Abstract
Almost 7 million children under the age 5 die each year, and most of these deaths are attributable to vaccine-preventable infections. Young infants respond poorly to infections and vaccines. In particular, dendritic cells secrete less IL-12 and IL-18, CD8pos T cells and NK cells have defective cytolysis and cytokine production, and CD4pos T cell responses tend to bias towards a Th2 phenotype and promotion of regulatory T cells (Tregs). The basis for these differences is not well understood and may be in part explained by epigenetic differences, as well as immaturity of the infant’s immune system. Here we present a third possibility, which involves active suppression by immune regulatory cells and place in context the immune suppressive pathways of mesenchymal stromal cells (MSC), myeloid-derived suppressor cells (MDSC), CD5pos B cells, and Tregs. The immune pathways that these immune regulatory cells inhibit are similar to those that are defective in the infant. Therefore, the immune deficiencies seen in infants could be explained, in part, by active suppressive cells, indicating potential new avenues for intervention.
Keywords: immune suppression, infant immunity, infant vaccines
1. Introduction
a. Overview of infant mortality rates due to infection
Infant mortality is a major global health problem, resulting in the commitment of 189 heads of state to achieve Millennium Development Goal (MDG) 4: to reduce the under-5 mortality rate by 2/3 between 1990 and 2015. Despite progress towards this goal, it is estimated that approximately 7 million children died before the age of 5 years in 2011;1 almost 2/3 of these deaths were due to infection. The overwhelming majority of under-5 deaths are in Sub-Saharan Africa, which has an infant mortality rate (102 deaths per 1,000 live births) that is approximately 15 times higher than in developing countries.2 The 3 infections that cause the most substantial morbidity and mortality are human immunodeficiency virus (HIV), tuberculosis (TB) and malaria, and all 3 of these infections disproportionately affect young children.3–5
Neonates and young children are known to respond poorly to infection. For example, neonatal and infant HSV- and CMV-specific CD4pos T cell responses are delayed and reduced as compared to adults with primary infection.6–10 Also, young infants are more likely to die or be hospitalized if infected with pertussis, respiratory syncytial virus (RSV), Enterovirus 71 or influenza than older children are.11–15 Furthermore, infants respond sub-optimally to most vaccines (reviewed in16–18). Sub-optimal responses to vaccinations and infection have traditionally been attributed to a combination of the immaturity of the neonatal/infant immune system and interference from passively transferred maternal antibodies. Here we will discuss data in support of a third potential mechanism: active suppression of early life responses.
b. Overview of poor response rates to vaccination
Infants are notorious for their reduced ability to respond to vaccination (reviewed in16–18), which is exacerbated by concomitant parasitic infection (reviewed in19). Antibody responses can be classified as T-cell-independent (TI) or T-cell-dependent (TD). In humans, TI responses do not reach adult levels until around 4–5 years and are absent before 3 months of age.20,21 TD responses are present early in life and can be induced by infection in utero. However, multiple immunizations are required in young infants in order to obtain protective immunity and these responses often wane without subsequent booster immunizations (reviewed in20). The reasons for this reduced ability to induce and maintain protective antibody titers are just beginning to be elucidated and limitations of T cell function early in life are likely contributors. Although infants are capable of mounting Th1 and CD8pos CTL responses to infection, the majority of vaccine-induced CD4pos T cell responses appear to be Th2-biased.22–25 Furthermore, upon stimulation through the TCR, CD4pos T cells will preferentially differentiate into Tregs rather than effector CD4pos T cells.26 However, this may be related to the type of antigens (mainly protein antigens)/adjuvants used for vaccination as live attenuated vaccines, such as bacillus Calmette-Guerin (BCG), which induce robust Th1 responses and CD8pos CTL responses in infants.27–31 It should be noted, though, that recent data show that cytokine profiles elicited by BCG vaccine can differ, dependent on the geographical region in which the infants reside.32,33 In addition, the mode of vaccine delivery may be important in determining Th bias, as use of attenuated Listeria monocytogenes as a vehicle to introduce antigen into the cytoplasm of APC has been shown to prime robust Th1 and CD8pos CTL responses in neonatal mice.34 Naïve T cells require dendritic cells (DC) for efficient priming. Of note, after stimulation, neonatal myeloid DC do not up-regulate as much CD80 or CD40 as adult DC,35,36 suggesting that they are inferior at providing sufficient co-stimulatory signals for both T cells and B cells. As a consequence of defective nucleosome remodeling,37 neonatal DCs also secrete less IL-12,24,38–40 which is required for both Th1 development and adequate NK cell responses.
2. Infant Immune Cell Immaturity
The following section summarizes some key observations regarding immaturity of the cellular immune response in neonates and young children. For a more extensive discussion of these developmental differences the reader is referred to a recent comprehensive review.18,41 Our review will highlight only a few aspects that we believe are important in the context of how infants respond to vaccination.
a. Dendritic cells
The neonatal immune system is Th2-biased due to an epigenetic predisposition for enhanced IL-4 and IL-13 production, as well as a delayed maturation of both IL-12- and type I IFN-producing dendritic cells (reviewed in24,42). It has been consistently shown that neonatal conventional DCs secrete less IL-12 and plasmacytoid DCs secrete less type I IFN in response to TLR stimulation.39 There is also a reduced ability to secrete IL-18, which acts in concert with IL-12 and type I IFN to activate NK cells.43 However, secretion of IL-1beta, IL-6, IL-23 and IL-10 is similar to or even higher than adult levels,38 suggesting that neonatal DCs do have the capacity to secrete cytokines but that their responses to stimulation differ from those of adults. Of note, combined TLR receptor stimulation appears to overcome the inability of neonatal DC to secrete IL-12,44 which has potential implications for enhancing infant vaccination efficacy.
b. Natural killer cells
Natural killer (NK) cells are lymphocytes that control initial infection through cytokine production and the killing of infected cells in an MHC-independent manner without prior sensitization.45,46 NK cells from umbilical cord blood consistently demonstrate poor cytotoxic function and generate reduced quantities of IFNγ and other cytokines when compared with NK cells obtained from adults (reviewed in47,48). We have demonstrated that cord blood contains increased frequencies of CD56 negative (CD56neg) NK cells with reduced expression of granzyme B and reduced production of IFNγ and the CC-class chemokines RANTES, MIP1α and MIP1β upon stimulation.49 Both CD56pos and CD56neg NK subpopulations showed impaired viral suppression in cord blood, with impairment most marked in the CD56neg subset. This NK cell subpopulation may reflect an immature NK cell subset, as has been suggested previously.50 Indeed, Gaddy et al have shown that incubation of CD56neg NK subpopulations with cytokines such as IL-12 and IL-15 matures these NK into adult-like cells with enhanced lytic ability.50 NK cell survival, proliferation and cytotoxicity are dependent on numerous cytokines including IL-12, IL-15 and IL-18. The reduced ability of neonatal DC to secrete IL-12 and IL-18 may account for the reduced maturation of NK cells and result in the accumulation of immature CD56neg NK subpopulations with impaired viral suppressive activity. It should be noted that there are conflicting data regarding the level of Granzyme B expression and the ability of neonatal NK cells to perform cytotoxicity, leading some groups to suggest that neonatal NK cells are not simply immature versions of adult NK cells.41 However, many of these other studies assessed NK cell activity in bulk populations (rather than separating the NK subpopulations) and used non-physiologic target cells (e.g., MHC-deficient K562 cells instead of autologous infected cells) making it difficult to assess how each subset contributes to lytic activity.
c. T cells
Neonatal CD4pos T cells secrete equivalent amounts of IL-2 and proliferate in vitro as well as in adult cells in response to strong stimuli, such as anti-CD3/anti-CD28 (51 and Gervassi et al., submitted). However, there is evidence that proliferative responses to physiologic stimuli may be more limited (reviewed in41). Unlike their ability to secrete IL-2, neonatal CD4pos T cells have a markedly reduced ability to secrete other cytokines, including IL-4, IL-5, and IFN-γ, among others.52,53 It is likely that the reduced ability of T cells to secrete cytokines is due to sub-optimal signal transduction events54–56 and to the fact that IFN-γ and IL-4 are only expressed in effector/memory cells, which are in low abundance in cord blood.57 Notably, immature DC, and in particular reduced IL-12 production, may be responsible for the limited ability of neonatal T cells to produce IFN-γ. This premise is supported by the fact that we have shown that short-term addition of IL-7 and IL-12 can enhance IFN-γ secretion in response to polyclonal stimulation (Gervassi et al., submitted). Additional evidence for a lack of adequate support from neonatal DC comes from a study that showed decreased measles-specific T cell proliferation in infants after vaccination when compared to older children or adults, but enhanced T cell proliferation and IFN-γ secretion when infant PBMC were stimulated in the presence of exogenous IL-12 and IL-15.58 There are multiple studies showing that neonatal T cells appear prone to differentiating into Th2 cells, rather than Th1 cells (reviewed in41). Indeed, higher antibody and Th2 CD4pos T cell memory responses are induced in neonates compared to adults.25 However, most studies used non-physiologic stimulation to activate neonatal T cells and such a skewing has not been observed when neonatal T cells are stimulated with allogeneic DC.59
Neonatal T cells also have reduced cytolytic ability.60,61 There are conflicting reports as to the level of perforin expressed in neonatal CD8pos T cells;62,63 however, there are data suggesting that perforin expression is tightly correlated with proliferative ability64 and thus it is plausible that neonatal T cells would express less perforin due to their limited proliferative ability.
d. B cells
The timeline of B cell response maturation differs depending on the requirement for cognate T cell help. Humoral responses to polysaccharide antigens (such as capsular polysaccharides from Haemophilus influenzae type b, Neisseria meningitis, or Streptococcus pneumonia) do not require T cell help (TI) and are mediated by IgMpos IgDpos CD27pos splenic marginal zone B cells. These responses are triggered by crosslinking of the Ig molecules on the B cell surface along with cytokines produced by marginal zone antigen presenting cell (APC) such as B cell-activating factor belonging to the TNF family (BAFF) and A proliferation-inducing ligand (APRIL).65 Newborns are exquisitely susceptible to infections and sepsis caused by these encapsulated bacteria and are non-responsive to polysaccharide vaccine antigens, due to a profound deficiency of TI B cell responses. These responses are undetectable before 3 months of age and do not fully develop until 4–5 years of age, partly due to the lack of maturity of the splenic marginal zone architecture66,67 and low expression levels of CD21.68 These deficiencies can be partially overcome by signaling through TLR9, leading to emigrant B cells acquiring the CD27pos IgMpos phenotype and differentiation into IgM producing plasma cells.69 Unlike TI B cell responses, TD B cell responses are present at birth, though of a lower magnitude when compared to older children or adults. These B cell responses are directed to protein antigens and are primed in the germinal centers with the help of follicular dendritic cells and follicular T helper cells. Lower TD responses could be attributable to the lower expression of CD40 ligand on CD4pos T cells,36,70 the delay in maturation of the follicular dendritic cell network,71 decreased somatic hypermutation limiting affinity maturation of antibodies,72,73 an inability for long-lived plasma cells to establish themselves in the bone marrow due to a lack of stromal cell support,74,75 and an overall inability of neonatal dendritic cells to prime effective T helper responses (see above). Vaccination to protein or conjugate antigens does prime protective B cell responses; however, the magnitude and duration of these responses is significantly lower compared to older children or adults, necessitating several boosters (reviewed in21). Nevertheless, some of the neonatal deficiencies that limit effective T-dependent B cell responses may also be circumvented by the use of specific adjuvants with the acquisition of adult-like B cell responses.76
3. Infant Immune Cell Suppression
Successful pregnancy relies on the establishment of a variety of immune-regulatory mechanisms to prevent HLA-mismatched inflammatory responses between fetus and mother. Bilateral transfer of nucleated cells occurs between the fetus and the mother during pregnancy,77,78 with the establishment of fetal-specific polyfunctional T cell responses.79 The establishment of several tolerance mechanisms to protect the fetus have been identified in the pregnant mother, and failure to establish tolerance has been associated with miscarriage and fetal resorption.80–84 Some of these maternal mechanisms include regulatory T cells,85–87 regulatory NK cells,88,89 regulatory dendritic cells,90 epigenetic silencing of T cell-attracting chemokines,91 indoleamine 2,3-dioxygenase (IDO),84,92 immunoregulatory hormones93–96 and regulatory molecule expression such as Human leukocyte antigen G (HLAG),90 galectin-1,97,98 CD137 (4-1BB),99 ICOS-L (B7h),100 PD1/PDL1,101–103 and Tim3.104 Less is known of the establishment of such mechanisms in the fetus, how these extend into neonatal/infancy and how they may modulate neonatal immune responses. Neonates can develop adult-like T cell responses under appropriate conditions (reviewed in105), and the inability to mount effective responses in infants may be due to the presence of specific suppressive mechanisms. Here we provide a summary of the fetal immune-suppressive mechanisms described thus far, their persistence into infancy and their potential to modulate vaccine-specific immune responses.
a. Regulatory T cells (Tregs)
Tregs have been shown to change in utero in the developing fetus in response to maternal alloantigens.78,106,107 Treg levels decrease with gestational age and attain adult-like levels by birth (107 and our unpublished data). Notably, these studies looked at bulk frequencies of Tregs and not whether antigen-specific subsets persist at elevated levels. Neonatal Tregs are potent suppressors of neonatal allogeneic T cell proliferative responses and depletion of these cells restores strong allo-proliferation.78,106,107 Neonatal Tregs also exert strong suppression of P. falciparum-specific T cell proliferation and IFN-γ production in infants exposed to malaria in utero.108 This suggests that neonatal T cells are functionally capable of proliferation and production of IFN-γ in response to specific antigenic stimulation, and that these responses are actively suppressed.
b. CD5pos B cells (B-1 cells)
CD5pos B cells comprise about 40% of B cells at birth, increasing during the first 4 months of life, after which they gradually decrease.109–111 They are an important source of low affinity IgM and have been suggested to be a first line of defense against infection.111,112 CD5pos B cells also have an immune-regulatory function and have been shown to skew the T cell response towards a Th2 phenotype in neonatal murine models. This effect is mediated by secretion of IL-10 by CD5pos B cells upon TLR stimulation and suppression of IL-12 production by dendritic cells. In these studies, purified neonatal DCs were shown to have the capacity to produce IL-12 and type-1 IFN in response to TLR engagement,113 but these responses were inhibited by CD5pos B cells. CD5pos T cells are also increased in neonates exposed to HIV with a concomitant increase in IL-10 production by mononuclear cells.114
c. Myeloid-derived suppressor cells (MDSC)
MDSC are a heterogeneous population of immature myeloid cells with suppressive function (reviewed in115,116). They can be characterized into monocytic-like (M-MDSC) or granulocytic-like (G-MDSC), dependent on specific surface phenotypes. In humans, M-MDSC are most often classified as CD33pos, CD11bpos, HLA-DRneg, and CD14lo/pos, whereas G-MDSC are CD33pos, CD11bpos, HLA-DRneg, CD14neg, and CD15pos, although many different marker combinations have been used (reviewed in117). MDSC are normally present at low frequency in healthy individuals but their frequencies increase in situations of persistent inflammation, such as chronic infection,118,119 autoimmunity120 and malignancy (reviewed in121). MDSC suppress T cell activation using a variety of mechanisms (reviewed in117,122) and have been shown to decrease efficacy of dendritic cell vaccines.123 MDSC have also been shown (in cancer models) to skew immunity to a Th2 response,124 which is a well-known characteristic of the infant immune response. Recently, our group and others125 have demonstrated that healthy neonates possess increased frequencies of G-MDSC. These neonatal MDSC potently suppress both T cell proliferation and IL-5, IL-17 and IFN-γ cytokine secretion (125 and Gervassi et al; submitted). Of note, we have observed elevated levels of G-MDSC up to 6-weeks of age and the increase in the frequency of these cells cannot be attributable to the transient increase in granulocytes that occurs at birth.
MDSC also inhibit NK cell cytotoxicity, IFN-γ secretion and NKG2D expression in mouse cancer models,126 raising the intriguing possibility that they may also suppress NK cell function in human neonates.
d. Mesenchymal stromal cells (MSC)
MSC are multipotent cells that can be isolated from almost every postnatal organ and tissue127 and are capable of differentiating into several mesodermal, endodermal and ectodermal lineages.128 They have gained much attention as tissue regenerative therapy for their self-renewal and differentiation capabilities and because they do not express MHC class II or CD40, CD80 or CD86 costimulatory molecules. These cells also inhibit a variety of innate and adaptive immune responses and are currently under clinical investigation/use to prevent graft failure, to promote hematopoietic stem cell transplantation and as treatment for certain inflammatory and auto-immune conditions.
MSC have not been investigated as potential immunemodulatory cells during gestation or infancy and to our knowledge there are no studies looking at the temporal frequency or specific location of these cells in infants. MSC are present at a high frequency in cord blood from gestational weeks 24–28, greatly decrease by gestational weeks 29–32 and are rarely identified in gestational weeks 37–40.129 They are found in full-term placental tissue, fetal membrane, and neonatal and infant thymus.130–132 MSC have multi-faceted immunomodulatory effects. They inhibit differentiation of monocytes into immature DC and differentiate DC into regulatory DC and M-MDSC.133–135 Regulatory DC, along with MSC, induce functional Foxp3pos Tregs from committed Th1 and Th17 cells.133,136–138 Furthermore, MSC skew the T helper response towards a Th2 phenotype,139 suppress the lytic activity of CD8pos T cells,140 suppress proliferation and induce anergy of both naïve and memory T cells.141,142 They suppress proliferation, cytotoxicity and cytokine production by NK cells.139,143,144 These immune regulatory effects are mediated by the production of prostaglandin E2 (PGE2), HLA-G, TGF-beta, indoleamine 2,3-dioxygenase (IDO), IL-10 and IL-6.134,136,139,144–147
4. Conclusions and Potential for Intervention
It will be critical to determine if Tregs, MDSC, MSC, CD5 B cells, or other regulatory cells are responsible for the reduced ability of infants to respond to vaccination/infection early in life. Observational studies aimed at addressing this issue are already underway. There is a possibility that regulatory cells could have beneficial effects, such as limiting inflammation induced during colonization of the human gut with commensal microbiota. However, the extreme heterogeneity in frequencies of MDSC that we have observed in healthy neonates argues that such a role may be limited.
Given the overwhelming data showing that the neonatal/young infant cellular immune system is immature and potentially actively suppressed, the use of novel adjuvants seems worth exploring. Many adjuvants are being specifically developed to stimulate through specific TLRs. However, the response of neonatal DC to TLR stimulation is very different than the responses seen in adults, as described above.38 A recent review of the data on use of adjuvants in infants suggests that many adjuvants may be safe and well tolerated148 but some, such as TLR2 agonists, may induce expression of IL-10 and lead to unresponsiveness to the vaccine. The use of TLR agonists as adjuvants may be attractive in infants because certain TLR agonists are known to inhibit the immunosuppressive function of MDSC (TLR 9 agonist CpG)131,149,150 and of MSC (TLR2 agonist Pam2Cys).151 However, caution should be employed as other TLR agonists (such as the TLR 7 agonist, imiquimod) have been shown to induce MDSC accumulation.152 There have been no studies to date determining if administration of specific adjuvants can overcome the Th2-bias of most protein-based vaccines antigens. Thus, more studies are required to assess the potential adjuvant effects of different TLR agonists. However, obviously, extensive safety monitoring will be required to assess potentially damaging inflammatory sequelae.
An alternative strategy is to use vitamin A supplementation at an earlier age than is currently recommended (6 months153). Vitamin A supplementation has been shown to reduce mortality and morbidity from some forms of diarrhea, measles, human immunodeficiency virus (HIV) infection, and malaria.154 The mechanism of its efficacy is not known but it is interesting that all-transretinoicacid (ATRA), the active metabolite of vitamin A, causes maturation of MDSC and improves response to vaccination and subsequent tumor regression in cancer patients,121,155–157 Thus, elimination of MDSC using vitamin A or ATRA may be a safe and effective means of enhancing infant immune responses.
A final approach is BCG vaccination. BCG has been shown to increase Th1- and Th2-responses to other vaccines such as HBV and oral polio vaccine.148 While this has been attributed to the potential of BCG to cause maturation of DC,158 it is possible that the numerous TLR agonists in BCG cause maturation/elimination of MDSC or other regulatory cells, thereby relieving immune suppression.
In summary, there are extensive data supporting the role of active immune suppression during early infancy. This active suppression may be responsible for the reduced ability of infants to respond to infection/vaccination. New approaches aimed at alleviating such immune suppression may enable infants to respond more appropriately to infection/vaccination and speed progress towards MDG4.
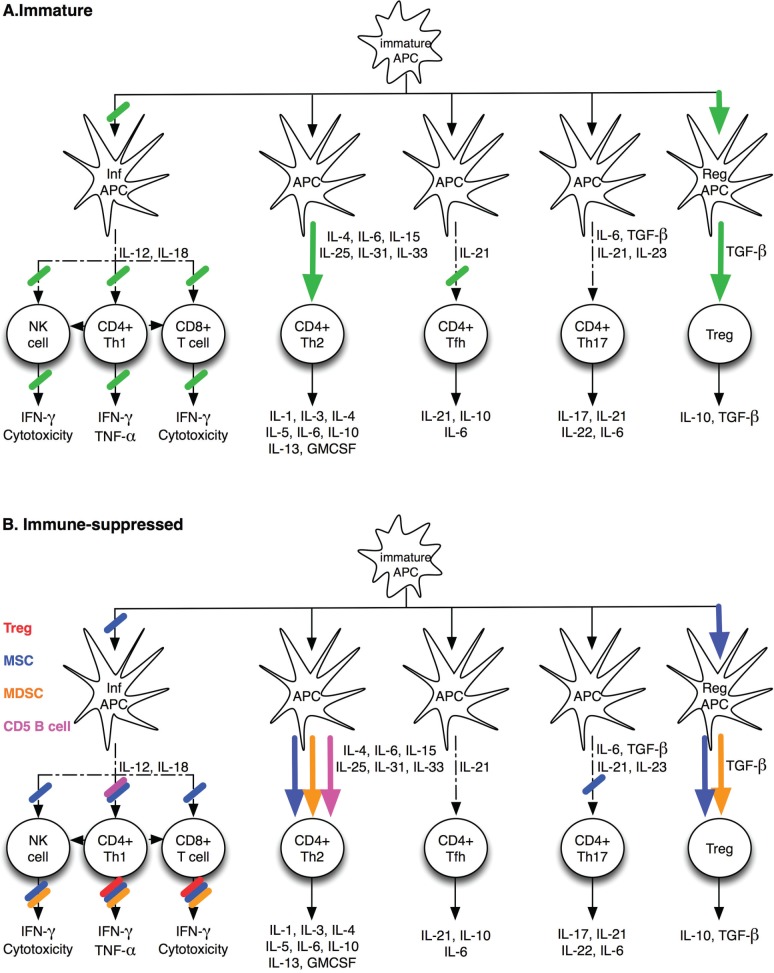
Figure 1.
Known characteristics of the infant’s immune responses. A. Immaturity of the infant’s immune system is dictated by the inability of inflammatory dendritic cells (inf APC) to produce IL-12 and IL-18. This leads to an impaired CD8+ T cell, NK cell and Th1 responses upon stimulation. The T-helper response is skewed toward Th2. The decreased ability of inf APC to effectively prime T helper responses leads to a decreased ability to provide the needed help for strong T dependent B cell responses. B. Possible involvement of specific regulatory cells in shaping the infant’s immune response described in A. Regulatory T cells (Tregs) suppress T cell proliferation and IFN-g secretion. CD5+ B cells decrease IL-12 production by inflammatory dendritic cells and skew the T helper response towards a Th2 phenotype. Myeloid derived suppressor cells (MDSC) suppress T cell proliferative responses, decrease IL-5, IL-17, and IFN-g production, and skew the T cell phenotype towards Th2. MDSC also inhibit IFN-g production by NK cells. Mesenchymal Stromal Cells (MSC) skew the dendritic cell differentiation towards a regulatory phenotype and induce MDSC. This induces the generation of Tregs from Th1 and Th17 cells. MSC induce T cell anergy and suppress NK cell proliferation, cytotoxicity and cytokine production.
Footnotes
Author Contributions
ALG and HH wrote the first draft of the manuscript. ALG and HH contributed to the writing of the manuscript. ALG and HH agree with manuscript conclusions. ALG and HH jointly developed the structure and arguments for the paper. ALG and HH made critical revisions and approved final version. ALG and HH reviewed and approved of the final manuscript.
DISCLOSURES AND ETHICS
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests. Provenance: the authors were invited to submit this paper.
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
REFERENCES
- 1.WHO . Child Mortality Report. Geneva: World Health Organization; 2012. [Google Scholar]
- 2.Lozano R, Wang H, Foreman KJ, et al. Progress towards Millennium Development Goals 4 and 5 on maternal and child mortality: an updated systematic analysis. Lancet. 2011;378(9797):1139–1165. doi: 10.1016/S0140-6736(11)61337-8. [DOI] [PubMed] [Google Scholar]
- 3.Newton SM, Brent AJ, Anderson S, Whittaker E, Kampmann B. Paediatric tuberculosis. Lancet Infect Dis. 2008;8(8):498–510. doi: 10.1016/S1473-3099(08)70182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snow RW, Korenromp EL, Gouws E. Pediatric mortality in Africa: plasmodium falciparum malaria as a cause or risk? Am J Trop Med Hyg. 2004;71(2 Suppl):16–24. [PubMed] [Google Scholar]
- 5.Chakraborty R. HIV-1 infection in children: a clinical and immunologic overview. Curr HIV Res. 2005;3(1):31–41. doi: 10.2174/1570162052773022. [DOI] [PubMed] [Google Scholar]
- 6.Tu W, Chen S, Sharp M, et al. Persistent and selective deficiency of CD4+ T cell immunity to cytomegalovirus in immunocompetent young children. J Immunol. 2004;172(5):3260–3267. doi: 10.4049/jimmunol.172.5.3260. [DOI] [PubMed] [Google Scholar]
- 7.Burchett SK, Corey L, Mohan KM, Westall J, Ashley R, Wilson CB. Diminished interferon-gamma and lymphocyte proliferation in neonatal and postpartum primary herpes simplex virus infection. J Infect Dis. 1992;165(5):813–818. doi: 10.1093/infdis/165.5.813. [DOI] [PubMed] [Google Scholar]
- 8.Gantt S, Muller WJ. The immunologic basis for severe neonatal herpes disease and potential strategies for therapeutic intervention. Clin Dev Immunol. 2013;2013:369172. doi: 10.1155/2013/369172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lidehäll AK, Engman ML, Sund F, et al. Cytomegalovirus-specific CD4 and CD8 T cell responses in infants and children. Scand J Immunol. 2013;77(2):135–143. doi: 10.1111/sji.12013. [DOI] [PubMed] [Google Scholar]
- 10.Sullender WM, Miller JL, Yasukawa LL, et al. Humoral and cell-mediated immunity in neonates with herpes simplex virus infection. J Infect Dis. 1987;155(1):28–37. doi: 10.1093/infdis/155.1.28. [DOI] [PubMed] [Google Scholar]
- 11.Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Jr, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med. 2000;342(4):225–231. doi: 10.1056/NEJM200001273420401. [DOI] [PubMed] [Google Scholar]
- 12.McIntyre P, Wood N. Pertussis in early infancy: disease burden and preventive strategies. Curr Opin Infect Dis. 2009;22(3):215–223. doi: 10.1097/QCO.0b013e32832b3540. [DOI] [PubMed] [Google Scholar]
- 13.Gantt S, Yao L, Kollmann TR, Casper C, Zhang J, Self SG. Implications of age-dependent immune responses to enterovirus-71 infection for disease pathogenesis and vaccine design. J Paed Infect Dis. 2013;2(2):162–170. doi: 10.1093/jpids/pit017. [DOI] [PubMed] [Google Scholar]
- 14.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ploin D, Chidiac C, Carrat F, et al. Fluco study group Complications and factors associated with severity of influenza in hospitalized children and adults during the pandemic wave of A(H1N1)pdm 2009 infections-The Fluco French cohort. J Clin Virol. 2013;58(1):114–119. doi: 10.1016/j.jcv.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 16.Demirjian A, Levy O. Safety and efficacy of neonatal vaccination. Eur J Immunol. 2009;39(1):36–46. doi: 10.1002/eji.200838620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood N, Siegrist CA. Neonatal immunization: where do we stand? Curr Opin Infect Dis. 2011;24(3):190–195. doi: 10.1097/QCO.0b013e328345d563. [DOI] [PubMed] [Google Scholar]
- 18.PrabhuDas M, Adkins B, Gans H, et al. Challenges in infant immunity: implications for responses to infection and vaccines. Nat Immunol. 2011;12(3):189–194. doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
- 19.Labeaud AD, Malhotra I, King MJ, King CL, King CH. Do antenatal parasite infections devalue childhood vaccination? PLoS Negl Trop Dis. 2009;3(5):e442. doi: 10.1371/journal.pntd.0000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson CB, Kollmann TR. Induction of antigen-specific immunity in human neonates and infants. Nestle Nutr Workshop Ser Pediatr Program. 2008;61:183–195. doi: 10.1159/000113493. [DOI] [PubMed] [Google Scholar]
- 21.Siegrist CA. The challenges of vaccine responses in early life: selected examples. J Comp Pathol. 2007;137(Suppl 1):S4–S9. doi: 10.1016/j.jcpa.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 22.van den Biggelaar AH, Pomat W, Bosco A, et al. Pneumococcal conjugate vaccination at birth in a high-risk setting: no evidence for neonatal T-cell tolerance. Vaccine. 2011;29(33):5414–5420. doi: 10.1016/j.vaccine.2011.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vekemans J, Ota MO, Wang EC, et al. T cell responses to vaccines in infants: defective IFNgamma production after oral polio vaccination. Clin Exp Immunol. 2002;127(3):495–498. doi: 10.1046/j.1365-2249.2002.01788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30(12):585–591. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ota MO, Vekemans J, Schlegel-Haueter SE, et al. Hepatitis B immunisation induces higher antibody and memory Th2 responses in new-borns than in adults. Vaccine. 2004;22(3–4):511–519. doi: 10.1016/j.vaccine.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Wang G, Miyahara Y, Guo Z, Khattar M, Stepkowski SM, Chen W. “Default” generation of neonatal regulatory T cells. J Immunol. 2010;185(1):71–78. doi: 10.4049/jimmunol.0903806. [DOI] [PubMed] [Google Scholar]
- 27.Ota MO, Vekemans J, Schlegel-Haueter SE, et al. Influence of Mycobacterium bovis bacillus Calmette-Guérin on antibody and cytokine responses to human neonatal vaccination. J Immunol. 2002;168(2):919–925. doi: 10.4049/jimmunol.168.2.919. [DOI] [PubMed] [Google Scholar]
- 28.Weir RE, Gorak-Stolinska P, Floyd S, et al. Persistence of the immune response induced by BCG vaccination. BMC Infect Dis. 2008;8:9. doi: 10.1186/1471-2334-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lalor MK, Smith SG, Floyd S, et al. Complex cytokine profiles induced by BCG vaccination in UK infants. Vaccine. 2010;28(6):1635–1641. doi: 10.1016/j.vaccine.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vekemans J, Amedei A, Ota MO, et al. Neonatal bacillus Calmette-Guérin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur J Immunol. 2001;31(5):1531–1535. doi: 10.1002/1521-4141(200105)31:5<1531::AID-IMMU1531>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 31.Tena-Coki NG, Scriba TJ, Peteni N, et al. CD4 and CD8 T-cell responses to mycobacterial antigens in African children. Am J RespirCrit Care Med. 2010;182(1):120–129. doi: 10.1164/rccm.200912-1862OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lalor MK, Ben-Smith A, Gorak-Stolinska P, et al. Population differences in immune responses to Bacille Calmette-Guérin vaccination in infancy. J Infect Dis. 2009;199(6):795–800. doi: 10.1086/597069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lalor MK, Floyd S, Gorak-Stolinska P, et al. BCG vaccination induces different cytokine profiles following infant BCG vaccination in the UK and Malawi. J Infect Dis. 2011;204(7):1075–1085. doi: 10.1093/infdis/jir515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kollmann TR, Reikie B, Blimkie D, et al. Induction of protective immunity to Listeria monocytogenes in neonates. J Immunol. 2007;178(6):3695–3701. doi: 10.4049/jimmunol.178.6.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Wit D, Tonon S, Olislagers V, et al. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J Autoimmun. 2003;21(3):277–281. doi: 10.1016/j.jaut.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Jullien P, Cron RQ, Dabbagh K, et al. Decreased CD154 expression by neonatal CD4+ T cells is due to limitations in both proximal and distal events of T cell activation. Int Immunol. 2003;15(12):1461–1472. doi: 10.1093/intimm/dxg145. [DOI] [PubMed] [Google Scholar]
- 37.Goriely S, Van Lint C, Dadkhah R, et al. A defect in nucleosome remodeling prevents IL-12(p35) gene transcription in neonatal dendritic cells. J Exp Med. 2004;199(7):1011–1016. doi: 10.1084/jem.20031272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kollmann TR, Crabtree J, Rein-Weston A, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183(11):7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renneson J, Dutta B, Goriely S, et al. IL-12 and type I IFN response of neonatal myeloid DC to human CMV infection. Eur J Immunol. 2009;39(10):2789–2799. doi: 10.1002/eji.200939414. [DOI] [PubMed] [Google Scholar]
- 40.Lavoie PM, Huang Q, Jolette E, et al. Profound lack of interleukin (IL)-12/IL-23p40 in neonates born early in gestation is associated with an increased risk of sepsis. J Infect Dis. 2010;202(11):1754–1763. doi: 10.1086/657143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewis DB. Developmental immunology and role of host defenses in fetal and neonatal susceptibility to infection. In: Klein JO, Wilson CB, Baker CJ, editors. Infectious Diseases of the Fetus and Newborn Infant. 6th Edition. Philadelphia: Elsevier Saunders; 2006. pp. 25–128. [Google Scholar]
- 42.Marodi L. Innate cellular immune responses in newborns. Clin Immunol. 2006;118(2–3):137–144. doi: 10.1016/j.clim.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 43.La Pine TR, Joyner JL, Augustine NH, Kwak SD, Hill HR. Defective production of IL-18 and IL-12 by cord blood mononuclear cells influences the T helper-1 interferon gamma response to group B Streptococci. Pediatr Res. 2003;54(2):276–281. doi: 10.1203/01.PDR.0000072515.10652.87. [DOI] [PubMed] [Google Scholar]
- 44.Krumbiegel D, Zepp F, Meyer CU. Combined Toll-like receptor agonists synergistically increase production of inflammatory cytokines in human neonatal dendritic cells. Hum Immunol. 2007;68(10):813–822. doi: 10.1016/j.humimm.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Zucchini N, Crozat K, Baranek T, Robbins SH, Altfeld M, Dalod M. Natural killer cells in immunodefense against infective agents. Expert Rev Anti Infect Ther. 2008;6(6):867–885. doi: 10.1586/14787210.6.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 47.Verneris MR, Miller JS. The phenotypic and functional characteristics of umbilical cord blood and peripheral blood natural killer cells. Br J Haematol. 2009;147(2):185–191. doi: 10.1111/j.1365-2141.2009.07768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guilmot A, Hermann E, Braud VM, Carlier Y, Truyens C. Natural killer cell responses to infections in early life. J Innate Immun. 2011;3(3):280–288. doi: 10.1159/000323934. [DOI] [PubMed] [Google Scholar]
- 49.Jacobson A, Bell F, Lejarcegui N, Mitchell C, Frenkel L, Horton H. Healthy Neonates Possess a CD56-Negative NK Cell Population with Reduced Anti-Viral Activity. PLoS One. 2013;8(6):e67700. doi: 10.1371/journal.pone.0067700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaddy J, Broxmeyer HE. Cord blood CD16+56- cells with low lytic activity are possible precursors of mature natural killer cells. Cell Immunol. 1997;180(2):132–142. doi: 10.1006/cimm.1997.1175. [DOI] [PubMed] [Google Scholar]
- 51.Hassan J, O’Neill S, O’Neill LA, Pattison U, Reen DJ. Signalling via CD28 of human naive neonatal T lymphocytes. Clin Exp Immunol. 1995;102(1):192–198. doi: 10.1111/j.1365-2249.1995.tb06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lewis DB, Yu CC, Meyer J, English BK, Kahn SJ, Wilson CB. Cellular and molecular mechanisms for reduced interleukin 4 and interferon-gamma production by neonatal T cells. J Clin Invest. 1991;87(1):194–202. doi: 10.1172/JCI114970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schultz C, Strunk T, Temming P, Matzke N, Härtel C. Reduced IL-10 production and -receptor expression in neonatal T lymphocytes. Acta Paediatr. 2007;96(8):1122–1125. doi: 10.1111/j.1651-2227.2007.00375.x. [DOI] [PubMed] [Google Scholar]
- 54.Ansart-Pirenne H, Soulimani N, Tartour E, Blot P, Sterkers G. Defective IL2 gene expression in newborn is accompanied with impaired tyrosine-phosphorylation in T cells. Pediatr Res. 1999;45(3):409–413. doi: 10.1203/00006450-199903000-00020. [DOI] [PubMed] [Google Scholar]
- 55.Oriss TB, McCarthy SA, Campana MA, Morel PA. Evidence of positive cross-regulation on Th1 by Th2 and antigen-presenting cells: effects on Th1 induced by IL-4 and IL-12. J Immunol. 1999;162(4):1999–2007. [PubMed] [Google Scholar]
- 56.Miscia S, Di Baldassarre A, Sabatino G, et al. Inefficient phospholipase C activation and reduced Lck expression characterize the signaling defect of umbilical cord T lymphocytes. J Immunol. 1999;163(5):2416–2424. [PubMed] [Google Scholar]
- 57.Fitzpatrick DR, Wilson CB. Methylation and demethylation in the regulation of genes, cells, and responses in the immune system. Clin Immunol. 2003;109(1):37–45. doi: 10.1016/s1521-6616(03)00205-5. [DOI] [PubMed] [Google Scholar]
- 58.Gans HA, Yasukawa LL, Zhang CZ, et al. Effects of interleukin-12 and interleukin-15 on measles-specific T-cell responses in vaccinated infants. Viral Immunol. 2008;21(2):163–172. doi: 10.1089/vim.2007.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen L, Cohen AC, Lewis DB. Impaired allogeneic activation and T-helper 1 differentiation of human cord blood naive CD4 T cells. Biol Blood Marrow Transplant. 2006;12(2):160–171. doi: 10.1016/j.bbmt.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 60.Barbey C, Irion O, Helg C, et al. Characterisation of the cytotoxic alloresponse of cord blood. Bone Marrow Transplant. 1998;22(Suppl 1):S26–S30. [PubMed] [Google Scholar]
- 61.Slavcev A, Stríz I, Ivasková E, Breur-Vriesendorp BS. Alloresponses of cord blood cells in primary mixed lymphocyte cultures. Hum Immunol. 2002;63(3):155–163. doi: 10.1016/s0198-8859(01)00383-4. [DOI] [PubMed] [Google Scholar]
- 62.Berthou C, Legros-Maïda S, Soulié A, et al. Cord blood T lymphocytes lack constitutive perforin expression in contrast to adult peripheral blood T lymphocytes. Blood. 1995;85(6):1540–1546. [PubMed] [Google Scholar]
- 63.Rukavina D, Laskarin G, Rubesa G, et al. Age-related decline of perforin expression in human cytotoxic T lymphocytes and natural killer cells. Blood. 1998;92(7):2410–2420. [PubMed] [Google Scholar]
- 64.Migueles SA, Laborico AC, Shupert WL, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002;3(11):1061–1068. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 65.Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17(3):282–289. doi: 10.1016/j.coi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 66.Weller S, Braun MC, Tan BK, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104(12):3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kruschinski C, Zidan M, Debertin AS, von Hörsten S, Pabst R. Age-dependent development of the splenic marginal zone in human infants is associated with different causes of death. Hum Pathol. 2004;35(1):113–121. doi: 10.1016/s0046-8177(03)00422-2. [DOI] [PubMed] [Google Scholar]
- 68.Timens W, Boes A, Rozeboom-Uiterwijk T, Poppema S. Immaturity of the human splenic marginal zone in infancy. Possible contribution to the deficient infant immune response. J Immunol. 1989;143(10):3200–3206. [PubMed] [Google Scholar]
- 69.Capolunghi F, Cascioli S, Giorda E, et al. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J Immunol. 2008;180(2):800–808. doi: 10.4049/jimmunol.180.2.800. [DOI] [PubMed] [Google Scholar]
- 70.Han P, McDonald T, Hodge G. Potential immaturity of the T-cell and antigen-presenting cell interaction in cord blood with particular emphasis on the CD40-CD40 ligand costimulatory pathway. Immunology. 2004;113(1):26–34. doi: 10.1111/j.1365-2567.2004.01933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pihlgren M, Tougne C, Bozzotti P, et al. Unresponsiveness to lymphoid-mediated signals at the neonatal follicular dendritic cell precursor level contributes to delayed germinal center induction and limitations of neonatal antibody responses to T-dependent antigens. J Immunol. 2003;170(6):2824–2832. doi: 10.4049/jimmunol.170.6.2824. [DOI] [PubMed] [Google Scholar]
- 72.Weitkamp JH, Lafleur BJ, Greenberg HB, Crowe JE. Natural evolution of a human virus-specific antibody gene repertoire by somatic hypermutation requires both hotspot-directed and randomly-directed processes. Hum Immunol. 2005;66(6):666–676. doi: 10.1016/j.humimm.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 73.Ridings J, Dinan L, Williams R, Roberton D, Zola H. Somatic mutation of immunoglobulin V(H)6 genes in human infants. Clin Exp Immunol. 1998;114(1):33–39. doi: 10.1046/j.1365-2249.1998.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pihlgren M, Friedli M, Tougne C, Rochat AF, Lambert PH, Siegrist CA. Reduced ability of neonatal and early-life bone marrow stromal cells to support plasmablast survival. J Immunol. 2006;176(1):165–172. doi: 10.4049/jimmunol.176.1.165. [DOI] [PubMed] [Google Scholar]
- 75.Belnoue E, Pihlgren M, McGaha TL, et al. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood. 2008;111(5):2755–2764. doi: 10.1182/blood-2007-09-110858. [DOI] [PubMed] [Google Scholar]
- 76.Mastelic B, Kamath AT, Fontannaz P, et al. Environmental and T cell-intrinsic factors limit the expansion of neonatal follicular T helper cells but may be circumvented by specific adjuvants. J Immunol. 2012;189(12):5764–5772. doi: 10.4049/jimmunol.1201143. [DOI] [PubMed] [Google Scholar]
- 77.Lo YM, Lo ES, Watson N, et al. Two-way cell traffic between mother and fetus: biologic and clinical implications. Blood. 1996;88(11):4390–4395. [PubMed] [Google Scholar]
- 78.Mold JE, Michaëlsson J, Burt TD, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322(5907):1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lissauer D, Piper K, Goodyear O, Kilby MD, Moss PA. Fetal-specific CD8+ cytotoxic T cell responses develop during normal human pregnancy and exhibit broad functional capacity. J Immunol. 2012;189(2):1072–1080. doi: 10.4049/jimmunol.1200544. [DOI] [PubMed] [Google Scholar]
- 80.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11(5):317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Inada K, Shima T, Nakashima A, Aoki K, Ito M, Saito S. Characterization of regulatory T cells in decidua of miscarriage cases with abnormal or normal fetal chromosomal content. J Reprod Immunol. 2013;97(1):104–111. doi: 10.1016/j.jri.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 82.Makrigiannakis A, Petsas G, Toth B, Relakis K, Jeschke U. Recent advances in understanding immunology of reproductive failure. J Reprod Immunol. 2011;90(1):96–104. doi: 10.1016/j.jri.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 83.Quinn KH, Parast MM. Decidual regulatory T cells in placental pathology and pregnancy complications. Am J Reprod Immunol. 2013;69(6):533–538. doi: 10.1111/aji.12077. [DOI] [PubMed] [Google Scholar]
- 84.Ban Y, Chang Y, Dong B, Kong B, Qu X. Indoleamine 2,3-dioxygenase levels at the normal and recurrent spontaneous abortion fetal-maternal interface. J Int Med Res. 2013;41(4):1135–1149. doi: 10.1177/0300060513487642. [DOI] [PubMed] [Google Scholar]
- 85.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490(7418):102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+ CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10(5):347–353. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- 87.Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112(1):38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fu B, Li X, Sun R, et al. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface. Proc Natl Acad Sci U S A. 2013;110(3):E231–E240. doi: 10.1073/pnas.1206322110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vacca P, Mingari MC, Moretta L. Natural killer cells in human pregnancy. J ReprodImmunol. 2013;97(1):14–19. doi: 10.1016/j.jri.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 90.Amodio G, Mugione A, Sanchez AM, et al. HLA-G expressing DC-10 and CD4(+) T cells accumulate in human decidua during pregnancy. Hum Immunol. 2013;74(4):406–411. doi: 10.1016/j.humimm.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nancy P, Tagliani E, Tay CS, Asp P, Levy DE, Erlebacher A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science. 2012;336(6086):1317–1321. doi: 10.1126/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 93.Lee JH, Lydon JP, Kim CH. Progesterone suppresses the mTOR pathway and promotes generation of induced regulatory T cells with increased stability. Eur J Immunol. 2012;42(10):2683–2696. doi: 10.1002/eji.201142317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bansal AS, Bora SA, Saso S, Smith JR, Johnson MR, Thum MY. Mechanism of human chorionic gonadotrophin-mediated immunomodulation in pregnancy. Expert Rev Clin Immunol. 2012;8(8):747–753. doi: 10.1586/eci.12.77. [DOI] [PubMed] [Google Scholar]
- 95.Mao G, Wang J, Kang Y, et al. Progesterone increases systemic and local uterine proportions of CD4+ CD25+ Treg cells during midterm pregnancy in mice. Endocrinology. 2010;151(11):5477–5488. doi: 10.1210/en.2010-0426. [DOI] [PubMed] [Google Scholar]
- 96.Schumacher A, Heinze K, Witte J, et al. Human chorionic gonadotropin as a central regulator of pregnancy immune tolerance. J Immunol. 2013;190(6):2650–2658. doi: 10.4049/jimmunol.1202698. [DOI] [PubMed] [Google Scholar]
- 97.Tirado-González I, Freitag N, Barrientos G, et al. Galectin-1 influences trophoblast immune evasion and emerges as a predictive factor for the outcome of pregnancy. Mol Hum Reprod. 2013;19(1):43–53. doi: 10.1093/molehr/gas043. [DOI] [PubMed] [Google Scholar]
- 98.Blois SM, Ilarregui JM, Tometten M, et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13(12):1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- 99.Kim KH, Choi BK, Kim JD, et al. 4-1BB signaling breaks the tolerance of maternal CD8+ T cells that are reactive with alloantigens. PLoS One. 2012;7(9):e45481. doi: 10.1371/journal.pone.0045481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Riella LV, Dada S, Chabtini L, et al. B7h (ICOS-L) maintains tolerance at the fetomaternal interface. Am J Pathol. 2013;182(6):2204–2213. doi: 10.1016/j.ajpath.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Taglauer ES, Yankee TM, Petroff MG. Maternal PD-1 regulates accumulation of fetal antigen-specific CD8+ T cells in pregnancy. J ReprodImmunol. 2009;80(1–2):12–21. doi: 10.1016/j.jri.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.D’Addio F, Riella LV, Mfarrej BG, et al. The link between the PDL1 costimulatory pathway and Th17 in fetomaternal tolerance. J Immunol. 2011;187(9):4530–4541. doi: 10.4049/jimmunol.1002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guleria I, Khosroshahi A, Ansari MJ, et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med. 2005;202(2):231–237. doi: 10.1084/jem.20050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chabtini L, Mfarrej B, Mounayar M, et al. TIM-3 regulates innate immune cells to induce fetomaternal tolerance. J Immunol. 2013;190(1):88–96. doi: 10.4049/jimmunol.1202176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4(7):553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 106.Godfrey WR, Spoden DJ, Ge YG, et al. Cord blood CD4(+) CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood. 2005;105(2):750–758. doi: 10.1182/blood-2004-06-2467. [DOI] [PubMed] [Google Scholar]
- 107.Takahata Y, Nomura A, Takada H, et al. CD25+ CD4+ T cells in human cord blood: an immunoregulatory subset with naive phenotype and specific expression of forkhead box p3 (Foxp3) gene. Exp Hematol. 2004;32(7):622–629. doi: 10.1016/j.exphem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 108.Mackroth MS, Malhotra I, Mungai P, Koech D, Muchiri E, King CL. Human cord blood CD4+ CD25hi regulatory T cells suppress prenatally acquired T cell responses to Plasmodium falciparum antigens. J Immunol. 2011;186(5):2780–2791. doi: 10.4049/jimmunol.1001188. [DOI] [PubMed] [Google Scholar]
- 109.Hannet I, Erkeller-Yuksel F, Lydyard P, Deneys V, DeBruyère M. Developmental and maturational changes in human blood lymphocyte subpopulations. Immunol Today. 1992;13215(6):218. doi: 10.1016/0167-5699(92)90157-3. [DOI] [PubMed] [Google Scholar]
- 110.Lundell AC, Björnsson V, Ljung A, et al. Infant B cell memory differentiation and early gut bacterial colonization. J Immunol. 2012;188(9):4315–4322. doi: 10.4049/jimmunol.1103223. [DOI] [PubMed] [Google Scholar]
- 111.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192(2):271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14(5):617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 113.Sun CM, Fiette L, Tanguy M, Leclerc C, Lo-Man R. Ontogeny and innate properties of neonatal dendritic cells. Blood. 2003;102(2):585–591. doi: 10.1182/blood-2002-09-2966. [DOI] [PubMed] [Google Scholar]
- 114.Borges-Almeida E, Milanez HM, Vilela MM, et al. The impact of maternal HIV infection on cord blood lymphocyte subsets and cytokine profile in exposed non-infected newborns. BMC Infect Dis. 2011;11:38. doi: 10.1186/1471-2334-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11(7):802–807. doi: 10.1016/j.intimp.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Poschke I, Kiessling R. On the armament and appearances of human myeloid-derived suppressor cells. Clin Immunol. 2012;144(3):250–268. doi: 10.1016/j.clim.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 118.Tacke RS, Lee HC, Goh C, et al. Myeloid suppressor cells induced by hepatitis C virus suppress T-cell responses through the production of reactive oxygen species. Hepatology. 2012;55(2):343–353. doi: 10.1002/hep.24700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vollbrecht T, Stirner R, Tufman A, et al. Chronic progressive HIV-1 infection is associated with elevated levels of myeloid-derived suppressor cells. AIDS. 2012;26(12):F31–F37. doi: 10.1097/QAD.0b013e328354b43f. [DOI] [PubMed] [Google Scholar]
- 120.Ioannou M, Alissafi T, Lazaridis I, et al. Crucial role of granulocytic myeloid-derived suppressor cells in the regulation of central nervous system autoimmune disease. J Immunol. 2012;188(3):1136–1146. doi: 10.4049/jimmunol.1101816. [DOI] [PubMed] [Google Scholar]
- 121.Montero AJ, Diaz-Montero CM, Kyriakopoulos CE, Bronte V, Mandruzzato S. Myeloid-derived suppressor cells in cancer patients: a clinical perspective. J Immunother. 2012;35(2):107–115. doi: 10.1097/CJI.0b013e318242169f. [DOI] [PubMed] [Google Scholar]
- 122.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Poschke I, Mao Y, Adamson L, Salazar-Onfray F, Masucci G, Kiessling R. Myeloid-derived suppressor cells impair the quality of dendritic cell vaccines. Cancer Immunol Immunother. 2012;61(6):827–838. doi: 10.1007/s00262-011-1143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Crosstalk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179(2):977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 125.Rieber N, Gille C, Köstlin N, et al. Neutrophilic myeloid-derived suppressor cells in cord blood modulate innate and adaptive immune responses. Clin Exp Immunol. 2013;174(1):45–52. doi: 10.1111/cei.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 2009;182(1):240–249. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 127.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(Pt 11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 128.Srikanth GV, Tripathy NK, Nityanand S. Fetal cardiac mesenchymal stem cells express embryonal markers and exhibit differentiation into cells of all three germ layers. World J Stem Cells. 2013;5(1):26–33. doi: 10.4252/wjsc.v5.i1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Javed MJ, Mead LE, Prater D, et al. Endothelial colony forming cells and mesenchymal stem cells are enriched at different gestational ages in human umbilical cord blood. Pediatr Res. 2008;64(1):68–73. doi: 10.1203/PDR.0b013e31817445e9. [DOI] [PubMed] [Google Scholar]
- 130.Nazarov I, Lee JW, Soupene E, et al. Multipotent stromal stem cells from human placenta demonstrate high therapeutic potential. Stem Cells Transl Med. 2012;1(5):359–372. doi: 10.5966/sctm.2011-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Karlsson H, Erkers T, Nava S, Ruhm S, Westgren M, Ringdén O. Stromal cells from term fetal membrane are highly suppressive in allogeneic settings in vitro. Clin Exp Immunol. 2012;167(3):543–555. doi: 10.1111/j.1365-2249.2011.04540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Siepe M, Thomsen AR, Duerkopp N, et al. Human neonatal thymus-derived mesenchymal stromal cells: characterization, differentiation, and immunomodulatory properties. Tissue Eng Part A. 2009;15(7):1787–1796. doi: 10.1089/ten.tea.2008.0356. [DOI] [PubMed] [Google Scholar]
- 133.Zhao ZG, Xu W, Sun L, et al. Immunomodulatory function of regulatory dendritic cells induced by mesenchymal stem cells. Immunol Invest. 2012;41(2):183–198. doi: 10.3109/08820139.2011.607877. [DOI] [PubMed] [Google Scholar]
- 134.Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105(5):2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 135.Chen HW, Chen HY, Wang LT, et al. Mesenchymal stem cells tune the development of monocyte-derived dendritic cells toward a myeloid-derived suppressive phenotype through growth-regulated oncogene chemokines. J Immunol. 2013;190(10):5065–5077. doi: 10.4049/jimmunol.1202775. [DOI] [PubMed] [Google Scholar]
- 136.Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+ CD25 high FOXP3+ regulatory T cells. Stem Cells. 2008;26(1):212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 137.Ghannam S, Pène J, Torcy-Moquet G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol. 2010;185(1):302–312. doi: 10.4049/jimmunol.0902007. [DOI] [PubMed] [Google Scholar]
- 138.Luz-Crawford P, Kurte M, Bravo-Alegría J, et al. Mesenchymal stem cells generate a CD4+ CD25+ Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4(3):65. doi: 10.1186/scrt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 140.Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76(8):1208–1213. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 141.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101(9):3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 142.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesen-chymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105(7):2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 143.Johann PD, Vaegler M, Gieseke F, et al. Tumour stromal cells derived from paediatric malignancies display MSC-like properties and impair NK cell cytotoxicity. BMC Cancer. 2010;10:501. doi: 10.1186/1471-2407-10-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111(3):1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 145.Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103(12):4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 146.Chen K, Wang D, Du WT, et al. Human umbilical cord mesenchymal stem cells hUC-MSCs exert immunosuppressive activities through a PGE2-dependent mechanism. Clin Immunol. 2010;135(3):448–458. doi: 10.1016/j.clim.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 147.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 148.Levy O, Goriely S, Kollmann TR. Immune response to vaccine adjuvants during the first year of life. Vaccine. 2013;31(21):2500–2505. doi: 10.1016/j.vaccine.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zoglmeier C, Bauer H, Nörenberg D, et al. CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice. Clin Cancer Res. 2011;17(7):1765–1775. doi: 10.1158/1078-0432.CCR-10-2672. [DOI] [PubMed] [Google Scholar]
- 150.Shirota Y, Shirota H, Klinman DM. Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J Immunol. 2012;188(4):1592–1599. doi: 10.4049/jimmunol.1101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lei J, Wang Z, Hui D, et al. Ligation of TLR2 and TLR4 on murine bone marrow-derived mesenchymal stem cells triggers differential effects on their immunosuppressive activity. Cell Immunol. 2011;271(1):147–156. doi: 10.1016/j.cellimm.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 152.Dang Y, Wagner WM, Gad E, et al. Dendritic cell-activating vaccine adjuvants differ in the ability to elicit antitumor immunity due to an adjuvant-specific induction of immunosuppressive cells. Clin Cancer Res. 2012;18(11):3122–3131. doi: 10.1158/1078-0432.CCR-12-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Benn CS. Combining vitamin A and vaccines: convenience or conflict? Dan Med J. 2012;59(1):B4378. [PubMed] [Google Scholar]
- 154.Villamor E, Fawzi WW. Effects of vitamin a supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev. 2005;18(3):446–464. doi: 10.1128/CMR.18.3.446-464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang WC, Ma G, Chen SH, Pan PY. Polarization and reprogramming of myeloid-derived suppressor cells. J Mol Cell Biol. 2013;5(3):207–209. doi: 10.1093/jmcb/mjt009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Najjar YG, Finke JH. Clinical perspectives on targeting of myeloid derived suppressor cells in the treatment of cancer. Front Oncol. 2013;3:49. doi: 10.3389/fonc.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mirza N, Fishman M, Fricke I, et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66(18):9299–9307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu E, Law HK, Lau YL. BCG promotes cord blood monocyte-derived dendritic cell maturation with nuclear Rel-B up-regulation and cytosolic I kappa B alpha and beta degradation. Pediatr Res. 2003;54(1):105–112. doi: 10.1203/01.PDR.0000069703.58586.8B. [DOI] [PubMed] [Google Scholar]