Highlights
-
•
Gastric parasitism was highest among inland freshwater alligators.
-
•
Crabs, fishes, and turtles are predicted intermediate hosts of alligator nematodes.
-
•
Use of multiple intermediate hosts may reflect host's generalist foraging behavior.
-
•
Results provide further evidence of the unique crocodilian-parasite dynamic.
Keywords: Alligator mississippiensis, Ascarididae, Georgia, Florida, Intermediate hosts, Stomach flushing
Graphical Abstract
Abstract
We examined the variation of stomach nematode intensity and species richness of Alligator mississippiensis from coastal estuarine and inland freshwater habitats in Florida and Georgia, and integrated prey content data to predict possible intermediate hosts. Nematode parasitism within inland freshwater inhabiting populations was found to have a higher intensity and species richness than those inhabiting coastal estuarine systems. This pattern potentially correlates with the difference and diversity of prey available between inland freshwater and coastal estuarine habitats. Increased consumption of a diverse array of prey was also correlated with increased nematode intensity in larger alligators. Parasitic nematodes Dujardinascaris waltoni, Brevimulticaecum tenuicolle, Ortleppascaris antipini, Goezia sp., and Contracaecum sp. were present in alligators from both habitat types. Dujardinascaris waltoni, B. tenuicolle, and O. antipini had a significantly higher abundance among inland inhabiting alligators than hosts from estuarine populations. Our findings also suggest that host specific nematode parasites of alligators may have evolved to infect multiple intermediate hosts, particularly fishes, crabs, and turtles, perhaps in response to the opportunistic predatory behaviors of alligators.
1. Introduction
The American alligator (Alligator mississippiensis) occupies a broad geographic range within the southeastern United States, where it inhabits freshwater swamps, wetlands, inland lakes, and rivers, in addition to coastal estuarine salt marshes and mangroves (Davis et al., 2005). The diversity of alligator habitats encompasses a broad range of prey that can directly influence the composition of parasites acquired via trophic transmission from prey hosts. Alligators are considered opportunistic generalist predators (Wolfe et al., 1987), whose food habits differ due to prey availability, sex, size, and the degree of individual specialization (Chabreck, 1971; Wolfe et al., 1987; Rootes et al., 1991; Delany et al., 1999; Gabrey, 2010; Rosenblatt et al., in press), however, the parasitic assemblage of the American alligator is similar across their distribution (Tellez, 2013). This contradicts a prevailing paradigm that parasitism of a definitive host species that is widely distributed should be heterogeneous due to variable abiotic and biotic factors, such as differential availability of suitable intermediate hosts (Marcogliese, 1997; Santoro et al., 2012). Explanations for the widespread similarity of alligator parasites include, (1) that the range of the intermediate hosts is congruent with that of the alligator definitive host, or (2) alligator parasites have evolved to infect multiple intermediate hosts across the range of habitats. If the latter is true, the use of various intermediate hosts may be an evolutionary response to the generalist foraging strategies and the diverse range of ecological communities inhabited by alligators, which is a common phenomenon among parasites of generalist predators (Anderson and Sukhdeo, 2011; Hatcher and Dunn, 2011; Sukhdeo and Hernandez, 2006). Such an adaptive pattern would create a mosaic of trophic interactions across the diverse habitats of the American alligator, while concurrently sustaining a similar assemblage of parasite species throughout the reptilian host's broad geographic distribution.
Unlike many other reptiles, stomach parasites of the American alligator are diverse and species rich (Aho, 1990; Tellez, 2010, 2013). Ten nematode genera, including 12 species, have been identified from alligators via stomach flushing or dissection (Tellez, 2010, 2013). Seven of the nematode species are identified as specific to crocodilians (Tellez, 2010, 2013). Although gastric nematodes of American alligators have been documented since 1819 (Rudolphi, 1819; Tellez, 2010, 2013), there is still relatively little known about the plausible intermediate hosts for the various parasite species (Gabrey et al., 2008; Waddle et al., 2009). Thus, to determine the plausible intermediate hosts and transmission pathways of alligator nematodes, the most basic procedure to conduct includes the exploration of the host's diet. Examination of stomach contents in correlation to parasite prevalence or abundance can provide essential information to identify intermediate hosts, in addition to the strength of connectivity between trophic links (Choudhury et al., 1996; Coman, 1972; Hatcher and Dunn, 2011; Knudsen et al., 2004; Petrić et al., 2011). Trophically transmitted parasites, such as alligator gastric nematodes, are potential biological indicators of food web connectance and of prey consumed over an extensive period of time (Doi et al., 2008; Johnson et al., 2004; Lafferty et al., 2008). Thus, the identification of intermediate hosts of alligator parasites could perhaps help evaluate alligator feeding behavior in the absence of prey content, and contribute further information about alligator–prey interactions in various habitats.
Here we examined gastric parasites of the American alligator from coastal estuarine and inland freshwater habitats in Florida and Georgia, and integrated prey content data to predict possible intermediate hosts among these populations. Since there is a lack of knowledge and data of the intermediate hosts used in the transmission pathways of alligator nematodes, we anticipated the comprehensive examination of parasitism and diet could help consolidate possible intermediate hosts. Even though the majority of alligator nematodes are ubiquitous across their host's range (Tellez, 2010, 2013), we expected a significant distinction of nematode abundance and species richness among inland freshwater and coastal estuarine alligator populations as a result of abiotic and biotic variation (i.e., salinity, water pH, prey diversity, etc.) between these aquatic environments, and differences in prey abundance. Secondly, we analyzed the relationship between alligator size and parasite abundance as well as species richness. Finally, we predicted that variation in environments and the opportunistic predatory behavior of the archaic reptilian host have influenced nematode species to evolve flexible life cycles over evolutionary time, causing the infection of multiple taxa as secondary intermediate hosts. Given that the study of crocodilian parasitism is still in its infancy, we anticipate the results from this study will establish a foundation of information on probable intermediate hosts to assist current and future researchers interested in the crocodilian–nematode dynamic.
2. Material and methods
2.1. Sample collection
Alligators (n = 48) were captured between May 2008 and August 2011 as a part of ongoing research in five locations (three coastal and two inland, Fig. 1). Estuarine coastal sites include Sapelo Island, Georgia (n = 11, May 2008–July 2009, 31.443483° N, 81.260272° W), Cape Canaveral (n = 8, April 2010, 28.499862° N, 80.601395° W), and Guana Lake, Florida (n = 5, July–August 2011, 30.080452° N, 81.340742° W). Inland sites include Lake Apopka (n = 12, April 2010, 28.626022° N, 81.625298° W) and Lake Woodruff (n = 12, April 2010, 29.097947° N, −81.417577° W), Florida. Alligators were captured using standard crocodilian capture techniques (Chabreck, 1963), and were subject to morphometric measurements and various tissue and fluid sampling techniques. In the case of 16 individuals captured in two coastal sites (n = 11, Sapelo Island and n = 5, Guana Lake) stomach contents were removed via gastric lavage (Fitzgerald, 1989). Stomach contents from all other individuals (n = 8, from Cape Canaveral and n = 24, from inland lakes) were removed during necropsy as a part of ongoing ecotoxicology and environmental contaminant studies. Collected contents were washed using fresh water to remove excess gastric enzymes then passed through a 1 mm mesh sieve (No. 18 USA Standard Test Sieve, Hogentogler and Co, Inc., Columbia, Maryland) to collect identifiable prey and gastric nematodes. Nematodes were collected from each alligator individual in our study (100% prevalence). Interestingly, 100% gastric parasitism among adult and large sub-adult alligators is a common phenomenon (Tellez, 2013, 2014; Tellez, unpublished data). The remaining portions were preserved in 70% ethyl alcohol until further processing. Stomach contents were separated first by broad taxonomic categories (e.g., vertebrates, invertebrates, vegetation) then identified to the lowest taxonomic subdivision possible, in many cases to the genus and/or species level. Numbers of individuals for each prey taxon were counted based on the presence of body portions specific to one individual for that particular taxon, e.g. the presence of an atlas bone for Actinopterygii, or the eye stalks for Decapoda. Gastric nematodes were placed in Petri dishes of glycerin alcohol for clearing, and identified via compound and stereo-microscopes.
Fig. 1.
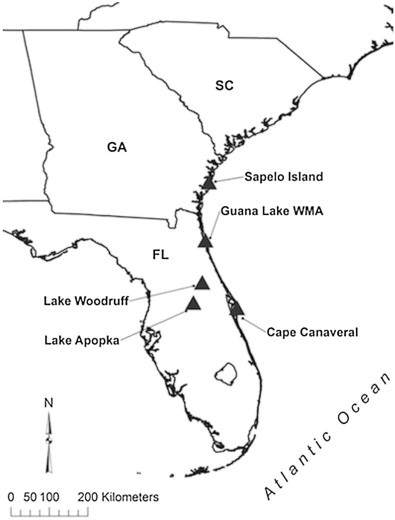
Collecting localities of alligators in Georgia and Florida.
It is pertinent to mention that given the time of data collection (May and August), it is possible that the particular prey contents gathered are not necessarily related to the nematodes collected simultaneously. However, some prey contents are slowly digested, possibly remaining in the alligator stomach for months prior to moving into the intestines (Garnett, 1984; Janes and Gutzke, 2002; Nifong et al., 2012). Thus, it is plausible that some of the nematodes collected in this study were transmitted by some of the prey identified. Additionally, the proportion and type of species consumed from 1986 to present among Florida and Georgia alligators in various habitats (which include some of our study sites) have remained relatively constant (Delany and Abercrombie, 1986; Delany, 1990, Delany et al., 1999; Shoop and Ruckdeschel, 1990; Barr, 1997; Rice, 2004; Nifong, 2014). Based on the findings cited earlier of prior alligator feeding ecology studies, we intend that our method of using diet data from alligators in our study is appropriate to estimate the general proportion and type of prey consumed by alligators. Concomitantly, we assume that correlating alligator diet data with parasite abundance is an appropriate method to predict intermediate hosts of alligator nematodes, particularly since the correlation of prey contents to parasite abundance is a fundamental strategy to determine intermediate hosts of parasite life cycles (Coman, 1972; Choudhury et al., 1996; Knudsen et al., 2004; Petrić et al., 2011; A. Kuris, pers. comm.).
All study techniques adhered to methods approved by the University of Florida and Institutional Animal Care and Use Committee (IACUC) (Protocol F-139 and 201005071) and Kennedy Space Center IACUC (Protocol GRD-06–044). All field collections were performed under Florida Fish and Wildlife (Scientific Collecting Permit No. SPGS-10-44R and SPGS-10-4) and Georgia Department of Natural Resources (Scientific Collecting Permit No. 29-WBH-08–178 and 29-WBH-09–56). Voucher specimens are deposited at the Manter Laboratory of Parasitology, Lincoln, Nebraska (Accession numbers: 68502–68507).
2.2. Statistical analyses
2.2.1. Variation of parasite abundance and species richness between inland freshwater and coastal estuarine alligator populations
To determine if parasite abundance and species richness significantly differed among coastal estuarine and inland freshwater alligator hosts, it was necessary to consider the SCA methods applied in obtaining nematode samples before statistically analyzing variation in parasitism between the two types of habitats. Although stomach flushing is a common method used in ecological and parasitological studies, not all stomach contents will be flushed out, particularly from large adult alligators. Examination of our raw data showed a lower average of mean parasite abundance among stomach flushed coastal estuarine alligators (n = 16) than necropsied coastal estuarine alligators (n = 8) or total necropsied alligators from both coastal estuarine and inland freshwater environments (n = 32). To control for this confounding variable, we first performed a negative binomial regression analysis to determine if location and a particular stomach content analysis method were significant predictor variables of nematode abundance. We also performed a negative binomial regression to determine if location and a particular SCA method were significant predictor variables of nematode species richness. Location and SCA method were associated with parasite abundance (intercept, p < 0.0001; location p = 0.03, SCA method, p < 0.001), and location was the only variable associated with parasite species richness (intercept, p < 0.01; location, p = 0.02; SCA method, p < 0.05). This suggests only location affects parasite species richness, and location and SCA method affect parasite abundance. As a result of the SCA method affecting parasite abundance, we performed a second negative binomial regression analysis using parasitic data from necropsy coastal estuarine and inland freshwater alligators to evaluate variation of parasite abundance between coastal estuarine and inland freshwater alligators. Variation of parasite species richness between the two habitats was examined with a Wilcox signed-rank test. Negative binomial regression analyses and Wilcox signed-rank test following the earlier protocol were also used to examine significant differences of identified nematode species between habitat types.
2.2.2. Correlation of alligator total length to parasite abundance and species richness, and consumption of taxonomic prey class
As alligator size is correlated with variation in prey types and also the amount of food consumed, and both can cause variation in trophically transmitted parasitism, Spearman rank correlation analyses were used to investigate the association of host size with parasite abundance and parasite species richness. A second series of Spearman rank correlation analyses examined the relationship between alligator size and taxonomic prey class to further propose the association between parasite abundance, and the proportion and type of prey consumed by larger alligators. We also performed Fisher's exact test to determine if there was a relationship between alligator size and habitat, as this could possibly result in false-positive results in the association of alligator size and parasitism, as well as the proportion and type of prey consumed.
2.2.3. Analysis of prey categories correlation to parasite abundance
Stomach contents of alligators were quantified and identified to lowest recognizable categories, family (n = 23), order (n = 18), or class (n = 10) (Table 1). Because the quantity of prey in each prey category was not normally distributed, we used non-parametric analyses to examine the relationship of prey categories to parasite abundance. Additionally, non-parametric tests were used to examine parasitism among hosts since parasites are aggregated among hosts (i.e., some hosts are heavily parasitized while other hosts are minimally or non-parasitized, creating an overdispersed distribution of parasitism). To identify probable intermediate hosts, a negative binomial regression was used to examine the relationship of parasite abundance (dependent variable) to prey categories (independent variables). We inferred that prey categories that have a strong relationship to parasite abundance were more likely candidates for alligator intermediate hosts. For this analysis, three negative binomial regressions were performed independently using taxonomic categories: family, order, class.
Table 1.
Taxonomic categories of prey items. Order and class are comprised of total number of prey items identified from that category. Prey contents identified only as Actinopterygii, Decapoda, Gastropoda, Insecta, Coeloptera, Mammalia, Rodentia, and Testudines marked (*) contained prey fragments identified to only these categories.
| Taxonomic categories | Total prey contents |
|---|---|
| Gastropoda (total) | 41 |
| Gastropoda* | 1 |
| Architaenioglossa | 40 |
| Ampullariidae | 40 |
| Annelida (total) | 1 |
| Hirudinea | 1 |
| Merostomata (total) | 30 |
| Xiphosura | 30 |
| Limulidae | 30 |
| Insecta (total) | 32 |
| Insecta* | 7 |
| Coeloptera | 18 |
| Coeloptera* | 15 |
| Carabidae | 1 |
| Dytiscidae | 2 |
| Hymneoptera | 3 |
| Odonata | 3 |
| Orthoptera | 1 |
| Malacostraca (total) | 182 |
| Decapoda | 182 |
| Decapoda* | 1 |
| Cambaridae | 156 |
| Menippidae | 1 |
| Palaemonidae | 14 |
| Panopeidae | 1 |
| Portunidae | 4 |
| Sesarmidae | 5 |
| Actinopterygii (total) | 58 |
| Actinopterygii* | 44 |
| Cyprinodontiformes | 5 |
| Cyprinodontidae | 4 |
| Poeciliidae | 1 |
| Perciformes | 3 |
| Centrarchidae | 1 |
| Sciaenidae | 2 |
| Mugiliformes | 1 |
| Mugilidae | 1 |
| Lepisosteifromes | 4 |
| Lepisosteidae | 4 |
| Siluriformes | 1 |
| Ariidae | 1 |
| Reptilia (total) | 11 |
| Crocodylia | 1 |
| Alligatoridae | 1 |
| Testudines | 7 |
| Testudines* | 6 |
| Kinosternidae | 1 |
| Squamata | 3 |
| Colubridae | 3 |
| Aves (total) | 13 |
| Mammalia (total) | 32 |
| Mammalia* | 14 |
| Carnivora | 7 |
| Procyonidae | 7 |
| Lagomorpha | 1 |
| Rodentia | 10 |
| Rodentia* | 4 |
| Muridae | 5 |
| Cricetidae | 1 |
2.2.4. Prediction of intermediate hosts of individual parasite species via negative binomial regression
Negative binomial regressions were also used to analyze the relationship of each particular nematode species to prey categories (independent variables) of likely intermediate hosts. We predicted that prey categories that have a strong relationship to a particular nematode species should comprise species that are likely intermediate hosts of alligator nematodes. For each analysis performed with a nematode species (dependent variable), three negative binomial regressions were performed independently of each other in accordance with taxonomic category (independent variables): family, order, class. Negative binomial regressions, Wilcox signed-rank test, Fisher's exact test, and Spearman rank correlations were performed with Program R 2.15.1 (R Development Core Team, 2012), and all tests were considered significant at p < 0.05. False discovery rates were calculated for each taxonomic category to analyze type I error of multiple comparisons. Statistical analyses performed in Sections 2.2.1–2.2.4 were instructed by the UCLA IDRE statistical consulting services.
2.2.5. Nematode and prey content species richness curves
Accumulation curves for prey content collected in estuarine and inland habitats, and nematode species were generated to evaluate sampling effectiveness by randomizing samples 1000 times using EstimateS 9.1.0 (Colwell, 2006).
3. Results
3.1. General data on component community of alligator gastric nematodes
A total of 17,199 nematodes comprised of five genera (Dujardinascaris waltoni, Brevimulticaecum tenuicolle, Ortleppascaris antipini, Goezia sp., Contracaecum sp.) from the families Ascarididae and Anisakidae were identified from both inland freshwater and coastal estuarine locations. The sampled nematode species accumulation curve reached its asymptote rapidly suggesting we collected all representative species of alligator gastric nematodes (Fig. 2). Overall, the comprehensive abundance and species richness of nematodes were highest among inland freshwater alligators relative to coastal estuarine populations (negative binominal regression, p = 0.04; W = 32, p = 0.004). Abundance of D. waltoni, B. tenuicolle, and O. antipini were found to be statistically higher among inland freshwater alligators relative to those inhabiting coastal estuarine habitats (negative binomial regression, p = 0.05; negative binomial regression, p = 0.05; W = 48, p = 0.017). Abundance of Contracaecum sp., and Goezia sp., did not statistically differ among habitats (W = 64, p = 0.07; W = 99, p = 0.83).
Fig. 2.
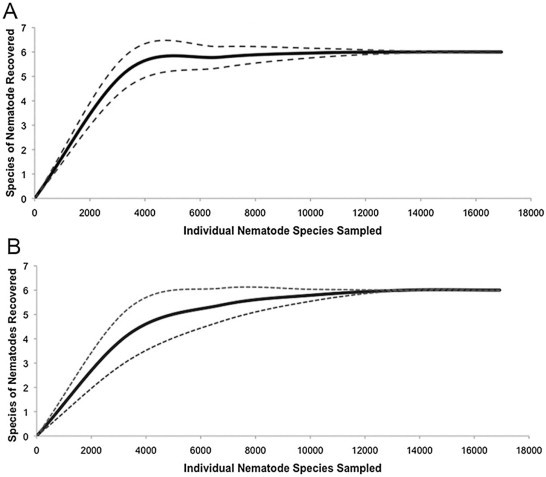
Nematode species richness accumulation curve (A) and Coleman rarefaction curve (B) based on 1000 randomizations using Estimate 9.1.0. The black broken line (- -) represents the upper 95% confidence level, and the broken dotted broken line (- ⋅ -) represents the lower 95% confidence level of the species accumulation curve (A). Upper and lower Coleman standard deviations are represented by solid black lines (B). Data obtained from stomach flushing or necropsy of American alligators from Florida and Georgia between 2008 and 2011. The rapid approach to the asymptote suggests we captured all possible species of alligator nematodes.
3.2. Correlation of alligator total length to parasitism and taxonomic prey class
A positive association between alligator length and the abundance of fish, birds, decapods, and reptiles consumed was found (statistical values summarized in Table 2). Alligator total length (TL) (mean ± SD, 254 ± 71 cm) ranged from 84 to 384 cm, spanning all size classes from juveniles (<0.9 m TL) to adults (>1.83 m TL). We found no significant association of parasite species richness with alligator size (p = 0.116, rs ≈ 0.229), however size was highly correlated with parasite abundance (p < 0.001, rs ≈ 0.592). Given the correlation of parasite abundance with host size, we then examined the association of alligator size to the abundance of the various species of nematodes. No relationship was evident for B. tenuicolle, O. antipini, Goezia sp., or Contracaecum sp. (p = 0.793, rs ≈ 0.04; p = 0.185, rs ≈ 0.195; p = 0.328, rs ≈ −0.144; p = 0.170, rs ≈ 0.201). However, the abundance of D. waltoni significantly increased and showed a strong association with alligator size (p < 0.001; rs ≈ 0.6).
Table 2.
Summary of Spearman rank correlations between alligator total length and taxonomic prey class.
| Taxonomic prey class | Spearman rank correlation coefficient |
|---|---|
| Actinopterygii | S = 13,195, p = 0.05, rs = 0.284 |
| Annelida | S = 20,461.06, p = 0.4544, rs = −0.111 |
| Aves | S = 11,525.53, p = 0.009, rs = 0.374 |
| Gastropoda | S = 14,130.05, p = 0.1109, rs = 0.233 |
| Insecta | S = 28,984.36, p < 0.001, rs = −0.573 |
| Malacostraca | S = 26,353.05, p = 0.002, rs = −0.43 |
| Mammalia | S = 21,863.23, p = 0.207, rs = −0.185 |
| Merostomata | S = 16,789.55, p = 0.549, rs = 0.089 |
| Reptilia | S = 10,545.68, p = 0.002, rs = 0.428 |
3.3. Accumulation curve for prey content
Five stomachs (10.4% of all samples), three from coastal habitats and two from inland freshwater lake alligators, contained no prey items. A species accumulation curve of coastal estuarine prey contents did not approach an asymptote (Fig. 3a). In this case, our data only provide a lower-bound estimate of species richness. The asymptote of the species accumulation curve of inland freshwater stomach contents suggests we collected the majority of possible prey of inland alligator populations (Fig. 3b). Further sampling of alligator stomachs should yield more species of alligator prey in both coastal estuarine and inland freshwater habitats. Overall, the majority of prey content occurrence from coastal estuarine alligators was comprised of invertebrates (89%), whereas prey content from inland freshwater alligators was mainly comprised of vertebrates (56%).
Fig. 3.
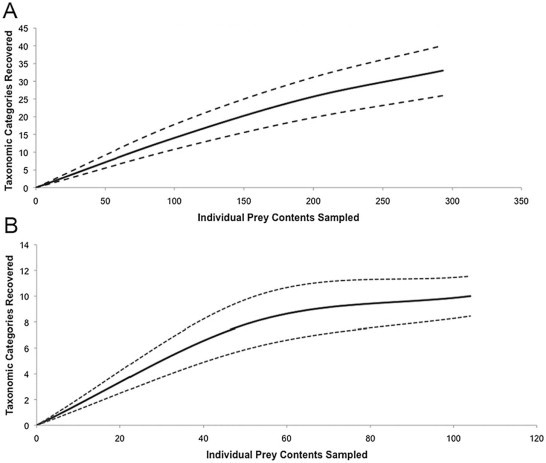
Prey content species richness accumulation curves based on 1000 randomizations using Estimate 9.1.0. Data obtained from stomach flushing or necropsy of American alligators from (A) coastal or (B) inland habitats between 2008 and 2011. The black broken line (- -) represents the upper 95% confidence level, and the broken dotted broken line (- ⋅ -) represents the lower 95% confidence level of the species accumulation curve. The slow approach to the asymptote in coastal habitats suggests prey contents of sampled alligators did not capture all probable prey. The asymptote of inland alligators slowly begins to plateau, which may suggest our sampling efforts were close to capturing most of the probable prey of alligators.
3.4. Identifying probable intermediate hosts of nematode species
In order to distinguish probable intermediate hosts of nematode species, we statistically analyzed prey contents at three taxonomic levels (family, order, class). Various taxonomic families were strong predictors of parasite abundance. Our model suggests that Poeciliidae has the strongest relationship with parasite abundance (p < 0.0001), followed by Panopeidae (p < 0.001), Portunidae (p = 0.002), Sesarmidae (p = 0.004), Colubridae (p = 0.003), Cyprinodontidae (p = 0.009), Procyonidae (p = 0.002), and Centrarchidae (p = 0.04). Taxonomic orders Decapoda, Testudines, Procyonidae, Rodentia, and Cyprinodontiformes also illustrated a strong relationship with parasite abundance (p = 0.008; p < 0.001; p = 0.002; p = 0.03; p < 0.001). In examining class, the most significant predictors of parasite abundance were Malacostraca (p = 0.002), Reptilia (p = 0.002), Mammalia (p = 0.004), and Actinopterygii (p = 0.05).
Several taxonomic families illustrated a strong relationship to Dujardinascaris waltoni. Poeciliidae (p < 0.001), Panopeidae (p < 0.001), Portunidae (p = 0.007), Sesarmidae (p = 0.007), Colubridae (p = 0.005), Cyprinodontidae (p = 0.01), Procyonidae (p = 0.008), and Centrarchidae (p = 0.05). Orders Cyprinodontiformes, Testudines, Procyonidae, Rodentia, and Decapoda were significant predictors of the presence of D. waltoni (p < 0.001; p = 0.003; p = 0.01; p = 0.05; p = 0.006). Classes Reptilia, Malacostraca, and Mammalia illustrated a strong relationship to the presence of D. waltoni (p = 0.001; p = 0.004; p = 0.007).
Brevimulticaecum tenuicolle only had a significant relationship with the order Testudines (p = 0.02), class categories Mammalia (p = 0.02), and Actinopterygii (p = 0.02). As for O. antipini, only order Decapoda showed a significant association with this particular stomach nematode (p = 0.002; p = 0.03). No significant interactions between Goezia, sp., Contracaecum sp., and ascarid larvae were found among any of the taxonomic categories (all p > 0.05). False discovery rates for the above significant statistical analyses are summarized in Table 3.
Table 3.
Summary of significant p values and their false discovery rate (FDR) for taxonomic categories in comparison to parasite intensity, parasite species richness. Dujardinascaris waltoni, Brevimulticaecum tenuicolle, and Ortleppascaris antipini. Prey contents identified only as Actinopterygii, Gastropoda, Insecta, Coeloptera, Mammalia, and Testudines marked (*) contained prey fragments identified to only these categories.
| p value | FDR | |
|---|---|---|
| Parasite intensity | ||
| Family | ||
| Centrarchidae | 0.04 | 0.07 |
| Colubridae | 0.003 | 0.008 |
| Cyprinodontidae | 0.009 | 0.02 |
| Dytiscidae | 0.04 | 0.07 |
| Panopeidae | 0.0002 | 0.002 |
| Poecillidae | <0.0001 | <0.001 |
| Portunidae | 0.002 | 0.008 |
| Procyonidae | 0.002 | 0.007 |
| Sesarmidae | 0.004 | 0.01 |
| Order | ||
| Carnivora | 0.002 | 0.003 |
| Cyprinodontiformes | <0.001 | 0.0004 |
| Decapoda | 0.002 | 0.03 |
| Rodentia | 0.03 | 0.07 |
| Testudines | <0.001 | 0.001 |
| Class | ||
| Actinopterygii | 0.05 | 0.11 |
| Malacostraca | 0.002 | 0.007 |
| Mammalia | 0.004 | 0.009 |
| Reptilia | 0.001 | 0.007 |
| Dujardinascaris waltoni | ||
| Family | ||
| Centrarchidae | 0.05 | 0.1 |
| Colubridae | 0.005 | 0.02 |
| Cyprinodontidae | 0.01 | 0.03 |
| Panopeidae | <0.001 | 0.003 |
| Poecillidae | <0.0001 | 0.001 |
| Portunidae | 0.007 | 0.02 |
| Procyonidae | 0.008 | 0.02 |
| Sesarmidae | 0.007 | 0.02 |
| Order | ||
| Carnivora | 0.01 | 0.03 |
| Cyprinodontiformes | <0.0001 | <0.001 |
| Decapoda | 0.006 | 0.02 |
| Rodentia | 0.05 | 0.1 |
| Testudines | <0.001 | 0.001 |
| Class | ||
| Malacostraca | 0.004 | 0.01 |
| Mammalia | 0.007 | 0.01 |
| Reptilia | 0.001 | 0.007 |
| Brevimulticaecum tenuicolle | ||
| Class | ||
| Actinopterygii | 0.02 | 0.06 |
| Mammalia | 0.03 | 0.06 |
| Order | ||
| Testudines | 0.02 | 0.2 |
| Ortleppascaris antipini | ||
| Order | ||
| Decapoda | 0.0312 | 0.3 |
4. Discussion
Understanding the trophic interactions of apex predators is an important goal for ecologists, as these species are known to exert strong top-down pressures on prey affecting community, as well as ecosystem structure and function (Duffy, 2002; Estes et al., 2011; Nifong and Silliman, 2013; Sergio et al., 2008). Alligators, in particular, serve as an interesting model species to study trophic interactions since alligators consume both terrestrial and aquatic prey. Given the influence parasites have on food web metrics and topology, the inclusion of alligator parasites in alligator feeding ecology studies could perhaps identify cryptic, yet influential, trophic links and nestedness among the local terrestrial and aquatic food web network (Arias-González and Morand, 2006; Lafferty et al., 2008; Thompson et al., 2005). Thus, in the event alligator parasites can successfully complete their life cycle using terrestrial and aquatic intermediate or paratenic hosts, the examination of alligator parasitism establishes a broader perspective of the food web network structure, and the interweaving of energy flow among the local terrestrial and aqueous ecosystems. In this study, we analyzed the prospect of alligator gastric nematodes evolving life-cycle pathways that included the use of multiple intermediate hosts. The use of multiple alligator prey as intermediate hosts could be a response to the variation of prey availability and abundance throughout the hosts' geographic distribution, in addition to the generalist foraging strategy of alligators. Because alligators are opportunistic predators, evolving to infect multiple prey species ensures successful completion of parasite transmission. Our results suggest multiple prey taxa, such as reptiles, crustaceans, and fish, are likely intermediate or paratenic hosts of alligator nematode parasites. These findings follow a trend of current crocodilian–parasite research, which illustrates a unique host–parasite relationship and patterns of distribution relative to fish, mammal, and other reptile host–parasite systems studied (Brooks, 1979; Huchzermeyer, 2003; Tellez, 2010; Tellez, 2013; Tellez, 2014; P. Martelli, pers. comm.).
4.1. Parasitism and alligator size
The opportunistic feeding behavior and diversity of prey species alligators consume expose these aquatic predators to a multitude of probable intermediate hosts, particularly as they grow in size. While the consumption of potential prey becomes more diverse as alligators grow in size (e.g., Wolfe et al., 1987, Gabrey, 2010), our study showed no variation of nematode species richness among sub-adults and adults. It is possible that nematode species richness reaches a threshold upon adult maturation as large sub-adults and adults may feed upon similar prey or intermediate hosts. In contrast to species richness, parasite abundance increased in accordance with alligator size. This phenomenon can potentially be explained by three factors. First, the higher consumption rate of larger alligators relative to smaller conspecifics likely elevates their rate of contact and feeding of intermediate hosts, thus preeminenting larger alligators to higher parasitic abundance. Secondly, the increase of gastric nematode abundance in our study, and analogous to previous studies (Delany et al., 1999; Goldberg et al., 1991; Tellez, 2014), parallels the onset of large sub-adult alligators broadening their diet. Thus, perhaps the increase of stomach nematode abundance reflects the expansion and opportunistic feeding behavior of alligators throughout maturation (i.e., the increase predation of reptiles, birds, fish, and decapods simultaneously), which emulates the use of multiple transmission pathways (i.e., various intermediate hosts) by parasites. Finally, the larger niche space of adult alligators allows a greater quantity of nematodes to access a common resource than stomachs from smaller alligators, which can lessen resource competition among parasites (Combes, 2001). Based on the earlier findings, we suggest the increase of nematode abundance parallels the augmentation of opportunistic feeding behavior of alligators throughout maturation. Therefore, examination of nematode parasitism could perhaps be a valuable tool to evaluate the ontogenetic dietary shifts among alligator individuals.
4.2. Parasitism between inland freshwater and coastal estuarine alligator populations, and identification of intermediate hosts
In this study, parasitism appeared to correlate to the consumption and proportion of vertebrate vs. invertebrate prey among inland freshwater and coastal estuarine alligators (Fig. 4). The larger portion of inland freshwater population diets consisted of fishes and higher vertebrate species, such as turtles, which our data suggest are the primary, vertebrate intermediate hosts of inland freshwater and coastal estuarine alligator populations. For example, our data suggest fishes may be primary obligate intermediate hosts of B. tenuicolle and D. waltoni. It is possible these nematode species share the same intermediate hosts. However, evolving to infect different intermediate hosts prevents competition of a similar resource, and ensures successful parasite transmission (Combes, 2001). Although we found no significant relationship with taxonomic fish orders or families to consolidate the plausible intermediate hosts of B. tenuicolle, our data suggest cyprinodonts (order Cyprinodontiformes, families Poeciliidae and Cyprinodontidae) are a good predictor of D. waltoni. Based on the few identifiable species of cyprinodonts from prey stomach contents, future experimental life cycles should primarily include mosquitofish (Gambusia affinis) and mummichog (Fundulus heteroclitus). The distribution of both these species is sympatric with the geographic range of alligators, which could explain the widespread distribution of D. waltoni (Tellez, 2010).
Fig. 4.
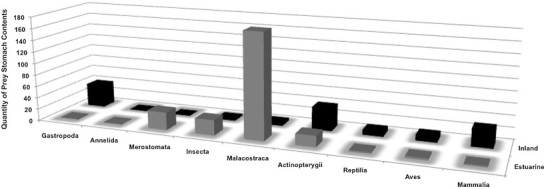
Prey stomach contents categorized to taxonomic class levels.
Besides fish, turtles (order Testudines) seem to be strong predictors of parasite intensity, and the presence of D. waltoni. Gabrey et al. (2008) considered it unlikely that reptiles function as intermediate hosts of the heavily fish-based diet of alligators. Yet, certain ascarid species utilize reptiles as obligate intermediate hosts (Kinne, 1985; Sprent, 1954). Furthermore, it is conceivable that the taxa used in life cycles vary given the stochasticity of prey abundance, and heterogeneity of environments inhabited by alligators throughout their distribution. For example, the large portion of fish, as well as blue crab, preyed upon by Louisiana alligators implies their preeminent position as intermediate host candidates in this region. However, as turtles make up a considerable portion of the diet of inland freshwater alligator populations in Florida (Delany and Abercrombie, 1986; Delany et al., 1999), we postulate alligator stomach nematodes have evolved to utilize turtles to successfully complete their life cycle. Larvae of Dujardinascaris spp. have been found in other reptiles, such as lizards and snakes (Bursey et al., 2005). As colubrids are not a consistent source of prey among alligators, particularly in comparison to turtles (Delany and Abercrombie, 1986; Delany et al., 1999; Rice, 2004), it is possible snakes are paratenic hosts of D. waltoni.
Paratenic hosts are a common phenomena among ascarid life cycles (Bush et al., 2001). From the perspective of the parasite, the inclusion of paratenic hosts is a favorable endeavor to ensure transmission success, particularly when the definitive host is an opportunistic predator. Mammals are known paratenic hosts of Dujardinascaris spp., Brevimulticaecum spp., and Ortleppascaris spp. (Anderson, 2000; Sprent, 1954; Villegas and González-Solís, 2009). Thus, the strong association between mammals with D. waltoni and B. tenuicolle in our study is most likely a result of their role as paratenic hosts for alligator gastric parasites. Overall, mammals represent a small portion of the prey biomass of alligators (Delany, 1990; Delany and Abercrombie, 1986; Delany et al., 1999; Nifong and Silliman, 2013; Rice, 2004). Thus, the use of various paratenic and intermediate vertebrate hosts is a common phenomenon among life cycles of ascarid species (Anderson, 2000; Bush et al., 2001), and may be a particularly favorable adaptive trait among alligator ascarids given the generalist predatory behavior of their definitive host.
In comparison to inland freshwater populations, the majority of the prey biomass of coastal estuarine inhabiting alligators are largely made up of invertebrates (Nifong and Silliman, 2013). Shrimp, crayfish, and horseshoe crabs are not associated intermediate hosts for these nematode genera (Anderson, 2000). However, crabs have been associated to the presence of Dujardinascaris spp. (Arya, 1980; Villegas and González-Solís, 2009; Tellez, unpublished data). Our data suggest a strong association with decapods, particularly crustaceans from the families Portunidae, Panopeidae, and Sesarmidae, to parasite abundance and the presence of D. waltoni. Similar to that of fish, crabs make up a large percentage of the American alligators' diet in juveniles, sub-adults, and adults throughout the host's geographic distribution (Delany and Abercrombie, 1986; Delany et al., 1999; Nifong and Silliman, 2013; Platt et al., 1990; Rice, 2004; Valentine et al., 1972), and have a strong role in the life cycle transmission of Dujardinascaris spp. (Arya, 1980; Villegas and González-Solís, 2009). Dujardinascaris petterae, a sister species to the alligator stomach nematode D. waltoni, was found infecting a crab in Africa (Tellez, unpublished data). Additionally, D. waltoni has been the only nematode species found in juvenile alligator stomachs in Louisiana (Tellez, unpublished data). Given the earlier field data, and statistical results from this study, we suggest future investigations of the life cycle of D. waltoni should include crabs.
In general, the large percent volume of invertebrate (particularly non-brachyuran) prey correlates to the lower stomach nematode abundance among coastal estuarine alligators. Based on the earlier results, we postulate that the higher abundance and predation of vertebrate prey strongly influence the greater abundance of nematode parasitism among inland freshwater alligators relative to coastal estuarine individuals.
4.3. Correlation of Goezia sp. and Contracaecum sp. to prey categories
Based on our data, Goezia sp. and Contracaecum sp. were not strongly associated with alligator prey. Neither of these two nematode species has been regularly identified from the stomach of alligators (Deardorff and Overstreet, 1979; Tellez, 2010, 2013). Both Goezia spp. and Contracaecum spp. are considered common parasites of fish-eating birds and marine mammals (Anderson, 2000; Sprent, 1954), thus it is plausible that infection among alligators is “accidental.”
4.4. Conclusion
In summary, alligator nematode abundance of inland freshwater alligators was higher than coastal estuarine alligators, which could reflect selective predation or variation of intermediate host availability between habitats. Variation in diet also seems to influence difference of nematode abundance among sizes of alligators as parasite intensity increases with alligator size in relation to ontogenetic diet shifts. Our data also suggest alligator stomach nematodes have evolved flexible life cycles in response to the opportunistic predatory behavior of alligators and geographic variation of prey abundance by infecting multiple intermediate hosts, particularly fishes, turtles, and crabs. The concept of crocodilian nematodes evolving flexible life cycles that include the use of multiple secondary hosts is not a novel theory (Huchzermeyer, 2003), yet conjectures of secondary hosts have primarily focused on fish (Gabrey et al., 2008; Hazen et al., 1978; Scott et al., 1999). The dissimilarity of diet and intermediate hosts throughout the alligator range may have generated the adaptive radiation of multiple transmission pathways of gastric nematodes. For example, it is possible D. waltoni has coevolved to infect turtles in Florida in contrast to Louisiana in response to the selective feeding habits of Florida alligators. Given the growing interest in crocodilian parasitology, we intend that our statistical findings can facilitate a foundation for future experiments of alligator nematode life cycles as a result of the consolidation of probable intermediate hosts, as well as assist researchers interested in all faucets of alligator parasite life cycles, particularly since there is a lack of research and knowledge in this host–parasite system. Further exploration of nematode life cycles could essentially reveal tightly linked trophic relationships between host specific nematodes, and their archaic host, as well as provide further insight on the evolutionary role of alligator nematodes in food webs.
Acknowledgements
We would like to thank Rachel L. Nifong, Nicholas Govsyeyev, Evan Whiting, Cameron Carter, Patrick Delany, Russ H. Lowers, Lou J. Guillette Jr., Arnold Brunell, Joan Ramos, and Ali Hagihghi for assistance during various aspects of this work. We would also like to acknowledge Donald Buth, Armand Kuris, Val Lance, Sherie Morrison, and Jamie Lloyd-Smith for their editorial contributions, and UCLA IDRE Statistical Consulting group for their statistical assistance. This research was conducted under the NSF-Graduate Research Fellowship Program Award No. DGE-0946816, IUCN/SSC-Crocodile SRAS, Chicago Herpetological Society Graduate Student Research in Herpetology Award, and an award from the Estuarine Reserves Division, Office of Ocean and Coastal Resource Management, National Ocean Service, National Oceanic and Atmospheric Administration, Award No. NA10NOS4200022 and Georgia Coastal Ecosystems LTER program under Grant Nos. OCE-0620959 and OCE-1237140.
References
- Aho J.M. Helminth communities of amphibians and reptiles: comparative approaches to understanding patterns and processes. In: Esch G.W., Bush A.O., Aho J.M., editors. Parasite Communities: Patterns and Processes. Chapman and Hall; New York: 1990. pp. 157–195. [Google Scholar]
- Anderson R.C. 2nd ed. CABI Publishing; London: 2000. Nematode Parasites of Vertebrates. [Google Scholar]
- Anderson T.K., Sukhdeo M.V.K. Host centrality in food web networks determines parasite diversity. PLoS ONE. 2011;6:1–9. doi: 10.1371/journal.pone.0026798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-González J.E., Morand S. Trophic functioning with parasites: a new insight for ecosystem analysis. Mar. Ecol. Prog. Ser. 2006;320:43–53. [Google Scholar]
- Arya S.N. On the larval forms of a nematode Dujardinascaris cybii Arya and Johnson, 1978. Rev. Bras. Biol. 1980;40:751–753. [Google Scholar]
- Barr, B.R., 1997. Food habits of the American alligator, Alligator mississippiensis, in the southern Everglades. PhD dissertation, University of Miami: ProQuest/UMI. (Publication No. 3472).
- Brooks D.R. Testing hypotheses of evolutionary relationships among parasites: the digeneans of crocodilians. Am. Zool. 1979;19:1225–1238. [Google Scholar]
- Bursey C.R., Goldberg S.R., Parmelee J.R. Gastrointestinal helminthes from 13 species of lizards from Reserva Cuzco Amazónico, Peru. Comp. Parasitol. 2005;72:50–68. [Google Scholar]
- Bush A.O., Fernandez J.C., Esch G.W., Seed J.R. Cambridge University Press; Cambridge: 2001. Parasitism: The Diversity and Ecology of Animal Parasites. [Google Scholar]
- Chabreck R.H. Methods of capturing, marking and sexing alligators. Proc. SE. Assoc. Game and Fish Comm. 1963;17:47–50. [Google Scholar]
- Chabreck R.H. The food and feeding habits of alligators from fresh and saline environments in Louisiana. Proc. SE. Assoc. Game and Fish Comm. 1971;25:117–124. [Google Scholar]
- Choudhury A., Bruch R., Dick T.A. Helminths and food habits of Lake Sturgeon Acipenser fulvescens from the Lake Winnebago System, Wisconsin. Am. Midl. Nat. 1996;135:274–282. [Google Scholar]
- Colwell R.K. EstimateS: statistical estimation of species richness and shared species from samples. Version 8.0.0. 2006. http://purl.oclc.org/estimates User's Guide and application published at: accessed 15.02.2013.
- Coman B.J. Helminth parasites of the dingo and feral dog in Victoria with some notes on the diet of the host. Aust. Vet. J. 1972;48:456–461. doi: 10.1111/j.1751-0813.1972.tb02281.x. [DOI] [PubMed] [Google Scholar]
- Combes C. University of Chicago Press; Chicago and London: 2001. Parasitism. The Ecology and Evolution of Intimate Interactions. [Google Scholar]
- Davis S.M., Childers D.L., Lorenz J.J., Wanless H.R., Hopkins T.E. A conceptual model of ecological interactions in the mangrove estuaries of the Florida Everglades. Wetlands. 2005;25:832–842. [Google Scholar]
- Deardorff T.L., Overstreet R.M. Goezia lacerticola sp. n. (Nematoda: Anisakidae) in Alligator mississippiensis from Florida. J. Helminthol. 1979;53:317–320. [Google Scholar]
- Delany M.F. Late summer diet of juvenile American alligators. J. Herpetol. 1990;24:418–421. [Google Scholar]
- Delany M.F., Abercrombie C.L. American alligator food habits in northcentral Florida. J. Wildlife. Manage. 1986;50:348–353. [Google Scholar]
- Delany M.F., Linda S.B., Moore C.T. Diet and condition of American alligators in 4 Florida lakes. Proc. SE. Assoc. Game and Fish Comm. 1999;53:375–389. [Google Scholar]
- Doi H., Yurlova N.I., Vodyanitskaya S.N., Kikuchi E., Shikano S., Yadrenkina E.N. Parasite-induced changes in nitrogen isotope signatures of host tissues. J. Parasitol. 2008;94:292–295. doi: 10.1645/GE-1228.1. [DOI] [PubMed] [Google Scholar]
- Duffy J.E. Biodiversity and ecosystem function: the consumer connection. Oikos. 2002;99:201–219. [Google Scholar]
- Estes J.A., Terborgh J., Brashares J.S., Power M.E., Berger J., Bond W.J. Trophic downgrading of planet Earth. Science. 2011;333:301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- Gabrey S., Franklin K., Bodri M. Preliminary investigation into the use of logistic regression to predict parasite intermediate hosts. Case study: Dujardinascaris waltoni (Nematoda: Ascarididae) in the American alligator (Alligator mississippiensis) Geor. J. Sci. 2008;66:87–95. [Google Scholar]
- Gabrey S.W. Demographic and geographic variation in food habits of American alligators (Alligator mississippiensis) in Louisiana. Herpetol. Conserv. Biol. 2010;5:241–250. [Google Scholar]
- Garnett S.T. The consequences of slow chitin digestion on crocodilian diet analyses. J. Herpetol. 1984;19:303–304. [Google Scholar]
- Goldberg S.R., Bursey C.R., Aquino-Shuster A.L. Gastric nematodes of the Paraguayan caiman, Caiman yacare (Alligatoridae) J. Parasitol. 1991;77:1009–1011. [PubMed] [Google Scholar]
- Hatcher M.J., Dunn A.M. Cambridge University Press; Cambridge and New York: 2011. Parasites in Ecological Communities: From Interactions to Ecosystems. [Google Scholar]
- Hazen T.C., Aho J.M., Murphy T.M., Esch G.W., Schmidt G.D. The parasite fauna of the American alligator (Alligator mississippiensis) in South Carolina. J. Wild. Dis. 1978;14:435–439. doi: 10.7589/0090-3558-14.4.435. [DOI] [PubMed] [Google Scholar]
- Huchzermeyer F.W. CABI Publishing; London: 2003. Crocodiles: Biology, Husbandry, and Diseases. [Google Scholar]
- Janes D., Gutzke W.H.N. Factors affecting retention time of turtle scutes in stomachs of American alligators, Alligator mississippiensis. Am. Midl. Nat. 2002;148:115–119. [Google Scholar]
- Johnson M.W., Hesslein R.H., Dick T.A. Host length, age, diet, parasite and stable isotopes as predictors of yellow perch (Perca flavescens Mitchill), trophic status in nutrient poor Canadian Shield lakes. Environ. Biol. Fishes. 2004;71:379–388. [Google Scholar]
- Kinne O. Biologische Anstalt Helgoland; Hamburg: 1985. Diseases of marine animals, V. IV, Part II. [Google Scholar]
- Knudsen R., Curtis M.A., Kristoffersen R. Aggregation of helminths: the role of feeding behavior of fish hosts. Jnl. Parastitol. 2004;90:1–7. doi: 10.1645/GE-3184. [DOI] [PubMed] [Google Scholar]
- Lafferty K.D., Allesina S., Arim M., Briggs C.J., de Leo G., Dobson A.P. Parasites in food webs: the ultimate missing links. Ecol. Lett. 2008;11:533–546. doi: 10.1111/j.1461-0248.2008.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcogliese D.J. Food webs and the transmission of parasites to marine fish. Parasitol. 1997;124:S83–S99. doi: 10.1017/s003118200200149x. [DOI] [PubMed] [Google Scholar]
- Nifong, J., 2014. Use of marine habitat and food resources by coastal inhabiting Alligator mississippiensis (American alligator): implications for food web and community dynamics. PhD dissertation, University of Florida, Gainesville.
- Nifong J., Silliman B. Impacts of a large-bodied, apex predator (Alligator mississippiensis Daudin 1801) on salt marsh food webs. J. Exp. Mar. Biol. Ecol. 2013;440:185–191. [Google Scholar]
- Nifong J., Rosenblatt A.E., Johnson N., Barichivich W., Silliman B.R., Heithaus M.R. American alligator digestion rate of blue crabs and its implications for stomach contents analysis. Copeia. 2012;3:419–423. [Google Scholar]
- Petrić M., Mladineo I., Sifner S.K. Insight into the short-finned squid Illex coindetti (Cephalopoda: Ommastrephidae) feeding ecology: is there a link between helminth parasites and food composition? J. Parasitol. 2011;97:55–62. doi: 10.1645/GE-2562.1. [DOI] [PubMed] [Google Scholar]
- Platt S.G., Brantley C.G., Hastings R.W. Food habits of juvenile American alligators in the upper Lake Ponchartrain estuary. NE. Gulf Science. 1990;11:123–130. [Google Scholar]
- R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2012. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Rice, A.N., 2004. Diet and condition of American Alligators (Alligator mississippiensis) in three central Florida Lakes. Master's Thesis, University of Florida.
- Rootes W.L., Chabreck R.H., Wright V.L., Brown B.W., Hess T.J. Growth rates of American alligators in estuarine and palustrine wetlands. Estuaries. 1991;14:489–494. [Google Scholar]
- Rudolphi K.K. Sumtibus Augusti Rucker; Berlin, Germany: 1819. Entozoorum synopsis cui accedunt mantissa duplex et indices locupletissimi. [Google Scholar]
- Santoro M., Mattiucci S., Nascetti G., Kinsella J.M., Di Prisco F., Troisi S. Helminth communities of owls (Strigiformes) indicate strong biological and ecological differences from birds of prey (Accipitriformes and Falconiformes) in southern Italy. PLoS ONE. 2012;7:e53375. doi: 10.1371/journal.pone.0053375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott T.P., Simicik S.R., Craig T.M. A key to some pentastome, nematode, and trematode parasites of the American alligator. Tex. J. Sci. 1999;51:127–138. [Google Scholar]
- Sergio F., Caro T., Brown D., Clucas B., Hunter J., Ketchum J. Top predators as conservation tools: ecological rationale, assumptions, and efficacy. Annu. Rev. Ecol. Evol. Syst. 2008;39:1–19. [Google Scholar]
- Shoop C.R., Ruckdeschel C.A. Alligators as predators on terrestrial mammals. Am. Midl. Nat. 1990;124:407–412. [Google Scholar]
- Sprent J.F.A. The life cycles of nematodes in the family Ascarididae Blanchard 1896. Jnl. Parasitol. 1954;40:608–617. [PubMed] [Google Scholar]
- Sukhdeo M.V.K., Hernandez A.D. Food web patterns and the parasite's perspective. In: Thomas F., Renaud F., Guégan J.-F., editors. Parasitism and Ecosystems. Oxford University Press Inc.; New York: 2006. [Google Scholar]
- Tellez, M., 2010. Crocodilians and their parasites: a database and evaluation of ecological and evolutionary effects of this host-parasite dynamic. Master's Thesis. University of California, Los Angeles.
- Tellez M. UC Press; Berkeley, California: 2013. A Checklist of Host-Parasite Interactions of the Order Crocodylia. [Google Scholar]
- Tellez, M., 2014. Alligator parasitism- the mysterious frontier unfolded: exploration of the ecological interaction between an archaic predator (Alligator mississippiensis) and its parasites. PhD dissertation, University of California, Los Angeles: ProQuest/UMI. (Publication No. 12328).
- Thompson R.M., Mouritsen K.N., Poulin R. Importance of parasites and their life cycle characteristics in determining the structure of a large marine food web. J. Anim. Ecol. 2005;74:77–85. [Google Scholar]
- Valentine J.M., Jr., Walther J.R., McCartney K.M., Ivy L.M. Alligator diets on the Sabine National Wildlife Refuge, Louisiana. J. Wildl. Manage. 1972;36:809–815. [Google Scholar]
- Villegas A., González-Solís D. Gastrointestinal helminth parasites of the American crocodile (Crocodylus acutus) in southern Quitana Roo, Mexico. Herpetol. Conserv. Biol. 2009;4:346–351. [Google Scholar]
- Waddle A.R., Kinsella J.M., Ross J.P., Rojas-Flores E., Percival H.F., Forrester D.J. Nematodes collected by gastric lavage from live American alligators, Alligator mississippiensis, in Florida. J. Parasitol. 2009;95:1237–1238. doi: 10.1645/GE-1989.1. [DOI] [PubMed] [Google Scholar]
- Wolfe J.L., Bradshaw D.K., Chabreck R.H. Alligator feeding habits: new data and a review. NE. Gulf Sci. 1987;9:1–8. [Google Scholar]


