increase in the hazard of cancer but, instead, was because of a decreasing hazard for death, graft failure, or retransplantation. In other words, improvements in clinical management have allowed recipients to live longer with a functioning graft, which provides an increased opportunity to develop cancer. Thus, cumulative incidence is useful when considering cancer prevention or screening strategies, because it appropriately accommodates for competing risks. We observed that cumulative incidence of select cancers among subgroups of transplantation recipients was as high or higher than that observed in the general population at ages for which cancer prevention and screening are recommended.
Except for nonmelanoma skin cancer, NHL is the most common cancer after transplantation. NHL comprises the malignant end of the spectrum of post-transplantation lymphoproliferative disorders (PTLDs).11 In transplantation recipients, poor immune control of Epstein-Barr virus (EBV) has been linked to the high incidence of NHL and PTLDs in general, especially for children who experience primary EBV infection after transplantation.12,13 Our study revealed subgroups at par ticular risk for developing NHL. The 5-year cumulative incidence, as might be expected, was high among the youngest recipients (ages 0–35 years) for every type of transplantation. In addition, lung and heart recipients aged >50 years were at high risk. Lung recipients had the highest cumulative incidence, especially at older ages, despite high mortality experienced by this group (ie, the 5-year survival for lung recipients aged >65 years who underwent transplantation between 2003 and 2008 was 41.7%; 2010 Annual Data Report available at: www.srtr.org [accessed March 7, 2013]). The possible benefits of decreasing immunosuppression in these high-risk recipient populations to reduce the cumulative incidence of NHL must be weighed against the risk of increasing rejection rates. Some transplantation centers closely monitor EBV viral load in children to identify recipients at highest risk of PTLDs,14,15 although the efficacy of this approach is not fully established. Similar monitoring may be warranted for lung recipients given their high cumulative incidence of NHL.
Lung cancer is the next most common malignancy among transplantation recipients.6 The 5-year cumulative incidence of lung cancer was highest in heart and lung recipients, which may be related to smoking as a contributor to end-stage heart and lung disease. Some lung recipients receive only a single lung, and cancers are most often diagnosed in the remaining native lung, reflecting the role of underlying pulmonary disease, including inflammatory processes and repeated infections, in the development of lung cancer.16–18 Our results highlight the importance of encouraging and facilitating transplantation recipients to quit tobacco smoking. Given the high rate of smoking resumption after transplantation,19,20 this issue should be monitored longitudinally. Recent findings from the National Lung Screening Trial suggest that annual computed tomography scan screening is effective in reducing overall mortality in a population with a 5-year cumulative incidence of lung cancer of at least 3.6%.21 Because this level of risk is on par with what we observed in heart and lung transplantation recipients aged >60 years, it is possible that lung cancer screening would benefit this subset of the transplantation population.
Kidney cancer is most common in kidney recipients and frequently arises in the native kidneys in association with acquired polycystic kidney disease.22,23 However, clinical practice guidelines for kidney recipients do not currently include kidney cancer screening.24 In a recent cost-effectiveness analysis of screening for kidney cancer using ultrasound,25 Wong et al observed that the cost per life-year saved was too high to recommend screening for all kidney recipients. Nonetheless, screening may be cost-effective for high-risk subgroups of kidney recipients, such as those with acquired polycystic kidney disease, a family history of kidney cancer, tobacco use, or certain genetic syndromes. We observed that the cumulative incidence of kidney cancer among kidney recipients increased steeply at age 35 years, suggesting that this may be an important age threshold.
The cumulative incidence of colorectal cancer also was high after transplantation, particularly for older recipients. For colorectal cancer, all organ recipients aged ≥50 years had higher cumulative incidence than the general US population at age 50 years (the recommended age for colorectal cancer screening).26 Because the utility of screening largely depends on the expected probability of developing cancer, our findings suggest that transplantation recipients aged >50 years should receive standard colorectal cancer screening.
Prostate cancer is the second most common cancer among American men after skin cancer. Nearly 66% of prostate cancer is diagnosed in men aged ≥65 years, and it is rare before age 40 years. Among transplantation recipients, there has been no demonstrated increased risk for prostate cancer compared with the general population.1,6 In addition, the competing risks of graft failure and death rise with age, which has the effect of decreasing the cumulative incidence of prostate cancer in older transplantation recipients. Approaches for prostate cancer screening include digital rectal examination and measurement of serum prostate-specific antigen; however, there are currently no definitive recommendations for the general population. Given the lack of an elevated risk in transplantation recipients and uncertainties in the sensitivity and specificity of screening tests in this population, it is unclear whether prostate cancer screening for transplantation recipients is warranted.
Breast cancer is the second most common cancer among American women, again, after skin cancer. Like prostate cancer in males, breast cancer risk in female transplantation recipients is not increased; and, in the Transplant Cancer Match Study, the risk was actually lower than that in the general population.6 Consequently, only heart recipients aged >60 years had 5-year cumulative incidence at least as high as women in the general population at age 50 years, and the 5-year cumulative incidence in recipients at age 50 years was more comparable to that in the general population at age 40 years. For women in the US general population, there is debate about whether mammography screening should begin at age 40 years or age 50 years.27,28 For transplantation recipients, current recommendations are to begin screening at age 50 years.24 Although an argument could be made for this approach, there is a lack of data on the sensitivity and specificity of screening mammography in the transplantation setting.
Indeed, the question of colorectal, prostate, and breast cancer screening among transplantation recipients is not straight forward. The cumulative incidence of cancer, although important, is only part of the equation, and the decision to screen also needs to consider that the sensitivity and specificity of screening modalities could be reduced in the transplantation setting, and risks associated with screening and treatment could be increased. Thus, applying current screening guidelines from the general population ultimately may not benefit transplantation recipients.29 For instance, the benefits of colorectal cancer screening must be weighed against potentially increased complications of invasive screening (eg, colonoscopy), including those related to the high burden of cardiovascular disease and delayed wound healing after biopsies. In the absence of clinical trials, there may be value in modeling the effects of various assumptions on the benefits and costs of screening or prevention approaches. To make informed recommendations for this population, it will be necessary to study the validity of extrapolating information about screening from the general population to transplantation recipients, the time course over which benefits and harms accrue, and the efficacy of interventions to treat cancer precursor lesions and cancer itself.
Cutaneous squamous cell cancers are the most frequent cancer after transplantation, and recipients are at greatly increased risk for these cancers.30,31 A limitation to our study was the lack of data for nonmelanoma skin cancers, because cancer registries do not capture information on these diagnoses. Sun protection by behavior, clothing, and daily sun screen application are the most effective preventive measures for skin cancer.32 To prevent and screen for squamous cell skin cancers, a multidisciplinary approach is advocated, beginning with education before transplantation, yearly dermatologist inspection after transplantation, and proactive treatment of in situ precursor lesions, such as actinic keratosis and Bowen disease.33,34 These screening guidelines also help in early detection of melanoma and other cutaneous malignancies.
Strengths of our study include its large size and representativeness of nearly half of the US transplantation population. Our choice of strata based on sex, age, and transplanted organ addresses major demographic and clinical characteristics related to cancer risk. Nonetheless, estimates of cancer risk ideally should account for such factors as smoking status, etiology of end-stage organ disease, other underlying medical conditions (eg, ulcerative colitis, hepatitis C status, EBV status), and family history. Information on some of these characteristics is not collected by the Scientific Registry of Transplant Recipients, and providing further stratification on other characteristics was beyond the scope of the current analyses.
In summary, our study quantified the absolute risk of cancer of solid organ transplantation recipients and compared this risk with the general population. We also identified some recipient subgroups at a high risk for specific cancers, including NHL for thoracic transplantation recipients at the extremes of age, lung cancer for older thoracic organ recipients, and kidney cancer for all but the youngest kidney recipients. These results suggest some avenues to target screening or prevention measures. Our results for colorectal, prostate, and breast cancers provide a context for considering the appropriateness of adapting general population screening guidelines to transplantation recipients. As the management of other transplantation-related conditions improves and the risk of competing events declines, morbidity and mortality from cancer will increase in recipients. More work is necessary to determine responsible prevention and screening protocols for these patients.
Acknowledgments
FUNDING SOURCES
This research was supported in part by the Intramural Research Program of the National Cancer Institute (E. A. Engels) and by training grant T32CA126607 (Clinical and Laboratory Research Training for Surgical Oncologists; to E. C. Hall). During the initial period when registry linkages were performed, the Scientific Registry of Transplant Recipients (SRTR) was managed by Arbor Research Collaborative for Health in Ann Arbor, Michigan (contract HHSH234200537009C); beginning in September 2010, the SRTR was managed by Minneapolis Medical Research Foundation in Minneapolis, Minnesota (HHSH250201000018C). The following cancer registries were supported by the National Program of Cancer Registries of the Centers for Disease Control and Prevention: California (agreement 1U58 DP000807-01), Colorado (U58 DP000848-04), Georgia (5U58DP000817-05), Illinois (5658DP 000805-04), Michigan (5U58DP000812-03), New Jersey (5U58/DP000808-05), New York (15-0351), North Carolina (U58DP000832), and Texas (5U58DP000824-04). The following cancer registries were supported by the SEER Program of the National Cancer Institute: California (contracts HHSN 261201000036C, HHSN261201000035C, and HHSN261201 000034C), Connecticut (HHSN261201000024C), Hawaii (HH SN261201000037C, N01-PC-35,137, and N01-PC-35,139), Iowa (N01-PC-35,143), New Jersey (HHSN261201000027C N01-PC-2012-00,027), Seattle-Puget Sound (N01-PC-35,142), and Utah (HHSN261201000026C). Additional support was provided by the states of California, Colorado, Connecticut, Illinois, Iowa, New Jersey, New York (Cancer Surveillance Improvement Initiative 14-2491), Texas, and Washington, as well as the Fred Hutchinson Cancer Research Center in Seattle, Washington.
We gratefully acknowledge the support and assistance provided by individuals at the Health Resources and Services Administration (including Monica Lin), the Scientific Registry of Transplant Recipients (Ajay Israni, Bertram Kasiske, Paul Newkirk, and Jon Snyder), and the following cancer registries: the states of California (Christina Clarke), Colorado (Jack Finch), Connecticut (Lou Gonsalves), Georgia (Rana Bayakly), Hawaii (Marc Goodman), Iowa (Charles Lynch), Illinois (Lori Koch), Michigan (Glenn Copeland), New Jersey (Karen Pawlish, Xiaoling Niu), New York (Amy Kahn), North Carolina (Chandrika Rao), Texas (Melanie Williams), and Utah (Janna Harrell) as well as the Seattle-Puget Sound area of Washington (Margaret Madeleine). We also thank analysts at Information Management Services for programming support (David Castenson and Ruth Parsons).
The views expressed in this article are those of the authors and should not be interpreted to reflect the views or policies of the National Cancer Institute, the Health Resources and Services Administration, the Scientific Registry of Transplant Recipients, cancer registries, or their contractors.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosup-pressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 2.Department of Health and Human Services. Health Resources and Services Administration . 2008 Annual Report of the US Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1998–2007. US Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; Rockville, MD: 2008. [Google Scholar]
- 3.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pintilie M. Competing Risks: A Practical Perspective. John Wiley & Sons, Ltd.; Chichester, United Kingdom: 2007. [Google Scholar]
- 5.Varadhan R, Weiss CO, Segal JB, Wu AW, Scharfstein D, Boyd C. Evaluating health outcomes in the presence of competing risks: a review of statistical methods and clinical applications. Med Care. 2010;48(6 suppl):S96–S105. doi: 10.1097/MLR.0b013e3181d99107. [DOI] [PubMed] [Google Scholar]
- 6.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–1901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks [serial online] Stata J. 2004;4:103e112. [Google Scholar]
- 8.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 9.Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK registry audit. Am J Transplant. 2010;10:1889–1896. doi: 10.1111/j.1600-6143.2010.03181.x. [DOI] [PubMed] [Google Scholar]
- 10.Villeneuve PJ, Schaubel DE, Fenton SS, Shepherd FA, Jiang Y, Mao Y. Cancer incidence among Canadian kidney transplant recipients. Am J Transplant. 2007;7:941–948. doi: 10.1111/j.1600-6143.2007.01736.x. [DOI] [PubMed] [Google Scholar]
- 11.Harris NL, Ferry JA, Swerdlow SH. Post-transplant lymphoproliferative disorders: summary of Society for Hematopathology workshop. Semin Diagn Pathol. 1997;14:8–14. [PubMed] [Google Scholar]
- 12.Dharnidharka VR, Lamb KE, Gregg JA, Meier-Kriesche HU. Associations between EBV serostatus and organ transplant type in PTLD risk: an analysis of the SRTR National Registry Data in the United States. Am J Transplant. 2012;12:976–983. doi: 10.1111/j.1600-6143.2011.03893.x. [DOI] [PubMed] [Google Scholar]
- 13.Opelz G, Daniel V, Naujokat C, Dohler B. Epidemiology of pre-transplant EBV and CMV serostatus in relation to post-transplant non-Hodgkin lymphoma. Transplantation. 2009;88:962–967. doi: 10.1097/TP.0b013e3181b9692d. [DOI] [PubMed] [Google Scholar]
- 14.Gregorek H, Jankowska I, Dzierzanowska-Fangrat K, et al. Long-term monitoring of Epstein-Barr virus DNA load and humoral parameter abnormalities in pediatric liver transplant recipients before development of malignancy. Pediatr Transplant. 2010;14:629–635. doi: 10.1111/j.1399-3046.2010.01293.x. [DOI] [PubMed] [Google Scholar]
- 15.Swerdlow AJ, Higgins CD, Hunt BJ, et al. Risk of lymphoid neoplasia after cardiothoracic transplantation. A cohort study of the relation to Epstein-Barr virus. Transplantation. 2000;69:897–904. doi: 10.1097/00007890-200003150-00039. [DOI] [PubMed] [Google Scholar]
- 16.Minai OA, Shah S, Mazzone P, et al. Bronchogenic carcinoma after lung transplantation: characteristics and outcomes. J Thorac Oncol. 2008;3:1404–1409. doi: 10.1097/JTO.0b013e31818e1259. [DOI] [PubMed] [Google Scholar]
- 17.Dickson RP, Davis RD, Rea JB, Palmer SM. High frequency of bronchogenic carcinoma after single-lung transplantation. J Heart Lung Transplant. 2006;25:1297–1301. doi: 10.1016/j.healun.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engels EA. Inflammation in the development of lung cancer: epidemiological evidence. Expert Rev Anticancer Ther. 2008;8:605–615. doi: 10.1586/14737140.8.4.605. [DOI] [PubMed] [Google Scholar]
- 19.Botha P, Peaston R, White K, Forty J, Dark JH, Parry G. Smoking after cardiac transplantation. Am J Transplant. 2008;8:866–871. doi: 10.1111/j.1600-6143.2007.02119.x. [DOI] [PubMed] [Google Scholar]
- 20.Vos R, De Vusser K, Schaevers V, et al. Smoking resumption after lung transplantation: a sobering truth. Eur Respir J. 2010;35:1411–1413. doi: 10.1183/09031936.00183509. [DOI] [PubMed] [Google Scholar]
- 21.National Lung Screening Trial Research Team. Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goh A, Vathsala A. Native renal cysts and dialysis duration are risk factors for renal cell carcinoma in renal transplant recipients. Am J Transplant. 2011;11:86–92. doi: 10.1111/j.1600-6143.2010.03303.x. [DOI] [PubMed] [Google Scholar]
- 23.Heinz-Peer G, Schoder M, Rand T, Mayer G, Mostbeck GH. Prevalence of acquired cystic kidney disease and tumors in native kidneys of renal transplant recipients: a prospective US study. Radiology. 1995;195:667–671. doi: 10.1148/radiology.195.3.7753991. [DOI] [PubMed] [Google Scholar]
- 24.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(suppl 3):S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 25.Wong G, Howard K, Webster AC, Chapman JR, Craig JC. Screening for renal cancer in recipients of kidney transplants. Nephrol Dial Transplant. 2011;26:1729–1739. doi: 10.1093/ndt/gfq627. [DOI] [PubMed] [Google Scholar]
- 26.US Preventive Services Task Force Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 27.US Preventive Services Task Force Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716–726. doi: 10.7326/0003-4819-151-10-200911170-00008. W-236. [DOI] [PubMed] [Google Scholar]
- 28.Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2010;60:99–119. doi: 10.3322/caac.20063. [DOI] [PubMed] [Google Scholar]
- 29.Kiberd BA, Keough-Ryan T, Clase CM. Screening for prostate, breast and colorectal cancer in renal transplant recipients. Am J Transplant. 2003;3:619–625. doi: 10.1034/j.1600-6143.2003.00118.x. [DOI] [PubMed] [Google Scholar]
- 30.Alam M, Brown RN, Silber DH, et al. Increased incidence and mortality associated with skin cancers after cardiac transplant. Am J Transplant. 2011;11:1488–1497. doi: 10.1111/j.1600-6143.2011.03598.x. [DOI] [PubMed] [Google Scholar]
- 31.Krynitz B, Edgren G, Lindelof B, et al. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008—A Swedish population-based study. Int J Cancer. 2013;132:1429–1438. doi: 10.1002/ijc.27765. [DOI] [PubMed] [Google Scholar]
- 32.Hofbauer GF, Anliker M, Arnold A, et al. Swiss clinical practice guidelines for skin cancer in organ transplant recipients. Swiss Med Wkly. 2009;139:407–415. doi: 10.4414/smw.2009.12725. [DOI] [PubMed] [Google Scholar]
- 33.Traywick C, O'Reilly FM. Management of skin cancer in solid organ transplant recipients. Dermatol Ther. 2005;18:12–18. doi: 10.1111/j.1529-8019.2005.05002.x. [DOI] [PubMed] [Google Scholar]
- 34.Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:263–279. doi: 10.1016/j.jaad.2010.11.063. quiz 280. [DOI] [PubMed] [Google Scholar]