Graphical Abstract
Keywords: T. gondii, Isolation, Genotyping, PCR/RFLP, Genetic markers, South America
Highlights
-
•
Toxoplasma gondii infections in animals are frequent, and the role of wildlife in its epidemiology is not well understood.
-
•
Samples from 226 wild animals from different Brazilian localities were submitted to mice bioassay and screened by PCR.
-
•
A total of 17 genotypes were identified, 13 identified for the first time and four already reported in published literature.
-
•
Most of genotypes here identified are different from previous studies in domestic animals and humans from Brazil.
Abstract
This study aimed to isolate and genotype T. gondii from Brazilian wildlife. For this purpose, 226 samples were submitted to mice bioassay and screened by PCR based on 18S rRNA sequences. A total of 15 T. gondii isolates were obtained, including samples from four armadillos (three Dasypus novemcinctus, one Euphractus sexcinctus), three collared anteaters (Tamandua tetradactyla), three whited-lipped peccaries (Tayassu pecari), one spotted paca (Cuniculus paca), one oncilla (Leopardus tigrinus), one hoary fox (Pseudalopex vetulus), one lineated woodpecker (Dryocopus lineatus) and one maned wolf (Chrysocyon brachyurus). DNA from the isolates, originated from mice bioassay, and from the tissues of the wild animal, designated as “primary samples”, were genotyped by PCR–restriction fragment length polymorphism (PCR/RFLP), using 12 genetic markers (SAG1, SAG2, alt.SAG2, SAG3, BTUB, GRA6, c22-8, c29-2, L258, PK1, CS3 and Apico). A total of 17 genotypes were identified, with 13 identified for the first time and four already reported in published literature. Results herein obtained corroborate previous studies in Brazil, confirming high diversity and revealing unique genotypes in this region. Given most of genotypes here identified are different from previous studies in domestic animals, future studies on T. gondii from wildlife is of interest to understand population genetics and structure of this parasite.
1. Introduction
Toxoplasma gondii is an intracellular protozoan parasite distributed worldwide capable of infecting virtually all warm-blooded animals, including birds, humans, livestock and marine mammals (Dubey, 2010). In Brazil, the prevalence of T. gondii infection in humans is especially high and can reach 100% in some areas (Bahia-Oliveira et al., 2003; Sobral et al., 2005; De Moura et al., 2006) and an average of 60% of the adult women have been exposed to this parasite (Neto et al., 1995). The interest in the evaluation of T. gondii infection has focused on domestic animals that cohabitate with or serves as food for humans, as these animals can act as reservoirs to human infections (Sogorb et al., 1972). Though wildlife may play an important role in transmission and maintenance of T. gondii in the environment, there is limited information on T. gondii circulating in wild animals (Yai et al., 2009; Dubey et al., 2011; Pena et al., 2011; Cabral et al., 2013; Cañón-Franco et al., 2013).
Genotypic studies on T. gondii from domestic animals in Brazil have shown high diversity of this parasite (Dubey et al., 2002, 2007a; Lehmann et al., 2006; Shwab et al., 2014). This genetic diversity is characterized by an epidemic population structure (Pena et al., 2008). Recent efforts to genetically characterize T. gondii isolates from the wildlife have shown that “exotic” or “atypical” strains are not insignificant anomalies in the population structure of this parasite, but rather important members of the gene pool that provide a much better representation of the vast host range utilized by this parasite. There is a need, therefore, to reconsider the established points of view on the population genetic structure and the relative roles of the various lifecycle stages of T. gondii in shaping the population biology of this important zoonotic pathogen (Wendte et al., 2011).
Constant human interference and the increasing urbanization of the Brazilian landscape have resulted in wildlife habitat lost and fragmentation, and in an increased interaction between humans, domestic and wild animals that can lead to a greater exchange of pathogens. Isolation of T. gondii from wildlife is difficult and time consuming because of several factors, including poor DNA material from naturally infected wildlife because of low density of T. gondii in tissues of asymptomatic animals, and difficulties in preserving and transporting tissue samples from remote areas (Dubey et al., 2011). In the present study, we successfully genotyped 22 T. gondii samples obtained from wildlife in different regions of Brazil, and provided new information on genetic diversity of the parasite.
2. Material and methods
2.1. Location and sampling
For three years (2009–2011), 226 samples (fragments of brain and heart) from free-living and captive wild animals were collected, by chance/convenience, from different locations in Brazil (Table 1). The locations were in four regions (North, Northeast, Midwest and Southeast), five states (Mato Grosso, Minas Gerais, Pará, Pernambuco and São Paulo) and covered the four major Brazilian ecosystems: Amazon Forest, Atlantic Forest, Cerrado and Pantanal. All sampling locations were on the Brazilian mainland, except for one on the island of Fernando de Noronha, 360 km off from the northeast cost. Wild animal samples were collected from both urban and rural areas, and each sample was from a single animal except for samples collected on Fernando de Noronha, which were each pooled tissues from five animals of the same species.
Table 1.
Sampling sites and animal data.
| Local (municipality, state) | Geographic coordinates | I/DNA | FL/C | IDs |
|---|---|---|---|---|
| Araraquara, SP | 21°47′41″ S, 48°10′36″ W | 1/0 | 1/0 | TgHoFBr1 |
| Confresa, MT | 10°38′40″ S, 51°34′4″ W | 0/1 | 1/0 | PS-TgSbaBr1 |
| Fernando de Noronha, PEa | 3°50′25″ S, 32°24′41″ W | 0/2 | 2/0 | PS-TgCaEgBr1; PS-TgCaEgBr2 |
| Jaborandi, SP | 20°54′0″ S, 47°16′0″ W | 1/0 | 1/0 | TgMWBr1 |
| Jaboticabal, SP | 21°15′19″ S, 48°19′21″ W | 1/1 | 1/1 | TgCantBr3; PS-TgTinBr1 |
| Recife, PE | 8°3′15″ S, 34°52′53″ W | 1/0 | 0/1 | TgOncBr1 |
| Santarém, PA | 2°26′22″ S, 54°41′55″ W | 9/2 | 11/0 | TgNbaBr1, 2, 3; TgCantBr1, 2; TgWlpBr1, 2, 3; TgSpPBr2; PS-TgSpPBr1; PS- TgNbaBr4 |
| São Paulo, SP | 23° 32′ 56″ S, 46° 38′ 20″ W | 1/1 | 1/1 | TgLWpBr1; PS-TgBHmBr1 |
| Uberlândia, MG | 18° 54′ 41″ S, 48° 15′ 44″ W | 0/1 | 1/0 | TgSbaBr2 |
I/DNA, T. gondii isolation in mice/DNA extracted directly from tissues of wild animals before mice bioassay; FL/C, free-living animals/captive animals.
Island located 360 km from the Northeast coast.
2.2. Bioassay
Fragments (brain and heart) of wild animal tissues, weighting from 5 to 50 grams (depending on the animal size), were mixed and homogenized, then digested in acidic pepsin and washed. Aliquots of homogenates were inoculated s.c. into five out-bred Swiss Webster (SW) mice (Dubey, 1998). Tissue imprints of lungs and brains of inoculated mice that died were examined for T. gondii tachyzoites (lungs) or tissue cysts (brain), by direct observation on microscope. Survivors were bled 45 days post infection (DPI) and a 1:25 dilution of serum was tested for T. gondii antibodies by the modified agglutination test (MAT) as described by Dubey and Desmonts (1987) in order to ensure that these animals were not infected with T. gondii. Mice were killed 60 DPI and their brains were examined for tissue cysts as previously described (Dubey, 2010). The inoculated mice were considered infected with T. gondii when tachyzoites or tissue cysts were detected in their tissues.
2.3. Molecular detection of T. gondii in wild animal tissues
DNA from 300 µL of the homogenate (prior to pepsin digestion) from tissues of wild animals (primary samples) was extracted with a commercial kit (Wizard® DNA Clean-Up System, Cat. A7280 – Promega, Madison, WI, USA), following manufacturer's instructions. Toxoplasma gondii was among the protozoans targeted with a nested PCR of 18S ribosomal DNA (PCR-18S) to detect parasites of the Sarcocystidae family in tissues of wild animals (data not published) performed using external primers Tg18s48F (5′CCATGCATGTCTAAGTATAAGC3′) and Tg18s359R (5′GTTACCCGTCACTGCCAC3′), and internal primers Tg18s58F (5′CTAAGTATAAGCTTTTATACGGC3′) and Tg18s348R (5′TGCCACGGTAGTCCAATAC3′) (Integrated DNA Technologies, USA). This amplification generates about 290 base pair (bp) product for Sarcocystis neurona, N. caninum, H. hammondi and T. gondii, and 310 bp for other Sarcocystis spp. The products of nested PCR were digested by two sets of restriction enzymes (set 1: AluI and HhaI, to differentiate S. tenella from T. gondii, N. caninum and H. hammondi; set 2: DdeI, Hpy188III and MspI, to differentiate all Sarcocystis species (da Silva et al., 2009). Twenty-eight positive samples for T. gondii were selected for genotyping analysis.
2.4. PCR/RFLP
DNA was extracted from lungs and brain of infected mice and from positive “primary samples” (tissue homogenate aliquots of wild animals). T. gondii strain genotyping was performed using the genetic markers SAG1, 5′ and 3′-SAG2, alt.SAG2, SAG3, BTUB, GRA6, c22–8, c29–2, L358, PK1, Apico and CS3 as described previously (Pena et al., 2008; Su et al., 2010). NeighborNet phylogenetic networks were inferred using the software SplitsTree4 (Huson, 1998; Huson and Bryant, 2006; Pena et al., 2008).
2.5. Animal ethics
This study was conducted after consultation with the Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA) and approval of the Ethical Committee of the Faculty of Veterinary Medicine of the University of São Paulo – USP (project no. 1588/2008). All experiments performed in mice were in accordance with the Ethical Principles in Animal Research adopted by the Brazilian College of Animal Experimentation. All sampled wild animals died from diverse causes, such as road kills and other sources of trauma or illness. No wild animals were killed for this research.
3. Results
3.1. Toxoplasma gondii isolation from wild animal tissues
Viable T. gondii was isolated from 15 out of the 226 wild animal samples. In general, isolates presented a profile of high pathogenicity for the infected mice. All infected mice died of acute toxoplasmosis. Mice death occurred between the 7th and 40th DPI, but most of the deaths occurred between the 11th and 18th DPI.
Regarding the origin of the isolates analyzed in the present study, 14 out of 15 (93.3%) were obtained from free-living animals. Only one sample (TgOncBr1), the isolate from an oncilla (Leopardus tigrinus), was from a captive animal. Thirteen isolates were from mammals and one isolate was from a bird. Details of viable T. gondii isolates obtained in this study and mice mortality are given in Table 2.
Table 2.
Isolation of Toxoplasma gondii in wild animals from Brazil.
| Sample ID | Species | Local | Origin | MAT titer* | Mice bioassaya | ||
|---|---|---|---|---|---|---|---|
| No. death/no. infected | % Death | Day of death (DPI) | |||||
| TgHoFBr1 | Hoary fox (Pseudalopex vetulus) | Araraquara | FL | 200 | 2/2 | 100 | 13, 18 |
| TgMWBr1 | Maned wolf (Chrysocyon brachyurus) | Jaborandi | FL | 400 | 3/3 | 100 | 23, 24, 28 |
| TgCantBr3 | Collared anteater (Tamandua tetradactyla) | Jaboticabal | FL | 400 | 3/3b | 100 | 17,18,18 |
| TgOncBr1 | Oncilla (Leopardus tigrinus) | Recife | C | ND | 5/5b | 100 | 15, 15, 15, 18, 23 |
| TgSpPBr2 | Spotted paca (Cuniculus paca) | Santarém | FL | ND | 5/5 | 100 | 14, 15, 16, 16, 16 |
| TgCantBr1 | Collared anteater (Tamandua tetradactyla) | Santarém | FL | ND | 5/5 | 100 | 13, 14, 14, 14, 29 |
| TgWlpBr1 | White-lipped peccary (Tayassu pecari) | Santarém | FL | ND | 5/5 | 100 | 14, 15, 16, 16, 17 |
| TgWlpBr2 | White-lipped peccary (Tayassu pecari) | Santarém | FL | ND | 3/3 | 100 | 15, 15, 34 |
| TgWlpBr3 | White-lipped peccary (Tayassu pecari) | Santarém | FL | ND | 4/4 | 100 | 14, 17, 21, 22 |
| TgNbaBr1 | Nine-banded armadillo (Dasypus novemcinctus) | Santarém | FL | ND | 5/5 | 100 | 10, 11, 11, 11, 13 |
| TgNbaBr2 | Nine-banded armadillo (Dasypus novemcinctus) | Santarém | FL | ND | 4/4 | 100 | 13, 14, 16, 16 |
| TgNbaBr3 | Nine-banded armadillo (Dasypus novemcinctus) | Santarém | FL | ND | 5/5 | 100 | 11, 11, 12, 13, 13 |
| TgCantBr1 | Collared anteater (Tamandua tetradactyla) | Santarém | FL | ND | 5/5 | 100 | 12, 12, 12, 18, 18 |
| TgLWpBr1 | Lineated woodpecker (Dryocopus lineatus) | São Paulo | FL | ND | 4/4b | 100 | 22, 23, 23, 24 |
| TgSbaBr2 | Six banded-armadillo (Euphractus sexcinctus) | Uberlândia | FL | 25 | 5/5b | 100 | 15,15, 17, 17, 30 |
FL, free-living; C, captive; neg, negative; ND, not done; DPI, days post-infection.
Wild animals MAT titer.
Five inoculated mice per group.
It was not possible to observe T. gondii in one or more dead mice, and infection was confirmed by serology.
3.2. Toxoplasma gondii molecular detection and genotyping
The genetic characterization of T. gondii by PCR/RFLP was performed on 15 isolates obtained from bioassay in mice and 28 DNA samples directly extracted from the tissues of the wild animal (primary samples) with positive results using the nested PCR of 18S ribosomal DNA protocol. Complete genetic characterization was successful in 22 samples; all 15 T. gondii isolates and seven of 28 primary samples, demonstrating a higher sensitivity of this method when applied to isolates rather than primary samples.
Genotypes obtained directly from wild animal tissues were identified with the letters “PS” (Primary Sample) prior to the regular identification in an attempt to differentiate from the genotypes obtained from T. gondii isolates (e.g., PS-TgSbaBr1 – PS = Primary Sample; Tg = Toxoplasma gondii; Sba = Six-banded armadillo [animal species]; Br1 = Brazil, isolate no.1).
Genetic characterization of 22 strains showed the presence of 17 distinct atypical genotypes, 13 of which were previously undescribed. Four genotypes had been previously reported in Brazil. One isolate (TgSbaBr2) belongs to the Brazilian clonal lineage BrI (#6), two samples (TgMWBr1 and PS-TgBHmBr1) belong to the Brazilian clonal lineage BrII (#11) (Pena et al., 2008), two samples (PS-TgCaEgBr1, PS-TgCaEgBr2) from Fernando de Noronha belong to genotype #146 previously described in chickens from the same island (Dubey et al., 2010), one sample (TgLWpBr1) belongs to genotype #175, which has been reported in capybara in Brazil (Yai et al., 2009). Genotyping data is summarized in Table 3.
Table 3.
Summary of Toxoplasma gondii PCR/RFLP alleles obtained from Brazilian wildlife.
| PCR-RFLP genotype | Species | Location | Origin | ToxoDB PCR-RFLP genotype |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample ID | SAG1 | 5′+ 3' SAG2 | alt. SAG2 | SAG3 | BTUB | GRA6 | c22-8 | c29-2 | L358 | PK1 | Apico | CS3 | ||||
| TgNbaBr1 | I | I | II | I | III | II | II | III | I | III | I | II | Nine-banded armadillo (Dasypus novemcinctus) | Santarém | FL | New, #231 |
| TgCantBr1 | Collared anteater (Tamandua tetradactyla) | Santarém | FL | |||||||||||||
| TgWlpBr1 | I | I | II | I | III | III | II | I | III | u-1 | III | II | White-lipped peccary (Tayassu pecari) | Santarém | FL | New, #232 |
| TgNbaBr2 | I | I | II | I | III | III | II | I | III | III | III | I | Nine-banded armadillo (Dasypus novemcinctus) | Santarém | FL | New, #195 |
| TgNbaBr3 | Nine-banded armadillo (Dasypus novemcinctus) | Santarém | FL | |||||||||||||
| PS-TgSpPBr1 | Spotted paca (Cuniculus paca) | Santarém | FL | |||||||||||||
| TgCantBr2 | I | I | II | I | III | III | II | I | III | III | I | I | Collared anteater (Tamandua tetradactyla) | Santarém | FL | New, #234 |
| TgOncBr1 | I | III | III | III | I | III | u-1 | I | I | I | III | I | Oncilla (Leopardus tigrinus) | Recife | C | New, #235 |
| TgHoFBr1 | I | III | III | III | III | III | I | III | I | u-1 | III | u-1 | Hoary fox (Pseudalopex vetulus) | Araraquara | FL | New, #237 |
| PS-TgNbaBr4 | I | I | II | I | III | III | I | I | III | III | I | II | Nine-banded armadillo (Dasypus novemcinctus) | Santarém | FL | New, #238 |
| PS-TgTinBr1 | I | I | I | III | I | II | I | III | I | II | III | III | Red-winged tinamou (Rhynchotus rufescens) | Jaboticabal | C | New, #239 |
| TgWlpBr2 | I | I | II | I | III | III | II | III | III | u-2 | I | u-3 | White-lipped peccary (Tayassu pecari) | Santarém | FL | New, #196 |
| PS-TgSbaBr1 | I | I | II | III | III | III | u-1 | I | I | III | III | u-1 | Six banded-armadillo (Euphractus sexcinctus) | Confresa | FL | New, #241 |
| TgSpPBr2 | I | I | II | I | III | III | II | I | I | u-2 | I | I | Spotted paca (Cuniculus paca) | Santarém | FL | New, #240 |
| TgWlpBr3 | I | I | II | I | III | III | III | I | III | III | III | II | White-lipped peccary (Tayassu pecari) | Santarém | FL | New, #233 |
| TgCantBr3 | u-1 | I | II | III | III | III | III | I | I | III | III | II | Collared anteater (Tamandua tetradactyla) | Jaboticabal | FL | New, #236 |
| TgLWpBr1 | u-1 | I | II | III | III | III | III | I | I | u-1 | I | II | Lineated woodpecker (Dryocopus lineatus) | São Paulo | FL | #175, TgCpBr25 (Yai et al., 2009) |
| TgSbaBr2 | I | I | I | III | I | II | u-1 | I | I | I | I | I | Six banded-armadillo (Euphractus sexcinctus) | Uberlândia | FL | BrI, #6 (Pena et al., 2008) |
| TgMWBr1 | I | I | II | III | III | III | I | III | I | II | III | I | Maned Wolf (Chrysocyon brachyurus) | Jaborandi | FL | BrII, #11 (Pena et al., 2008) |
| PS-TgBHmBr1 | Black Howler monkey (Alouatta caraya) | São Paulo | C | |||||||||||||
| PS-TgCaEgBr1 | I | I | I | III | II | II | I | III | III | II | III | III | Cattle Egret (5 bird pool) (Bubulcus ibis) | Fernando de Noronha | FL | #146 TgCkBr210 (Dubey et al., 2010) |
| PS-TgCaEgBr2 | Cattle Egret (5 bird pool) (Bubulcus ibis) | Fernando de Noronha | FL | |||||||||||||
I, II, III are the alleles found in the clonal lineages type I, type II and type III; u-1 is the new allele that is different from the clonal type alleles.
FL, free-living; C, captive.
Of the 22 genotyped samples, four were from birds and 18 were from mammals. From this collection, 19 (86.4%) originated from free-living animals (three birds and 16 mammals) and three samples (13.6%) were from captive animals (one bird and two mammals).
Phylogenetic network analysis of the 22 genotypes is summarized in Fig. 1 and geographic distribution of these genotypes is summarized in Fig. 2. Eleven isolates, all from Amazon region (Santarém, PA) are clustered into one group, suggesting they are closely related. The other samples assumed a random distribution in the phylogenetic net.
Fig. 1.
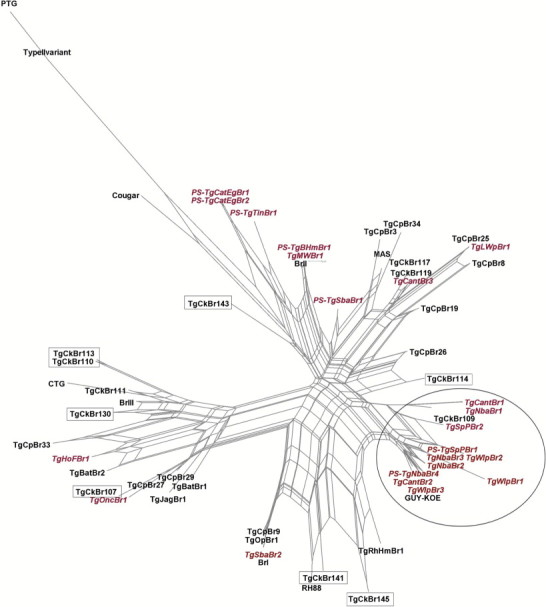
Phylogenetic network analysis of Toxoplasma gondii from wildlife in Brazil. Genotype ID and the representative strain are listed for each taxonomic branch. Reference strains are in black, the strains from this study are in red, and the Amazonic reference strains that did not cluster together are in boxes. Inside the circle are listed all the genotypes obtained from the Amazon region which are in the same branch as other isolates from this biome.
Fig. 2.
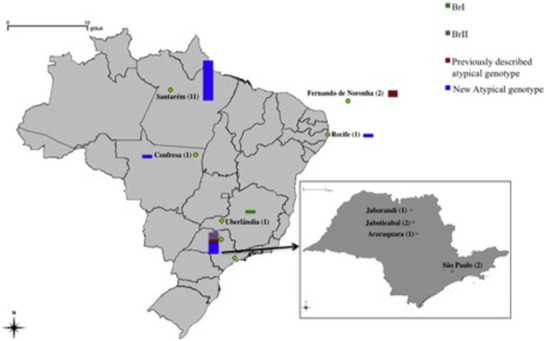
Geographical distribution of the genotypes of Toxoplasma gondii from wildlife in Brazil. Samples are grouped by states. Sample size is represented by the size of the bar and the number written in brackets. The smallest bar represents one genotype. Color code: green, purple, red and blue are for BrI, BrII, previously described atypical genotypes and new atypical genotypes, respectively.
4. Discussion
4.1. Biology and prevalence
Isolation of T. gondii from free-living wild animals provides valuable information on its population structure in wildlife. There are a few data in the literature on the isolation of T. gondii from wild animals, particularly in South American wildlife. This study is the largest collection of T. gondii isolates from South American wildlife and reports, for the first time, the isolation of this parasite in these twelve host species.
Although all the isolates were virulent and lethal to the mice it is not accurate to make inference on virulence without knowing the dosage of the inoculum. However, the pathogenic profile here observed is indicative of high virulence and highlights biological differences between South American isolates and those from the Northern Hemisphere.
In birds, isolation in mice is an important tool to detect T. gondii because many serologic tests for toxoplasmosis may be relatively insensitive compared to mammals (Frenkel, 1981). This insensitivity was observed in crested caracaras (Caracara plancus), red-legged partridges (Alectoris rufa) and pigeons (Columba livia), all experimentally infected with T. gondii. Most of the infected birds had a sharp decrease in antibody titers and some birds became serologically negative after a short period post-infection (Martínez-Carrasco et al., 2004; Mineo et al., 2009; Vitaliano et al., 2010). In the present study, T. gondii was isolated from a lineated woodpecker. To our knowledge this is the first isolation report in Brazilian wild birds, although it has been detected in birds by serology and molecular techniques (Gondim et al., 2010; Vitaliano et al., 2010; Costa et al., 2012). Regarding the source of infection for the lineated woodpecker, environmental contamination with T. gondii oocysts could have played a significant role, since this species have insectivore feeding habits.
T. gondii was isolated from one maned wolf and one hoary fox, both free-living animals. In fact, this is the first report of T. gondii isolation from South American wild canids. Until now, there was only serological evidence of T. gondii infection in these species (Vitaliano et al., 2004; André et al., 2010). Wild canids here are omnivore, so the prevalence of T. gondii in these animals likely indicates consumption of infected prey and/or environmental contamination with oocysts.
Very little information is available concerning T. gondii infection in xenarthrans in Brazil and South America. These animals are insectivores (e.g., anteaters) and sometimes omnivores (e.g., armadillos). Despite the difference on feeding habits, xenarthrans share their foraging habits, seeking food on the ground. For this reason, T. gondii infection in these animals can be considered indicative of contamination with oocysts. Additionally, in the omnivore species the consumption of infected carrion is likely to be an important source of infection. Sogorb et al. (1977), isolated viable T. gondii from a giant armadillo (Priodontes giganteus). da Silva et al. (2009) attempted, without success, to isolate T. gondii from armadillos; however, in the six-banded armadillo samples, some of the inoculated mice were positive in the direct agglutination test, indicating infection. Here, we isolated for the first time viable T. gondii from three collared anteaters, one six-banded armadillo and three nine-banded armadillos.
Wild felids, as well as domestic cats, are central to transmission of T. gondii since felids are the only definitive hosts of this parasite. Unfortunately, there is little information on the isolation of T. gondii from Brazilian wild felids. The only report of isolation available is from a captive animal, a jaguarondi (Puma yagouaroundi) inhabitant of a zoo in Northeast Brazil (Pena et al., 2011). The oncilla in the present study was captive. Although this is the first report of T. gondii isolation from an oncilla, it is from a captive animal and infection was likely acquired from a domestic source although this genotype has never been identified in a domestic animal.
Rodents can play a central role in the epidemiology of T. gondii since they are commonly prey for cats, both domestic and wild, including the jaguar (Panthera onca), which preys on capybaras (Hydrochoerus hydrochaeris), the world's largest rodents. In the present study, T. gondii was isolated from a free-living spotted paca from Santarém, PA, and was previously isolated from free-ranging and captive capybaras (Yai et al., 2008).
In the present study, T. gondii was isolated from three free-living white-lipped peccaries from Santarém, PA. Currently, there is no other report of T. gondii isolation from South American wild suids. In France, T. gondii was isolated from 21 hunted wild boars (Sus scrofa) from two different regions (Richomme et al., 2009). Peccaries have omnivorous habits normally foraging for roots, vegetation and small amounts of animal matter from the ground and, for this reason, may became infected with T. gondii from both animal and environmental sources. Wild suids in South America may have an important role in the transmission of T. gondii as their main predators are jaguars and pumas (Puma concolor).
4.2. Genetic types and phylogenetic analysis
Genetic analysis of 22 wild animal samples revealed an extremely high diversity of T. gondii from Brazilian wildlife. This diversity was evidenced by the presence of 17 different genotypes in these samples. Our findings are in agreement with several studies realized on this continent that demonstrate high diversity within and between T. gondii populations (Pena et al., 2008, 2011; Yai et al., 2009; Rajendran et al., 2012; Cañón-Franco et al., 2013). Due to this great genetic diversity and insufficient amount of genotyping data from wildlife, it is not clear if there are genotypes that are exclusive or more common in wild animals compared to domestic animals and humans.
From a total of 22 complete genotypes, 15 were obtained from mice isolates and seven were obtained direct from wild animals tissues. Complete genetic characterization of primary samples is more difficult relative to the isolates from mice because, in chronic or subclinical cases, the amount of T. gondii DNA in the tissues may be lower than 250 µg/100 g of tissue (Dubey et al., 2004), but in case of wild animal samples, which are difficult to have access to, especially endangered species, the attempt is valid. Genetic characterization of T. gondii in primary samples from wild felids in Brazil has been reported, although the sensitivity of detection was lower relative to isolates in mice (Cañón-Franco et al., 2013), as was observed in the present study.
The genetic relationship among the 22 T. gondii genotypes is presented as a NeighborNet phylogenetic network (Fig. 1). In this network it was possible to observe the presence of an Amazonic phylogenetic branch, in which all samples from Santarém region, which belongs to Legal Amazon, have clustered together. Although the data have not shown the existence of a unique Amazonic cluster, it suggests that this branch may be dominant in Amazonic area. It is noteworthy that there is not a local cluster form Santarém either, as one of the reference strains (GUY-KOE) that clustered in the branch originated from French Guiana. More studies are necessary to evaluate the existence of an Amazon cluster, and its relationship with severe cases of toxoplasmosis in immunocompetent patients, as observed in French Guiana (Carme et al., 2002; Ajzenberg et al., 2004; Wendte et al., 2011).
4.3. Genetic diversity and epidemiology
T. gondii populations in South America, and particularly in this study in Brazil, have an extremely high genetic diversity (Dubey et al., 2011). In wildlife, genetic diversity is probably greater than in anthropized environments (Dubey et al., 2007b; Boothroyd, 2009; Dubey, 2010), possibly due to a larger range of hosts. The assumption was supported in French Guiana by Mercier et al. (2010); a greater genetic diversity was reported in the rainforest than in the anthropized environment. Nonetheless, genetic differences are not the only feature between these two populations from both hemispheres, as South American populations are also biologically different from T. gondii populations found in the Northern hemisphere. In southern populations recombination plays a significant role in strains diversification (Pena et al., 2008). Although T. gondii infection is asymptomatic or subclinical in most immunocompetent hosts, it is known that severe cases of toxoplasmosis can be caused especially by atypical genotypes (Carme et al., 2002; Ajzenberg et al., 2004; Wendte et al., 2011).
Results of the present study and other recent reports from Brazil indicate the presence of diverse genotypes in Brazilian wildlife in several different regions of the country (Yai et al., 2009; Pena et al., 2011; Cañón-Franco et al., 2013). We also detected the presence of Brazilian clonal lineages (BrI and BrII) observed in previous studies (Yai et al., 2009; Pena et al., 2011; Cañón-Franco et al., 2013). Although hunting is not permitted in Brazil, wild animals are hunted in rural areas, and they can serve as a source of infection to humans and domestic animals. Also, urbanization is characterized by the expansion of human settlement, which invades the natural habitats of animals and increases the chance of interactions between wild and domestic animals and lead to an increased risk of infection in humans. This can enhance the transmission of wildlife T. gondii strains to domestic animals and humans.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgements
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). S.N. Vitaliano possessed a scholarship from FAPESP (project no. 2009/00175). S.M. Gennari, R.M. Soares and H.F.J. Pena are in receipt of productivity scholarships from CNPq.
References
- Ajzenberg D., Bañuls A.L., Su C., Dumètre A., Demar M., Carme B. Genetic diversity, clonality and sexuality in Toxoplasma gondii. Int. J. Parasitol. 2004;34:1185–1196. doi: 10.1016/j.ijpara.2004.06.007. [DOI] [PubMed] [Google Scholar]
- André M.R., Adania C.H., Teixeira R.H.F., Silva K.F., Jusi M.M.G., Machado S.T.Z. Antibodies to Toxoplasma gondii and Neospora caninum in captive neotropical and exotic wild canids and felids. J. Parasitol. 2010;96:1007–1009. doi: 10.1645/GE-2502.1. [DOI] [PubMed] [Google Scholar]
- Bahia-Oliveira L.M.G., Jones J.L., Azevedo-Silva J., Alves C.C.F., Oréfice F., Addiss D.G. Highly endemic, waterborne toxoplasmosis in north Rio de Janeiro State, Brazil. Emerg. Infect. Dis. 2003;9:55–62. doi: 10.3201/eid0901.020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothroyd J.C. Expansion of host range as a driving force in the evolution of Toxoplasma. Mem. Inst. Oswaldo Cruz. 2009;104:179–184. doi: 10.1590/s0074-02762009000200009. [DOI] [PubMed] [Google Scholar]
- Cabral A.D., Gama A.R., Sodré M.M., Savani E.S.M.M., Galvão-Dias M.A., Jordão L.R. First isolation and genotyping of Toxoplasma gondii from bats (Mammalia: Chiroptera) Vet. Parasitol. 2013;193:100–104. doi: 10.1016/j.vetpar.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Cañón-Franco W.A., Araújo F.A., López-Orozco N., Jardim M.M., Keid L.B., Dalla-Rosa C. Toxoplasma gondii in free-ranging wild small felids from Brazil: molecular detection and genotypic characterization. Vet. Parasitol. 2013;197:462–469. doi: 10.1016/j.vetpar.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Carme B., Bissuel F., Ajzenberg D., Bouyne R., Aznar C., Demar M. Severe acquired toxoplasmosis in immunocompetent adult patients in French Guiana. J. Clin. Microbiol. 2002;40:4037–4044. doi: 10.1128/JCM.40.11.4037-4044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa D.G.C., Marvulo M.F.V., Silva J.S.A., Santana S.C., Magalhães F.J.R., Lima-Filho C.D.F. Seroprevalence of Toxoplasma gondii in domestic and wild animals from Fernando de Noronha, Brazil. J. Parasitol. 2012;98:679–680. doi: 10.1645/GE-2910.1. [DOI] [PubMed] [Google Scholar]
- da Silva R.C., Su C., Langoni H. First identification of Sarcocystis tenella (Railliet, 1886) Moulé, 1886 (Protozoa: Apicomplexa) by PCR in naturally infected sheep from Brazil. Vet. Parasitol. 2009;165:332–336. doi: 10.1016/j.vetpar.2009.07.016. [DOI] [PubMed] [Google Scholar]
- De Moura L., Bahia-Oliveira L.M.G., Wada M.Y., Jones J.L., Tuboi S.H., Carmo E.H. Waterborne outbreak of toxoplasmosis, Brazil, from field to gene. Emerg. Infect. Dis. 2006;12:326–329. doi: 10.3201/eid1202.041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J.P. Refinement of pepsin digestion method for isolation of Toxoplasma gondii from infected tissues. Vet. Parasitol. 1998;74:75–77. doi: 10.1016/s0304-4017(97)00135-0. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. second ed. CRC Press; Boca Raton, Florida: 2010. Toxoplasmosis of Animals and Humans. [Google Scholar]
- Dubey J.P., Desmonts G. Serological responses of equids fed Toxoplasma gondii oocysts. Equine Vet. J. 1987;19:337–339. doi: 10.1111/j.2042-3306.1987.tb01426.x. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Graham D.H., Blackston C.R., Lehmann T., Gennari S.M., Ragozo A.M.A. Biological and genetic characterization of Toxoplasma gondii isolates from chickens (Gallus domesticus) from São Paulo, Brazil: unexpected findings. Int. J. Parasitol. 2002;32:99–105. doi: 10.1016/s0020-7519(01)00364-2. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Graham D.H., de Young R.W., Dahl E., Eberhard M.L., Nace E.K. Molecular and biologic characteristics of Toxoplasma gondii isolates from wildlife in the United States. J. Parasitol. 2004;90:67–71. doi: 10.1645/GE-110R. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Gennari S.M., Sundar N., Vianna M.C.B., Bandini L.M., Yai L.E.O. Diverse and atypical genotypes identified in Toxoplasma gondii from dogs in São Paulo, Brazil. J. Parasitol. 2007;93:60–64. doi: 10.1645/GE-972R.1. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Sundar N., Nolden C.A., Samuel M.D., Velmurugan G.V., Bandini L.A. Characterization of Toxoplasma gondii from raccoons (Procyon lotor), coyotes (Canis latrans), and striped skunks (Mephitis mephitis) in Wisconsin identified several atypical genotypes. J. Parasitol. 2007;93:1524–1527. doi: 10.1645/GE-1245.1. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Rajendran C., Costa D.G.C., Ferreira L.R., Kwok O.C.H., Qu D. New Toxoplasma gondii genotypes isolated from free-range chickens from the Fernando de Noronha, Brazil: unexpected findings. J. Parasitol. 2010;96:709–712. doi: 10.1645/GE-2425.1. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Velmurugan G.V., Rajendran C., Yabsley M.J., Thomas N.J., Beckmen K.B. Genetic characterisation of Toxoplasma gondii in wildlife from North America revealed widespread and high prevalence of the fourth clonal type. Int. J. Parasitol. 2011;41:1139–1147. doi: 10.1016/j.ijpara.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Frenkel J.K. False-negative serologic test for Toxoplasma in birds. J. Parasitol. 1981;76:952–953. [PubMed] [Google Scholar]
- Gondim L.S.Q., Abe-Sandes K., Uzêda R.S., Silva M.S.A., Santos S.L., Mota R.A. Toxoplasma gondii and Neospora caninum in sparrows (Passer domesticus) in the northeast of Brazil. Vet. Parasitol. 2010;168:121–124. doi: 10.1016/j.vetpar.2009.09.055. [DOI] [PubMed] [Google Scholar]
- Huson D.H. Splits Tree: a program for analyzing and visualizing evolutionary data. Bioinformatics. 1998;14:68–73. doi: 10.1093/bioinformatics/14.1.68. [DOI] [PubMed] [Google Scholar]
- Huson D.H., Bryant D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- Lehmann T., Marcet P.L., Graham D.H., Dahl E.R., Dubey J.P. Globalization and the population structure of Toxoplasma gondii. PNAS. 2006;103:11423–11428. doi: 10.1073/pnas.0601438103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Carrasco C., Ortiz J.M., Bernabé A., Ruiz De Ybáñez M.R., Garijo M., Alonso F.D. Serologic response of red-legged partridges (Alectoris rufa) after oral inoculation with Toxoplasma gondii oocysts. Vet. Parasitol. 2004;121:143–149. doi: 10.1016/j.vetpar.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Mercier A., Devillard S., Ngoubangoye B., Bonnabu H., Bañuls A.L., Durand P. Additional haplogroups of Toxoplasma gondii out of Africa: population structure and mouse-virulence of strains from Gabon. PLoS Negl. Trop. Dis. 2010;4:876. doi: 10.1371/journal.pntd.0000876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo T.P.W., Carrasco A.O.T., Marciano J.A., Werther K., Pinto A.A., Machado R.Z. Pigeons (Columba livia) are a suitable experimental model for Neospora caninum infection in birds. Vet. Parasitol. 2009;159:149–153. doi: 10.1016/j.vetpar.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Neto V.A., Medeiros E.A.S., Levi G.C., Duarte M.I.S. fourth ed. Sarvier; São Paulo: 1995. Toxoplasmose; p. 154. [Google Scholar]
- Pena H.F., Gennari S.M., Dubey J.P., Su C. Population structure and mouse-virulence of Toxoplasma gondii in Brazil. Int. J. Parasitol. 2008;38:561–569. doi: 10.1016/j.ijpara.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Pena H.F., Marvulo M.F., Horta M.C., Silva M.A., Silva J.C., Siqueira D.B. Isolation and genetic characterisation of Toxoplasma gondii from a red-handed howler monkey (Alouatta belzebul), a jaguarundi (Puma yagouaroundi), and a black-eared opossum (Didelphis aurita) from Brazil. Vet. Parasitol. 2011;175:377–381. doi: 10.1016/j.vetpar.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Rajendran C., Su C., Dubey J.P. Molecular genotyping of Toxoplasma gondii from Central and South America revealed high diversity within and between populations. Infect. Genet. Evol. 2012;12:359–368. doi: 10.1016/j.meegid.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Richomme C., Aubert D., Gilot-Fromont E., Ajzenberg D., Mercier A., Ducrot C. Genetic characterization of Toxoplasma gondii from wild boar (Sus scrofa) in France. Vet. Parasitol. 2009;164:296–300. doi: 10.1016/j.vetpar.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Shwab E.K., Zhu X.Q., Majumdar D., Pena H.F., Gennari S.M., Dubey J.P. Geographical patterns of Toxoplasma gondii genetic diversity revealed by multilocus PCR-RFLP genotyping. Parasitology. 2014;141:453–461. doi: 10.1017/S0031182013001844. [DOI] [PubMed] [Google Scholar]
- Sobral C.A., Amendoeira M.R.R., Teva A., Patel B.N., Klein C.H. Seroprevalence of infection with Toxoplasma gondii in indigenous Brazilian populations. Am. J. Trop. Med. Hyg. 2005;72:37–41. [PubMed] [Google Scholar]
- Sogorb F.S., Jamra L.F., Guimarães E.C., Deane M.P. Toxoplasmose espontânea em animais domésticos e silvestres, em São Paulo. Rev. Inst. Med. Trop. Sao Paulo. 1972;14:314–320. [PubMed] [Google Scholar]
- Sogorb F.S., Jamra L.F., Guimarães E.C. Toxoplasmose em animais de São Paulo, Brasil. Rev. Inst. Med. Trop. Sao Paulo. 1977;19:191–194. [PubMed] [Google Scholar]
- Su C., Shwab E.K., Zhou P., Zhu X.Q., Dubey J.P. Moving towards an integrated approach to molecular detection and identification of Toxoplasma gondii. Parasitology. 2010;137:1–11. doi: 10.1017/S0031182009991065. [DOI] [PubMed] [Google Scholar]
- Vitaliano S.N., Silva D.A.O., Mineo T.W.P., Ferreira R.A., Bevilacqua E., Mineo J.R. Seroprevalence of Toxoplasma gondii and Neospora caninum in captive maned wolves (Chrysocyon brachyurus) from southeastern and midwestern regions of Brazil. Vet. Parasitol. 2004;122:253–260. doi: 10.1016/j.vetpar.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Vitaliano S.N., Mineo T.W.P., André M.R., Machado R.Z., Mineo J.R., Werther K. Experimental infection of crested caracara (Caracara plancus) with Toxoplasma gondii simulating natural conditions. Vet. Parasitol. 2010;172:71–75. doi: 10.1016/j.vetpar.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Wendte J.M., Gibson A.K., Grigg M.E. Population genetics of Toxoplasma gondii: new perspectives from parasite genotypes in wildlife. Vet. Parasitol. 2011;182:96–111. doi: 10.1016/j.vetpar.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yai L.E.O., Ragozo A.M.A., Aguiar D.M., Damaceno J.T., Oliveira L.N., Dubey J.P. Isolation of Toxoplasma gondii from capybaras (Hydrochaeris hydrochaeris) from São Paulo state, Brazil. J. Parasitol. 2008;94:1060–1063. doi: 10.1645/GE-1548.1. [DOI] [PubMed] [Google Scholar]
- Yai L.E.O., Ragozo A.M., Soares R.M., Pena H.F., Su C., Gennari S.M. Genetic diversity among capybara (Hydrochaeris hydrochaeris) isolates of Toxoplasma gondii from Brazil. Vet. Parasitol. 2009;162:332–333. doi: 10.1016/j.vetpar.2009.03.007. [DOI] [PubMed] [Google Scholar]


