Abstract
Inherited retinopathies (IR) are common untreatable blinding conditions. Most of them are inherited as monogenic disorders, due to mutations in genes expressed in retinal photoreceptors (PR) and in retinal pigment epithelium (RPE). The retina’s compatibility with gene transfer has made transduction of different retinal cell layers in small and large animal models via viral and non-viral vectors possible. The ongoing identification of novel viruses as well as modifications of existing ones based either on rational design or directed evolution have generated vector variants with improved transduction properties. Dozens of promising proofs of concept have been obtained in IR animal models with both viral and non-viral vectors, and some of them have been relayed to clinical trials. To date, recombinant vectors based on the adeno-associated virus (AAV) represent the most promising tool for retinal gene therapy, given their ability to efficiently deliver therapeutic genes to both PR and RPE and their excellent safety and efficacy profiles in humans. However, AAVs’ limited cargo capacity has prevented application of the viral vector to treatments requiring transfer of genes with a coding sequence larger than 5 kb. Vectors with larger capacity, i.e. nanoparticles, adenoviral and lentiviral vectors are being exploited for gene transfer to the retina in animal models and, more recently, in humans. This review focuses on the available platforms for retinal gene therapy to fight inherited blindness, highlights their main strengths and examines the efforts to overcome some of their limitations.
Keywords: Gene therapy, Adeno-associated virus, Lentivirus, Adenovirus, Non-viral vectors, Inherited retinopathies
1. Inherited retinopathies
Untreatable blinding diseases include: i. common conditions like age-related macular degeneration, diabetic retinopathy or glaucoma which are inherited as complex traits in which the disease is the result of the interplay between environmental and genetic factors; ii. inherited retinopathies (IR) which are almost exclusively due to single gene defects and are very rare if considered as single entities but represent all together a significant cause of blindness, affecting about 1:2000 people worldwide (Berger et al., 2010). This review will focus on gene therapy of IR. IR are monogenic diseases caused by mutations in one of more than 200 genes (Cepko, 2012) mainly expressed in photoreceptor cells (PR, rods and cones) and to a lesser extent in the retinal pigment epithelium (RPE). IR are generally classified based on the genetic defect (when identified), the inheritance pattern (either autosomal dominant, recessive or X-linked), the main cell type affected, the onset and type of visual dysfunction, the disease progression rate, and the appearance of peculiar ocular fundus abnormalities (Berger et al., 2010). Among the most frequent and severe forms of IR are retinitis pigmentosa (RP), Leber congenital amaurosis (LCA), Star-gardt disease (STGD) and achromatopsia (Lipinski et al., 2013). Retinitis pigmentosa presents either as isolated or as a syndrome. The most frequent syndromic RP is Usher syndrome (USH), which also affects hearing ability (Millan et al., 2011).
2. The eye as a target for gene therapy
Effective treatments for IR are currently unavailable. However, in the past three decades, the identification of many IR-causing genes has paved the way for the development of many gene therapy-based strategies. The eye’s unique features make it ideal for gene therapy (Sahel and Roska, 2013). Specifically, it is a small, enclosed compartment, thus enabling small amounts of vector to act, keeping the risk of toxic reactions to a minimum. The eye is also an immune-privileged site given the presence of tight junctions between RPE cells, the blood–retina barrier, local inhibition of immune responses by the unique intraocular microenvironment and systemic induction of immunosuppressive regulatory T cells via eye-specific mechanisms (Caspi, 2010; Willett and Bennett, 2013). These features keep the vector from disseminating systemically and the immune system from reacting against either the vector components or the transgene product. Together these advantages reduce the risk of adverse systemic effects. Retinal cell types are post-mitotic, and thus sustained long-term gene expression can be achieved even in the absence of transgene integration. Various IR animal models are available, which may facilitate preclinical assessment of the efficacy of therapy. The eye’s structure allows to evaluate treatment progress; the transparency of the ocular media and the availability of recently developed in vivo imaging techniques allow for non-invasive and consistent monitoring of the effects of gene delivery in both animal models and patients. Additionally, given that many ocular diseases are bilaterally symmetrical, we can take advantage of this characteristic to compare the effects of vector/gene delivery in one eye to disease progression in the contralateral eye. Lastly, the eye’s accessibility from the exterior allows surgical procedures to be adapted to transfer genetic material into specific ocular compartments and to preferentially target a particular ocular cell type, with minimal risk to patients undergoing surgical procedures. The two most common methods of intraocular delivery are intravitreal and subretinal injections (Liang et al., 2000). Intravitreal injections consist of the release of the therapeutic agent in the vitreous and result in the exposure of the anterior retina. Subretinal injections, alternatively, deposit the vector into a virtual space between the RPE and the PR, inducing a regional and reversible detachment called a “bleb”. The intravitreal injection allows a more widespread distribution of the therapeutic agent over the retina than that of subretinal delivery, using a less challenging and invasive procedure. However, the diffusion of the therapeutic agent to the PR and RPE after intra-vitreal delivery is limited by several physical barriers, such as the vitreous, the inner limiting membrane (ILM), and the inner retina. Thus, subretinal vector delivery is currently considered to be the most efficient route for targeting PR and RPE cells in the outer retina, the target cells for the treatment of most IR.
3. Gene therapy approaches for IR
Various strategies can be applied to IR gene therapy according to the effect of the mutation underlying the disease. Gene replacement is employed for disorders due to loss-of-function mutations and is based on the delivery of a correct copy of the defective gene without removal of the endogenous mutant one. Gene silencing inhibits the expression of the mutated gene via modification of messenger RNA (mRNA) and is applied to disorders caused by gain-of-function mutations. Given the high number of genes involved in IR and the common apoptotic pathways leading to PR cell death and degeneration, mutation-independent strategies may be designed to either restore photosensitivity by converting surviving retinal cells into photosensors (optogenetics, see Section 5.3.5.) (Mei and Zhang, 2012) or to slow down retinal degeneration (neuro-protection) (Colella and Auricchio, 2010). Neuroprotection prolongs the lifespan of the PR rather than correcting a gene-specific defect. This is typically accomplished by genetically supplying naturally occurring low molecular weight growth factors, which when present at high concentrations, elicit neuroprotective effects. This approach is advantageous because such factors are highly diffusible, and thus the RPE or retinal ganglion cells (RGC) can be converted into factories for secretion of the neurotrophic factor by simple gene transfer techniques. Irrespective of IR mutation-dependent and -independent strategies, vectors are key for successful gene therapy in the eye. There are two major classes of vectors for gene therapy, non-viral and viral. Non-viral vectors include DNA, which can be left naked or combined with either chemicals such as liposomes, polymers and compacted nano-particles, or physical methods such as electroporation or iontophoresis in order to increase transduction efficiency (Tamboli et al., 2011). Unlike non-viral vectors, viral vectors deliver nucleic acids into the host cell’s nucleus after specific interaction with a membrane receptor and internalization (Nonnenmacher and Weber, 2012). Various viral vectors have been tested in the retina; among them, those based on lentivirus (LV), adenovirus (Ad) and adeno-associated virus (AAV) (Table 1) are the most used (Kumar-Singh, 2008; Lipinski et al., 2013). This review will highlight both viral and non-viral strategies, which are emerging as promising retinal treatments.
Table 1.
Features of viral vectors for retinal gene transfer.
| Vector | AAV | LV | Ad |
|---|---|---|---|
| Cloning Capacity (kb) | ≤4.7 | ≤8 | ≤36 |
| Particle size (nm) | 25 | 80–100 | 100 |
| Genome | ssDNA | ssRNA | dsDNA |
| Integration | No | Yes | No |
AAV: adeno-associated virus; LV: lentivirus; Ad: adenovirus; ss: single-stranded; ds: double-stranded.
4. Non-viral vectors
Administration of nucleic acids both naked or combined with physical or chemical methods can accomplish non-viral gene delivery to ocular tissues. Non-viral vectors are useful in gene therapy given their high safety due to low immunogenicity, low risk of insertional mutagenesis, and capacity to be produced at large scale (Charbel Issa and MacLaren, 2012). Most importantly, non-viral vectors’ large cargo capacity allows for delivery of large trans-genes and entire genomic DNA fragments such as gene regulatory elements and intronic sequences, which yield optimal expression efficiency. On the other hand, non-viral vectors need to overcome natural barriers that viral vectors have evolved to pass including: (i) extracellular DNA degradation and immune responses, (ii) cell membrane, (iii) lysosome- or endosome-mediated degradation, (iv) cytoplasmic degradative enzymes, (v) the nuclear envelope, which is difficult to cross in post-mitotic retinal cells. The presence of physical barriers in the eye (such as the vitreous, inner/outer limiting membranes and the inter-PR matrix) and the high concentration of glycosaminoglycans, which sequester DNA, further limit cellular access (Lipinski et al., 2013). Physical and chemical methods have been developed to increase efficiency of naked nucleic acid delivery through these barriers (Table 2).
Table 2.
Non-viral gene delivery to the retina.
| Target cell | Preclinical POC in IR | References | ||
|---|---|---|---|---|
| Physical methods | Electroporation | RPE, PR, RGC | BDNF in RCS rat | 1,2,3,4,5,6 |
| Electron avalanche transfection | RPE, PR | np | 7 | |
| Supra-choroidal electrotransfer | RPE, PR | np | 8 | |
| Trans-palpebral iontophoresis | PR | PDE6B in rd1 mouse | 9 | |
| Trans-scleral iontophoresis | PR | PDE6B in rd1 mouse | 10 | |
| Chemical methods | Liposomes | RPE | np | 1 |
| PEI polyplexes | RGC | np | 11 | |
| Oligochitosan polyplexes | RPE, PR, RGC | np | 12,13 | |
| Solid lipid nanoparticles | RPE, PR, RGC | np | 14 | |
| TransIT-TKO | RPE, RGC | np | 15,16 | |
| VP22 particles | RPE, RGC | np | 17 | |
| POD nanoparticles | RPE, PR | GDNF in light-induced retinal damage | 18,19,20,21 | |
| CK30PEG nanoparticles | RPE, PR, RGC |
PRPH2 in rds mouse ABCA4 in Abca4−/− mouse RPE65 in Rpe65−/− mouse |
22,23,24,25,26 |
POC: proof-of-concept; IR: inherited retinopathies; np: not performed; RPE: retinal pigment epithelium; PR: photoreceptors; RGC: retinal ganglion cells; PEI: poly-ethylenimine; POD: peptides for ocular delivery; CK30PEG: polyethylene glycol-substituted 30-mer lysine peptides. References cited in the table:
4.1. Physical methods to improve non-viral retinal delivery
Physical methods used to improve non-viral retinal delivery include electroporation and iontophoresis (reviewed in Charbel Issa and MacLaren, 2012). Electroporation consists of applying electrical pulses to cellular membranes to increase permeability, and thus facilitate the penetration of naked DNA molecules. Electroporation after intravitreal injection of DNA resulted in transfection of adult rat RGC (Dezawa et al., 2002; Ellouze et al., 2008). In order to transfect the outer retina, electroporation is applied following subretinal delivery of DNA. In newborn rodents, this procedure resulted in transgene expression in a significant fraction of the neurosensory retina (Matsuda and Cepko, 2004). In adult rodents however, Kachi et al. (2005) and Johnson et al. (2008a) found that electroporation following subretinal delivery of naked plasmids enhanced transfection efficiency only in the RPE. However, by using the electron avalanche transfection method, the RPE, and also some PR, were targeted after DNA subretinal injection in the rabbit retina (Chalberg et al., 2006). This technique places an array of microelectrodes behind the eyeball to produce a localized electric field in proximity to cells targeted for transfection. Electroporation has been applied to preclinical studies to treat IR, in the Royal College of Surgeons (RCS) rat model. Zhang et al. (2009a) showed that increased PR survival can be achieved in this model using brain-derived neurotrophic factor (BDNF) gene transfection in RPE after a combination of subretinal injection and electroporation.
A second physical approach is iontophoresis, a technique whereby charged molecules move according to an applied low voltage electrical current through a tissue within an electrical field. Transpalpebral iontophoresis was coupled to intravitreal injection of oligonucleotides (ODNs) in order to treat rd1 pups (Andrieu-Soler et al., 2007), an RP mouse model with a very rapid and severe PR degeneration due to a mutation in the PDE6B gene (Pittler and Baehr, 1991), encoding for the beta subunit of the rod-specific cyclic-GMP phosphodiesterase 6. Improvement of both outer nuclear layer (ONL) cell number and rhodopsin and PDE6B immunoreactivity was observed around twenty-days post-injection (Andrieu-Soler et al., 2007). Interestingly, Souied et al. observed that 3 consecutive transscleral iontophoresis applications of a plasmid containing the PDE6B-GFP expressing cassette in adult C57/BL6 mice, without the need of performing invasive intraocular injection, successfully transfected the outer segment (OS) of PR. Notably, authors also observed that this technique resulted in a thicker ONL and a detectable scotopic electroretinogram (ERG) measurement in rd1 mice (Souied et al., 2008). In addition, no adverse effects were observed in ocular tissues after repeated applications once the iontophoresis conditions had been optimized. Another transfection method that does not require globe penetrative procedures is suprachoroidal electrotransfer, which combines administration of plasmid DNA into the suprachoroidal space with the application of an electrical field. This approach resulted in targeting choroidal cells, RPE and potentially PR in the adult rat (Touchard et al., 2012).
To date, gene delivery to outer retinal cells by electrotransfer means have shown relatively low efficiency and sometimes cell death, most likely due to loss of cytoplasmic materials after prolonged permeabilization of the cell membrane or as a result of thermal damage (Kachi et al., 2005; Pliquett et al., 2002).
4.2. Chemical methods
Liposomes, polymers such as polypeptides and polysaccharides, and compacted nanoparticles are among the chemical methods currently available for non-viral gene delivery. These form a complex with DNA and allow passage through/into cell membranes, in some instances in a receptor-mediated manner (Chen et al., 2008b). Liposomes are self-assembled vesicular structures made up of amphiphilic lipid-molecules, such as phospholipids and cholesterol. Liposomes are biodegradable and remain localized at the site of injection (Naik et al., 2009). Transfection of RPE cells, but not of PR, was achieved by subretinal delivery of these vectors (Kachi et al., 2005). Cationic polymers spontaneously form the so-called “polyplexes” with negatively charged DNA. Numerous cationic polymers, such as polyethylenimine (PEI), poly-L-lysine, chitosan, polyamidoamine starburst dendrimers, and their derivatives, are used. PEI polyplexes have been used to transfect inner retinal cells following intravitreal injection (Liao and Yau, 2007). However, potential toxic effects have been observed with both liposomes and polyplexes when injected into the subretinal space (Charbel Issa and MacLaren, 2012; Kachi et al., 2005; Prow et al., 2008).
Subretinal administration of oligochitosan polyplexes transfected mainly RPE (Puras et al., 2013a; Puras et al., 2013b) and PR (Puras et al., 2013b), whereas intravitreal delivery transfected cells in the ganglion cell layer, blood vessels in the inner layers of the retina and PR (Puras et al., 2013b).
Solid lipid nanoparticles (SLN) conjugated with dextran and protamine, have also been tested after subretinal and intravitreal injection. Expression of GFP mediated by SLN injected subretinally was observed mainly in the RPE, and some expression was reported in PR (Delgado et al., 2012). After intravitreal injection, high levels of gene expression were found mainly in RGC (Delgado et al., 2012). On the other hand, TransIT-TKO reagent is a cationic proprietary polymer/lipid formulation, it is non-liposomal and was used to deliver siRNA subretinally to mouse RPE (Reich et al., 2003b) and intravitreally to RGC cells predominantly (Turchinovich et al., 2010).
An alternative system, which uses the C-terminal portion of VP22, a structural protein of herpes simplex virus, has been described to bind fluorescent labelled ODNs, which led to the formation of spherical particles of 0.3–1 μm in diameter that could be internalized by cells to later be released after illumination (Normand et al., 2001). With this technique, ODNs were observed in Lewis rats’ RPE, inner nuclear layer and RGC 24 h after intra-vitreal injection (Normand et al., 2005). The exact mechanism of light-induced ODN release is poorly understood but requires fluorochrome to be covalently attached to either ODN or the protein, and it is possible that thermal effects from the absorbance of light by the fluorochrome may cause particle disruption and ODN release (Normand et al., 2001). Additional studies have not been performed to gain further insight into this technique. Compacted nano-particles (NP) contain a single DNA molecule and positively-charged peptide molecules which may exhibit properties similar to those of DNA–histone complexes. The most relevant NP tested so far are peptides for ocular delivery (POD) and CK30PEG-NP. POD is a cell-penetrating peptide that was demonstrated to enter retinal cells in vivo (Read et al., 2010b), and POD nanoparticles localize to RPE and PR following subretinal injection (Johnson et al., 2008b, 2010). POD nanoparticles conjugated with polyethylene glycol (PEG–POD) containing an expression cassette for GDNF and injected subretinally result in reduction of ONL apoptosis induced by blue-light (Read et al., 2010a). To date, CK30PEG NP have yielded the most successful results of all NP. CK30PEG NP consist of a DNA molecule compacted by polyethylene glycol (PEG)-substituted 30-mer lysine peptides (Liu et al., 2003). Their minimal diameter is much smaller (usually < 25 nm) than that of other NP, which allows them to travel across nuclear pores without active transport, thus remaining episomal. Moreover, CK30PEG NP directly bind to the cell surface receptor nucleolin, which delivers them to the nucleus via nucleo-cytoplasmatic transport (Chen et al., 2008b). CK30PEG NP are well tolerated, even after repeated injections (Cai et al., 2010, 2009), result in retinal transduction in the absence of an inflammatory response after delivery to the eye (Ding et al., 2009; Farjo et al., 2006) and are non-toxic and non-immunogenic in humans (Konstan et al., 2004). Most interestingly, they present a large transgene capacity up to 14 kb (Han et al., 2012). CK30PEG NP showed persistent transgene expression (up to 1 year) after sub-retinal injection in adult mice, without extraocular diffusion (Han et al., 2012). When CK30PEG NP carrying the peripherin gene (PRPH2) were injected subretinally in the rds+/− mouse model of RP, a phenotypic improvement was observed for 120 days after injection (Cai et al., 2010, 2009). Han et al. (2012) have recently tested CK30PEG NP for the delivery of the ABCA4 gene (encoding for the ATP binding cassette transporter) in the Abca4−/− mouse model of STGD. They detected ABCA4 transgene expression for up to 8 months after injection and found both improved recovery of dark adaptation and reduced lipofuscin accumulation. These results represent the first evidence of effective non-viral vector-mediated delivery of large genes, such as ABCA4, to the adult PR cell layer.
4.3. Additional strategies to improve non-viral vector-mediated transgene expression
Although CK30PEG-mediated gene expression was long-lasting in the mouse retina, non-virally delivered genes usually undergo gene silencing. Differential methylation and potentially immunogenic CpGs, which are present in prokaryotic plasmid backbones, may promote gene silencing (Chen et al., 2004). Minicircle DNA vectors devoid of bacterial DNA were built in order to circumvent this problem, resulting in persistent and high-level transgene expression in vivo (Chen et al., 2003; Gill et al., 2009; Gracey Maniar et al., 2013). Even if the retinal cell population is post-mitotic, meaning that episomal non-viral vectors should be retained, integrase from bacteriophage ΦC31 has been demonstrated to confer genomic integration of plasmid DNA and long-term expression in mammalian cells, including RPE (Chalberg et al., 2005). In this study, subretinal injection followed by electrotransfer of a plasmid encoding the ΦC31 integrase, co-injected with a reporter plasmid led to expression in rat RPE cells for 4–5 months, compared to 3–4 weeks in the absence of the plasmid coding for the ΦC31 integrase. Consistently, addition of the Sleeping Beauty transposon results in stable expression of pigment epithelium-derived factor (PEDF) in ARPE19 cells (Johnen et al., 2012). Recently it has been shown that the total levels of transgene expression mediated by PEG–POD NP containing plasmids with a scaffold matrix attachment region (S/MAR) and/or CpG depletion were not significantly different from expression levels of PEG–POD NP, suggesting that the S/MARs or the deletion of CpGs were playing no significant role in extending the duration of transgene expression (Binder et al., 2013). In contrast, the data showed that there is an inverse correlation between concentration of DNA and longevity of transgene expression: while smaller amounts of total DNA led to less transgene expression, the duration of such expression was extended (up to 10 weeks post-injection), suggesting that the duration of transgene expression from PEG–POD containing S/MAR and CpG depletion may be concentration-dependent. However, the S/MAR and CpG modifications of the plasmid backbone influenced the stability of transgene expression. When levels of transgene expression from PEG–POD DNA NP were compared to their respective non-compacted plasmid DNA controls, there was a consistent relative difference that fluctuated over the 10-week period, with a peak at 5 weeks. No such relative peak of gene expression was observed from PEG–POD with S/MAR and CpG depleted NP, suggesting that these elements lead to a more stable transgene expression (Binder et al., 2013). The NP expressing GDNF led to improvements in light-induced PR degeneration models up to 77 days post-injection (Binder et al., 2013). In another work, S/MAR was incorported in a plasmid containing a vitelliform macular dystrophy 2 (VMD2) promoter driving the expression of EGFP. The construct was shown to target the RPE driving long-term expression that lasted for up to two and a half years (the last timepoint of the analysis) when delivered either naked or by means of CK30PEG NP (Koirala et al., 2013). Transgene expression levels were several fold higher than with plasmid alone or with NP without S/MARs. Furthermore, S/ MAR-coupled NP or plasmid containing the VMD2 promoter driving the expression of hRPE65, encoding for the RPE all-trans retinol isomerase, led to retinal improvement in the Rpe65−/− mouse model (Koirala et al., 2013).
These promising data indicating long-term rescue after using CK30PEG deserve further investigation in large animal models and other forms of IR.
5. Adeno-associated viral vectors
The adeno-associated virus (AAV) is a small (25 nm), non-enveloped, icosahedral virus that packages a linear single-stranded DNA genome of ~4.7 kb (Berns and Giraud, 1996). It belongs to the Parvoviridae family, genus Dependovirus, and receives its name from the ability to replicate in the presence of helper viruses such as adeno-, herpes-, or papilloma-viruses. The wild-type AAV genome includes the following open reading frames: rep, required for DNA replication, and cap, which encodes for both the three structural proteins that form the icosahedral capsid and for a protein essential for capsid assembly within the nucleolus (Sonntag et al., 2010).
The AAV genome is flanked by two 145 bp long palindromic inverted terminal repeats (ITRs) which form hairpin-loop secondary structures at the strand termini. The only viral sequences that are retained in cis in the recombinant AAV vector genome are the ITRs. Rep and cap, which are exchanged with the exogenous DNA, are provided in trans together with the adenoviral helper functions during the AAV production process to the packaging cells (Daya and Berns, 2008).
AAV are currently the most favoured vectors for gene therapy of IR given their ability to efficiently target various retinal layers, most likely due to their relatively small size and ability to spread after subretinal delivery. In addition, AAV have an excellent safety profile and low immunogenicity which allows for (i) long-term expression of the therapeutic gene after a single administration, a desirable feature when treating a chronic condition like IR and (ii) re-administration of AAV to the subretinal space (Bennett et al., 2012). Thus, AAV have been used in a number of successful pre-clinical and clinical studies (see Section 5.3). Lastly, dozens of different AAV variants have been identified thus far, either isolated as infectious or as molecular clones. Each serotype has its own external capsid protein. The availability of different capsids and the versatility of the AAV production system allows exchange of capsids among various AAV to generate hybrid vectors containing a genome with the same ITRs (i.e. from AAV2) and the capsid from a different variant (Auricchio, 2003). AAV obtained through this trans-capsidation system are named AAV2/n, where the first number refers to the ITRs and the second to the capsid (Surace and Auricchio, 2008).
5.1. AAV transduction properties in the retina
5.1.1. Naturally-occurring AAV serotypes/variants
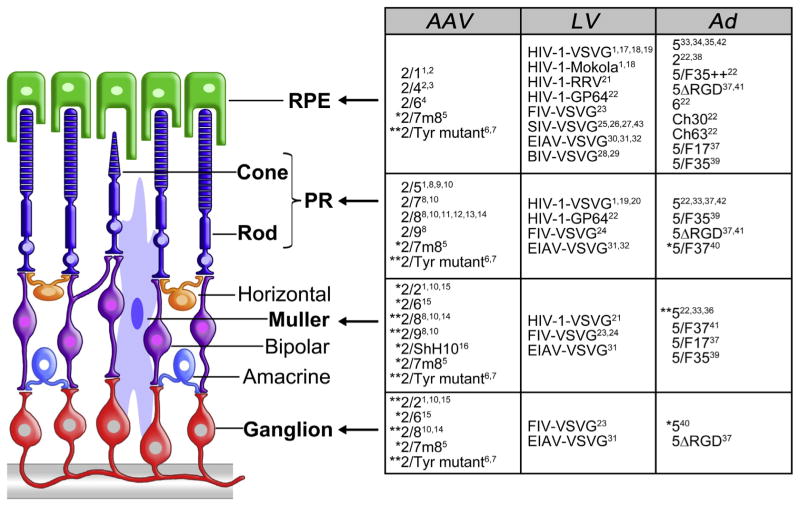
Each AAV serotype has unique transduction characteristics (i.e target cells, kinetic of transgene expression) because different AAV capsids interact with different receptors on target cells and/or impact the AAV post-entry transduction steps (Sanlioglu et al., 2001). This allows the user to select the most appropriate AAV serotype to transduce the retinal cell layer of interest (Fig. 1; Allocca et al., 2007; Auricchio et al., 2001; Lebherz et al., 2008). Cell transduction and targeting are additionally influenced by the route of vector administration. Following subretinal delivery, virtually all AAV serotypes tested efficiently transduce RPE, with AAV2/1, AAV2/ 4 and AAV2/6 being the most specific in various animal models (Auricchio et al., 2001; Dinculescu et al., 2005; Rabinowitz et al., 2002; Weber et al., 2003; Yang et al., 2002). This may result from (i) the RPE accessibility from the subretinal space into which vectors are injected, (ii) an inherent permissiveness of the RPE to AAV infection (specifically, the presence of AAV receptors and co-receptors at the RPE cell membrane), and/or lastly, (iii) the phagocytic properties of the RPE that may facilitate entry of AAV particles (Vandenberghe and Auricchio, 2012). Conversely, the levels of PR transduction vary significantly among different sero-types. AAV2/5, 2/7, 2/8 and 2/9 have all been demonstrated to efficiently transduce PR, in addition to RPE (Allocca et al., 2007; Auricchio et al., 2001; Lebherz et al., 2008; Lotery et al., 2003), with AAV2/8 being the most efficient serotype in mice (Allocca et al., 2007), pigs (Manfredi et al., 2013; Mussolino et al., 2011a), dogs (Stieger et al., 2008) and non-human primates (Vandenberghe et al., 2011). Particular attention has been recently directed towards the identification of AAV vectors that efficiently transduce cone PR in addition to rods, which make up the majority of PR in the retina. This is required for gene therapy of cone and cone-rod dystrophies, such as Stargardt disease, achromatopsia and age-related macular degeneration. These diseases are primarily characterized by cone involvement, with possible concomitant loss of rods. The AAV serotypes’ ability to transduce cones has been evaluated in the pig (Manfredi et al., 2013) and non-human primate (Vandenberghe et al., 2013) retinas, which present cone density and distribution more similar to humans than mice. Among these, serotypes AAV2/5 (Alexander et al., 2007; Komaromy et al., 2010; Mancuso et al., 2009), AAV2/8 (Manfredi et al., 2013) and AAV2/9 (Vandenberghe et al., 2013) were found to present the highest cone transduction properties.
Fig. 1.
Retinal tropism of AAV, Ad, and LV. Arrows point at target cells (in bold) transduced after subretinal injection of the vectors indicated in the corresponding rows of the adjacent table. *: transduces the indicated cell type after intravitreal injection. **: transduces the indicated cell type after both intravitreal or subretinal injection. Tyr: tyrosine. References cited in the figure: 1. Auricchio et al., 2001; 2. Rabinowitz et al., 2002; 3. Weber et al., 2003; 4. Yang et al., 2002; 5. Dalkara et al., 2013; 6. Petrs-Silva et al., 2009; 7. Petrs-Silva et al., 2011; 8. Allocca et al., 2007; 9. Lotery et al., 2003; 10. Lebherz et al., 2008; 11. Mussolino et al., 2011a; 12. Vandenberghe et al., 2011; 13. Stieger et al., 2008; 14. Igarashi et al., 2013; 15. Hellstrom et al., 2009; 16. Klimczak et al., 2009; 17. Bainbridge et al., 2001; 18. Bemelmans et al., 2005; 19. Miyoshi et al., 1997; 20. Lipinski et al., 2014; 21. Greenberg et al., 2007; 22. Puppo et al., 2014; 23. Cheng et al., 2005; 24. Lotery et al., 2002; 25. Duisit et al., 2002; 26. Ikeda et al., 2003; 27. Miyazaki et al., 2003; 28. Molina et al., 2004b; 29. Takahashi et al., 2002; 30. Balaggan et al., 2006; 31. Binley et al., 2013; 32. Zallocchi et al., 2014; 33. Bennett et al., 1994; 34. Li et al., 1994; 35. Kreppel et al., 2002; 36. Lamartina et al., 2007; 37. Cashman et al., 2007; 38. Jomary et al., 1994; 39. Mallam et al., 2004; 40. Von Seggern et al., 2003; 41. Sweigard et al., 2010; 42. Wu et al., 2011; 43. Ikeda et al., 2009b.
Intravitreal delivery is the preferred route for inner retina targeting. AAV2/2, AAV2/6 and AAV2/8 are currently the serotypes showing the highest transduction efficiency of retinal cells from the vitreous, with a pattern mainly restricted to RGC and Müller cells (Ali et al., 1998; Hellstrom et al., 2009; Igarashi et al., 2013; Lebherz et al., 2008). The inability of AAV vectors to transduce the outer retina from the vitreous side appears to be mainly due to the presence of a thick ILM, especially in large animals, and the relative abundance of AAV receptors that capture vectors after intravitreal administration (Dalkara et al., 2009). Indeed, if the retinal architecture is altered by a degenerative process (Kolstad et al., 2010; Park et al., 2009; Vacca et al., 2014), or by enzymatic digestion with Pronase E (Dalkara et al., 2009), the diffusion of AAV viral particles to the outer retina from the vitreous side is enhanced. When the ILM is disrupted, AAV serotypes 2/1, 2/2, 2/5, 2/8, and 2/9 transduce the various retinal cell layers to a variable degree, with AAV2/5 being the most efficient of all (Dalkara et al., 2009).
5.1.2. Modified AAV capsids
In addition to the large portfolio of naturally-occurring AAV serotypes, novel AAV capsid variants with enhanced gene transfer efficiency and altered tropism have been generated in recent years by either rational design or directed evolution (Vandenberghe et al., 2009). Rational design is a method that alters the capsid in silico and in vitro based on knowledge of the structure–function relationship of the virion. Conversely, directed evolution does not require a full understanding of the structure–function biology of AAV capsids for successful modification, since it is based on random mutagenesis of wild-type AAV capsids that are then screened by applying selective conditions.
Successful examples of rational design are the tyrosine-mutant capsids developed by the group of Srivastava. Phosphorylation of specific tyrosine residues within the AAV capsid is thought to be responsible for AAV targeting to proteasome and degradation (Zhong et al., 2008a). Therefore, the authors sought to evaluate whether the exchange of conserved tyrosine residues with phenylalanine in AAV capsids of different serotypes results in higher levels of transduction compared to wild-type AAV capsids, presumably allowing AAV particles to escape the proteasome and reach the nucleus. Indeed the AAV tyrosine-mutants have increased gene transfer efficiency both in vitro and in vivo (Petrs-Silva et al., 2009; Zhong et al., 2008b). In particular AAV2-Y444F, AAV2-Y730F, AAV8-Y733F and AAV9-Y446F have: (i) an efficacy similar to that of wild-type AAV at lower doses; (ii) broader tropism within the neuronal retina and enhanced diffusion across the retina (Petrs-Silva et al., 2009). Importantly, the AAV8-Y733F variant has been successfully used to drive long-term expression of PDE6B and improvement of the retinal phenotype in rd10 mice, a model of autosomal recessive RP, which is challenging to achieve given the model’s aggressive and severe PR degeneration (Pang et al., 2011). These initial findings pushed the generation of other capsid mutants, among which, quadruple and pentuple tyrosine-mutant AAV2 vectors have confirmed efficient transduction of murine PR following intravitreal delivery (Petrs-Silva et al., 2011). A further mutation involving a threonine residue (T491V) in the quadruple AAV2/2 capsid resulted in a 3.5-fold increase in PR transduction efficiency after intravitreal injection (Kay et al., 2013). However, preliminary results in the adult canine retina, which presents robust physical barriers between the vitreous and inner retina, have shown that transduction efficiency of the quadruple AAV2/2 mutant following intravitreal delivery is more limited than that in the murine retina (Mowat et al., 2014). This was confirmed by the more widespread and efficient transduction of both PR and RPE achieved in the same study using intravitreal injection in the juvenile canine retina, which presents fewer barriers to AAV penetration, than that of the adult.
Directed evolution has been pursued as a means to select AAV derived from combinatorial libraries. Using this approach, the labs of David Schaffer and John Flannery identified AAV with improved glial tropism, and among these, a variant of AAV6 named ShH10, whose intravitreal administration in a rat model results in efficient and selective Müller glia transduction (Klimczak et al., 2009). Interestingly, ShH10-mediated delivery of GDNF was found to significantly ameliorate degeneration and retinal function in a rat model of RP (Dalkara et al., 2011). Another example of successful identification of AAV variants through directed evolution is the 7m8 mutant of AAV2/2, recently identified by Dalkara and coworkers (Dalkara et al., 2013). This mutant has demonstrated widespread transduction of PR and RPE after intravitreal delivery in the murine retina. This rescued the phenotype of the Rs1h−/− and rd12 mouse models of IR. In particular, the rescue achieved in the rd12 mice, whose phenotype results from mutations in the RPE65 gene expressed in the RPE, further proves this vector’s ability to reach the RPE from the vitreous. However, differently from what was observed in mice, intravitreal delivery of doses as high as 5 × 1012 viral particles of 7m8 AAV to the nonhuman primate retina resulted in gene expression in both punctuated regions across the retina and inside the fovea, but not in the RPE. This appears to confirm that intravitreal administration of AAV does not overcome the physical barriers from the vitreous side as efficiently in the primate retina as it does in the murine, where such barriers are less substantial. In addition, inflammation was observed in retinal areas expressing high levels of potentially immunogenic EGFP.
5.1.3. Strategies to modulate AAV-mediated transgene expression
The use of different administrative routes, as well as alternative serotypes can influence the spatial distribution of the vector within the retina. However, since tight spatial control of transgene expression can represent a crucial requirement for effective retinal gene therapy (Lipinski et al., 2013; Stieger et al., 2011), particularly in cases in which ectopic transgene expression might be detrimental, the use of promoters or microRNA target regions to restrict transgene expression can be a valid option. The majority of gene therapy studies use strong ubiquitous viral promoters, such as the immediate early cytomegalovirus (CMV) promoter or the chimeric chicken beta-actin (CBA)/CMV enhancer promoter. However, AAV specificity can be controlled by cell-type-specific promoters. RPE-specific cell targeting can be achieved with either the human RPE65 (Nicoletti et al., 1998) or the VMD2 promoter (Esumi et al., 2004). Conversely, PR can be transduced by either the human rhodopsin (RHO) (Flannery et al., 1997) or the rhodopsin kinase promoter (RHOK) (Young et al., 2003). Notably, the RHOK promoter has been found to achieve an efficient transduction of both rods and cones PR in mice (Khani et al., 2007) and nonhuman primates (Boye et al., 2012). Recently, high levels of combined cone and rod transduction have been achieved with either the ubiquitous CMV (Manfredi et al., 2013) or the PR-specific IRBP (Beltran et al., 2012) promoter. In contrast, cone-specific targeting can be achieved with the arrestin-3 promoter (Carvalho et al., 2011), while different cone subtypes might be targeted with different opsin promoters (Alexander et al., 2007; Fei, 2003; Komaromy et al., 2008). The mouse glial fibrillary acidic protein (GFAP) promotor has been successfully used to target Müller cells (Kuzmanovic et al., 2003) as factory cells to produce neurotrophic factors (Dorrell et al., 2009). It is important to take into account that although cell-specific promoters allow greater spatial control, they often yield lower levels of expression than ubiquitous promoters (Lipinski et al., 2013). A possible solution is the inclusion of binding sites for differentially-expressed microRNAs (miRNAs) in the 3′UTR of the transgene. Indeed, miRNAs can bind to defined sequences within the 3′UTRs of mammalian mRNAs, resulting in translational suppression or silencing (Lipinski et al., 2013). Including miRNA-124 or -204 target sites in the transgene expression cassette results in efficient silencing in either the RPE or PR, respectively (Karali et al., 2011). However, higher doses of AAV vectors containing miRNA target sites may saturate the corresponding endogenous miRNA and lead to variable off-target expression (Karali et al., 2011).
5.2. Challenges in the use of AAV vectors in the retina
Two major limitations of AAVs in the retina are its slow onset of transgene expression due to second strand synthesis required for transcription, and the vector’s limited cargo capacity. The first limitation has been overcome with the use of self-complementary AAV (scAAV) (reviewed in McCarty, 2008). These vectors package a single genome designed to include two copies of the transgene cassette, one in sense and the other in reverse complement orientation, so that the complementary strands can anneal to form double-stranded AAV genomes in the nucleus (Lipinski et al., 2013). scAAV2/2, 2/5 and 2/8 have shown faster onset and stronger levels of transgene expression than their single-stranded counterparts in the retina of mice (Kong et al., 2010; Natkunarajah et al., 2008; Yokoi et al., 2007) and dogs (Petersen-Jones et al., 2009), theoretically allowing the delivery of lower vector doses to obtain similar levels of transgene expression. The reduction in the time required to obtain the transduction peak may be relevant in developing treatment of diseases with a rapid retinal degeneration. Indeed, scAAV have been successfully used to substantially restore visual function within 4 days of treatment in two genetically distinct models of Leber Congenital amaurosis due to RPE65-deficiency (Pang et al., 2010). Importantly, scAAV vectors have been used in conjunction with capsid mutants to restore vision in the Aipl1−/− mouse model of early onset LCA4. Subretinal delivery of scAAV encoding for AIPL1 was found to be more efficacious than single stranded vectors, especially when administered during the active phase of retinal degeneration (Ku et al., 2011). However, the need to package double-stranded genomes reduces the already-limited AAV cargo capacity. This limits the number of diseases that can be treated with scAAV, although small genes and RNA-based therapies can still be accommodated. In addition, it is not clear whether the scAAV vectors’ ability to achieve similar levels of transgene expression with lower vector doses in comparison to ssAAV is due to the inherent properties of scAAV or to a systematic erroneous titration (Fagone et al., 2012) resulting in the administration of higher-than-expected doses of scAAV.
The major limitation of using AAV for gene replacement in PR is the insufficient cargo capacity of 4.7 kb. Certain forms of IR are caused by mutations in genes whose cDNA exceeds 5 kb (such as ABCA4, MYO7A, or CEP290, mutated in STGD, USH1B, and LCA10, respectively), thus making it hard to treat them with AAV. Different strategies to overcome AAV cargo limitations have been developed. One is based on packaging oversize genomes, i.e. larger than 5 kb (Allocca et al., 2008; Grieger and Samulski, 2005; Hirsch et al., 2010). Notably, oversize AAV have been found to successfully express full-length proteins in vitro and in the retina of mouse models of IR, resulting in significant and stable morphological and functional improvement of the IR phenotype (Allocca et al., 2008; Lopes et al., 2013). However, the genomes contained in oversize AAV vectors appear highly heterogeneous in size and are predominantly shorter than expected (Dong et al., 2010; Hirsch et al., 2010, 2013; Lai et al., 2010; Wu et al., 2010). This limits their use in clinical settings. Alternatively, the ability of heterologous AAV genomes to form intermolecular concatemers in target cell nuclei (Duan et al., 1998) can be exploited to express large genes, which can then be split into two halves, independently packaged in two different (dual) AAV vectors (Ghosh and Duan, 2007). Various dual AAV strategies [referred to as trans-splicing (Yan et al., 2000), overlapping (Duan et al., 2001) and hybrid dual-vector strategies (Ghosh et al., 2008); see Fig. 2] have been described, and they have been used to efficiently deliver large genes to different tissues. In the trans-splicing approach the 5′-half vector has a splice donor (SD) signal at the 3′ end of the AAV genome, while the 3′-half vector carries a splice acceptor (SA) signal at the 5′ end of the AAV genome. This allows splicing of a single large mRNA molecule following head-to-tail concatemerization of the two AAV (Yan et al., 2000). In the overlapping approach, the dual AAV genomes share overlapping sequences, thus the reconstitution of the large gene expression cassette relies on homologous recombination (Duan et al., 2001). The hybrid dual AAV approach is a combination of the two previous approaches and it is based on adding a highly recombinogenic exogenous sequence to the trans-splicing vectors in order to increase recombination efficiency (Ghosh et al., 2011, 2008; Trapani et al., 2014). This recombinogenic sequence is placed downstream of the SD signal in the 5′-half vector and upstream of the SA signal in the 3′-half vector, so that it is spliced out from the mRNA after recombination. The recombinogenic sequences used to induce the recombination between dual AAV hybrid vectors have thus far derived from regions of either the alkaline phosphatase gene (AP, Ghosh et al., 2008, 2011) or the F1 phage genome (AK, Trapani et al., 2014). We have recently compared side-by-side the efficiency of the various oversize, dual AAV overlapping, trans-splicing and hybrid strategies for AAV-mediated large gene transduction in vitro and in the mouse retina (Trapani et al., 2014). We found that dual AAV vectors are more efficient than oversize vectors, both in vitro and in the murine retina. However, different groups have found different efficiency of PR transduction with dual AAV overlapping vectors; we found that dual AAV overlapping vectors drive efficient transgene expression in RPE, but not PR, in mice and pigs (Trapani et al., 2014). Others reported low levels of dual AAV overlapping-mediated MYO7A expression and mouse phenotype correction (Lopes et al., 2013), and subsequently found these levels to be more robust (Dyka et al., 2014). Interestingly, different groups have reported that dual AAV trans-splicing and hybrid vectors efficiently reconstitute various transgenes in mouse (Dyka et al., 2014; Lopes et al., 2013; Reich et al., 2003a; Trapani et al., 2014) and pig PR (Colella et al., 2014; Trapani et al., 2014). Consequently, subretinal administration of dual AAV trans-splicing and hybrid AK vectors encoding for ABCA4 and MYO7A improves the phenotype in mouse models of STGD and USH1B, respectively (Trapani et al., 2014). Notably, these observations provide the first evidence of efficacy of dual AAV strategies for gene therapy of blinding conditions, which require large gene transfer to PR as well as RPE. Remarkably, differences in the design of the oversize and dual AAV vectors and in the transgene used seems to have a strong impact on the relative efficacy of the AAV-based systems for large gene transfer; for example in a recent study which uses the luciferase reporter gene, the oversize platform outperforms the trans-splicing in the mouse retina (Hirsch et al., 2013). In conclusion, the genome discrete nature and efficiency of dual AAV vectors make them preferable over oversize vector strategies for further clinical application.
Fig. 2.
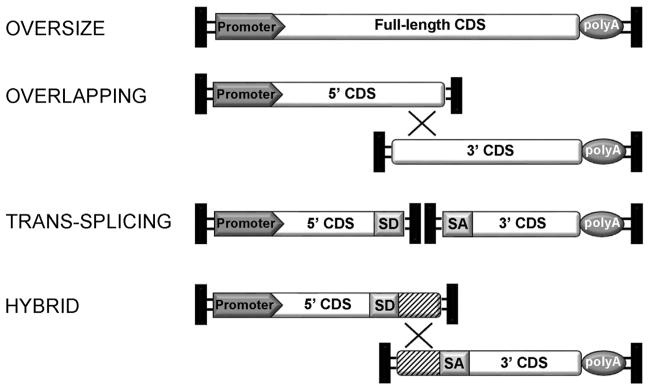
Schematic representation of AAV-based strategies for large gene transduction. X shows overlapping regions for homologous recombination. The striped boxes depict the exogenous recombinogenic sequence used in the dual hybrid approach. CDS: coding sequence; SD: splicing donor signal; SA: splicing acceptor signal; polyA: poly-adenylation signal.
5.3. AAV as a tool for treatment of IR
AAV serotypes are available in dozens of different variants able to transduce the various cell layers in the retina. This makes them useful in gene-dependent and -independent approaches in various animal models of IR. Thus, some of these promising pre-clinical applications have been translated into successful IR clinical trials, as reviewed below.
5.3.1. AAV for gene replacement in RPE
Most forms of IR are recessively inherited and are determined by loss-of-function mutations. These mutations can be tackled by gene replacement or addition directed to PR or RPE. Historically, diseases originating in the RPE have been found to be more amenable to gene therapy than diseases due to mutation in genes expressed in PR. This could be due to more efficient AAV transduction in RPE than in PR as discussed in Section 5.1.1., or to the different trend of retinal degeneration, that is faster in diseases originating in PR than in those originating in RPE (Smith et al., 2009).
Several proof-of-concept studies of AAV-mediated correction of RPE-originating IR have been provided in the last decade. Among them, the most effective are those for IR due to mutations in either MERTK, encoding for the mer receptor tyrosine kinase, or in RPE65, both of which have been translated into human clinical trials. MERTK is required for efficient phagocytosis of PR OS by the RPE, and its deficiency leads to profound retinal degeneration (Herron et al., 1969). Indeed, a MERTK mutation was also identified in the well known RCS rat model of RP (D’Cruz et al., 2000). AAV2/2 vectors have been subretinally delivered to transfer MERTK to the RPE of RCS rats, leading to a decrease in OS debris formation, prolonged PR survival and transient visual function improvement, for up to 9 weeks (Smith et al., 2003). The use of an AAV2/8 tyrosine mutant has been recently associated with improved recovery (Deng et al., 2012). AAV2/8 tyrosine mutant-mediated MERTK delivery to the RCS rat has indeed shown to preserve retinal structure and function for up to at least 8 months after treatment, probably due to the increased efficiency of the AAV and the earlier injection. Successful preclinical data using an AAV2/2-VMD2-hMERTK vector (Conlon et al., 2013) have finally led to a phase I clinical trial (NCT01482195). Three patients have been treated with subretinal injection, and no adverse events have been reported thus far (Boye et al., 2013a).
Currently, RPE65 gene delivery represents the most successful example of ocular gene therapy. Indeed, LCA caused by a mutation in RPE65 is the first IR for which retinal gene therapy has been developed up to phase I/II clinical trials, thanks to the sound results obtained in preclinical studies in small and large animal models. A pioneering study in the Briard dog model of LCA2 carrying an homozygous RPE65 mutation demonstrated that a single subretinal injection of an AAV2/2 vector carrying the canine RPE65 gene under the control of a strong CBA promoter was sufficient to restore visual function in affected dogs, as assessed by ERG and behavioural testing (Acland et al., 2001). This result has been replicated by several other groups (Annear et al., 2011; Le Meur et al., 2007; Narfstrom et al., 2003) and has opened the doors to the first clinical trials testing the safety and efficacy of subretinal administrations of AAV2/2 in LCA2 patients (NCT00516477; NCT00643747; NCT00481546). The longest follow up reported is 3 years (Jacobson et al., 2012; Testa et al., 2013). Because of the many different variables in the three studies (regulatory sequences, method of vector production and characterization, age of LCA2 patients and outcome measures), it is difficult to directly compare the results from one study to the other. However, some important conclusions can be drawn from these studies: (i) AAV2/ 2 subretinal administration is safe and well tolerated (Bainbridge et al., 2008; Cideciyan et al., 2009a, 2009b; Hauswirth et al., 2008; Jacobson et al., 2012; Maguire et al., 2009, 2008; Simonelli et al., 2010); (ii) AAV2/2 subretinal administrations resulted in improvement of retinal and visual function in all trials (Bainbridge et al., 2008; Cideciyan et al., 2008, 2009a, 2009b; Hauswirth et al., 2008; Jacobson et al., 2012; Maguire et al., 2009, 2008; Simonelli et al., 2010), although at different levels, with reactivation of the visual cortex (Ashtari et al., 2011); (iii) one trial has shown evidence that the maximal efficacy was obtained in the youngest LCA2 patients, presumably with better retinal preservation (Maguire et al., 2009); (iv) readministration of AAV2/ 2 to the subretinal space is safe and effective (Bennett et al., 2012). The LCA2 trials, however, have raised some concerns over the invasiveness of the subretinal vector delivery to a diseased retina, especially in the foveal region (Jacobson et al., 2005; Maguire et al., 2008). In the 3-year study (Jacobson et al., 2012) approximately half of the patients who experienced a vector bleb that detached the fovea during surgery lost foveal thickness. This suggests that vector delivery to the fovea should be avoided, while delivery to the extrafoveal region with greater ONL thickness preservation (Jacobson et al., 2005, 2008) may be a valid alternative to achieve significant visual improvement. In addition, Cideciyan et al. (2013) have recently published that, although gene therapy leads to long-term improvement of vision in LCA2 patients, it does not halt the degenerative process, as the thickness of the PR cell layer continued to diminish. The authors hypothesized that this was due to irreversible accumulation of changes in the patients’ retina at the time of the injection, which could not be reverted by AAV mediated RPE65 delivery (Cepko and Vandenberghe, 2013). Efforts to initiate a phase III clinical trial of AAV2/2-RPE65 are currently underway (NCT00999609) (Boye et al., 2013a). In addition, Le Meur and colleagues have exploited the capacity of an AAV2/4 vector to target transgene expression specifically to the RPE, in conjunction with a specific RPE65 promoter, to restore vision in the Briard dog model (Le Meur et al., 2007). This set up should increase the specificity and safety of the therapy. They demonstrated rescue of rod and cone function in these animals, which was at least as efficient as AAV2/2 treatment in dogs. This has led to further evaluation of AAV2/4 in a clinical trial for LCA2 (NCT01496040), the results of which have not yet been published.
5.3.2. AAV for gene replacement in PR
Gene transfer to PR has seen enormous improvement in recent years thanks to the development of more efficient AAV vectors. Indeed, in the last years, effective gene delivery has been developed in animal models of RP and LCA due to mutation in genes including: PDE6B, PRPH2, GNAT2 encoding for alpha transducin subunit, CNGB3 encoding for the cyclic nucleotide gated channel subunit beta, GUCY2D encoding for the retinal guanylate cyclase 2D, AIPL1 encoding for the aryl hydrocarbon receptor-interacting protein like 1, RPGRIP1 encoding for the Retinitis pigmentosa GTPase regulator interacting protein 1, RS1 encoding for retinoschisin (reviewed in Stieger et al., 2011). Historically, AAV-mediated delivery of the PDE6B gene represents the first evidence of the benefits as well as limitations of gene replacement in mouse PR (Jomary et al., 1997). Indeed, intravitreal delivery of AAV2/2 vectors encoding for PDE6B in the rd1 mouse resulted in very limited rescue of the phenotype. This revealed the necessity for AAV vectors that transduce PR more efficiently. Indeed, better but transient rescue has been achieved in rd10 mice, yielding slightly slower PR degeneration with AAV2/5 (Pang et al., 2008) and AAV2/8 (Allocca et al., 2011) than with AAV2/ 2. As previously discussed, more effective and long-term rescue has been recently achieved with AAV8-Y733F in rd10 mice (Pang et al., 2011), although a direct comparison cannot be made as the animals in this study were dark-reared and this protects from PR degeneration. Recently, AAV2/5 and AAV2/8 mediated PDE6B gene delivery has, for the first time, showed long-term restoration of retinal function and vision in the rcd1 dog model of PDE6B deficiency, providing great promise for human translation (Petit et al., 2012). AAV2/8 and its tyrosine mutant have also shown their efficacy in the Aipl1−/− mouse model of LCA4 caused by AIPL1 deficiency (Ku et al., 2011; Sun et al., 2010; Tan et al., 2009; Testa et al., 2011). Notably, this mouse model has the most severe PR degeneration of any other model, and its rescue provides strong evidence that this generation of AAV vectors could be a very effective tool in achieving therapeutic levels of transduction in PR.
Both AAV2/5 and AAV2/8 have been successfully applied to the retina of animal models of cone-specific or cone–rod dystrophies (Colella and Auricchio, 2012; Stieger et al., 2011). Mice (Alexander et al., 2007; Carvalho et al., 2011; Michalakis et al., 2010; Pang et al., 2012), dogs (Komaromy et al., 2010) and squirrel monkeys (Mancuso et al., 2009) with achromatopsia or color blindness have been successfully treated long-term after single subretinal administrations of AAV. A promising proof-of-concept of AAV-mediated gene replacement has also been obtained in mouse models of LCA1 caused by mutations in GUCY2D encoding for the guanylate cyclase-1 gene. GUCY2D represents one of the most frequently mutated LCA genes. The LCA1 preserved retinal structure further suggested that this could be a very good candidate for gene replacement therapy. AAV2/5 and AAV2/8 (either wild-type or in its Y733F version) have been found to provide long-term rescue of the guanylate cyclase 1 knock-out mouse model, which exhibits loss of cone function and morphology (Boye et al., 2010, 2011; Manfredi et al., 2013; Mihelec et al., 2011). These positive results were recently confirmed in the double guanylate cyclase 1 and 2 knock-out mouse model (Boye et al., 2013b), which lacks both rod and cone function. However, given the high density of cones in the foveal region and the issues associated with subretinal injection in regions close to the fovea, the identification of vectors capable of transducing PR following intravitreal delivery would be an important step toward the clinical translation of gene delivery to the cone-enriched macula.
AAV have also demonstrated efficacy in conditions affecting multiple retinal tissues, such as choroideremia (CHM), an X-linked degeneration of choroid, PR and RPE (Coussa and Traboulsi, 2012). CHM is caused by loss-of-function mutations in the CHM/REP1 gene that encodes the Rab escort protein 1 (REP1). Tolmachova et al. (2013) have recently demonstrated AAV2/2’s ability to efficiently deliver REP1 to both PR and RPE of CHM mice and human retinal explants. Notably, the levels achieved were able to rescue the phenotype of the CHM mouse model. In 2011 a phase I/II clinical trial with AAV2/2 was started (NCT01461213) in order to determine whether high levels of transduction could be reached. The initial results of the trial have been recently published and have confirmed that subretinal administration of AAV2/2 is well tolerated in humans. In addition, recovery of visual acuity and improvement in maximal retinal sensitivity in the eyes treated was observed despite retinal detachment (Maclaren et al., 2014).
5.3.3. AAV for gene replacement in RGC
Successful gene replacement has been recently obtained in a disease that requires transduction of RGC. Leber hereditary optic neuropathy (LHON) is caused by mutations in several genes encoding subunits of the mitochondrial respiratory NADH-ubiquinone oxidoreductase complex (complex I). This results in RGC death and loss of vision in young adults (Tonska et al., 2010). Development of gene therapies for LHON has been limited by the need to deliver therapies to mitochondria, however it has been found that inserting an N-terminal mitochondrial targeting sequence in wild-type proteins mediates efficient delivery into RGC mitochondria (Qi et al., 2007). Approximately half of LHON cases are due to a mutation in the mitochondrial gene encoding subunit 4 of NADH dehydrogenase (ND4). Allotopic AAV2/2-mediated expression in mice of human ND4 fused to a mitochondrial targeting sequence was found to result in proper ND4 localization to the RGC mitochondria in the absence of cell death (Guy et al., 2009). Notably, AAV2/2-ND4 has been further evaluated for its transduction abilities in ex vivo human eyes, for its efficacy in a rodent model of LHON, and for its safety in nonhuman primate retinas (Koilkonda et al., 2014). These evaluations confirmed the positive results obtained in mice. Effective rescue of an induced murine model of optic neuropathy has also been recently achieved by intravitreal injection of an AAV2/2 expressing the nuclear gene NADH-quinone oxidoreductase (NDI1) taken from yeast (Saccharomyces cerevisiae), which encodes a single subunit complex I equivalent containing an endogenous signal for mitochondria import (Chadderton et al., 2013). The use of mitochondrial targeting sequences alone, however, could limit the import of allotopic protein to the mitochondria because of the lack of a specific 3′UTR, which can cooperate with the mitochondrial targeting signal. To overcome this issue, the group of Corral-Debrinski developed an approach that combines the use of both mitochondrial targeting signals and 3′UTRs from nuclear genes whose mRNA has been found to localize to the mitochondrial surface (Kaltimbacher et al., 2006). This was found to result in targeting of the hybrid-mRNA to the mitochondrial surface and in the increase in the amount of protein fully imported in mitochondria compared with the presence of the mitochondrial targeting signal alone. Preliminary results have shown that optimized allotopic expression of the ND4 gene by transfection prevents mitochondrial dysfunction in both human fibroblasts (Bonnet et al., 2008) and in a LHON rat model (Ellouze et al., 2008). Further investigation of AAV2-mediated delivery of the optimized genes in the LHON rat model and non-human primate retinas seems to confirm robust, long-duration gene expression, and safety (Cwerman-Thibault et al., 2014). These successful examples of gene therapy for a mitochondrial disease have been translated into two ongoing clinical trials for LHON (NCT01267422, NCT02064569).
Another promising proof-of-concept for the treatment of diseases characterized by mitochondrial impairment has been recently obtained by the group of Corral-Debrinski. This group has shown that AAV2/2-mediated intravitreal delivery of neuroglobin, a powerful neuroprotectant, prevents respiratory chain impairment and preserves RGC and their axons from degeneration in the Harlequin (Hq) mouse model of human neurodegenerative diseases due to respiratory chain impairment (Lechauve et al., 2014).
5.3.4. AAV for gene silencing
Forty percent of IR patients with a recognizable pattern of inheritance are affected by dominant forms of the disease (Berger et al., 2010). More than 150 different mutations associated with dominant RP have been described in the RHO gene alone which encodes for rhodopsin. The first RHO mutation to be identified encoded a proline-to-histidine substitution at position 23 (P23H) (Dryja et al., 1990). This mutation accounts for 12% of all adRP cases in the US (Dryja et al., 1991). P23H RHO mutants are retained in the endoplasmic reticulum and are unable to associate with 11-cis-retinal. AAV vectors have been used to silence RHO expression using both allele-dependent and -independent strategies. Several groups have attempted to preferentially suppress the production of mutant alleles using small RNA technology including ribozymes (LaVail et al., 2000; Lewin et al., 1998) and short-hairpin (sh) RNAs (Tessitore et al., 2006). Ribozymes are catalytic RNA molecules with tertiary structures that bind and directly cleave complementary mRNA sequences, while shRNAs are short RNA sequences that bind target mRNAs and trigger their degradation (Burnett and Rossi, 2012). While, this last strategy was found insufficient to prevent or block PR degeneration (Tessitore et al., 2006), AAV-mediated delivery of ribozymes results in partial but long-term rescue of retinal degeneration in P23H RHO transgenic rats (LaVail et al., 2000; Lewin et al., 1998). However, because of the great RHO allelic heterogeneity, allele-specific ribozymes need to be specifically developed for each mutation, making this an expensive and challenging option. As an alternative, mutation-independent approaches in which ribozymes or shRNA target sequences common to both mutant and wild-type RHO alleles have been developed. As all endogenous RHO is down-regulated, this approach requires the simultaneous delivery of a replacement copy of RHO that is resistant to silencing. The development of allele-independent ribozymes (Gorbatyuk et al., 2007a, 2005) or shRNA (Chadderton et al., 2009; Gorbatyuk et al., 2007b) has been successfully exploited. Notably, the allele-independent strategies provide more potent (up to 80%) silencing than those allele-specific. An alternative approach has been developed to achieve repression of the RHO gene expression in a mutation-independent manner by targeting artificial zinc-finger-based transcriptional repressors to the RHO promoter (Mussolino et al., 2011b). In this interesting approach the target of silencing is the locus of the gene itself, which is present in only two copies per diploid cell, as opposed to the RHO transcript, which is very abundant since rhodopsin represents >70% of the total rod PR OS proteins (Roof et al., 1982). For this same reason, achieving sufficient RHO replacement is challenging. To date, the most advanced proof-of-concept of the “suppression-replacement” strategy for dominant RHO mutations has been provided using a single AAV vector that co-delivers both a shRNA directed to RHO and a silencing-resistant RHO transgene. This strategy resulted in significant delay of PR cell loss in RHO P23H mice (Mao et al., 2012; O’Reilly et al., 2007) and in increased rod function (Mao et al., 2012). The use of two separate AAV vectors, one that delivers shRNA, and the other the RHO transgene, allows RHO suppression and replacement to be modulated and optimized by varying the doses of the two vectors (Millington-Ward et al., 2011). As both high and low levels of RHO have been shown to be deleterious for rods (Gorbatyuk et al., 2005; Mao et al., 2011), further studies are required to optimize this complex approach in order to achieve consistent therapeutic efficacy and avoid toxicity. However, the “suppress and supplant” approach may not be necessary for treatment of some dominant RHO mutations. Indeed, the simple AAV delivery of a wild-type mouse cDNA has been recently reported to significantly retard retinal degeneration of heterozygous transgenic P23H RHO mice in a Rho+/+ background (Mao et al., 2011). This raises the possibility that P23H exerts a dominant-negative rather than toxic gain-of-function effect, and that a treatment could be developed by altering the balance between mutant and wild-type forms of RHO. However, no benefit was observed following RHO gene delivery in the P347S mouse model of RHO-adRP (Millington-Ward et al., 2011).
5.3.5. AAV for mutation-independent strategies
Since PR apoptosis is the endpoint of most IR, independently of the specific causative gene (Wright et al., 2010), therapies based on “supply” of growth, neurotrophic, anti-apoptotic, anti-oxidative, and anti-inflammatory molecules have been widely exploited to sustain PR survival (Colella and Auricchio, 2010; Lipinski et al., 2013). Neurotrophic factors delivered by intraocular gene transfer with AAV vectors include the fibroblast and lens-epithelium-derived growth factors (FGF and LEDGF, respectively), the ciliary-derived neurotrophic factors (CNTF), BDNF, GDNF, PEDF, erythropoietin (EPO) and its non-erythropoietic derivatives, the X-linked inhibitor of apoptosis (XIAP), and heme oxygenase 1 (HO-1) (reviewed in Colella and Auricchio, 2010). The data collected so far showed that protective factors are able to delay PR loss in a mutation-independent manner and, thus, could represent valid therapeutic options for IR treatment. However, the non-physiological expression of such factors with growth and anti-apoptotic properties raises safety concerns. Indeed, reductions in ERG amplitude were reported in a phase I trial (NCT00063765) testing the safety of CNTF intraocular release in IR patients using encapsulated cell implants (Sieving et al., 2006). EPO is one of the most attractive candidates for non-specific treatment of retinal degeneration (Grimm et al., 2005; Rex et al., 2004). Importantly, the safety concerns related to EPO erythropoietic activity may have been partly overcome by the discovery of EPO mutants, which lack erythropoietic activity while retaining neuroprotective effects (Leist et al., 2004). AAV-mediated intraocular delivery of these derivatives in IR animal models has shown promising results (Colella et al., 2011; Sullivan and Rex, 2011).
Optogenetic therapy is emerging as a potential therapeutic option for those forms of blindness in which PR are mostly degenerated but other parts of the retinal circuitry remain intact. This strategy, which is independent of the mutation underlying the disease, relies on delivery of genes encoding light-activated ion channels or pumps to retinal neurons such as remaining PR, bipolar cells or RGC to stimulate retinal activity. The two most studied optogenetic proteins are channelrhodopsin-2 (ChR2), from the algae Chlamydomona reinhardtii (Boyden et al., 2005; Nagel et al., 2003), and halorhodopsin (NpHR), from the archaebacterium Natronomas pharaonis (Zhang et al., 2007). Both ChR2 and NpHR are photosensitive and can be activated at specific light wavelengths. ChR2, an optogenetic activator, is a non-selective cation channel that provides neurons with excitatory currents, while NpHR, an optogenetic inhibitor, is a chloride pump that generates inhibitory currents. Effective optogenetic therapies should be designed taking the following into account: cells stimulated in greater proximity to the PR in the neuronal chain will yield a more natural retinal processing; the optogenetic sensor-evoked activity should match the natural activity of the stimulated cell. Based on these ideas and on which cell types have been spared in the patient, optogenetic approaches have been thus far targeted to PR, bipolar interneurons or RGC (Cepko, 2012). Roska and colleagues have used AAV-mediated delivery of NpHR to convert the light-insensitive cones of two mouse models of RP into light-sensitive cells capable of reactivating cortical circuits and mediating visually-guided behaviour (Busskamp et al., 2010). Notably, this strategy also reactivated PR in human retinal explants (Busskamp et al., 2010). However, the sensitivity of the currently used NpHR alleles is sufficient for vision in bright outdoor light but not in dimmer light conditions (e.g. room light), thus this strategy needs further optimization for human applications. Attempts to enhance light sensitivity have been made through the use of specialized sets of glasses with an array of light-sensitive detectors to activate NpHR that has been transduced into cones using AAV (Grossman et al., 2010). This technique is moving towards a Phase I clinical trial which will test AAV encoding NpHR from a human cone promoter (Cepko, 2012). Unlike NpHR, ChR2 has been delivered to ON bipolar cells via AAV2/8 tyrosine-mutants to restore light-induced electrical activity in the retina of RP mouse models (Doroudchi et al., 2011). Targeted expression of ChR2 resulted in electrophysiological ON responses in post-synaptic RGC and significant improvement in visually-guided behaviour, including motion detection and light–dark discrimination, for up to 10 months post-injection. Several independent groups have restored sensitization to light with optogenetic targeting of spared inner retinal neurons. Bi et al. (2006) used intra-vitreal delivery of AAV2/2 to permanently express ChR2 in RGC in rd1 mice and demonstrated restored photosensitivity at the retinal and cortical levels. These results have also been confirmed in marmosets and rats (Ivanova et al., 2010; Ivanova and Pan, 2009; Tomita et al., 2007). Another option under study is the use of endogenous channels chemically-modified to be light-sensitive. Light-sensitive channels based on glutamate receptors (LiGluR) were successfully delivered to RGC and were found to restore light sensitivity in the rd1 mouse retina (Caporale et al., 2011). Also, as an alternative to ChR2 delivery, Lin et al. used intravitreal delivery of AAV expressing melanopsin (Opn4), a rhabdomeric opsin involved in circadian rhythm and physiologically expressed in approximately 0.5% of RGC in the mouse, to transduce non-melanopsin expressing RGC (Lin et al., 2008). RP mice treated with Opn4 showed restored retinal photosensitivity and improvement in visually-guided behaviour. However, further applicability of melanopsin is limited by slow kinetics. In addition, direct optical stimulation of RGC bypasses the important step of stimulus propagation to the inner retinal circuitry, in which bipolar and amacrine cells shape the spatial and temporal properties. Zhang et al. (2009b) have used a more complex approach, using AAV-mediated co-delivery of NpHR and ChR2 to RGC. This produces ON, OFF, and even ON–OFF responses, depending on the wavelength of the light stimulus. However, despite these promising proof-of-concept studies, further translation of optogenetic strategies requires the optimization of some critical still unsolved aspects which are reviewed in Busskamp et al. (2012).
6. Lentiviral vectors
Lentiviruses (LV) are enveloped retroviruses containing a positive, single-stranded RNA genome, capable of infecting both dividing and non-dividing cells. LV vectors integrate their genome in the chromosomes of target cells and thus can provide long-term expression in dividing cells. Even if the adult retina is made of post-mitotic neurons, in which episomal genomes would not be lost by cell division, integration may support long-term expression in peripheral RPE cells, which despite a very slow turn-over, have the capacity to enter the cell cycle and complete cellular division in rodents (Al-Hussaini et al., 2008; Loewen et al., 2003). Most viral genes are deleted in the recombinant LV genome, leaving room for transgenes and sequences of upto 8 kb in size. This relatively large capacity has been explored for the generation of bicistronic, bipartite or dual-promoter LV vectors, which have been successfully used to deliver multiple therapeutic proteins to the retina (Semple-Rowland and Berry, 2014; Semple-Rowland et al., 2010, 2007; Verrier et al., 2011). LV do not appear to induce adverse immunological responses after intraocular delivery (Azzouz et al., 2004). Moreover, LV are highly versatile because they can use foreign viral envelope glycoproteins in a process called pseudotyping, which greatly expands their tropism. Some drawbacks of LV vectors include potential insertional mutagenesis, complexity of production, and their large diameter (~80–100 nm), which may influence their distribution due to steric hindrance. The LV genome has been manipulated in order to improve its biosafety. These efforts include the inactivation of the 3′LTR to generate self-inactivating (SIN) vectors (Zufferey et al., 1998), as well as the development of third-generation packaging systems, which rely on transfection of four different plasmids, thus minimizing homology regions potentially responsible for replication-competent viral reconstitution. LV integration patterns have been analysed in rodent eyes, revealing a close to random frequency of integration into genes and gene spare long interspersed nuclear elements (LINE), suggesting its safety for clinical applications (Bartholomae et al., 2011). Moreover, subretinal injections of high titre LV vectors induced no ocular tumours in p53−/− mice, which are highly susceptible to intraocular malignant transformation (Balaggan et al., 2012). In order to substantially reduce the risk of LV insertional mutagenesis, integration-deficient vectors have been used and shown to mediate efficient and sustained transgene expression in rodent RPE for upto 8 weeks (Yanez-Munoz et al., 2006).
6.1. HIV-1-based LV vectors
The recombinant human immunodeficiency virus type 1 (HIV-1) has been used as a blueprint for the development of LV vectors (Naldini et al., 1996) and was the first LV to be tested intraocularly (Miyoshi et al., 1997). Other LV vectors that have been tested in the eye derive from the primate HIV-2, from simian (SIV), bovine (BIV), feline immunodeficiency viruses (FIV), or from equine infectious anaemia virus (EIAV) (Fig. 1). Most ocular studies have used vesicular stomatitis virus glycoprotein (VSVG)-pseudotyped vectors. Intravitreal delivery of LV vectors typically fails to produce efficient intraocular expression (Bainbridge et al., 2001; Greenberg et al., 2007; Yanez-Munoz et al., 2006), however, partial transduction of RPE and neuroretina has been observed near the injection site in some studies (Harvey et al., 2002). Subretinal administration of HIV-1-VSVG vectors containing ubiquitous or PR-specific promoters such as RHO in neonatal rodents resulted in efficient PR targeting (Miyoshi et al., 1997). Subretinal injection of HIV-1-VSVG in the pups (post natal day 2–5) of the rd1 mouse model of IR mediated a PR morphological rescue which lasted 24 weeks (Takahashi et al., 1999). Transduction with LV expressing PDE6B resulted in histologic and functional rescue of PR in mutant mice containing the PDE6B-H620Q missense allele (Davis et al., 2008). LV-mediated knockdown of GUCY2E or CNGA1 via shRNA in the PDE6B-H620Q retina counteracted loss of PDE6B function by increasing visual function and PR survival (Tosi et al., 2011). In addition, LV treatment of GUCY1B-deficient chicken embryos, a model of LCA1, partially restored the optokinetic reflexes (Verrier et al., 2011) as well as ERG responses and volitional visual behaviours (Williams et al., 2006). Interestingly, the MYO7A gene, whose size exceeds the canonical AAV cargo capacity, was successfully delivered to the neonatal murine retina of shaker1 mice with LV and resulted in correction of the shaker1 RPE and PR phenotype (Hashimoto et al., 2007). In adult animals, subretinal administration of LV vectors predominantly resulted in RPE transduction with some PR transduced with variable efficiency and distribution (Auricchio et al., 2001; Bainbridge et al., 2001; Bemelmans et al., 2005; Miyoshi et al., 1997). Indeed, rescue of IR caused by defects in genes expressed in the RPE was also achieved in juvenile and adult rodent models using HIV-1-VSVG vectors (Bemelmans et al., 2006; Kostic et al., 2011; Tolmachova et al., 2012; Tschernutter et al., 2005; Yanez-Munoz et al., 2006). Moreover, subretinal injection of a LV-VSVG vector containing the Müller specific CD44 promoter in postnatal day 21 Sprague–Dawley rats showed EGFP expression for more than 6 months in Müller glia, which represents a useful target for continuous production and secretion of universal neuroprotective factors (Greenberg et al., 2007).
Several groups including ours have tried to identify LV pseudotypes with improved PR tropism. However, HIV-1-Mokola and Ross River Virus (HIV-1-RRV) were shown to transduce only RPE after subretinal delivery (Auricchio et al., 2001; Bemelmans et al., 2005; Greenberg et al., 2007). Recently, HIV-1-based vectors pseudotyped with the Venezuelan Equine Encephalitis Virus-derived glycoprotein (VEEVG) were shown to transduce murine RPE after subretinal delivery (Lipinski et al., 2014), although degeneration of the ONL was observed with this pseudotype. A LV vector based on the HIV-2 genome was reported to transduce PR after subretinal injection in adult rabbits (Cheng et al., 2002). However, no preclinical experiments or tests in larger animals were performed with any of these. We have recently tested side-by-side seven different pseudotypes of HIV-1-based LV vectors in the adult murine retina and found that although baculovirus glycoprotein 64 (GP64)-pseudotyped LV tends to transduce more PR than the VSVG pseudotype, none of them provides widespread efficient PR transduction (Puppo et al., 2014).
It has been proposed that the large dimension of LV (80–100 nm) (Desmaris et al., 2001) might impose steric effects, hampering the virus’ diffusion to PR. Thus, following a subretinal injection, which delivers the vector in close proximity to PR, physical barriers such as the interphotoreceptor matrix and outer limiting membrane (OLM) might prevent LV from interacting with their cognate receptors on the target cells. Transduction and rescue of PR in neonatal retinas, in which the anatomical barriers are not yet fully formed, but not in adult retinas, indirectly suggests that the large LV particle size may play a role in transduction. Experiments in favour of this hypothesis were carried out using enzymatic treatments that weaken the interphotoreceptor matrix, such as Neuraminidase X, whose simultaneous subretinal injection with LV increased the number of transduced PR cells (Gruter et al., 2005). Similarly the efficiency of LV-transduced PR appears higher in the degenerating rd mouse retina which presents more permissive physical barriers (Pang et al., 2006). Besides, a higher number of Müller cells were transduced by LV vectors in S334Ter−/− rats (Greenberg et al., 2007) and by LV-Mokola in Rho−/− retinas than in wild-type, indicating that retinal remodelling may modify the tropism of LV vectors during the progression of degeneration (Calame et al., 2011).
However, the low PR tropism of LV in the adult retina has not been fully understood yet. Although LV-VSVG vector binding seemed independent of specific cell surface receptors (Coil and Miller, 2004), studies carried out in adult murine retinal explants, which lack physical barriers since they are compromised during the process of explantation, have shown that LV-VSVG cannot transduce PR (Lipinski et al., 2014). Surprisingly, limited PR transduction was observed in a human retinal explant transduced with LV-VSVG, suggesting that transduction may be species-dependent (Lipinski et al., 2014).
6.2. Non-HIV-1-based LV vectors
The non-HIV-1-based FIV, SIV, BIV and EIAV LV have been developed to potentially reduce risks associated with the use of vectors derived from HIV-1. In addition, they may exhibit a different retinal tropism than that of HIV-1-based LV (Fig. 1). FIV-mediated β-galactosidase expression was observed in RPE (Cheng et al., 2005; Loewen et al., 2003), RGC (Cheng et al., 2005) and PR OS (Loewen et al., 2004) in rodents, while in rabbit FIV-based LV transduced RPE, RGC and Müller cells upon subretinal administration (Cheng et al., 2005). In non-human primates the pattern of transduction included Müller cells, PR and sporadic RPE (Lotery et al., 2002). Second-generation FIV vectors mediated expression in the rat RPE for up to 25 months after subretinal administration (Cheng et al., 2005). SIV-based vectors pseudotyped with VSVG (Duisit et al., 2002; Ikeda et al., 2003; Miyazaki et al., 2003), Mokola, amphotropic murine leukemia virus envelope (4070A-Env), influenza A virus hemagglutinin (HA) or lymphocytic choriomeningitis virus glycoprotein (LCMV) (Duisit et al., 2002) targeted the rat RPE when delivered subretinally. In addition, gene transfer of PEDF using this vector was shown to protect RCS rats from retinal degeneration and functional defects (Miyazaki et al., 2003). SIV-mediated gene transfer was shown to be safe (Ikeda et al., 2009a) and stable in the non-human primate retina for up to 4 years (Ikeda et al., 2009b), and a Phase I clinical study of a third-generation SIV-based LV vector carrying human PEDF to treat patients with retinitis pigmentosa is ongoing (Yasuhiro Ikeda. Dept of Ophthalmology, Kyushu University, Fukuoka, Japan; ARVO 2014, Program Number: 2707). Vectors based on BIV pseudotyped with VSVG or GP64 after subretinal injection transduced rodent RPE (Molina et al., 2004a, 2004b; Takahashi et al., 2002) and occasional Müller cells (Takahashi et al., 2002), but not PR.
EIAV-based LV vectors were reported to transduce RPE and PR in neonatal mice (Nicoud et al., 2007) and almost exclusively RPE in adult mice (Balaggan et al., 2006). Recently, adult rabbit and macaque PR transduction was reported to be safe after subretinal administration of EIAV vectors (Binley et al., 2013; Zallocchi et al., 2014). EIAV vectors have been recently used to deliver the large ABCA4 gene (6,8 kb) to newborn Abca4−/− mice (Kong et al., 2008) and to deliver the MYO7A gene (6,7 kb) to the newborn retina of the shaker 1 mouse model of USH1B (Zallocchi et al., 2014). Notably, the PR transduction efficiency was sufficient to rescue the phenotype of these mouse models. Based on these results, clinical trials delivering EIAV vectors pseudotyped with VSVG via subretinal delivery are currently being carried out for USH1B (NCT01505062, NCT02065011) and STGD (NCT01367444, NCT01736592).
7. Adenoviral vectors
Adenoviruses (Ad) contain a linear, double-stranded DNA genome contained in an icosahedral non-enveloped capsid with fiber-like projections from each of its 12 vertices (Russell, 2000). Ad efficiently infect post-mitotic cells with a fast onset of expression, which is detectable in vivo 48 h post-injection (Bennett et al., 1994, 1998; Lamartina et al., 2007). Ad vectors are episomal and offer minimal risks of insertional mutagenesis. In addition, among viral vectors used for gene therapy, Ad vectors offer one of the largest capacities for delivering transgenes (up to 37 kb) (Parks et al., 1996). Additionally, highly pure and concentrated vector preps can be routinely manufactured (Kay et al., 2001). Another major advantage that Ad vectors offer is their high versatility, including over 50 different human Ad serotypes and Ad from other species that may show different tropism (Fig. 1). On the other hand, Ad’s large capacity depends on its particle size (~100 nm), which may negatively impact its journey towards target cells due to the presence of physical barriers in the subretinal space (Pang et al., 2004). Another drawback of Ad vectors are the immune reactions that they can elicit, even in an immune privileged organ such as the eye, as discussed below. Ad vectors may be classified as either first generation (Ad, deleted of the early region E1/E3 genes), second generation (further deleted of the E2/E4 genes) or as helper-dependent (HdAd, deleted of all viral genes and containing only the cis sequences required for vector genome encapsidation). Current vectors are primarily derived from Ad serotype 5 (Ad5). When tested subretinally in rodents, Ad5-based vectors mainly transduce RPE (Bennett et al., 1994; Kreppel et al., 2002; Li et al., 1994; Wu et al., 2011), as does the closely related Ad2 (Jomary et al., 1994; Zhang and Bergelson, 2005), and to a lesser extent PR (Puppo et al., 2014). Indeed, RPE65 and MERTK were successfully delivered to the RPE via subretinal administration, preventing cone loss in Rpe65−/− mice (Chen et al., 2006) and correcting the retinal dystrophy phenotype of the RCS rat (Vollrath et al., 2001), respectively. As with LV, neonatal delivery significantly increases the efficiency of Ad-mediated PR transduction (Li et al., 1994; Pang et al., 2004). Also similarly to LV transduction, Ad5-mediated transduction of PR was more efficient in the degenerated murine rd retina than in wild-type (Pang et al., 2004). Furthermore, Ad-mediated reporter gene expression was higher in two models of proliferative retinopathy than in normal mice (Mori et al., 2002). Two studies using recombinant Ad containing wild-type PDE6B injected in neonatal mice resulted in histological rescue of the degenerating rd PR, lasting 6 and 12 weeks respectively (Bennett et al., 1996b; Kumar-Singh and Farber, 1998). Since Ad entry requires high-affinity binding to cell receptors via the knob portion of the fiber, improvement in tropism towards PR by exchanging the Ad knob (K) or fiber (F) proteins among different serotypes or subgroups has been sought after. While Ad5 recognizes the Coxsackie adenovirus receptor (CAR) for initial attachment, followed by internalization through an interaction between the RGD motif in the Ad penton base with αvβ3–5 integrins, other viruses such as Ad35 and Ad37 interact with CD46 (Gaggar et al., 2003) and sialic acid (Arnberg et al., 2002), respectively, for entry. Recognition of alternative receptors potentially results in modified tropism. An improvement in PR targeting was observed with the Ad5/F35 vector (Ad5 capsid containing fibers from Ad35) (Mallam et al., 2004) and Ad5/F37 (Von Seggern et al., 2003), even though in this study the vector had been delivered via intravitreal delivery. A study compared the transduction profiles following subretinal injection of a vector bearing a deletion of the RGD domain in the Ad penton base (Ad5ΔRGD), which had been reported to target PR (Cashman et al., 2007), to Ad5/F35 and Ad5/F37 vectors, and found Ad5ΔRGD to be the most efficient for PR targeting (Sweigard et al., 2010). However, a comprehensive study comparing the retinal transduction profiles of different naturally-occurring or mutant Ad serotypes suggests that none of them significantly outperforms Ad5 in adult murine PR transduction (Puppo et al., 2014).
Growth and neurotrophic factors have been delivered to protect retinal PR from degeneration in animal models. Ad vectors expressing bFGF or CNTF were delivered to the retina of the RCS rat model and this resulted in PR protection (Akimoto et al., 1999; Huang et al., 2004). Gene transfer of CNTF has also shown to reduce PR loss and improve ERG a- and b-wave amplitudes in the rds mouse model (Cayouette et al., 1998). Moreover, the intravitreal injection of Ad expressing CNTF increased the number of PR nuclei in the rd mouse model of IR (Cayouette and Gravel, 1997). Intravitreal injection of Ad expressing PEDF rescued PR from light-induced cell death (Imai et al., 2005), and resulted in neuroprotection in rats after ischemic injury (Takita et al., 2003). Gene expression was shown to be prolonged following intravitreal administration of an Ad35-compared to an Ad5-based vector, and Ad35-mediated expression of PEDF led to improvements in a complex retinal disease model (Hamilton et al., 2008). Ad5 was also shown to target Müller cells after intravitreal delivery in rats and to preserve the structure and function of light damaged PR through BDNF expression (Gauthier et al., 2005).
Transgene expression in preclinical studies, mediated by first and second generation Ad, is generally short-lived (Reichel et al., 1998), even if the subretinal space is an immune-privileged site (Bennett, 2003; Bennett et al., 1996a; Hoffman et al., 1997). It has been demonstrated that cytotoxic T lymphocytes mediate removal of the transduced cells, in which expression of viral genes takes place (Reichel et al.,1998). In order to diminish immune responses elicited by Ad vectors, HdAd vectors were developed (Parks et al.,1996). The only viral sequences retained in HdAd are the viral ITRs and the packaging recognition signal. Production of HdAd vectors relies on administration of a first generation Ad helper, which provides the Ad viral functions required for replication but does not get assembled for a conditional deletion in the packaging signal. Indeed, HdAd-reduced immunogenicity has led to multi-year HdAd-mediated expression in the liver of animal models (Brunetti-Pierri et al., 2013). Subretinal administration of HdAd5 resulted in long-term transgene expression in immunocompetent rats (Kreppel et al., 2002) and mice (Puppo et al., 2014). An HdAd5 injected intravitreally transduced Müller cells for up to one year (Lamartina et al., 2007), and persistent expression of PEDF was observed also after intravitreal delivery of HdAd (Chen et al., 2008a). Because HdAd vectors have the same capsid proteins as Ad, acute toxicity may still be present (Brunetti-Pierri et al., 2004). To date, no clinical trials based on Ad have been performed to treat IR, although two clinical trials have been performed using Ad5 intraocularly in order to treat age-related macular degeneration (Campochiaro et al., 2006) and retinoblastoma (Chevez-Barrios et al., 2005).
8. Conclusions and future directions
Identifying vectors that efficiently transduce various retinal cell layers has allowed significant advances in both pre-clinical and clinical retinal gene therapy (Table 3).
Table 3.
Number of publications on proof-of-concept studies in IR animal models and humans using AAV-, LV- and Ad-based vectors.
| AAV | LV | Ad | |
|---|---|---|---|
| Preclinical studies | ~70 | ~15 | ~10 |
| Clinical trials | 12 | 4 | 0 |
Source for preclinical studies: Pubmed as of May 2014. The numbers in the columns derive from the analysis of publications selected using the following keywords: adeno-associated, retina; adenovirus, retina; lentivirus, retina. Source for clinical trials: clinicaltrials.gov as of May 2014.
The development of non-viral vectors for gene delivery has been largely encouraged because of its high safety and theoretically unlimited packaging delivery. Indeed, the high-capacity of CK30PEG NP has enabled long-term rescue of a mouse model of IR due to mutations in a large gene expressed in PR (Han et al., 2012). However, further investigation in large animal models is warranted in order to assess their potential for clinical applications.
LV and Ad viral vectors have demonstrated their potential to effectively deliver large genes to RPE. However, the ability of these large particles to efficiently transduce PR in the highly compacted adult mammalian retina remains controversial (Bemelmans et al., 2005; Bennett et al., 1994; Binley et al., 2013; Cashman et al., 2007; Kreppel et al., 2002; Li et al., 1994; Mallam et al., 2004; Miyoshi et al., 1997; Puppo et al., 2014; Sweigard et al., 2010; Zallocchi et al., 2014). The outcome of the currently ongoing clinical trials based on the use of EIAV-VSVG LV vectors will hopefully help to understand the efficiency of these high capacity vectors for use in adult human PR transduction.
The demonstration of the safety and efficacy of intraocular AAV administration in humans has provided a unique momentum for retinal gene therapy and established AAV as the most promising platform for retinal gene therapy to date (Table 3). Dual AAV-mediated large gene delivery in mice (Dyka et al., 2014; Lopes et al., 2013; Reich et al., 2003a; Trapani et al., 2014) and in pigs (Colella et al., 2014) seems to overcome AAV’s limited cargo capacity, extending its applicability to IR due to mutations in large genes. The additional development of AAV mutants that transduce the outer retina from the vitreous support AAV’s ability to treat IR. These next generation gene transfer tools perfectly match new therapeutic strategies, which go beyond gene replacement in RPE or PR. Optogenetics, for instance, requires transfer of light-sensitive molecules to inner retinal cells. Some challenges remain to be solved; the next generation of AAV vectors must be able to transduce the retina of a large animal as efficiently as it does that of a small rodent. Moreover, while some barriers appear difficult to penetrate, AAV has been reported to spread to neurons of the central nervous system following subretinal administration (Stieger et al., 2008). Although this has not been consistently observed (Vandenberghe et al., 2011), it poses safety concerns that can be overcome by the use of cell-specific promoters to avoid misexpression of the transgene. In addition, independently of the barriers, serotype tropism may vary among different species (Vandenberghe and Auricchio, 2012). Particular care should be taken in assessing both the timing of injection with respect to the age of onset of the disease (Cideciyan et al., 2013; Maguire et al., 2009) and the site of injection of the vector in order to avoid fovea damage.
In conclusion, despite continuous challenges, the always expanding portfolio of vectors provides improved tools for retinal gene therapy and warrants a bright future for treatment of blindness.
Acknowledgments
We thank Alexis Burton and Graciana Diez-Roux (Scientific Office, TIGEM, Naples, Italy) for the critical reading of this manuscript. The following funding support is gratefully acknowledged: the European Research Council/ERC Grant agreement n° 282085 “RetGeneTx”; the NIH (grant R24 RY019861-01A); the Italian Telethon Foundation (grant TGM11MT1).
References
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, Jacobson SG, Hauswirth WW, Bennett J. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- Akimoto M, Miyatake S, Kogishi J, Hangai M, Okazaki K, Takahashi JC, Saiki M, Iwaki M, Honda Y. Adenovirally expressed basic fibroblast growth factor rescues photoreceptor cells in RCS rats. Invest Ophthalmol Vis Sci. 1999;40:273–279. [PubMed] [Google Scholar]
- Al-Hussaini H, Kam JH, Vugler A, Semo M, Jeffery G. Mature retinal pigment epithelium cells are retained in the cell cycle and proliferate in vivo. Mol Vis. 2008;14:1784–1791. [PMC free article] [PubMed] [Google Scholar]
- Alexander JJ, Umino Y, Everhart D, Chang B, Min SH, Li Q, Timmers AM, Hawes NL, Pang JJ, Barlow RB, Hauswirth WW. Restoration of cone vision in a mouse model of achromatopsia. Nat Med. 2007;13:685–687. doi: 10.1038/nm1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali RR, Reichel MB, De Alwis M, Kanuga N, Kinnon C, Levinsky RJ, Hunt DM, Bhattacharya SS, Thrasher AJ. Adeno-associated virus gene transfer to mouse retina. Hum Gene Ther. 1998;9:81–86. doi: 10.1089/hum.1998.9.1-81. [DOI] [PubMed] [Google Scholar]
- Allocca M, Doria M, Petrillo M, Colella P, Garcia-Hoyos M, Gibbs D, Kim SR, Maguire A, Rex TS, Di Vicino U, Cutillo L, Sparrow JR, Williams DS, Bennett J, Auricchio A. Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J Clin Invest. 2008;118:1955–1964. doi: 10.1172/JCI34316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allocca M, Manfredi A, Iodice C, Di Vicino U, Auricchio A. AAV-mediated gene replacement, either alone or in combination with physical and pharmacological agents, results in partial and transient protection from photoreceptor degeneration associated with betaPDE deficiency. Invest Ophthalmol Vis Sci. 2011;52:5713–5719. doi: 10.1167/iovs.10-6269. [DOI] [PubMed] [Google Scholar]
- Allocca M, Mussolino C, Garcia-Hoyos M, Sanges D, Iodice C, Petrillo M, Vandenberghe LH, Wilson JM, Marigo V, Surace EM, Auricchio A. Novel adeno-associated virus serotypes efficiently transduce murine photoreceptors. J Virol. 2007;81:11372–11380. doi: 10.1128/JVI.01327-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrieu-Soler C, Halhal M, Boatright JH, Padove SA, Nickerson JM, Stodulkova E, Stewart RE, Ciavatta VT, Doat M, Jeanny JC, de Bizemont T, Sennlaub F, Courtois Y, Behar-Cohen F. Single-stranded oligonucleotide-mediated in vivo gene repair in the rd1 retina. Mol Vis. 2007;13:692–706. [PMC free article] [PubMed] [Google Scholar]
- Annear MJ, Bartoe JT, Barker SE, Smith AJ, Curran PG, Bainbridge JW, Ali RR, Petersen-Jones SM. Gene therapy in the second eye of RPE65-deficient dogs improves retinal function. Gene Ther. 2011;18:53–61. doi: 10.1038/gt.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnberg N, Pring-Akerblom P, Wadell G. Adenovirus type 37 uses sialic acid as a cellular receptor on Chang C cells. J Virol. 2002;76:8834–8841. doi: 10.1128/JVI.76.17.8834-8841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtari M, Cyckowski LL, Monroe JF, Marshall KA, Chung DC, Auricchio A, Simonelli F, Leroy BP, Maguire AM, Shindler KS, Bennett J. The human visual cortex responds to gene therapy-mediated recovery of retinal function. J Clin Invest. 2011;121:2160–2168. doi: 10.1172/JCI57377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auricchio A. Pseudotyped AAV vectors for constitutive and regulated gene expression in the eye. Vis Res. 2003;43:913–918. doi: 10.1016/s0042-6989(02)00676-4. [DOI] [PubMed] [Google Scholar]
- Auricchio A, Kobinger G, Anand V, Hildinger M, O’Connor E, Maguire AM, Wilson JM, Bennett J. Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum Mol Genet. 2001;10:3075–3081. doi: 10.1093/hmg/10.26.3075. [DOI] [PubMed] [Google Scholar]
- Azzouz M, Kingsman SM, Mazarakis ND. Lentiviral vectors for treating and modeling human CNS disorders. J Gene Med. 2004;6:951–962. doi: 10.1002/jgm.600. [DOI] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Bainbridge JW, Stephens C, Parsley K, Demaison C, Halfyard A, Thrasher AJ, Ali RR. In vivo gene transfer to the mouse eye using an HIV-based lentiviral vector; efficient long-term transduction of corneal endothelium and retinal pigment epithelium. Gene Ther. 2001;8:1665–1668. doi: 10.1038/sj.gt.3301574. [DOI] [PubMed] [Google Scholar]
- Balaggan KS, Binley K, Esapa M, Iqball S, Askham Z, Kan O, Tschernutter M, Bainbridge JW, Naylor S, Ali RR. Stable and efficient intraocular gene transfer using pseudotyped EIAV lentiviral vectors. J Gene Med. 2006;8:275–285. doi: 10.1002/jgm.845. [DOI] [PubMed] [Google Scholar]
- Balaggan KS, Duran Y, Georgiadis A, Thaung C, Barker SE, Buch PK, MacNeil A, Robbie S, Bainbridge JW, Smith AJ, Ali RR. Absence of ocular malignant transformation after sub-retinal delivery of rAAV2/2 or integrating lentiviral vectors in p53-deficient mice. Gene Ther. 2012;19:182–188. doi: 10.1038/gt.2011.194. [DOI] [PubMed] [Google Scholar]
- Bartholomae CC, Arens A, Balaggan KS, Yanez-Munoz RJ, Montini E, Howe SJ, Paruzynski A, Korn B, Appelt JU, Macneil A, Cesana D, Abel U, Glimm H, Naldini L, Ali RR, Thrasher AJ, von Kalle C, Schmidt M. Lentiviral vector integration profiles differ in rodent postmitotic tissues. Mol Ther. 2011;19:703–710. doi: 10.1038/mt.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran WA, Cideciyan AV, Lewin AS, Iwabe S, Khanna H, Sumaroka A, Chiodo VA, Fajardo DS, Roman AJ, Deng WT, Swider M, Aleman TS, Boye SL, Genini S, Swaroop A, Hauswirth WW, Jacobson SG, Aguirre GD. Gene therapy rescues photoreceptor blindness in dogs and paves the way for treating human X-linked retinitis pigmentosa. Proc Natl Acad Sci U S A. 2012;109:2132–2137. doi: 10.1073/pnas.1118847109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemelmans AP, Bonnel S, Houhou L, Dufour N, Nandrot E, Helmlinger D, Sarkis C, Abitbol M, Mallet J. Retinal cell type expression specificity of HIV-1-derived gene transfer vectors upon subretinal injection in the adult rat: influence of pseudotyping and promoter. J Gene Med. 2005;7:1367–1374. doi: 10.1002/jgm.788. [DOI] [PubMed] [Google Scholar]
- Bemelmans AP, Kostic C, Crippa SV, Hauswirth WW, Lem J, Munier FL, Seeliger MW, Wenzel A, Arsenijevic Y. Lentiviral gene transfer of RPE65 rescues survival and function of cones in a mouse model of Leber congenital amaurosis. PLoS Med. 2006;3:e347. doi: 10.1371/journal.pmed.0030347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. Immune response following intraocular delivery of recombinant viral vectors. Gene Ther. 2003;10:977–982. doi: 10.1038/sj.gt.3302030. [DOI] [PubMed] [Google Scholar]
- Bennett J, Ashtari M, Wellman J, Marshall KA, Cyckowski LL, Chung DC, McCague S, Pierce EA, Chen Y, Bennicelli JL, Zhu X, Ying GS, Sun J, Wright JF, Auricchio A, Simonelli F, Shindler KS, Mingozzi F, High KA, Maguire AM. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med. 2012;4:120ra115. doi: 10.1126/scitranslmed.3002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J, Pakola S, Zeng Y, Maguire A. Humoral response after administration of E1-deleted adenoviruses: immune privilege of the subretinal space. Hum Gene Ther. 1996a;7:1763–1769. doi: 10.1089/hum.1996.7.14-1763. [DOI] [PubMed] [Google Scholar]
- Bennett J, Tanabe T, Sun D, Zeng Y, Kjeldbye H, Gouras P, Maguire AM. Photoreceptor cell rescue in retinal degeneration (rd) mice by in vivo gene therapy. Nat Med. 1996b;2:649–654. doi: 10.1038/nm0696-649. [DOI] [PubMed] [Google Scholar]
- Bennett J, Wilson J, Sun D, Forbes B, Maguire A. Adenovirus vector-mediated in vivo gene transfer into adult murine retina. Invest Ophthalmol Vis Sci. 1994;35:2535–2542. [PubMed] [Google Scholar]
- Bennett J, Zeng Y, Bajwa R, Klatt L, Li Y, Maguire AM. Adenovirus-mediated delivery of rhodopsin-promoted bcl-2 results in a delay in photore-ceptor cell death in the rd/rd mouse. Gene Ther. 1998;5:1156–1164. doi: 10.1038/sj.gt.3300733. [DOI] [PubMed] [Google Scholar]
- Berger W, Kloeckener-Gruissem B, Neidhardt J. The molecular basis of human retinal and vitreoretinal diseases. Prog Retin Eye Res. 2010;29:335–375. doi: 10.1016/j.preteyeres.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Berns KI, Giraud C. Biology of adeno-associated virus. Curr Top Microbiol Immunol. 1996;218:1–23. doi: 10.1007/978-3-642-80207-2_1. [DOI] [PubMed] [Google Scholar]
- Bi A, Cui J, Ma YP, Olshevskaya E, Pu M, Dizhoor AM, Pan ZH. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron. 2006;50:23–33. doi: 10.1016/j.neuron.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder C, Cashman SM, Kumar-Singh R. Extended duration of transgene expression from pegylated POD nanoparticles enables attenuation of photoreceptor degeneration. PLoS One. 2013;8:e82295. doi: 10.1371/journal.pone.0082295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley K, Widdowson P, Loader J, Kelleher M, Iqball S, Ferrige G, de Belin J, Carlucci M, Angell-Manning D, Hurst F, Ellis S, Miskin J, Fernandes A, Wong P, Allikmets R, Bergstrom C, Aaberg T, Yan J, Kong J, Gouras P, Prefontaine A, Vezina M, Bussieres M, Naylor S, Mitrophanous KA. Transduction of photoreceptors with equine infectious anemia virus lentiviral vectors: safety and biodistribution of StarGen for Stargardt disease. Invest Ophthalmol Vis Sci. 2013;54:4061–4071. doi: 10.1167/iovs.13-11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet C, Augustin S, Ellouze S, Benit P, Bouaita A, Rustin P, Sahel JA, Corral-Debrinski M. The optimized allotopic expression of ND1 or ND4 genes restores respiratory chain complex I activity in fibroblasts harboring mutations in these genes. Biochim Biophys Acta. 2008;1783:1707–1717. doi: 10.1016/j.bbamcr.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Boye SE, Alexander JJ, Boye SL, Witherspoon CD, Sandefer KJ, Conlon TJ, Erger K, Sun J, Ryals R, Chiodo VA, Clark ME, Girkin CA, Hauswirth WW, Gamlin PD. The human rhodopsin kinase promoter in an AAV5 vector confers rod- and cone-specific expression in the primate retina. Hum Gene Ther. 2012;23:1101–1115. doi: 10.1089/hum.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye SE, Boye SL, Lewin AS, Hauswirth WW. A comprehensive review of retinal gene therapy. Mol Ther. 2013a;21:509–519. doi: 10.1038/mt.2012.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye SE, Boye SL, Pang J, Ryals R, Everhart D, Umino Y, Neeley AW, Besharse J, Barlow R, Hauswirth WW. Functional and behavioral restoration of vision by gene therapy in the guanylate cyclase-1 (GC1) knockout mouse. PLoS One. 2010;5:e11306. doi: 10.1371/journal.pone.0011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye SL, Conlon T, Erger K, Ryals R, Neeley A, Cossette T, Pang J, Dyka FM, Hauswirth WW, Boye SE. Long-term preservation of cone photoreceptors and restoration of cone function by gene therapy in the guanylate cyclase-1 knockout (GC1KO) mouse. Invest Ophthalmol Vis Sci. 2011;52:7098–7108. doi: 10.1167/iovs.11-7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye SL, Peshenko IV, Huang WC, Min SH, McDoom I, Kay CN, Liu X, Dyka FM, Foster TC, Umino Y, Karan S, Jacobson SG, Baehr W, Dizhoor A, Hauswirth WW, Boye SE. AAV-mediated gene therapy in the guanylate cyclase (RetGC1/RetGC2) double knockout mouse model of Leber congenital amaurosis. Hum Gene Ther. 2013b;24:189–202. doi: 10.1089/hum.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Ng T, Iannitti D, Cioffi W, Stapleton G, Law M, Breinholt J, Palmer D, Grove N, Rice K, Bauer C, Finegold M, Beaudet A, Mullins C, Ng P. Transgene expression up to 7 years in nonhuman primates following hepatic transduction with helper-dependent adenoviral vectors. Hum Gene Ther. 2013;24:761–765. doi: 10.1089/hum.2013.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Palmer DJ, Beaudet AL, Carey KD, Finegold M, Ng P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum Gene Ther. 2004;15:35–46. doi: 10.1089/10430340460732445. [DOI] [PubMed] [Google Scholar]
- Burnett JC, Rossi JJ. RNA-based therapeutics: current progress and future prospects. Chem Biol. 2012;19:60–71. doi: 10.1016/j.chembiol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busskamp V, Duebel J, Balya D, Fradot M, Viney TJ, Siegert S, Groner AC, Cabuy E, Forster V, Seeliger M, Biel M, Humphries P, Paques M, Mohand-Said S, Trono D, Deisseroth K, Sahel JA, Picaud S, Roska B. Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science. 2010;329:413–417. doi: 10.1126/science.1190897. [DOI] [PubMed] [Google Scholar]
- Busskamp V, Picaud S, Sahel JA, Roska B. Optogenetic therapy for retinitis pigmentosa. Gene Ther. 2012;19:169–175. doi: 10.1038/gt.2011.155. [DOI] [PubMed] [Google Scholar]
- Cai X, Conley SM, Nash Z, Fliesler SJ, Cooper MJ, Naash MI. Gene delivery to mitotic and postmitotic photoreceptors via compacted DNA nano-particles results in improved phenotype in a mouse model of retinitis pigmentosa. FASEB J. 2010;24:1178–1191. doi: 10.1096/fj.09-139147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Nash Z, Conley SM, Fliesler SJ, Cooper MJ, Naash MI. A partial structural and functional rescue of a retinitis pigmentosa model with compacted DNA nanoparticles. PLoS One. 2009;4:e5290. doi: 10.1371/journal.pone.0005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calame M, Cachafeiro M, Philippe S, Schouwey K, Tekaya M, Wanner D, Sarkis C, Kostic C, Arsenijevic Y. Retinal degeneration progression changes lentiviral vector cell targeting in the retina. PLoS One. 2011;6:e23782. doi: 10.1371/journal.pone.0023782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campochiaro PA, Nguyen QD, Shah SM, Klein ML, Holz E, Frank RN, Saperstein DA, Gupta A, Stout JT, Macko J, DiBartolomeo R, Wei LL. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: results of a phase I clinical trial. Hum Gene Ther. 2006;17:167–176. doi: 10.1089/hum.2006.17.167. [DOI] [PubMed] [Google Scholar]
- Caporale N, Kolstad KD, Lee T, Tochitsky I, Dalkara D, Trauner D, Kramer R, Dan Y, Isacoff EY, Flannery JG. LiGluR restores visual responses in rodent models of inherited blindness. Mol Ther. 2011;19:1212–1219. doi: 10.1038/mt.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho LS, Xu J, Pearson RA, Smith AJ, Bainbridge JW, Morris LM, Fliesler SJ, Ding XQ, Ali RR. Long-term and age-dependent restoration of visual function in a mouse model of CNGB3-associated achromatopsia following gene therapy. Hum Mol Genet. 2011;20:3161–3175. doi: 10.1093/hmg/ddr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman SM, McCullough L, Kumar-Singh R. Improved retinal transduction in vivo and photoreceptor-specific transgene expression using adenovirus vectors with modified penton base. Mol Ther. 2007;15:1640–1646. doi: 10.1038/sj.mt.6300203. [DOI] [PubMed] [Google Scholar]
- Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010;120:3073–3083. doi: 10.1172/JCI42440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayouette M, Behn D, Sendtner M, Lachapelle P, Gravel C. Intraocular gene transfer of ciliary neurotrophic factor prevents death and increases responsiveness of rod photoreceptors in the retinal degeneration slow mouse. J Neurosci. 1998;18:9282–9293. doi: 10.1523/JNEUROSCI.18-22-09282.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayouette M, Gravel C. Adenovirus-mediated gene transfer of ciliary neurotrophic factor can prevent photoreceptor degeneration in the retinal degeneration (rd) mouse. Hum Gene Ther. 1997;8:423–430. doi: 10.1089/hum.1997.8.4-423. [DOI] [PubMed] [Google Scholar]
- Cepko CL. Emerging gene therapies for retinal degenerations. J Neurosci. 2012;32:6415–6420. doi: 10.1523/JNEUROSCI.0295-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko CL, Vandenberghe LH. Retinal gene therapy coming of age. Hum Gene Ther. 2013;24:242–244. doi: 10.1089/hum.2013.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderton N, Millington-Ward S, Palfi A, O’Reilly M, Tuohy G, Humphries MM, Li T, Humphries P, Kenna PF, Farrar GJ. Improved retinal function in a mouse model of dominant retinitis pigmentosa following AAV-delivered gene therapy. Mol Ther. 2009;17:593–599. doi: 10.1038/mt.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderton N, Palfi A, Millington-Ward S, Gobbo O, Overlack N, Carrigan M, O’Reilly M, Campbell M, Ehrhardt C, Wolfrum U, Humphries P, Kenna PF, Farrar GJ. Intravitreal delivery of AAV-NDI1 provides functional benefit in a murine model of Leber hereditary optic neuropathy. Eur J Hum Genet. 2013;21:62–68. doi: 10.1038/ejhg.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalberg TW, Genise HL, Vollrath D, Calos MP. phiC31 integrase confers genomic integration and long-term transgene expression in rat retina. Invest Ophthalmol Vis Sci. 2005;46:2140–2146. doi: 10.1167/iovs.04-1252. [DOI] [PubMed] [Google Scholar]
- Chalberg TW, Vankov A, Molnar FE, Butterwick AF, Huie P, Calos MP, Palanker DV. Gene transfer to rabbit retina with electron avalanche transfection. Invest Ophthalmol Vis Sci. 2006;47:4083–4090. doi: 10.1167/iovs.06-0092. [DOI] [PubMed] [Google Scholar]
- Charbel Issa P, MacLaren RE. Non-viral retinal gene therapy: a review. Clin Exp Ophthalmol. 2012;40:39–47. doi: 10.1111/j.1442-9071.2011.02649.x. [DOI] [PubMed] [Google Scholar]
- Chen P, Hamilton M, Thomas CA, Kroeger K, Carrion M, Macgill RS, Gehlbach P, Brough DE, Wei LL, King CR, Bruder JT. Persistent expression of PEDF in the eye using high-capacity adenovectors. Mol Ther. 2008a;16:1986–1994. doi: 10.1038/mt.2008.211. [DOI] [PubMed] [Google Scholar]
- Chen X, Kube DM, Cooper MJ, Davis PB. Cell surface nucleolin serves as receptor for DNA nanoparticles composed of pegylated polylysine and DNA. Mol Ther. 2008b;16:333–342. doi: 10.1038/sj.mt.6300365. [DOI] [PubMed] [Google Scholar]
- Chen Y, Moiseyev G, Takahashi Y, Ma JX. RPE65 gene delivery restores isomerohydrolase activity and prevents early cone loss in Rpe65−/− mice. Invest Ophthalmol Vis Sci. 2006;47:1177–1184. doi: 10.1167/iovs.05-0965. [DOI] [PubMed] [Google Scholar]
- Chen ZY, He CY, Ehrhardt A, Kay MA. Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Mol Ther. 2003;8:495–500. doi: 10.1016/s1525-0016(03)00168-0. [DOI] [PubMed] [Google Scholar]
- Chen ZY, He CY, Meuse L, Kay MA. Silencing of episomal transgene expression by plasmid bacterial DNA elements in vivo. Gene Ther. 2004;11:856–864. doi: 10.1038/sj.gt.3302231. [DOI] [PubMed] [Google Scholar]
- Cheng L, Chaidhawangul S, Wong-Staal F, Gilbert J, Poeschla E, Toyoguchi M, El-Bradey M, Bergeron-Lynn G, Soules KA, Freeman WR. Human immunodeficiency virus type 2 (HIV-2) vector-mediated in vivo gene transfer into adult rabbit retina. Curr Eye Res. 2002;24:196–201. doi: 10.1076/ceyr.24.3.196.8302. [DOI] [PubMed] [Google Scholar]
- Cheng L, Toyoguchi M, Looney DJ, Lee J, Davidson MC, Freeman WR. Efficient gene transfer to retinal pigment epithelium cells with long-term expression. Retina. 2005;25:193–201. doi: 10.1097/00006982-200502000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevez-Barrios P, Chintagumpala M, Mieler W, Paysse E, Boniuk M, Kozinetz C, Hurwitz MY, Hurwitz RL. Response of retinoblastoma with vitreous tumor seeding to adenovirus-mediated delivery of thymidine kinase followed by ganciclovir. J Clin Oncol. 2005;23:7927–7935. doi: 10.1200/JCO.2004.00.1883. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, Pang JJ, Sumaroka A, Windsor EA, Wilson JM, Flotte TR, Fishman GA, Heon E, Stone EM, Byrne BJ, Jacobson SG, Hauswirth WW. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci U S A. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, Windsor EA, Conlon TJ, Sumaroka A, Pang JJ, Roman AJ, Byrne BJ, Jacobson SG. Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum Gene Ther. 2009a;20:999–1004. doi: 10.1089/hum.2009.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, Windsor EA, Conlon TJ, Sumaroka A, Roman AJ, Byrne BJ, Jacobson SG. Vision 1 year after gene therapy for Leber’s congenital amaurosis. N Engl J Med. 2009b;361:725–727. doi: 10.1056/NEJMc0903652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Jacobson SG, Beltran WA, Sumaroka A, Swider M, Iwabe S, Roman AJ, Olivares MB, Schwartz SB, Komaromy AM, Hauswirth WW, Aguirre GD. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci U S A. 2013;110:E517–E525. doi: 10.1073/pnas.1218933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coil DA, Miller AD. Phosphatidylserine is not the cell surface receptor for vesicular stomatitis virus. J Virol. 2004;78:10920–10926. doi: 10.1128/JVI.78.20.10920-10926.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colella P, Auricchio A. AAV-mediated gene supply for treatment of degenerative and neovascular retinal diseases. Curr Gene Ther. 2010;10:371–380. doi: 10.2174/156652310793180670. [DOI] [PubMed] [Google Scholar]
- Colella P, Auricchio A. Gene therapy of inherited retinopathies: a long and successful road from viral vectors to patients. Hum Gene Ther. 2012;23:796–807. doi: 10.1089/hum.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colella P, Iodice C, Di Vicino U, Annunziata I, Surace EM, Auricchio A. Non-erythropoietic erythropoietin derivatives protect from light-induced and genetic photoreceptor degeneration. Hum Mol Genet. 2011;20:2251–2262. doi: 10.1093/hmg/ddr115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colella P, Trapani I, Cesi G, Sommella A, Manfredi A, Puppo A, Iodice C, Rossi S, Simonelli F, Giunti M, Bacci ML, Auricchio A. Efficient gene delivery to the cone-enriched pig retina by dual AAV vectors. Gene Ther. 2014;21:450–456. doi: 10.1038/gt.2014.8. [DOI] [PubMed] [Google Scholar]
- Conlon TJ, Deng WT, Erger K, Cossette T, Pang JJ, Ryals R, Clement N, Cleaver B, McDoom I, Boye SE, Peden MC, Sherwood MB, Abernathy CR, Alkuraya FS, Boye SL, Hauswirth WW. Preclinical potency and safety studies of an AAV2-mediated gene therapy vector for the treatment of MERTK associated retinitis pigmentosa. Hum Gene Ther Clin Dev. 2013;24:23–28. doi: 10.1089/humc.2013.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussa RG, Traboulsi EI. Choroideremia: a review of general findings and pathogenesis. Ophthalmic Genet. 2012;33:57–65. doi: 10.3109/13816810.2011.620056. [DOI] [PubMed] [Google Scholar]
- Cwerman-Thibault H, Augustin S, Ellouze S, Sahel JA, Corral-Debrinski M. Gene therapy for mitochondrial diseases: Leber Hereditary Optic Neuropathy as the first candidate for a clinical trial. C R Biol. 2014;337:193–206. doi: 10.1016/j.crvi.2013.11.011. [DOI] [PubMed] [Google Scholar]
- D’Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet. 2000;9:645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- Dalkara D, Byrne LC, Klimczak RR, Visel M, Yin L, Merigan WH, Flannery JG, Schaffer DV. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med. 2013;5:189ra176. doi: 10.1126/scitranslmed.3005708. [DOI] [PubMed] [Google Scholar]
- Dalkara D, Kolstad KD, Caporale N, Visel M, Klimczak RR, Schaffer DV, Flannery JG. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol Ther. 2009;17:2096–2102. doi: 10.1038/mt.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalkara D, Kolstad KD, Guerin KI, Hoffmann NV, Visel M, Klimczak RR, Schaffer DV, Flannery JG. AAV mediated GDNF secretion from retinal glia slows down retinal degeneration in a rat model of retinitis pigmentosa. Mol Ther. 2011;19:1602–1608. doi: 10.1038/mt.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ, Tosi J, Janisch KM, Kasanuki JM, Wang NK, Kong J, Tsui I, Cilluffo M, Woodruff ML, Fain GL, Lin CS, Tsang SH. Functional rescue of degenerating photoreceptors in mice homozygous for a hypomorphic cGMP phosphodiesterase 6 b allele (Pde6bH620Q) Invest Ophthalmol Vis Sci. 2008;49:5067–5076. doi: 10.1167/iovs.07-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daya S, Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 2008;21:583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado D, del Pozo-Rodriguez A, Solinis MA, Aviles-Triqueros M, Weber BH, Fernandez E, Gascon AR. Dextran and protamine-based solid lipid nanoparticles as potential vectors for the treatment of X-linked juvenile retinoschisis. Hum Gene Ther. 2012;23:345–355. doi: 10.1089/hum.2011.115. [DOI] [PubMed] [Google Scholar]
- Deng WT, Dinculescu A, Li Q, Boye SL, Li J, Gorbatyuk MS, Pang J, Chiodo VA, Matthes MT, Yasumura D, Liu L, Alkuraya FS, Zhang K, Vollrath D, LaVail MM, Hauswirth WW. Tyrosine-mutant AAV8 delivery of human MERTK provides long-term retinal preservation in RCS rats. Invest Ophthalmol Vis Sci. 2012;53:1895–1904. doi: 10.1167/iovs.11-8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmaris N, Bosch A, Salaun C, Petit C, Prevost MC, Tordo N, Perrin P, Schwartz O, de Rocquigny H, Heard JM. Production and neurotropism of lentivirus vectors pseudotyped with lyssavirus envelope glycoproteins. Mol Ther. 2001;4:149–156. doi: 10.1006/mthe.2001.0431. [DOI] [PubMed] [Google Scholar]
- Dezawa M, Takano M, Negishi H, Mo X, Oshitari T, Sawada H. Gene transfer into retinal ganglion cells by in vivo electroporation: a new approach. Micron. 2002;33:1–6. doi: 10.1016/s0968-4328(01)00002-6. [DOI] [PubMed] [Google Scholar]
- Dinculescu A, Glushakova L, Min SH, Hauswirth WW. Adeno-associated virus-vectored gene therapy for retinal disease. Hum Gene Ther. 2005;16:649–663. doi: 10.1089/hum.2005.16.649. [DOI] [PubMed] [Google Scholar]
- Ding XQ, Quiambao AB, Fitzgerald JB, Cooper MJ, Conley SM, Naash MI. Ocular delivery of compacted DNA-nanoparticles does not elicit toxicity in the mouse retina. PLoS One. 2009;4:e7410. doi: 10.1371/journal.pone.0007410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B, Nakai H, Xiao W. Characterization of genome integrity for oversized recombinant AAV vector. Mol Ther. 2010;18:87–92. doi: 10.1038/mt.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroudchi MM, Greenberg KP, Liu J, Silka KA, Boyden ES, Lockridge JA, Arman AC, Janani R, Boye SE, Boye SL, Gordon GM, Matteo BC, Sampath AP, Hauswirth WW, Horsager A. Virally delivered channelrhodopsin-2 safely and effectively restores visual function in multiple mouse models of blindness. Mol Ther. 2011;19:1220–1229. doi: 10.1038/mt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell MI, Aguilar E, Jacobson R, Yanes O, Gariano R, Heckenlively J, Banin E, Ramirez GA, Gasmi M, Bird A, Siuzdak G, Friedlander M. Antioxidant or neurotrophic factor treatment preserves function in a mouse model of neovascularization-associated oxidative stress. J Clin Invest. 2009;119:611–623. doi: 10.1172/JCI35977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja TP, Hahn LB, Cowley GS, McGee TL, Berson EL. Mutation spectrum of the rhodopsin gene among patients with autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991;88:9370–9374. doi: 10.1073/pnas.88.20.9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja TP, McGee TL, Reichel E, Hahn LB, Cowley GS, Yandell DW, Sandberg MA, Berson EL. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990;343:364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- Duan D, Sharma P, Yang J, Yue Y, Dudus L, Zhang Y, Fisher KJ, Engelhardt JF. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J Virol. 1998;72:8568–8577. doi: 10.1128/jvi.72.11.8568-8577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Yue Y, Engelhardt JF. Expanding AAV packaging capacity with trans-splicing or overlapping vectors: a quantitative comparison. Mol Ther. 2001;4:383–391. doi: 10.1006/mthe.2001.0456. [DOI] [PubMed] [Google Scholar]
- Duisit G, Conrath H, Saleun S, Folliot S, Provost N, Cosset FL, Sandrin V, Moullier P, Rolling F. Five recombinant simian immunodeficiency virus pseudotypes lead to exclusive transduction of retinal pigmented epithelium in rat. Mol Ther. 2002;6:446–454. doi: 10.1006/mthe.2002.0690. [DOI] [PubMed] [Google Scholar]
- Dyka FM, Boye SL, Chiodo VA, Hauswirth WW, Boye SE. Dual adeno-associated virus vectors result in efficient in vitro and in vivo expression of an oversized gene, MYO7A. Hum Gene Ther Methods. 2014;25:166–177. doi: 10.1089/hgtb.2013.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellouze S, Augustin S, Bouaita A, Bonnet C, Simonutti M, Forster V, Picaud S, Sahel JA, Corral-Debrinski M. Optimized allotopic expression of the human mitochondrial ND4 prevents blindness in a rat model of mitochondrial dysfunction. Am J Hum Genet. 2008;83:373–387. doi: 10.1016/j.ajhg.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esumi N, Oshima Y, Li Y, Campochiaro PA, Zack DJ. Analysis of the VMD2 promoter and implication of E-box binding factors in its regulation. J Biol Chem. 2004;279:19064–19073. doi: 10.1074/jbc.M309881200. [DOI] [PubMed] [Google Scholar]
- Fagone P, Wright JF, Nathwani AC, Nienhuis AW, Davidoff AM, Gray JT. Systemic errors in quantitative polymerase chain reaction titration of self-complementary adeno-associated viral vectors and improved alternative methods. Hum Gene Ther Methods. 2012;23:1–7. doi: 10.1089/hgtb.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farjo R, Skaggs J, Quiambao AB, Cooper MJ, Naash MI. Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PLoS One. 2006;1:e38. doi: 10.1371/journal.pone.0000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei Y. Development of the cone photoreceptor mosaic in the mouse retina revealed by fluorescent cones in transgenic mice. Mol Vis. 2003;9:31–42. [PubMed] [Google Scholar]
- Flannery JG, Zolotukhin S, Vaquero MI, LaVail MM, Muzyczka N, Hauswirth WW. Efficient photoreceptor-targeted gene expression in vivo by recombinant adeno-associated virus. Proc Natl Acad Sci U S A. 1997;94:6916–6921. doi: 10.1073/pnas.94.13.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- Gauthier R, Joly S, Pernet V, Lachapelle P, Di Polo A. Brain-derived neurotrophic factor gene delivery to muller glia preserves structure and function of light-damaged photoreceptors. Invest Ophthalmol Vis Sci. 2005;46:3383–3392. doi: 10.1167/iovs.05-0362. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Duan D. Expanding adeno-associated viral vector capacity: a tale of two vectors. Biotechnol Genet Eng Rev. 2007;24:165–177. doi: 10.1080/02648725.2007.10648098. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Yue Y, Duan D. Efficient transgene reconstitution with hybrid dual AAV vectors carrying the minimized bridging sequences. Hum Gene Ther. 2011;22:77–83. doi: 10.1089/hum.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Yue Y, Lai Y, Duan D. A hybrid vector system expands adeno-associated viral vector packaging capacity in a transgene-independent manner. Mol Ther. 2008;16:124–130. doi: 10.1038/sj.mt.6300322. [DOI] [PubMed] [Google Scholar]
- Gill DR, Pringle IA, Hyde SC. Progress and prospects: the design and production of plasmid vectors. Gene Ther. 2009;16:165–171. doi: 10.1038/gt.2008.183. [DOI] [PubMed] [Google Scholar]
- Gorbatyuk M, Justilien V, Liu J, Hauswirth WW, Lewin AS. Preservation of photoreceptor morphology and function in P23H rats using an allele independent ribozyme. Exp Eye Res. 2007a;84:44–52. doi: 10.1016/j.exer.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbatyuk M, Justilien V, Liu J, Hauswirth WW, Lewin AS. Suppression of mouse rhodopsin expression in vivo by AAV mediated siRNA delivery. Vis Res. 2007b;47:1202–1208. doi: 10.1016/j.visres.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbatyuk MS, Pang JJ, Thomas J, Jr, Hauswirth WW, Lewin AS. Knockdown of wild-type mouse rhodopsin using an AAV vectored ribozyme as part of an RNA replacement approach. Mol Vis. 2005;11:648–656. [PubMed] [Google Scholar]
- Gracey Maniar LE, Maniar JM, Chen ZY, Lu J, Fire AZ, Kay MA. Mini-circle DNA vectors achieve sustained expression reflected by active chromatin and transcriptional level. Mol Ther. 2013;21:131–138. doi: 10.1038/mt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg KP, Geller SF, Schaffer DV, Flannery JG. Targeted transgene expression in muller glia of normal and diseased retinas using lentiviral vectors. Invest Ophthalmol Vis Sci. 2007;48:1844–1852. doi: 10.1167/iovs.05-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger JC, Samulski RJ. Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps. J Virol. 2005;79:9933–9944. doi: 10.1128/JVI.79.15.9933-9944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Hermann DM, Bogdanova A, Hotop S, Kilic U, Wenzel A, Kilic E, Gassmann M. Neuroprotection by hypoxic preconditioning: HIF-1 and erythropoietin protect from retinal degeneration. Semin Cell Dev Biol. 2005;16:531–538. doi: 10.1016/j.semcdb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Grossman N, Poher V, Grubb MS, Kennedy GT, Nikolic K, McGovern B, Berlinguer Palmini R, Gong Z, Drakakis EM, Neil MA, Dawson MD, Burrone J, Degenaar P. Multisite optical excitation using ChR2 and micro-LED array. J Neural Eng. 2010;7:16004. doi: 10.1088/1741-2560/7/1/016004. [DOI] [PubMed] [Google Scholar]
- Gruter O, Kostic C, Crippa SV, Perez MT, Zografos L, Schorderet DF, Munier FL, Arsenijevic Y. Lentiviral vector-mediated gene transfer in adult mouse photoreceptors is impaired by the presence of a physical barrier. Gene Ther. 2005;12:942–947. doi: 10.1038/sj.gt.3302485. [DOI] [PubMed] [Google Scholar]
- Guy J, Qi X, Koilkonda RD, Arguello T, Chou TH, Ruggeri M, Porciatti V, Lewin AS, Hauswirth WW. Efficiency and safety of AAV-mediated gene delivery of the human ND4 complex I subunit in the mouse visual system. Invest Ophthalmol Vis Sci. 2009;50:4205–4214. doi: 10.1167/iovs.08-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MM, Byrnes GA, Gall JG, Brough DE, King CR, Wei LL. Alternate serotype adenovector provides long-term therapeutic gene expression in the eye. Mol Vis. 2008;14:2535–2546. [PMC free article] [PubMed] [Google Scholar]
- Han Z, Conley SM, Makkia R, Guo J, Cooper MJ, Naash MI. Comparative analysis of DNA nanoparticles and AAVs for ocular gene delivery. PLoS One. 2012;7:e52189. doi: 10.1371/journal.pone.0052189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AR, Kamphuis W, Eggers R, Symons NA, Blits B, Niclou S, Boer GJ, Verhaagen J. Intravitreal injection of adeno-associated viral vectors results in the transduction of different types of retinal neurons in neonatal and adult rats: a comparison with lentiviral vectors. Mol Cell Neurosci. 2002;21:141–157. doi: 10.1006/mcne.2002.1168. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Gibbs D, Lillo C, Azarian SM, Legacki E, Zhang XM, Yang XJ, Williams DS. Lentiviral gene replacement therapy of retinas in a mouse model for Usher syndrome type 1B. Gene Ther. 2007;14:584–594. doi: 10.1038/sj.gt.3302897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, Conlon TJ, Boye SL, Flotte TR, Byrne BJ, Jacobson SG. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom M, Ruitenberg MJ, Pollett MA, Ehlert EM, Twisk J, Verhaagen J, Harvey AR. Cellular tropism and transduction properties of seven adeno-associated viral vector serotypes in adult retina after intravitreal injection. Gene Ther. 2009;16:521–532. doi: 10.1038/gt.2008.178. [DOI] [PubMed] [Google Scholar]
- Herron WL, Riegel BW, Myers OE, Rubin ML. Retinal dystrophy in the rat – a pigment epithelial disease. Invest Ophthalmol. 1969;8:595–604. [PubMed] [Google Scholar]
- Hirsch ML, Agbandje-McKenna M, Samulski RJ. Little vector, big gene transduction: fragmented genome reassembly of adeno-associated virus. Mol Ther. 2010;18:6–8. doi: 10.1038/mt.2009.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch ML, Li C, Bellon I, Yin C, Chavala S, Pryadkina M, Richard I, Samulski RJ. Oversized AAV transductifon is mediated via a DNA-PKcs-independent, Rad51C-dependent repair pathway. Mol Ther. 2013;21:2205–2216. doi: 10.1038/mt.2013.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman LM, Maguire AM, Bennett J. Cell-mediated immune response and stability of intraocular transgene expression after adenovirus-mediated delivery. Invest Ophthalmol Vis Sci. 1997;38:2224–2233. [PubMed] [Google Scholar]
- Huang SP, Lin PK, Liu JH, Khor CN, Lee YJ. Intraocular gene transfer of ciliary neurotrophic factor rescues photoreceptor degeneration in RCS rats. J Biomed Sci. 2004;11:37–48. doi: 10.1007/BF02256547. [DOI] [PubMed] [Google Scholar]
- Igarashi T, Miyake K, Asakawa N, Miyake N, Shimada T, Takahashi H. Direct comparison of administration routes for AAV8-mediated ocular gene therapy. Curr Eye Res. 2013;38:569–577. doi: 10.3109/02713683.2013.779720. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Goto Y, Yonemitsu Y, Miyazaki M, Sakamoto T, Ishibashi T, Tabata T, Ueda Y, Hasegawa M, Tobimatsu S, Sueishi K. Simian immunodeficiency virus-based lentivirus vector for retinal gene transfer: a preclinical safety study in adult rats. Gene Ther. 2003;10:1161–1169. doi: 10.1038/sj.gt.3301973. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Yonemitsu Y, Miyazaki M, Kohno R, Murakami Y, Murata T, Goto Y, Tabata T, Ueda Y, Ono F, Suzuki T, Ageyama N, Terao K, Hasegawa M, Sueishi K, Ishibashi T. Acute toxicity study of a simian immunodeficiency virus-based lentiviral vector for retinal gene transfer in nonhuman primates. Hum Gene Ther. 2009a;20:943–954. doi: 10.1089/hum.2009.048. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Yonemitsu Y, Miyazaki M, Kohno R, Murakami Y, Murata T, Tabata T, Ueda Y, Ono F, Suzuki T, Ageyama N, Terao K, Hasegawa M, Sueishi K, Ishibashi T. Stable retinal gene expression in nonhuman primates via subretinal injection of SIVagm-based lentiviral vectors. Hum Gene Ther. 2009b;20:573–579. doi: 10.1089/hum.2009.009. [DOI] [PubMed] [Google Scholar]
- Imai D, Yoneya S, Gehlbach PL, Wei LL, Mori K. Intraocular gene transfer of pigment epithelium-derived factor rescues photoreceptors from light-induced cell death. J Cell Physiol. 2005;202:570–578. doi: 10.1002/jcp.20155. [DOI] [PubMed] [Google Scholar]
- Ivanova E, Hwang GS, Pan ZH, Troilo D. Evaluation of AAV-mediated expression of Chop2-GFP in the marmoset retina. Invest Ophthalmol Vis Sci. 2010;51:5288–5296. doi: 10.1167/iovs.10-5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, Pan ZH. Evaluation of the adeno-associated virus mediated long-term expression of channelrhodopsin-2 in the mouse retina. Mol Vis. 2009;15:1680–1689. [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Aleman TS, Cideciyan AV, Sumaroka A, Schwartz SB, Windsor EA, Traboulsi EI, Heon E, Pittler SJ, Milam AH, Maguire AM, Palczewski K, Stone EM, Bennett J. Identifying photoreceptors in blind eyes caused by RPE65 mutations: prerequisite for human gene therapy success. Proc Natl Acad Sci U S A. 2005;102:6177–6182. doi: 10.1073/pnas.0500646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Aleman TS, Sumaroka A, Windsor EA, Schwartz SB, Heon E, Stone EM. Photoreceptor layer topography in children with leber congenital amaurosis caused by RPE65 mutations. Invest Ophthalmol Vis Sci. 2008;49:4573–4577. doi: 10.1167/iovs.08-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Ratnakaram R, Heon E, Schwartz SB, Roman AJ, Peden MC, Aleman TS, Boye SL, Sumaroka A, Conlon TJ, Calcedo R, Pang JJ, Erger KE, Olivares MB, Mullins CL, Swider M, Kaushal S, Feuer WJ, Iannaccone A, Fishman GA, Stone EM, Byrne BJ, Hauswirth WW. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol. 2012;130:9–24. doi: 10.1001/archophthalmol.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnen S, Izsvak Z, Stocker M, Harmening N, Salz AK, Walter P, Thumann G. Sleeping Beauty transposon-mediated transfection of retinal and iris pigment epithelial cells. Invest Ophthalmol Vis Sci. 2012;53:4787–4796. doi: 10.1167/iovs.12-9951. [DOI] [PubMed] [Google Scholar]
- Johnson CJ, Berglin L, Chrenek MA, Redmond TM, Boatright JH, Nickerson JM. Technical brief: subretinal injection and electroporation into adult mouse eyes. Mol Vis. 2008a;14:2211–2226. [PMC free article] [PubMed] [Google Scholar]
- Johnson LN, Cashman SM, Kumar-Singh R. Cell-penetrating peptide for enhanced delivery of nucleic acids and drugs to ocular tissues including retina and cornea. Mol Ther. 2008b;16:107–114. doi: 10.1038/sj.mt.6300324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LN, Cashman SM, Read SP, Kumar-Singh R. Cell penetrating peptide POD mediates delivery of recombinant proteins to retina, cornea and skin. Vis Res. 2010;50:686–697. doi: 10.1016/j.visres.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomary C, Piper TA, Dickson G, Couture LA, Smith AE, Neal MJ, Jones SE. Adenovirus-mediated gene transfer to murine retinal cells in vitro and in vivo. FEBS Lett. 1994;347:117–122. doi: 10.1016/0014-5793(94)00512-5. [DOI] [PubMed] [Google Scholar]
- Jomary C, Vincent KA, Grist J, Neal MJ, Jones SE. Rescue of photoreceptor function by AAV-mediated gene transfer in a mouse model of inherited retinal degeneration. Gene Ther. 1997;4:683–690. doi: 10.1038/sj.gt.3300440. [DOI] [PubMed] [Google Scholar]
- Kachi S, Oshima Y, Esumi N, Kachi M, Rogers B, Zack DJ, Campochiaro PA. Nonviral ocular gene transfer. Gene Ther. 2005;12:843–851. doi: 10.1038/sj.gt.3302475. [DOI] [PubMed] [Google Scholar]
- Kaltimbacher V, Bonnet C, Lecoeuvre G, Forster V, Sahel JA, Corral-Debrinski M. mRNA localization to the mitochondrial surface allows the efficient translocation inside the organelle of a nuclear recoded ATP6 protein. RNA. 2006;12:1408–1417. doi: 10.1261/rna.18206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karali M, Manfredi A, Puppo A, Marrocco E, Gargiulo A, Allocca M, Corte MD, Rossi S, Giunti M, Bacci ML, Simonelli F, Surace EM, Banfi S, Auricchio A. MicroRNA-restricted transgene expression in the retina. PLoS One. 2011;6:e22166. doi: 10.1371/journal.pone.0022166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay CN, Ryals RC, Aslanidi GV, Min SH, Ruan Q, Sun J, Dyka FM, Kasuga D, Ayala AE, Van Vliet K, Agbandje-McKenna M, Hauswirth WW, Boye SL, Boye SE. Targeting photoreceptors via intravitreal delivery using novel, capsid-mutated AAV vectors. PLoS One. 2013;8:e62097. doi: 10.1371/journal.pone.0062097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7:33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- Khani SC, Pawlyk BS, Bulgakov OV, Kasperek E, Young JE, Adamian M, Sun X, Smith AJ, Ali RR, Li T. AAV-mediated expression targeting of rod and cone photoreceptors with a human rhodopsin kinase promoter. Invest Ophthalmol Vis Sci. 2007;48:3954–3961. doi: 10.1167/iovs.07-0257. [DOI] [PubMed] [Google Scholar]
- Klimczak RR, Koerber JT, Dalkara D, Flannery JG, Schaffer DV. A novel adeno-associated viral variant for efficient and selective intravitreal transduction of rat Muller cells. PLoS One. 2009;4:e7467. doi: 10.1371/journal.pone.0007467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koilkonda RD, Yu H, Chou TH, Feuer WJ, Ruggeri M, Porciatti V, Tse D, Hauswirth WW, Chiodo V, Boye SL, Lewin AS, Neuringer M, Renner L, Guy J. Safety and effects of the vector for the leber hereditary optic neuropathy gene therapy clinical trial. JAMA Ophthalmol. 2014;132:409–420. doi: 10.1001/jamaophthalmol.2013.7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koirala A, Makkia RS, Conley SM, Cooper MJ, Naash MI. S/MAR-containing DNA nanoparticles promote persistent RPE gene expression and improvement in RPE65-associated LCA. Hum Mol Genet. 2013;22:1632–1642. doi: 10.1093/hmg/ddt013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolstad KD, Dalkara D, Guerin K, Visel M, Hoffmann N, Schaffer DV, Flannery JG. Changes in adeno-associated virus-mediated gene delivery in retinal degeneration. Hum Gene Ther. 2010;21:571–578. doi: 10.1089/hum.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaromy AM, Alexander JJ, Cooper AE, Chiodo VA, Glushakova LG, Acland GM, Hauswirth WW, Aguirre GD. Targeting gene expression to cones with human cone opsin promoters in recombinant AAV. Gene Ther. 2008;15:1049–1055. doi: 10.1038/gt.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaromy AM, Alexander JJ, Rowlan JS, Garcia MM, Chiodo VA, Kaya A, Tanaka JC, Acland GM, Hauswirth WW, Aguirre GD. Gene therapy rescues cone function in congenital achromatopsia. Hum Mol Genet. 2010;19:2581–2593. doi: 10.1093/hmg/ddq136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F, Li W, Li X, Zheng Q, Dai X, Zhou X, Boye SL, Hauswirth WW, Qu J, Pang JJ. Self-complementary AAV5 vector facilitates quicker transgene expression in photoreceptor and retinal pigment epithelial cells of normal mouse. Exp Eye Res. 2010;90:546–554. doi: 10.1016/j.exer.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Kim SR, Binley K, Pata I, Doi K, Mannik J, Zernant-Rajang J, Kan O, Iqball S, Naylor S, Sparrow JR, Gouras P, Allikmets R. Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Ther. 2008;15:1311–1320. doi: 10.1038/gt.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstan MW, Davis PB, Wagener JS, Hilliard KA, Stern RC, Milgram LJ, Kowalczyk TH, Hyatt SL, Fink TL, Gedeon CR, Oette SM, Payne JM, Muhammad O, Ziady AG, Moen RC, Cooper MJ. Compacted DNA nanoparticles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution. Hum Gene Ther. 2004;15:1255–1269. doi: 10.1089/hum.2004.15.1255. [DOI] [PubMed] [Google Scholar]
- Kostic C, Crippa SV, Pignat V, Bemelmans AP, Samardzija M, Grimm C, Wenzel A, Arsenijevic Y. Gene therapy regenerates protein expression in cone photoreceptors in Rpe65(R91W/R91W) mice. PLoS One. 2011;6:e16588. doi: 10.1371/journal.pone.0016588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreppel F, Luther TT, Semkova I, Schraermeyer U, Kochanek S. Long-term transgene expression in the RPE after gene transfer with a high-capacity adenoviral vector. Invest Ophthalmol Vis Sci. 2002;43:1965–1970. [PubMed] [Google Scholar]
- Ku CA, Chiodo VA, Boye SL, Goldberg AF, Li T, Hauswirth WW, Ramamurthy V. Gene therapy using self-complementary Y733F capsid mutant AAV2/8 restores vision in a model of early onset Leber congenital amaurosis. Hum Mol Genet. 2011;20:4569–4581. doi: 10.1093/hmg/ddr391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar-Singh R. Barriers for retinal gene therapy: separating fact from fiction. Vis Res. 2008;48:1671–1680. doi: 10.1016/j.visres.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar-Singh R, Farber DB. Encapsidated adenovirus mini-chromosome-mediated delivery of genes to the retina: application to the rescue of photo-receptor degeneration. Hum Mol Genet. 1998;7:1893–1900. doi: 10.1093/hmg/7.12.1893. [DOI] [PubMed] [Google Scholar]
- Kuzmanovic M, Dudley VJ, Sarthy VP. GFAP promoter drives Muller cell-specific expression in transgenic mice. Invest Ophthalmol Vis Sci. 2003;44:3606–3613. doi: 10.1167/iovs.02-1265. [DOI] [PubMed] [Google Scholar]
- Lai Y, Yue Y, Duan D. Evidence for the failure of adeno-associated virus serotype 5 to package a viral genome > or = 8.2 kb. Mol Ther. 2010;18:75–79. doi: 10.1038/mt.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamartina S, Cimino M, Roscilli G, Dammassa E, Lazzaro D, Rota R, Ciliberto G, Toniatti C. Helper-dependent adenovirus for the gene therapy of proliferative retinopathies: stable gene transfer, regulated gene expression and therapeutic efficacy. J Gene Med. 2007;9:862–874. doi: 10.1002/jgm.1083. [DOI] [PubMed] [Google Scholar]
- LaVail MM, Yasumura D, Matthes MT, Drenser KA, Flannery JG, Lewin AS, Hauswirth WW. Ribozyme rescue of photoreceptor cells in P23H transgenic rats: long-term survival and late-stage therapy. Proc Natl Acad Sci U S A. 2000;97:11488–11493. doi: 10.1073/pnas.210319397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Meur G, Stieger K, Smith AJ, Weber M, Deschamps JY, Nivard D, Mendes-Madeira A, Provost N, Pereon Y, Cherel Y, Ali RR, Hamel C, Moullier P, Rolling F. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Ther. 2007;14:292–303. doi: 10.1038/sj.gt.3302861. [DOI] [PubMed] [Google Scholar]
- Lebherz C, Maguire A, Tang W, Bennett J, Wilson JM. Novel AAV sero-types for improved ocular gene transfer. J Gene Med. 2008;10:375–382. doi: 10.1002/jgm.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechauve C, Augustin S, Cwerman-Thibault H, Reboussin E, Roussel D, Lai-Kuen R, Saubamea B, Sahel JA, Debeir T, Corral-Debrinski M. Neuroglobin gene therapy prevents optic Atrophy and preserves Durably visual function in harlequin mice. Mol Ther. 2014;22:1096–1109. doi: 10.1038/mt.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, Savino C, Bianchi M, Nielsen J, Gerwien J, Kallunki P, Larsen AK, Helboe L, Christensen S, Pedersen LO, Nielsen M, Torup L, Sager T, Sfacteria A, Erbayraktar S, Erbayraktar Z, Gokmen N, Yilmaz O, Cerami-Hand C, Xie QW, Coleman T, Cerami A, Brines M. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- Lewin AS, Drenser KA, Hauswirth WW, Nishikawa S, Yasumura D, Flannery JG, LaVail MM. Ribozyme rescue of photoreceptor cells in a transgenic rat model of autosomal dominant retinitis pigmentosa. Nat Med. 1998;4:967–971. doi: 10.1038/nm0898-967. [DOI] [PubMed] [Google Scholar]
- Li T, Adamian M, Roof DJ, Berson EL, Dryja TP, Roessler BJ, Davidson BL. In vivo transfer of a reporter gene to the retina mediated by an adenoviral vector. Invest Ophthalmol Vis Sci. 1994;35:2543–2549. [PubMed] [Google Scholar]
- Liang FQ, Anand V, Maguire AM, Bennett J. Intraocular delivery of recombinant virus. In: Rakoczy PE, editor. Methods in Molecular Medicine: Ocular Molecular Biology Protocols. Humana Press. Inc; Totowa, NJ: 2000. pp. 125–139. [DOI] [PubMed] [Google Scholar]
- Liao HW, Yau KW. In vivo gene delivery in the retina using poly-ethylenimine. Biotechniques. 2007;42:285–286. 288. doi: 10.2144/000112404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Koizumi A, Tanaka N, Panda S, Masland RH. Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc Natl Acad Sci U S A. 2008;105:16009–16014. doi: 10.1073/pnas.0806114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski DM, Barnard AR, Charbel Issa P, Singh MS, De Silva SR, Trabalza A, Eleftheriadou I, Ellison SM, Mazarakis ND, Maclaren RE. Vesicular stomatitis virus glycoprotein- and venezuelan equine encephalitis virus-derived glycoprotein-pseudotyped lentivirus vectors differentially transduce corneal endothelium, trabecular meshwork, and human photoreceptors. Hum Gene Ther. 2014;25:50–62. doi: 10.1089/hum.2013.009. [DOI] [PubMed] [Google Scholar]
- Lipinski DM, Thake M, MacLaren RE. Clinical applications of retinal gene therapy. Prog Retin Eye Res. 2013;32:22–47. doi: 10.1016/j.preteyeres.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Liu G, Li D, Pasumarthy MK, Kowalczyk TH, Gedeon CR, Hyatt SL, Payne JM, Miller TJ, Brunovskis P, Fink TL, Muhammad O, Moen RC, Hanson RW, Cooper MJ. Nanoparticles of compacted DNA transfect postmitotic cells. J Biol Chem. 2003;278:32578–32586. doi: 10.1074/jbc.M305776200. [DOI] [PubMed] [Google Scholar]
- Loewen N, Leske DA, Cameron JD, Chen Y, Whitwam T, Simari RD, Teo WL, Fautsch MP, Poeschla EM, Holmes JM. Long-term retinal transgene expression with FIV versus adenoviral vectors. Mol Vis. 2004;10:272–280. [PubMed] [Google Scholar]
- Loewen N, Leske DA, Chen Y, Teo WL, Saenz DT, Peretz M, Holmes JM, Poeschla EM. Comparison of wild-type and class I integrase mutant-FIV vectors in retina demonstrates sustained expression of integrated transgenes in retinal pigment epithelium. J Gene Med. 2003;5:1009–1017. doi: 10.1002/jgm.447. [DOI] [PubMed] [Google Scholar]
- Lopes VS, Boye SE, Louie CM, Boye S, Dyka F, Chiodo V, Fofo H, Hauswirth WW, Williams DS. Retinal gene therapy with a large MYO7A cDNA using adeno-associated virus. Gene Ther. 2013;20:824–833. doi: 10.1038/gt.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotery AJ, Derksen TA, Russell SR, Mullins RF, Sauter S, Affatigato LM, Stone EM, Davidson BL. Gene transfer to the nonhuman primate retina with recombinant feline immunodeficiency virus vectors. Hum Gene Ther. 2002;13:689–696. doi: 10.1089/104303402317322258. [DOI] [PubMed] [Google Scholar]
- Lotery AJ, Yang GS, Mullins RF, Russell SR, Schmidt M, Stone EM, Lindbloom JD, Chiorini JA, Kotin RM, Davidson BL. Adeno-associated virus type 5: transduction efficiency and cell-type specificity in the primate retina. Hum Gene Ther. 2003;14:1663–1671. doi: 10.1089/104303403322542301. [DOI] [PubMed] [Google Scholar]
- Maclaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, Clark KR, During MJ, Cremers FP, Black GC, Lotery AJ, Downes SM, Webster AR, Seabra MC. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–1137. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, Mingozzi F, Bennicelli JL, Ying GS, Rossi S, Fulton A, Marshall KA, Banfi S, Chung DC, Morgan JI, Hauck B, Zelenaia O, Zhu X, Raffini L, Coppieters F, De Baere E, Shindler KS, Volpe NJ, Surace EM, Acerra C, Lyubarsky A, Redmond TM, Stone E, Sun J, McDonnell JW, Leroy BP, Simonelli F, Bennett J. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell’Osso L, Hertle R, Ma JX, Redmond TM, Zhu X, Hauck B, Zelenaia O, Shindler KS, Maguire MG, Wright JF, Volpe NJ, McDonnell JW, Auricchio A, High KA, Bennett J. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Eng J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallam JN, Hurwitz MY, Mahoney T, Chevez-Barrios P, Hurwitz RL. Efficient gene transfer into retinal cells using adenoviral vectors: dependence on receptor expression. Invest Ophthalmol Vis Sci. 2004;45:1680–1687. doi: 10.1167/iovs.03-0730. [DOI] [PubMed] [Google Scholar]
- Mancuso K, Hauswirth WW, Li Q, Connor TB, Kuchenbecker JA, Mauck MC, Neitz J, Neitz M. Gene therapy for red-green colour blindness in adult primates. Nature. 2009;461:784–787. doi: 10.1038/nature08401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredi A, Marrocco E, Puppo A, Cesi G, Sommella A, Della Corte M, Rossi S, Giunti M, Craft CM, Bacci ML, Simonelli F, Surace EM, Auricchio A. Combined rod and cone transduction by adeno-associated virus 2/8. Hum Gene Ther. 2013;24:982–992. doi: 10.1089/hum.2013.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H, Gorbatyuk MS, Rossmiller B, Hauswirth WW, Lewin AS. Long-term rescue of retinal structure and function by rhodopsin RNA replacement with a single adeno-associated viral vector in P23H RHO transgenic mice. Hum Gene Ther. 2012;23:356–366. doi: 10.1089/hum.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H, James T, Jr, Schwein A, Shabashvili AE, Hauswirth WW, Gorbatyuk MS, Lewin AS. AAV delivery of wild-type rhodopsin preserves retinal function in a mouse model of autosomal dominant retinitis pigmentosa. Hum Gene Ther. 2011;22:567–575. doi: 10.1089/hum.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Cepko CL. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc Natl Acad Sci U S A. 2004;101:16–22. doi: 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty DM. Self-complementary AAV vectors; advances and applications. Mol Ther. 2008;16:1648–1656. doi: 10.1038/mt.2008.171. [DOI] [PubMed] [Google Scholar]
- Mei Y, Zhang F. Molecular tools and approaches for optogenetics. Biol Psychiatry. 2012;71:1033–1038. doi: 10.1016/j.biopsych.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalakis S, Muhlfriedel R, Tanimoto N, Krishnamoorthy V, Koch S, Fischer MD, Becirovic E, Bai L, Huber G, Beck SC, Fahl E, Buning H, Paquet-Durand F, Zong X, Gollisch T, Biel M, Seeliger MW. Restoration of cone vision in the CNGA3−/− mouse model of congenital complete lack of cone photoreceptor function. Mol Ther. 2010;18:2057–2063. doi: 10.1038/mt.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihelec M, Pearson RA, Robbie SJ, Buch PK, Azam SA, Bainbridge JW, Smith AJ, Ali RR. Long-term preservation of cones and improvement in visual function following gene therapy in a mouse model of leber congenital amaurosis caused by guanylate cyclase-1 deficiency. Hum Gene Ther. 2011;22:1179–1190. doi: 10.1089/hum.2011.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan JM, Aller E, Jaijo T, Blanco-Kelly F, Gimenez-Pardo A, Ayuso C. An update on the genetics of usher syndrome. J Ophthalmol. 2011;2011:417217. doi: 10.1155/2011/417217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millington-Ward S, Chadderton N, O’Reilly M, Palfi A, Goldmann T, Kilty C, Humphries M, Wolfrum U, Bennett J, Humphries P, Kenna PF, Farrar GJ. Suppression and replacement gene therapy for autosomal dominant disease in a murine model of dominant retinitis pigmentosa. Mol Ther. 2011;19:642–649. doi: 10.1038/mt.2010.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M, Ikeda Y, Yonemitsu Y, Goto Y, Sakamoto T, Tabata T, Ueda Y, Hasegawa M, Tobimatsu S, Ishibashi T, Sueishi K. Simian lentiviral vector-mediated retinal gene transfer of pigment epithelium-derived factor protects retinal degeneration and electrical defect in Royal College of Surgeons rats. Gene Ther. 2003;10:1503–1511. doi: 10.1038/sj.gt.3302028. [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Takahashi M, Gage FH, Verma IM. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci U S A. 1997;94:10319–10323. doi: 10.1073/pnas.94.19.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina RP, Ye HQ, Brady J, Mech CA, Kaleko M, Tianci L. Baculovirus GP64 pseudotyped bovine immunodeficiency virus-based lentiviral vectors efficiently transduce retinal cells in vivo. Mol Ther. 2004a;9:S277. [Google Scholar]
- Molina RP, Ye HQ, Brady J, Zhang J, Zimmerman H, Kaleko M, Luo T. A synthetic Rev-independent bovine immunodeficiency virus-based packaging construct. Hum Gene Ther. 2004b;15:865–877. doi: 10.1089/hum.2004.15.865. [DOI] [PubMed] [Google Scholar]
- Mori K, Gehlbach P, Ando A, Wahlin K, Gunther V, McVey D, Wei L, Campochiaro PA. Intraocular adenoviral vector-mediated gene transfer in proliferative retinopathies. Invest Ophthalmol Vis Sci. 2002;43:1610–1615. [PubMed] [Google Scholar]
- Mowat FM, Gornik KR, Dinculescu A, Boye SL, Hauswirth WW, Petersen-Jones SM, Bartoe JT. Tyrosine capsid-mutant AAV vectors for gene delivery to the canine retina from a subretinal or intravitreal approach. Gene Ther. 2014;21:96–105. doi: 10.1038/gt.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussolino C, della Corte M, Rossi S, Viola F, Di Vicino U, Marrocco E, Neglia S, Doria M, Testa F, Giovannoni R, Crasta M, Giunti M, Villani E, Lavitrano M, Bacci ML, Ratiglia R, Simonelli F, Auricchio A, Surace EM. AAV-mediated photoreceptor transduction of the pig cone-enriched retina. Gene Ther. 2011a;18:637–645. doi: 10.1038/gt.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussolino C, Sanges D, Marrocco E, Bonetti C, Di Vicino U, Marigo V, Auricchio A, Meroni G, Surace EM. Zinc-finger-based transcriptional repression of rhodopsin in a model of dominant retinitis pigmentosa. EMBO Mol Med. 2011b;3:118–128. doi: 10.1002/emmm.201000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik R, Mukhopadhyay A, Ganguli M. Gene delivery to the retina: focus on non-viral approaches. Drug Discov Today. 2009;14:306–315. doi: 10.1016/j.drudis.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Narfstrom K, Katz ML, Bragadottir R, Seeliger M, Boulanger A, Redmond TM, Caro L, Lai CM, Rakoczy PE. Functional and structural recovery of the retina after gene therapy in the RPE65 null mutation dog. Invest Ophthalmol Vis Sci. 2003;44:1663–1672. doi: 10.1167/iovs.02-0595. [DOI] [PubMed] [Google Scholar]
- Natkunarajah M, Trittibach P, McIntosh J, Duran Y, Barker SE, Smith AJ, Nathwani AC, Ali RR. Assessment of ocular transduction using single-stranded and self-complementary recombinant adeno-associated virus sero-type 2/8. Gene Ther. 2008;15:463–467. doi: 10.1038/sj.gt.3303074. [DOI] [PubMed] [Google Scholar]
- Nicoletti A, Kawase K, Thompson DA. Promoter analysis of RPE65, the gene encoding a 61-kDa retinal pigment epithelium-specific protein. Invest Ophthalmol Vis Sci. 1998;39:637–644. [PubMed] [Google Scholar]
- Nicoud M, Kong J, Iqball S, Kan O, Naylor S, Gouras P, Allikmets R, Binley K. Development of photoreceptor-specific promoters and their utility to investigate EIAV lentiviral vector mediated gene transfer to photoreceptors. J Gene Med. 2007;9:1015–1023. doi: 10.1002/jgm.1115. [DOI] [PubMed] [Google Scholar]
- Nonnenmacher M, Weber T. Intracellular transport of recombinant adeno-associated virus vectors. Gene Ther. 2012;19:649–658. doi: 10.1038/gt.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normand N, Valamanesh F, Savoldelli M, Mascarelli F, BenEzra D, Courtois Y, Behar-Cohen F. VP22 light controlled delivery of oligonucleotides to ocular cells in vitro and in vivo. Mol Vis. 2005;11:184–191. [PubMed] [Google Scholar]
- Normand N, van Leeuwen H, O’Hare P. Particle formation by a conserved domain of the herpes simplex virus protein VP22 facilitating protein and nucleic acid delivery. J Biol Chem. 2001;276:15042–15050. doi: 10.1074/jbc.M010294200. [DOI] [PubMed] [Google Scholar]
- O’Reilly M, Palfi A, Chadderton N, Millington-Ward S, Ader M, Cronin T, Tuohy T, Auricchio A, Hildinger M, Tivnan A, McNally N, Humphries MM, Kiang AS, Humphries P, Kenna PF, Farrar GJ. RNA interference-mediated suppression and replacement of human rhodopsin in vivo. Am J Hum Genet. 2007;81:127–135. doi: 10.1086/519025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang J, Boye SE, Lei B, Boye SL, Everhart D, Ryals R, Umino Y, Rohrer B, Alexander J, Li J, Dai X, Li Q, Chang B, Barlow R, Hauswirth WW. Self-complementary AAV-mediated gene therapy restores cone function and prevents cone degeneration in two models of Rpe65 deficiency. Gene Ther. 2010;17:815–826. doi: 10.1038/gt.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang J, Cheng M, Haire SE, Barker E, Planelles V, Blanks JC. Efficiency of lentiviral transduction during development in normal and rd mice. Mol Vis. 2006;12:756–767. [PubMed] [Google Scholar]
- Pang J, Cheng M, Stevenson D, Trousdale MD, Dorey CK, Blanks JC. Adenoviral-mediated gene transfer to retinal explants during development and degeneration. Exp Eye Res. 2004;79:189–201. doi: 10.1016/j.exer.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Boye SL, Kumar A, Dinculescu A, Deng W, Li J, Li Q, Rani A, Foster TC, Chang B, Hawes NL, Boatright JH, Hauswirth WW. AAV-mediated gene therapy for retinal degeneration in the rd10 mouse containing a recessive PDEbeta mutation. Invest Ophthalmol Vis Sci. 2008;49:4278–4283. doi: 10.1167/iovs.07-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Dai X, Boye SE, Barone I, Boye SL, Mao S, Everhart D, Dinculescu A, Liu L, Umino Y, Lei B, Chang B, Barlow R, Strettoi E, Hauswirth WW. Long-term retinal function and structure rescue using capsid mutant AAV8 vector in the rd10 mouse, a model of recessive retinitis pigmentosa. Mol Ther. 2011;19:234–242. doi: 10.1038/mt.2010.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Deng WT, Dai X, Lei B, Everhart D, Umino Y, Li J, Zhang K, Mao S, Boye SL, Liu L, Chiodo VA, Liu X, Shi W, Tao Y, Chang B, Hauswirth WW. AAV-mediated cone rescue in a naturally occurring mouse model of CNGA3-achromatopsia. PLoS One. 2012;7:e35250. doi: 10.1371/journal.pone.0035250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TK, Wu Z, Kjellstrom S, Zeng Y, Bush RA, Sieving PA, Colosi P. Intravitreal delivery of AAV8 retinoschisin results in cell type-specific gene expression and retinal rescue in the Rs1-KO mouse. Gene Ther. 2009;16:916–926. doi: 10.1038/gt.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks RJ, Chen L, Anton M, Sankar U, Rudnicki MA, Graham FL. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci U S A. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen-Jones SM, Bartoe JT, Fischer AJ, Scott M, Boye SL, Chiodo V, Hauswirth WW. AAV retinal transduction in a large animal model species: comparison of a self-complementary AAV2/5 with a single-stranded AAV2/5 vector. Mol Vis. 2009;15:1835–1842. [PMC free article] [PubMed] [Google Scholar]
- Petit L, Lheriteau E, Weber M, Le Meur G, Deschamps JY, Provost N, Mendes-Madeira A, Libeau L, Guihal C, Colle MA, Moullier P, Rolling F. Restoration of vision in the pde6beta-deficient dog, a large animal model of rod-cone dystrophy. Mol Ther. 2012;20:2019–2030. doi: 10.1038/mt.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrs-Silva H, Dinculescu A, Li Q, Deng WT, Pang JJ, Min SH, Chiodo V, Neeley AW, Govindasamy L, Bennett A, Agbandje-McKenna M, Zhong L, Li B, Jayandharan GR, Srivastava A, Lewin AS, Hauswirth WW. Novel properties of tyrosine-mutant AAV2 vectors in the mouse retina. Mol Ther. 2011;19:293–301. doi: 10.1038/mt.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrs-Silva H, Dinculescu A, Li Q, Min SH, Chiodo V, Pang JJ, Zhong L, Zolotukhin S, Srivastava A, Lewin AS, Hauswirth WW. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther. 2009;17:463–471. doi: 10.1038/mt.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittler SJ, Baehr W. Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase beta-subunit gene of the rd mouse. Proc Natl Acad Sci U S A. 1991;88:8322–8326. doi: 10.1073/pnas.88.19.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliquett UF, Martin GT, Weaver JC. Kinetics of the temperature rise within human stratum corneum during electroporation and pulsed high-voltage iontophoresis. Bioelectrochemistry. 2002;57:65–72. doi: 10.1016/s1567-5394(01)00177-3. [DOI] [PubMed] [Google Scholar]
- Prow TW, Bhutto I, Kim SY, Grebe R, Merges C, McLeod DS, Uno K, Mennon M, Rodriguez L, Leong K, Lutty GA. Ocular nanoparticle toxicity and transfection of the retina and retinal pigment epithelium. Nano-medicine. 2008;4:340–349. doi: 10.1016/j.nano.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puppo A, Cesi Giulia, Marrocco Elena, Piccolo Pasquale, Jacca Sarah, Shayakhmetov Dmitry M, Parks Robin J, Davidson Beverly L, Colloca Stefano, Brunetti-Pierri Nicola, Ng Philip, Donofrio Gaetano, Auricchio Alberto. Retinal transduction profiles by high-capacity viral vectors. Gene Ther. 2014 doi: 10.1038/gt.2014.57. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puras G, Zarate J, Aceves M, Murua A, Diaz AR, Aviles-Triguero M, Fernandez E, Pedraz JL. Low molecular weight oligochitosans for non-viral retinal gene therapy. Eur J Pharm Biopharm. 2013a;83:131–140. doi: 10.1016/j.ejpb.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Puras G, Zarate J, Diaz-Tahoces A, Aviles-Trigueros M, Fernandez E, Pedraz JL. Oligochitosan polyplexes as carriers for retinal gene delivery. Eur J Pharm Sci. 2013b;48:323–331. doi: 10.1016/j.ejps.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Qi X, Sun L, Lewin AS, Hauswirth WW, Guy J. The mutant human ND4 subunit of complex I induces optic neuropathy in the mouse. Invest Ophthalmol Vis Sci. 2007;48:1–10. doi: 10.1167/iovs.06-0789. [DOI] [PubMed] [Google Scholar]
- Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, Samulski RJ. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read SP, Cashman SM, Kumar-Singh R. POD nanoparticles expressing GDNF provide structural and functional rescue of light-induced retinal degeneration in an adult mouse. Mol Ther. 2010a;18:1917–1926. doi: 10.1038/mt.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read SP, Cashman SM, Kumar-Singh R. A poly(ethylene) glycolylated peptide for ocular delivery compacts DNA into nanoparticles for gene delivery to post-mitotic tissues in vivo. J Gene Med. 2010b;12:86–96. doi: 10.1002/jgm.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich SJ, Auricchio A, Hildinger M, Glover E, Maguire AM, Wilson JM, Bennett J. Efficient trans-splicing in the retina expands the utility of adeno-associated virus as a vector for gene therapy. Hum Gene Ther. 2003a;14:37–44. doi: 10.1089/10430340360464697. [DOI] [PubMed] [Google Scholar]
- Reich SJ, Fosnot J, Kuroki A, Tang W, Yang X, Maguire AM, Bennett J, Tolentino MJ. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol Vis. 2003b;9:210–216. [PubMed] [Google Scholar]
- Reichel MB, Ali RR, Thrasher AJ, Hunt DM, Bhattacharya SS, Baker D. Immune responses limit adenovirally mediated gene expression in the adult mouse eye. Gene Ther. 1998;5:1038–1046. doi: 10.1038/sj.gt.3300691. [DOI] [PubMed] [Google Scholar]
- Rex TS, Allocca M, Domenici L, Surace EM, Maguire AM, Lyubarsky A, Cellerino A, Bennett J, Auricchio A. Systemic but not intraocular Epo gene transfer protects the retina from light-and genetic-induced degeneration. Mol Ther. 2004;10:855–861. doi: 10.1016/j.ymthe.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Roof DJ, Korenbrot JI, Heuser JE. Surfaces of rod photoreceptor disk membranes: light-activated enzymes. J Cell Biol. 1982;95:501–509. doi: 10.1083/jcb.95.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell WC. Update on adenovirus and its vectors. J Gen Virol. 2000;81:2573–2604. doi: 10.1099/0022-1317-81-11-2573. [DOI] [PubMed] [Google Scholar]
- Sahel JA, Roska B. Gene therapy for blindness. Annu Rev Neurosci. 2013;36:467–488. doi: 10.1146/annurev-neuro-062012-170304. [DOI] [PubMed] [Google Scholar]
- Sanlioglu S, Monick MM, Luleci G, Hunninghake GW, Engelhardt JF. Rate limiting steps of AAV transduction and implications for human gene therapy. Curr Gene Ther. 2001;1:137–147. doi: 10.2174/1566523013348788. [DOI] [PubMed] [Google Scholar]
- Semple-Rowland SL, Berry J. Use of lentiviral vectors to deliver and express bicistronic transgenes in developing chicken embryos. Methods. 2014;66:466–473. doi: 10.1016/j.ymeth.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple-Rowland SL, Coggin WE, Geesey M, Eccles KS, Abraham L, Pachigar K, Ludlow R, Khani SC, Smith WC. Expression characteristics of dual-promoter lentiviral vectors targeting retinal photoreceptors and Muller cells. Mol Vis. 2010;16:916–934. [PMC free article] [PubMed] [Google Scholar]
- Semple-Rowland SL, Eccles KS, Humberstone EJ. Targeted expression of two proteins in neural retina using self-inactivating, insulated lentiviral vectors carrying two internal independent promoters. Mol Vis. 2007;13:2001–2011. [PubMed] [Google Scholar]
- Sieving PA, Caruso RC, Tao W, Coleman HR, Thompson DJ, Fullmer KR, Bush RA. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A. 2006;103:3896–3901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, Rossi S, Marshall K, Banfi S, Surace EM, Sun J, Redmond TM, Zhu X, Shindler KS, Ying GS, Ziviello C, Acerra C, Wright JF, McDonnell JW, High KA, Bennett J, Auricchio A. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther. 2010;18:643–650. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Bainbridge JW, Ali RR. Prospects for retinal gene replacement therapy. Trends Genet. 2009;25:156–165. doi: 10.1016/j.tig.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Schlichtenbrede FC, Tschernutter M, Bainbridge JW, Thrasher AJ, Ali RR. AAV-Mediated gene transfer slows photoreceptor loss in the RCS rat model of retinitis pigmentosa. Mol Ther. 2003;8:188–195. doi: 10.1016/s1525-0016(03)00144-8. [DOI] [PubMed] [Google Scholar]
- Sonntag F, Schmidt K, Kleinschmidt JA. A viral assembly factor promotes AAV2 capsid formation in the nucleolus. Proc Natl Acad Sci U S A. 2010;107:10220–10225. doi: 10.1073/pnas.1001673107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souied EH, Reid SN, Piri NI, Lerner LE, Nusinowitz S, Farber DB. Non-invasive gene transfer by iontophoresis for therapy of an inherited retinal degeneration. Exp Eye Res. 2008;87:168–175. doi: 10.1016/j.exer.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger K, Colle MA, Dubreil L, Mendes-Madeira A, Weber M, Le Meur G, Deschamps JY, Provost N, Nivard D, Cherel Y, Moullier P, Rolling F. Subretinal delivery of recombinant AAV serotype 8 vector in dogs results in gene transfer to neurons in the brain. Mol Ther. 2008;16:916–923. doi: 10.1038/mt.2008.41. [DOI] [PubMed] [Google Scholar]
- Stieger K, Cronin T, Bennett J, Rolling F. Adeno-associated virus mediated gene therapy for retinal degenerative diseases. Methods Mol Biol. 2011;807:179–218. doi: 10.1007/978-1-61779-370-7_8. [DOI] [PubMed] [Google Scholar]
- Sullivan T, Rex TS. Systemic gene delivery protects the photoreceptors in the retinal degeneration slow mouse. Neurochem Res. 2011;36:613–618. doi: 10.1007/s11064-010-0272-6. [DOI] [PubMed] [Google Scholar]
- Sun X, Pawlyk B, Xu X, Liu X, Bulgakov OV, Adamian M, Sandberg MA, Khani SC, Tan MH, Smith AJ, Ali RR, Li T. Gene therapy with a promoter targeting both rods and cones rescues retinal degeneration caused by AIPL1 mutations. Gene Ther. 2010;17:117–131. doi: 10.1038/gt.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Surace EM, Auricchio A. Versatility of AAV vectors for retinal gene transfer. Vis Res. 2008;48:353–359. doi: 10.1016/j.visres.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Sweigard JH, Cashman SM, Kumar-Singh R. Adenovirus vectors targeting distinct cell types in the retina. Invest Ophthalmol Vis Sci. 2010;51:2219–2228. doi: 10.1167/iovs.09-4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Luo T, Saishin Y, Sung J, Hackett S, Brazzell RK, Kaleko M, Campochiaro PA. Sustained transduction of ocular cells with a bovine immunodeficiency viral vector. Hum Gene Ther. 2002;13:1305–1316. doi: 10.1089/104303402760128531. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Miyoshi H, Verma IM, Gage FH. Rescue from photoreceptor degeneration in the rd mouse by human immunodeficiency virus vector-mediated gene transfer. J Virol. 1999;73:7812–7816. doi: 10.1128/jvi.73.9.7812-7816.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takita H, Yoneya S, Gehlbach PL, Duh EJ, Wei LL, Mori K. Retinal neuroprotection against ischemic injury mediated by intraocular gene transfer of pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2003;44:4497–4504. doi: 10.1167/iovs.03-0052. [DOI] [PubMed] [Google Scholar]
- Tamboli V, Mishra GP, Mitrat AK. Polymeric vectors for ocular gene delivery. Ther Deliv. 2011;2:523–536. doi: 10.4155/tde.11.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MH, Smith AJ, Pawlyk B, Xu X, Liu X, Bainbridge JB, Basche M, McIntosh J, Tran HV, Nathwani A, Li T, Ali RR. Gene therapy for retinitis pigmentosa and Leber congenital amaurosis caused by defects in AIPL1: effective rescue of mouse models of partial and complete Aipl1 deficiency using AAV2/2 and AAV2/8 vectors. Hum Mol Genet. 2009;18:2099–2114. doi: 10.1093/hmg/ddp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A, Parisi F, Denti MA, Allocca M, Di Vicino U, Domenici L, Bozzoni I, Auricchio A. Preferential silencing of a common dominant rhodopsin mutation does not inhibit retinal degeneration in a transgenic model. Mol Ther. 2006;14:692–699. doi: 10.1016/j.ymthe.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Testa F, Maguire AM, Rossi S, Pierce EA, Melillo P, Marshall K, Banfi S, Surace EM, Sun J, Acerra C, Wright JF, Wellman J, High KA, Auricchio A, Bennett J, Simonelli F. Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital Amaurosis type 2. Ophthalmology. 2013;120:1283–1291. doi: 10.1016/j.ophtha.2012.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa F, Surace EM, Rossi S, Marrocco E, Gargiulo A, Di Iorio V, Ziviello C, Nesti A, Fecarotta S, Bacci ML, Giunti M, Della Corte M, Banfi S, Auricchio A, Simonelli F. Evaluation of Italian patients with leber congenital amaurosis due to AIPL1 mutations highlights the potential applicability of gene therapy. Invest Ophthalmol Vis Sci. 2011;52:5618–5624. doi: 10.1167/iovs.10-6543. [DOI] [PubMed] [Google Scholar]
- Tolmachova T, Tolmachov OE, Barnard AR, de Silva SR, Lipinski DM, Walker NJ, Maclaren RE, Seabra MC. Functional expression of Rab escort protein 1 following AAV2-mediated gene delivery in the retina of cho-roideremia mice and human cells ex vivo. J Mol Med (Berl) 2013;91:825–837. doi: 10.1007/s00109-013-1006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolmachova T, Tolmachov OE, Wavre-Shapton ST, Tracey-White D, Futter CE, Seabra MC. CHM/REP1 cDNA delivery by lentiviral vectors provides functional expression of the transgene in the retinal pigment epithelium of choroideremia mice. J Gene Med. 2012;14:158–168. doi: 10.1002/jgm.1652. [DOI] [PubMed] [Google Scholar]
- Tomita H, Sugano E, Yawo H, Ishizuka T, Isago H, Narikawa S, Kugler S, Tamai M. Restoration of visual response in aged dystrophic RCS rats using AAV-mediated channelopsin-2 gene transfer. Invest Ophthalmol Vis Sci. 2007;48:3821–3826. doi: 10.1167/iovs.06-1501. [DOI] [PubMed] [Google Scholar]
- Tonska K, Kodron A, Bartnik E. Genotype-phenotype correlations in Leber hereditary optic neuropathy. Biochim Biophys Acta. 2010;1797:1119–1123. doi: 10.1016/j.bbabio.2010.02.032. [DOI] [PubMed] [Google Scholar]
- Tosi J, Sancho-Pelluz J, Davis RJ, Hsu CW, Wolpert KV, Sengillo JD, Lin CS, Tsang SH. Lentivirus-mediated expression of cDNA and shRNA slows degeneration in retinitis pigmentosa. Exp Biol Med (Maywood) 2011;236:1211–1217. doi: 10.1258/ebm.2011.011053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchard E, Berdugo M, Bigey P, El Sanharawi M, Savoldelli M, Naud MC, Jeanny JC, Behar-Cohen F. Suprachoroidal electrotransfer: a nonviral gene delivery method to transfect the choroid and the retina without detaching the retina. Mol Ther. 2012;20:1559–1570. doi: 10.1038/mt.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani I, Colella P, Sommella A, Iodice C, Cesi G, de Simone S, Marrocco E, Rossi S, Giunti M, Palfi A, Farrar GJ, Polishchuk R, Auricchio A. Effective delivery of large genes to the retina by dual AAV vectors. EMBO Mol Med. 2014;6:194–211. doi: 10.1002/emmm.201302948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschernutter M, Schlichtenbrede FC, Howe S, Balaggan KS, Munro PM, Bainbridge JW, Thrasher AJ, Smith AJ, Ali RR. Long-term preservation of retinal function in the RCS rat model of retinitis pigmentosa following lentivirus-mediated gene therapy. Gene Ther. 2005;12:694–701. doi: 10.1038/sj.gt.3302460. [DOI] [PubMed] [Google Scholar]
- Turchinovich A, Zoidl G, Dermietzel R. Non-viral siRNA delivery into the mouse retina in vivo. BMC Ophthalmol. 2010;10:25. doi: 10.1186/1471-2415-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca O, Darche M, Schaffer DV, Flannery JG, Sahel JA, Rendon A, Dalkara D. AAV-mediated gene delivery in Dp71-null mouse model with compromised barriers. Glia. 2014;62:468–476. doi: 10.1002/glia.22617. [DOI] [PubMed] [Google Scholar]
- Vandenberghe LH, Auricchio A. Novel adeno-associated viral vectors for retinal gene therapy. Gene Ther. 2012;19:162–168. doi: 10.1038/gt.2011.151. [DOI] [PubMed] [Google Scholar]
- Vandenberghe LH, Bell P, Maguire AM, Cearley CN, Xiao R, Calcedo R, Wang L, Castle MJ, Maguire AC, Grant R, Wolfe JH, Wilson JM, Bennett J. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci Transl Med. 2011;3:88ra54. doi: 10.1126/scitranslmed.3002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe LH, Bell P, Maguire AM, Xiao R, Hopkins TB, Grant R, Bennett J, Wilson JM. AAV9 targets cone photoreceptors in the nonhuman primate retina. PLoS One. 2013;8:e53463. doi: 10.1371/journal.pone.0053463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe LH, Wilson JM, Gao G. Tailoring the AAV vector capsid for gene therapy. Gene Ther. 2009;16:311–319. doi: 10.1038/gt.2008.170. [DOI] [PubMed] [Google Scholar]
- Verrier JD, Madorsky I, Coggin WE, Geesey M, Hochman M, Walling E, Daroszewski D, Eccles KS, Ludlow R, Semple-Rowland SL. Bicistronic lentiviruses containing a viral 2A cleavage sequence reliably co-express two proteins and restore vision to an animal model of LCA1. PLoS One. 2011;6:e20553. doi: 10.1371/journal.pone.0020553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath D, Feng W, Duncan JL, Yasumura D, D’Cruz PM, Chappelow A, Matthes MT, Kay MA, LaVail MM. Correction of the retinal dystrophy phenotype of the RCS rat by viral gene transfer of Mertk. Proc Natl Acad Sci U S A. 2001;98:12584–12589. doi: 10.1073/pnas.221364198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Seggern DJ, Aguilar E, Kinder K, Fleck SK, Gonzalez Armas JC, Stevenson SC, Ghazal P, Nemerow GR, Friedlander M. In vivo transduction of photoreceptors or ciliary body by intravitreal injection of pseudotyped adenoviral vectors. Mol Ther. 2003;7:27–34. doi: 10.1016/s1525-0016(02)00030-8. [DOI] [PubMed] [Google Scholar]
- Weber M, Rabinowitz J, Provost N, Conrath H, Folliot S, Briot D, Cherel Y, Chenuaud P, Samulski J, Moullier P, Rolling F. Recombinant adeno-associated virus serotype 4 mediates unique and exclusive long-term trans-duction of retinal pigmented epithelium in rat, dog, and nonhuman primate after subretinal delivery. Mol Ther. 2003;7:774–781. doi: 10.1016/s1525-0016(03)00098-4. [DOI] [PubMed] [Google Scholar]
- Willett K, Bennett J. Immunology of AAV-mediated gene Transfer in the eye. Front Immunol. 2013;4:261. doi: 10.3389/fimmu.2013.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ML, Coleman JE, Haire SE, Aleman TS, Cideciyan AV, Sokal I, Palczewski K, Jacobson SG, Semple-Rowland SL. Lentiviral expression of retinal guanylate cyclase-1 (RetGC1) restores vision in an avian model of childhood blindness. PLoS Med. 2006;3:e201. doi: 10.1371/journal.pmed.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat Rev Genet. 2010;11:273–284. doi: 10.1038/nrg2717. [DOI] [PubMed] [Google Scholar]
- Wu L, Lam S, Cao H, Guan R, Duan R, van der Kooy D, Bremner R, Molday RS, Hu J. Subretinal gene delivery using helper-dependent adenoviral vectors. Cell Biosci. 2011;1:15. doi: 10.1186/2045-3701-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Yang H, Colosi P. Effect of genome size on AAV vector packaging. Mol Ther. 2010;18:80–86. doi: 10.1038/mt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Zhang Y, Duan D, Engelhardt JF. Trans-splicing vectors expand the utility of adeno-associated virus for gene therapy. Proc Natl Acad Sci U S A. 2000;97:6716–6721. doi: 10.1073/pnas.97.12.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez-Munoz RJ, Balaggan KS, MacNeil A, Howe SJ, Schmidt M, Smith AJ, Buch P, MacLaren RE, Anderson PN, Barker SE, Duran Y, Bartholomae C, von Kalle C, Heckenlively JR, Kinnon C, Ali RR, Thrasher AJ. Effective gene therapy with nonintegrating lentiviral vectors. Nat Med. 2006;12:348–353. doi: 10.1038/nm1365. [DOI] [PubMed] [Google Scholar]
- Yang GS, Schmidt M, Yan Z, Lindbloom JD, Harding TC, Donahue BA, Engelhardt JF, Kotin R, Davidson BL. Virus-mediated transduction of murine retina with adeno-associated virus: effects of viral capsid and genome size. J Virol. 2002;76:7651–7660. doi: 10.1128/JVI.76.15.7651-7660.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi K, Kachi S, Zhang HS, Gregory PD, Spratt SK, Samulski RJ, Campochiaro PA. Ocular gene transfer with self-complementary AAV vectors. Invest Ophthalmol Vis Sci. 2007;48:3324–3328. doi: 10.1167/iovs.06-1306. [DOI] [PubMed] [Google Scholar]
- Young JE, Vogt T, Gross KW, Khani SC. A short, highly active photoreceptor-specific enhancer/promoter region upstream of the human rhodopsin kinase gene. Invest Ophthalmol Vis Sci. 2003;44:4076–4085. doi: 10.1167/iovs.03-0197. [DOI] [PubMed] [Google Scholar]
- Zallocchi M, Binley K, Lad Y, Ellis S, Widdowson P, Iqball S, Scripps V, Kelleher M, Loader J, Miskin J, Peng YW, Wang WM, Cheung L, Delimont D, Mitrophanous KA, Cosgrove D. EIAV-based retinal gene therapy in the shaker1 mouse model for usher syndrome type 1B: development of UshStat. PLoS One. 2014;9:e94272. doi: 10.1371/journal.pone.0094272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- Zhang M, Mo X, Fang Y, Guo W, Wu J, Zhang S, Huang Q. Rescue of photoreceptors by BDNF gene transfer using in vivo electroporation in the RCS rat of retinitis pigmentosa. Curr Eye Res. 2009a;34:791–799. doi: 10.1080/02713680903086018. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bergelson JM. Adenovirus receptors. J Virol. 2005;79:12125–12131. doi: 10.1128/JVI.79.19.12125-12131.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ivanova E, Bi A, Pan ZH. Ectopic expression of multiple microbial rhodopsins restores ON and OFF light responses in retinas with photoreceptor degeneration. J Neurosci. 2009b;29:9186–9196. doi: 10.1523/JNEUROSCI.0184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Li B, Jayandharan G, Mah CS, Govindasamy L, Agbandje-McKenna M, Herzog RW, Weigel-Van Aken KA, Hobbs JA, Zolotukhin S, Muzyczka N, Srivastava A. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology. 2008a;381:194–202. doi: 10.1016/j.virol.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, Herzog RW, Zolotukhin I, Warrington KH, Jr, Weigel-Van Aken KA, Hobbs JA, Zolotukhin S, Muzyczka N, Srivastava A. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci U S A. 2008b;105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]