Abstract
Objective
To test the association between docosahexaenoic acid (DHA)supplementation and perceived stress and cortisol response to a stressor during pregnancy in a sample of African American women living in low-income environments.
Methods
Sixty-four African American women were enrolled at 16–21 weeks of gestation. Power calculations were computed using published standard deviations for the Perceived Stress Scale and the Trier Social Stress Test. Participants were randomized to either 450 mg per day of DHA(n=43) or placebo (n=21).At baseline, 24, and 30 weeks of gestation, perceived stress was assessed by self-report. Cortisol response to a controlled stressor, the Trier Social Stress Test (TSST) was measured from saliva samples collected upon arrival to the laboratory and after the completion of the TSST.
Results
Women in the DHA supplementation group reported lower levels of perceived stress at 30 weeks of gestation, controlling for depression and negative life events (mean = 27.4 versus 29.5, (F [3, 47] = 5.06, p = .029, cohen’s d = .65). Women in the DHA supplementation had lower cortisol output in response to arriving to the laboratory and a more modulated response to the stressor (F [1.78, 83.85] = 6.24, p = .004, cohen’s d = .76).
Conclusions
Pregnant women living in urban low-income environments who received DHA reported reduced perceived stress and lower levels of stress hormones in the third trimester. DHA supplementation may be a method for attenuating the effects of maternal stress during late pregnancy and improving the uterine environment with regard to fetal exposure to glucocorticoids.
Introduction
Consistent with the prenatal programming hypothesis,1 there is now evidence from multiple studies using a variety of methodologies and across different species that the mother’s level of psychosocial stress during pregnancy is significantly associated with suboptimal developmental outcomes in their offspring including disturbances in attention,2–3 impaired learning and disruption in neurogenesis,4–5 and increased anxiety-like behaviors.2 The strength of the causal claim is based on rigorous controlled experiments in which the prenatal effect is distinguished from postnatal effects by using methods such as cross-fostering or nursery rearing. The pattern of findings in humans closely mirrors those from controlled animal studies.6–9 The strongest candidate for the mechanism by which prenatal stress confers risk to the offspring is the maternal hypothalamic-pituitary-adrenal (HPA) axis,. Prenatal stress causes long-term alterations in the functioning of the offspring’s HPA axis,10–11 and each of the phenotypic outcomes identified above can be linked with disruptions in the HPA axis.
A few investigators have examined the effects of DHA supplementation during pregnancy on later developmental functioning. Neuroprotective effects of DHA supplementation during pregnancy on the offspring has been reported in controlled animal studies12–14 In humans, fatty acid supplementation is also associated with reductions in stress reactivity in controlled studies.15–18 These data converge to support the hypothesis that prenatal DHA supplementation among women living in stressful environments would lead to reductions in perceived stress and greater modulation of the activation of the HPA axis in response to stress.
Materials and Methods
Pregnant women were recruited from Obstetrics Clinics within the University of Pittsburgh Medical Center from 2010–2012. Sample size was determined based on power calculations using existing data on perceived stress and cortisol response to the Trier Social Stress Test (TSST) among pregnant women. Given the goals of the study, only demographically eligible women were approached for screening. Demographic eligibility included: Medicaid insurance or Medicaid eligible, African American race, age between 20 and 30 years, and 16–21 weeks of gestation.
In addition to African American women being disproportionately represented among families living in inner-city poverty in the U.S. and having higher rates of suboptimal birth outcomes,19 there are race differences in cortisol reactivity to stress and pregnancy.24–26 In order to control for these group differences either adequate numbers of participants of different races would need to be included or the study would have to be limited to a single race. Given the scope of the study, we chose to study African Americans only. We limited the sample to ages 20–30, which comprises over 60% of pregnancies among African American.27 This range excluded younger and older ages of mothers during which the risk for suboptimal pregnancy outcomes increases.
Power calculations were computed using published data: the standard deviation (SD) of perceived stress using the Perceived Stress Scale was estimated to be 7.4.28 Forty participants in the supplement group and 20 in the placebo group yielded 80% power to detect a difference in stress levels of approximately 4 between the two groups using a two-sided test at the 0.05 significance level. The SD of the cortisol response to the TSST at 20 minutes was estimated to the equal to 8 nmol/L.29 Forty participants in the supplement group and 20 in the placebo group yielded 80% power to detect a difference in peak response of approximately 6 nmol/l between the two groups using a two-sided test at the 0.05 significance level.
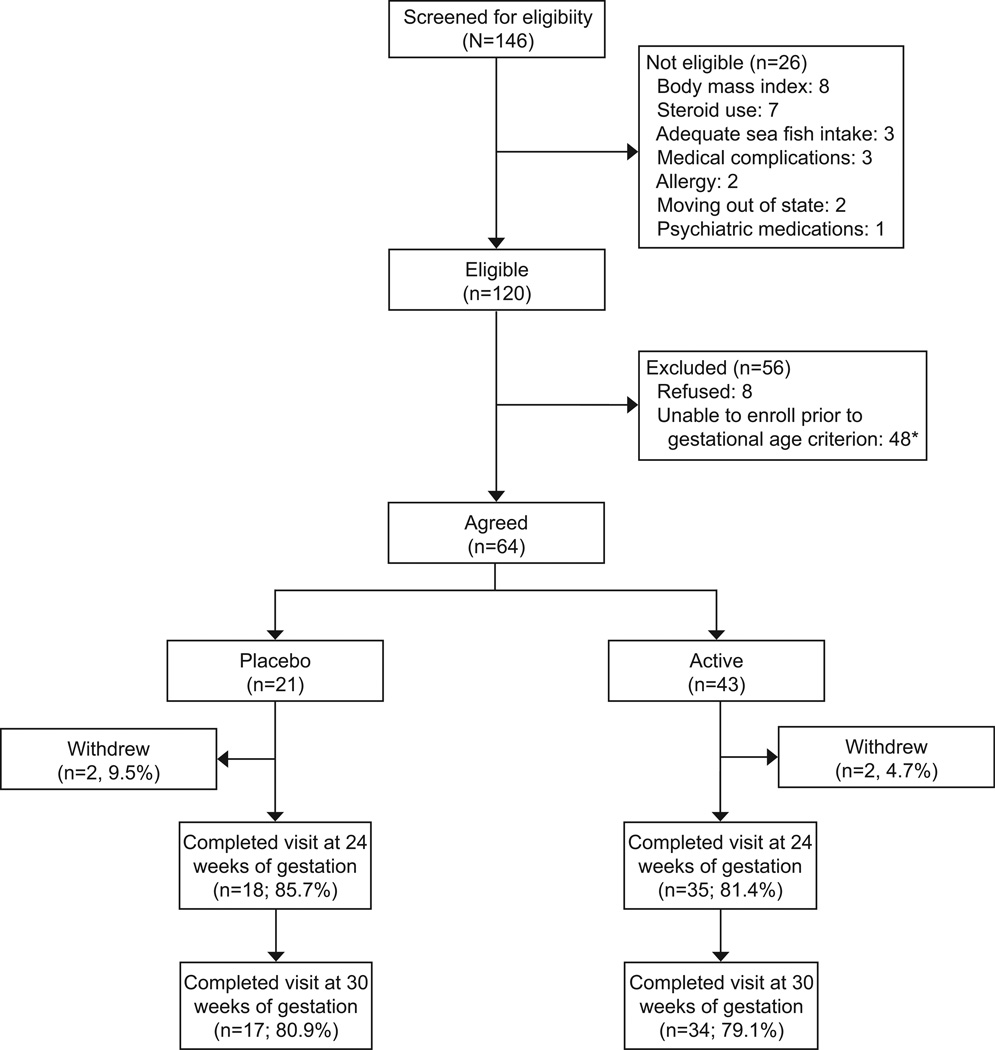
Women were recruited using two methods. First, research assistants attended obstetric clinics and provided fliers to patients that listed the demographic inclusion criteria (i.e., African American race, Medicaid insurance, gestational age between 16 and 21 weeks, and maternal age between 20 and 30 years) asking those eligible to complete the screening. Second, demographically eligible patients identified through electronic medical records were contacted by mail and phone to assess interest in the study. Telephone screenings were then conducted to assess eligibility. Exclusion criteria included2 or more servings of sea fish per week, known medical complications (gestational diabetes, pre-eclampsia, subchorionic hematoma), regular use of steroid medications, regular alcohol use, cigarettes or use of illegal substances (by maternal report), use of blood thinners or anti-coagulants, use of psychotropic medications, BMI >40, and allergy to iodine or soy. One hundred forty-six participated in screening for eligibility. Of those screened, 26 were ineligible, 64 were eligible and enrolled, 48 were eligible at time of screening but could not be enrolled prior to the enrollment window (i.e., 16–21 weeks of gestation), and 8 refused to participate (see Figure 1).
Figure 1.
Nutrition and Pregnancy Study participation rates. *Eligible at time of screening; but, unable to schedule baseline within gestational age criterion.
Participants were reimbursed on an accelerated schedule with $40 for their first visit and an increase in payments of $10 for each subsequent visit. The Institutional Review Boards of the University of Chicago and University of Pittsburgh approved all study procedures. This study also was a registered clinical trial (NCT01158976).
Once enrolled, women were randomly assigned on a 2:1 ratio to receive the omega-3 nutritional supplement (n = 43) or a corn and soybean oil placebo (n = 21) beginning at enrollment and up through the end of pregnancy. We expected greater variability in the dependent measures (e.g., stress reactivity) among the experimental participants than the control patients. Thus, in order to optimize power to test the hypotheses, we enrolled a higher number of participants in the experimental group to adequately capture that variability. Women randomized to the study group received the supplement via two gel capsules providing: 450 mg of DHA; 40 mg of docospentaenoic acid and eicosatetranoic acid; 90 mg of eicosapentaenoic acid; and 15 IU of vitamin E,, supplied by Nordic Naturals. Women receiving the placebo received two capsules supplied by Nordic Naturals that were matched in size, color, and smell to the study drug. The pharmacist at the University of Pittsburgh used computer generated random assignment of ID numbers to active supplement or placebo in blocks of nine: six ID numbers were randomized to active supplement and three to placebo. In order to maintain the double-blind, the pharmacist divided each randomization block of nine ID numbers into groups of three: three ID numbers were assigned to group A (placebo) and the remaining six were assigned to either group Bor C, both of which received identical doses of active supplement. This approach allowed the pharmacist to randomize on a 2:1 ratio without having the unbalanced design break the blind. Once the study was complete, the blind was broken and the two groups receiving identical doses of active supplement were combined for analyses.
Cortisol response to a social evaluative stressor was measured at baseline, 24 and 30 weeks of gestation. Participants completed questionnaires covering stressful life events, perceived stress, and symptoms of depression at baseline, 24, and 30 weeks of gestation. Research assistants contacted participants by phone 3 times per week to ask the time of day that the supplement was taken, and gathered data on perception of taste, and possible gastrointestinal side effects to increase compliance.
Symptoms of depression were assessed using the Edinburgh Postnatal Depression Scale,30 a 10-item measure designed to assess pre- and postnatal depression without confounding the assessment of depression with somatic symptoms of pregnancy (e.g., weight gain, loss of energy). Internal consistency of in this sample was high: alpha = .88 at baseline; .86 at 24 weeks; .87 at 30 weeks. The Difficult Life Circumstances Scale31 is a set of 28 yes-no questions about difficult circumstances at home or work that may be a problem for the primary caregiver. The measure was designed to include items that would be applicable to women living in poverty such as difficulty with finances and housing. The internal consistency of the scale as measured by alpha ranged from .73 to .80. The Perceived Stress Scale32 is a 14-item scale designed to measure the degree to which situations in one’s life are appraised as stressful. The internal consistency of the scale as measured by alpha was high: .85 at baseline; .88 at 24 weeks;.87 at 30 weeks.
Following published recommendations for assessing physiological stress reactivity during pregnancy,33 the Trier Social Stress Test (TSST)34 was used as a psychological stressor. The TSST procedure consists of a two-minute preparation time, followed by a 5-minute speech (as if for a job interview), and then a 5-minute mental arithmetic task. The latter two tasks are performed in front of a video camera and an audience. The TSST typically elicits individual differences in cortisol reactivity, even during pregnancy.29,35
Saliva was collected at three time points at the baseline, 24 and 30 week assessments: 20 minutes following arrival to the lab, and 20, 45 minutes post-TSST. To collect saliva samples, an absorbent, unflavored dental roll was applied to the tongue, cheek, and gums for several minutes. The dental roll was then placed in a labeled plastic salivette. Samples were immediately transferred to a freezer and stored at −20°C until assayed. On the day of testing, samples were thawed, centrifuged at 3,000 rpm for 10 minutes allowing for a clear sample to be pipetted into appropriate test wells. All samples from each subject were assayed in the same batch to minimize variability, and assayed with reagents from the same lot. Samples with sufficient saliva were assayed in duplicate using the Salimetrics HS Salivary Cortisol EIA Kit for unbound cortisol. This assay has a lower limit of sensitivity from .007 to 1.2 µg/dL. The average between-assay variance is 3.9% and 7.1%, and the average within-assay variance is 6.7% and 6.9% for high and low concentrations, respectively. The correlation between saliva and serum using the Salimetrics HS Salivary Cortisol EIA Kit and the Coat-a-Count Serum Cortisol RIA kit is .96, p < .0001. Analyses were conducted with log10-transformed cortisol values, but are presented as untransformed µg/dL for ease of interpretation.
Results
Of the 64 participants who completed the baseline assessment, four (6.3%) withdrew from the study: two participants from each study arm. Two participants randomized to placebo withdrew due to adverse events: one miscarried and the other withdrew due to mood changes. Two participants randomized to active supplement withdrew due to adverse events: one reported headaches and the other upset stomach. Forty-seven participants (73.4%) attended all three sessions. Descriptive statistics are presented in Table 1 for depression, negative life events and perceived stress at baseline, 24, and 30 weeks of gestation. Data collected from all participants, including those who withdrew, are included in the analyses. Baseline self-report data for one participant in the active group was lost. One participant, with undetectable cortisol values for five of the nine samples, was not included in the analyses of cortisol response.
Table 1.
Descriptive statistics for depression, negative life events, and perceived stress scores at baseline, 24 weeks, and 30 weeks for total sample and the active and placebo groups
| Baseline | 24 weeks | 30 weeks | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total N = 63 Mean (SD) |
Active N = 20 Mean (SD) |
Placebo N = 43 Mean (SD) |
Total N = 53 Mean (SD) |
Active N = 35 Mean (SD) |
Placebo N = 18 Mean (SD) |
Total N = 51 Mean (SD) |
Active N = 34 Mean (SD) |
Placebo N = 17 Mean (SD) |
|
| Depression | 12.97 (4.9) | 13.05 (5.3) | 12.80 (4.2) | 12.92 (4.8) | 13.3 (5.1) | 12.11 (4.1) | 11.86 (4.9) | 12.12 (4.8) | 11.35 (5.1) |
| Negative Events | 5.03 (3.4) | 5.16 (3.4) | 4.75 (3.6) | 4.08 (3.5) | 4.57 (3.9) | 3.11 (2.4) | 3.80 (0.51) | 3.91 (0.63) | 3.59 (0.89) |
| Perceived stress | 28.16 (2.4) | 27.77 (2.2) | 29.00 (2.7) | 27.94 (3.0) | 28.06 (3.5) | 27.72 (2.0) | 28.08 (3.4) | 27.47 (3.4) | 29.29 (3.1) |
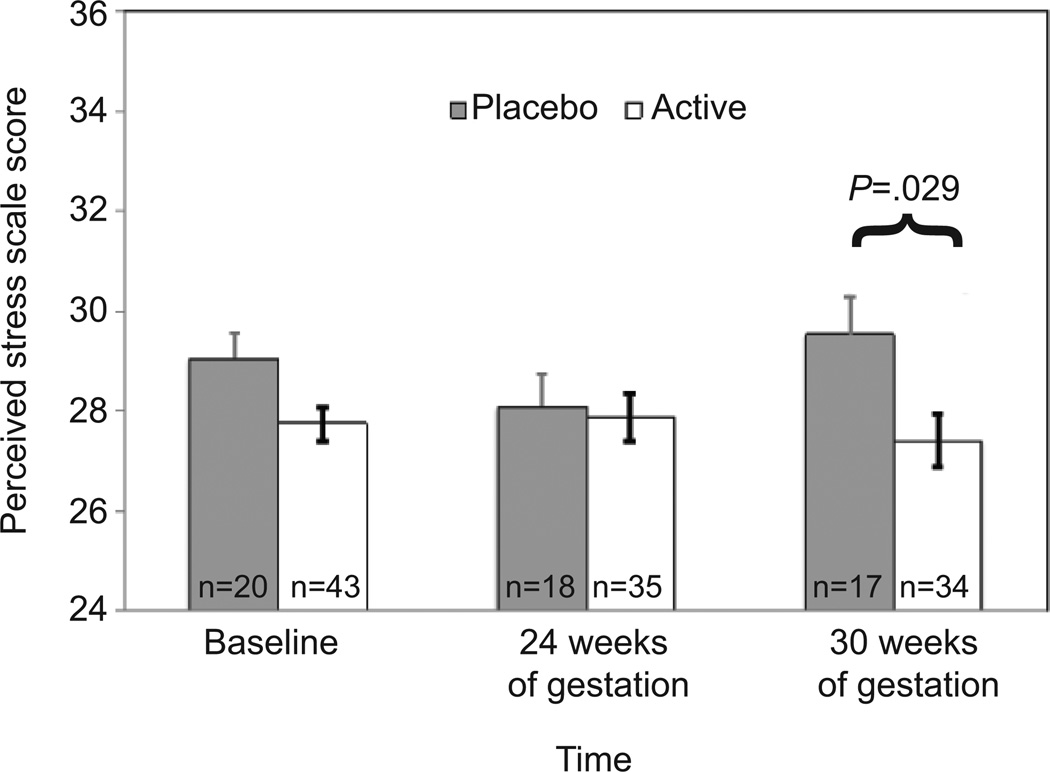
The first goal was to examine group differences in level of depression, negative life events, and perceived stress by conducting analyses of variance for each measure, controlling for the other two measures. At baseline and 24 weeks of gestation, there were no group differences in any of the three measures. At 30 weeks, perceived stress was significantly lower among the participants receiving supplementation (mean = 27.4) compared to those receiving placebo (mean = 29.5), controlling for negative life events and depression scores at 30 weeks (F [3, 47] = 5.06, p = .029, cohen’s d = .65 (Figure 2), a difference of more than half of a standard deviation for the sample.
Figure 2.
Effect of omega-3 supplementation on perceived stress score, controlling for negative life events and depression scores.
Cortisol response to the TSST was examined as a function of group status (supplementation versus placebo) at baseline, 24 and 30 weeks of gestation by repeated measures analysis of variance, using a Greenhouse-Geisser correction to account for lack of sphericity. Time of day was significantly associated with initial cortisol levels at baseline, but not at 24 and 30 weeks; time of day was controlled in analyses involving cortisol levels at baseline.
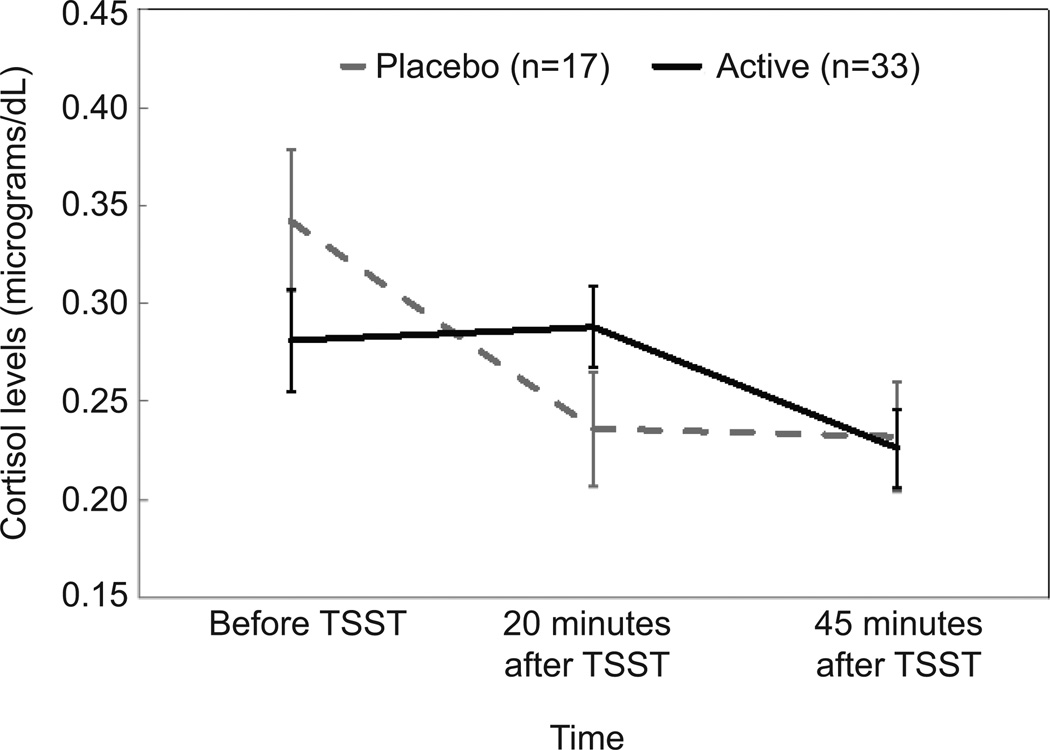
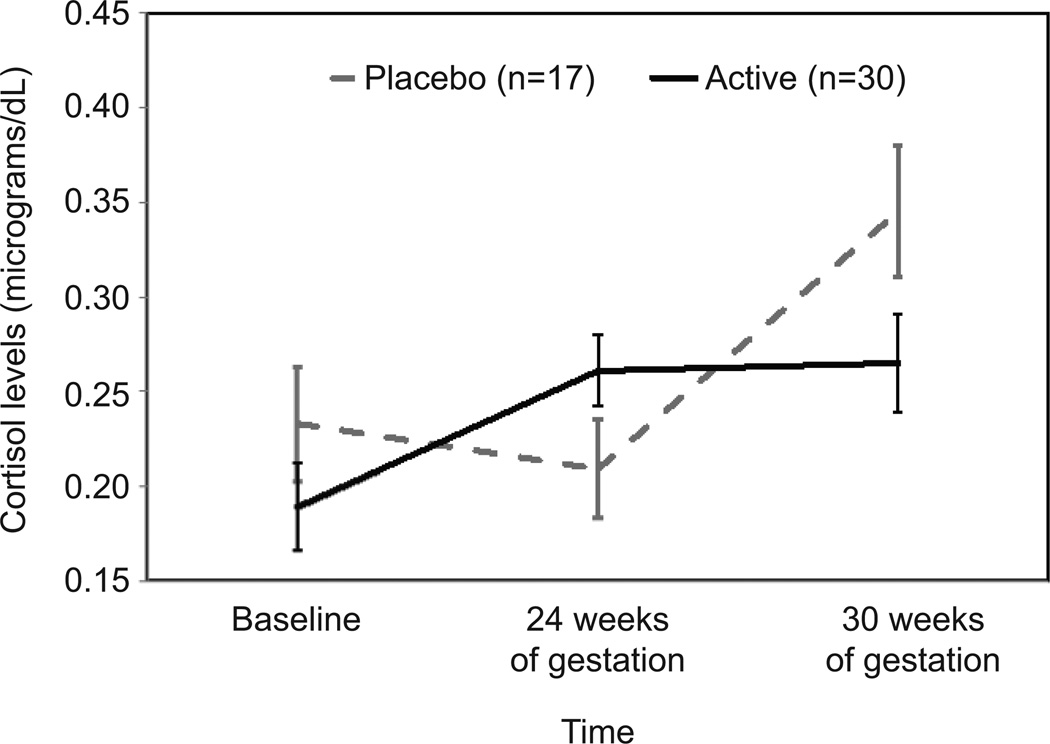
Cortisol levels before and after the TSST did not vary as a function of supplementation at baseline or at 24 weeks. At 30 weeks, cortisol levels over time significantly differed as a function of supplementation (F [1.78, 83.85] = 6.24, p = .004, cohen’s d = .76). As shown in Figure 3, women who received placebo had higher levels of cortisol upon arrival to the lab compared to women receiving omega-3 supplementation. Levels for women receiving placebo were characterized by a relatively steep decline, whereas levels for women receiving omega-3 supplement evidenced a slight increase in response to the stressor, on average, followed by decline during the period of recovery. To further probe the differences in levels upon arrival to the laboratory, the two groups were compared at baseline, 24 weeks, and 30 weeks of gestation. As shown in Figure 4, cortisol levels upon arrival to the lab were similar for the two groups at baseline, but diverged over time such that by 30 weeks of gestation women who received placebo had levels that were on average 20% higher than women receiving supplement (mean = 0.35 versus 0.27) (F [1.74, 74.63] = 3.51, p = .041, cohen’s d = .56).
Figure 3.
Cortisol levels before and after the Trier Social Stress Test (TSST) at 30 weeks of gestation. F(1.78, 83.85) =6.24, P=.004; cohen’s d=.76; error bars indicate standard error at each time point within each group.
Figure 4.
Cortisol levels 20 minutes following arrival to laboratory at baseline, at 24 weeks of gestation, and at 30 weeks of gestation. F(1.74, 74.63), P=.041; cohen’s d=.56; error bars indicate standard error at each time point within each group.
Discussion
The present study was conducted from the perspective of the prenatal programming hypothesis.1 Specifically, a primary hypothesized mechanism by which prenatal stress affects offspring development is through exposure of the fetus to high levels of glucocorticoids released by the mother. This exposure, in turn, affects the fetal stress architecture in part by adjusting the set point for mounting a stress response and interfering with the feedback mechanisms for maintaining homeostasis. Although the postpartum environment continues to affect brain development, it is plausible that this initial insult sets the stage for a poor developmental trajectory that begins with poorly modulated response to stress in infancy.
Results from the present study provide preliminary evidence that changes in prenatal nutrition may be one way to interrupt the suboptimal prenatal programming of the fetus developing in the context of high levels of maternal stress. Pregnant women living in high stress, low-income, urban environment who received a fatty acid supplement reported lower levels of perceived stress than women who received placebo, despite a lack of change in exposure to stressors. Moreover, the reduction of perceived stress was independent of depression, which was not associated with fatty acid supplementation. The lack of an association between DHA supplementation and depression symptoms is consistent with the literature on perinatal depression. In several randomized controlled trials, DHA was not associated with depression scores,36–37 but supplementation did appear to enhance the efficacy of psychopharmacologic treatment for depression,38 suggesting that systems associated with depressed mood and affect neurotransmission, such as the HPA-axis, are impacted by fatty acid supplementation.
In fact, evidence from the present study suggests that DHA supplementation does affect the functioning of the HPA-axis by modulating response to a social stressor. This was demonstrated primarily via a lower cortisol response to arriving at the laboratory. By 30 weeks of gestation, participants who received DHA supplementation had lower levels of cortisol upon arrival to the laboratory, and on average demonstrated a slight increase in cortisol in response to the stressor, a pattern typically observed late in pregnancy.29,33 In contrast, participants who had received placebo evidenced high levels of cortisol upon arrival to the laboratory, followed by a steep decrease in cortisol levels during and after exposure to the TSST. One interpretation of this pattern is that an exaggerated response to anticipatory stress leads to a less flexible response to the stressor. The high levels of cortisol upon arrival to the lab followed by a lack of responsiveness to the stressor has been described as a pattern of dysregulated HPA axis functioning associated with depression both in adults39 and children.40 Lack of cortisol response to a social stressor also has been observed among adults reporting high levels of early life adversity, especially females.41 DHA supplementation appears to protect pregnant women living in low-income environments from manifesting this type of dysregulation.
Demonstrating associations between of fatty acid supplementation and lower prenatal stress, however, is not sufficient. The next step will be to test the hypothesis that alterations in maternal stress levels via fatty acid supplementation improves developmental outcomes in the offspring. Data from other studies support the testing of this hypothesis. For example, Bolten and colleagues35 reported that cortisol reactivity to the TSST at 32–34 weeks of gestation, but not basal cortisol levels, was associated with infant self-regulation. Similarly, Werner and colleagues42 found that infants of women with high levels of maternal cortisol 25 minutes after arrival to the lab during late gestation were more likely to be classified as “high reactive”(i.e. more motor activity and crying) in response to novel stimuli.
Thus, several important links among prenatal stress, cortisol levels, and infant neurodevelopment have been demonstrated. Data from our preliminary study are compelling in terms of the potential impact on health disparities in maternal and infant health, a significant public health problem that is poorly understood. The findings, however, are in need of replication. In addition, a number of limitations should be noted. First, the small sample size and attrition of participants over time likely impact the reliability of the findings, and thus replication and timing of effects need to be explored in a larger study population. Although retention across study visits was comparable for the two groups, there may have been some differential selection bias across the two groups. Second, given the preliminary nature of the study, we did not control for multiple testing, and of the 13 tests of significance, we could expect that one may be due to chance. The effect sizes for the significant results were medium to large, however, providing some indication that the results are not spurious. Third, we relied on maternal report of uptake as opposed to a rigorous assessment of blood levels of fatty acids across pregnancy. In addition to the possibility that uptake varied, blood levels may have varied among individuals with the same level of supplementation due to other dietary factorsor individual differences in metabolism. Fourth, although we did not re-administer the TSST after 30 weeks, it would be important to examine whether differences in cortisol levels as a function of supplementation are maintained. Moreover, in the context of improving infant outcomes, it may be important to supplement earlier in gestation. The third trimester is a period of fetal development that may be particularly sensitive to prenatal stress given the increase in maternal cortisol and decrease in placental 11β-HSD2, yet exposure earlier in gestation may also confer risk.43
In conclusion, the results reported here extend earlier work on the association between fatty acid supplementation and improved obstetric outcomes, and complement existing research on prenatal stress and offspring neurodevelopment by providing preliminary evidence for nutritional moderation of prenatal stress.
Acknowledgments
Supported by National Institutes of Health grant R21 HD058269 to Dr. Keenan and by the University of Chicago Institute for Translational Medicine (UL1 TR000430). Nordic Naturals provided the nutritional supplement and placebo.
The authors thank Joelle Tighe and Suzanne Pierce for their assistance in recruitment and data collection, Kristen Wroblewski for statistical consultation, and pharmacist Ray Cefola, who conducted the randomization.
Abbreviations
- DHA
docosahexaenoic acid
- TSST
Trier Social Stress Test
- HPA-Axis
hypothalamic pituitary adrenal axis
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Contributor Information
Kate Keenan, Department of Psychiatry and Behavioral Neuroscience, University of Chicago
Alison E. Hipwell, Department of Psychiatry, University of Pittsburgh
Jenna Bortner, Department of Psychiatry, University of Pittsburgh
Amy Hoffmann, Department of Psychiatry, University of Pittsburgh
Rose McAloon, Department of Psychiatry, University of Pittsburgh
References
- 1.Barker DJ. Intrauterine programming of adult disease. Mol Med Today. 1995;1:418–423. doi: 10.1016/s1357-4310(95)90793-9. [DOI] [PubMed] [Google Scholar]
- 2.Schneider ML, Moore CF, Kraemer GW, Roberts AD, DeJesus OT. The impact of prenatal stress, fetal alcohol exposure, or both on development: Perspectives from a primate model. Psychoneuroendocrinology. 2002;27:285–298. doi: 10.1016/s0306-4530(01)00050-6. [DOI] [PubMed] [Google Scholar]
- 3.Keenan K, Bartlett TQ, Nijland M, Rodriquez J, Nathanliez P, Zurcher N. Poor nutrition during pregnancy and lactation negatively impacts neurodevelopment of the offspring: Evidence from a translational primate model. Amer J Clin Nutrition. 2013;98:396–402. doi: 10.3945/ajcn.112.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapillon P, Patin V, Roy V, Vincent A, Caston J. Effects of pre-and postnatal stimulation on developmental, emotional, and cognitive aspects in rodents: A review. Devel Psychobiol. 2002;41:373–387. doi: 10.1002/dev.10066. [DOI] [PubMed] [Google Scholar]
- 5.Coe CL, Lulbach GR, Schneider ML. Prenatal disturbance alters the size of the corpus callosum in young monkeys. Devel Psychol. 2002;41:178–185. doi: 10.1002/dev.10063. [DOI] [PubMed] [Google Scholar]
- 6.Huizink AC, Robles de Medina PG, Mulder EJH, Visser GHA, Buitelaar JK. Psychological measures of prenatal stress as predictors of infant temperament. J Amer Acad Child Adolesc Psychiatry. 2002;41:1078–1085. doi: 10.1097/00004583-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, Golding J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369:578–585. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- 8.Kohlboeck G, Glaser C, Tiesler C, Demmelmair H, Standl M, Romanos M, Koletzko B, Lehmann I, Heinrich J LISA plus Study Group. Effect of fatty acid status in cord blood serum on children's behavioral difficulties at 10 y of age: results from the LISA plus Study. Am J Clin Nutr. 2011;94:1592–1599. doi: 10.3945/ajcn.111.015800. [DOI] [PubMed] [Google Scholar]
- 9.Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics. 2003;111:39–44. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- 10.Henry C, Kabbaj M, Simon H, Le Moal M, Maccari S. Prenatal stress increases the hypothalamic–pituitary–adrenal axis response to stress in young and adult rats. J Endocrinol. 1994;6:341–345. doi: 10.1111/j.1365-2826.1994.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 11.Weinstock M. Does prenatal stress impair coping and regulation of hypothalamic-pituitary-adrenal axis? Neuroscience Biobehav Rev. 1997;21:1–10. doi: 10.1016/s0149-7634(96)00014-0. [DOI] [PubMed] [Google Scholar]
- 12.Sable PS, Dangat KD, Joshi AA, Joshi SR. Maternal omega 3 fatty acid supplementation during pregnancy to a micronutrient-imbalanced diet protects postnatal reduction of brain neurotrophins in the rat offspring. Neuroscience. 2012;217:46–55. doi: 10.1016/j.neuroscience.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Jayashankar S, Glover CN, Folven KI, Brattelid T, Hogstrand C, Lundebye AK. Cerebral gene expression and neurobehavioural responses in mice pups exposed to methylmercury and docosahexaenoic acid through the maternal diet. Environ Toxicol Pharmacol. 2012;33:26–38. doi: 10.1016/j.etap.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Del Bigio MR, Weiler HA. Maternal arachidonic acid supplementation improves neurodevelopment in young adult offspring from rat dams with and without diabetes. Prostaglandins Leukot Essent Fatty Acids. 2011;84:63–70. doi: 10.1016/j.plefa.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Maes M, Christophe A, Bosmans E, Lin A, Neels H. In humans, serum polyunsaturated fatty acid levels predict the response of proinflammatory cytokines to psychologic stress. Biol Psychiatry. 2000;47:910–920. doi: 10.1016/s0006-3223(99)00268-1. [DOI] [PubMed] [Google Scholar]
- 16.Hellhammer J, Hero T, Franz N, Contreras C, Schubert M. Omega-3fatty acids administered in phosphatidylserine improved certain aspects of high chronic stress in men. Nutr Res. 2012;32:241–250. doi: 10.1016/j.nutres.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Yehuda S, Rabinovitz S, Mostofsky DI. Mixture of essential fatty acids lowers test anxiety. Nutritional Neuroscience. 2005;8:265–267. doi: 10.1080/10284150500445795. [DOI] [PubMed] [Google Scholar]
- 18.Delarue J, Matzinger O, Binnert C, Schneiter P, Chiolero R, Tappy L. Fish oil prevents the adrenal activation elicited by mental stress in healthy men. Diabetes Metabolism. 2003;29:289–295. doi: 10.1016/s1262-3636(07)70039-3. [DOI] [PubMed] [Google Scholar]
- 19.Giscombé CL, Lobel M. Explaining disproportionately high rates of adverse birth outcomes among African Americans: The impact of stress, racism, and related factors in pregnancy. Psychol Bull. 2005;131:662–683. doi: 10.1037/0033-2909.131.5.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev Sci. 2007;10:464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 21.Reiss F. Socioeconomic inequalities and mental health problems in children and adolescents: A systematic review. Soc Sci Med. 2013:24–31. doi: 10.1016/j.socscimed.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Stark KD, Beblo S, Murthy M, Buda-Abela M, Janisse J, Rockett H, Whitty JE, Martier SS, Sokol RJ, Hannigan JH, Salem N. Comparison of bloodstream fatty acid composition from African-American women at gestation, delivery, and postpartum. J Lipid Res. 2005;4:516–525. doi: 10.1194/jlr.M400394-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Kent ST, McClure LA, Zaitchik BF, Gohlke JM. Area-level risk factors for adverse birth outcomes: Trends in urban and rural settings. BMC Pregnancy Childbirth. 2013;13:129. doi: 10.1186/1471-2393-13-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J Adol Health. 2007;41:3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Glynn LM, Schetter CD, Chicz-DeMet A, Hobel CJ, Sandman CA. Ethnic differences in adrenocorticotropic hormone, cortisol and corticotropin-releasing hormone during pregnancy. Peptides. 2007;28:1155–1161. doi: 10.1016/j.peptides.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Wilcox S, Bopp M, Wilson DK, Fulk LJ, Hand GA. Race differences in cardiovascular and cortisol responses to an interpersonal challenge in women who are family caregivers. Ethnic Dis. 2005;15:17–24. (2005). [PubMed] [Google Scholar]
- 27.Martin JA, Hamilton BE, Ventura SJ, Osterman MJK, Matthews TJ. National vital statistics reports. 1. Vol. 62. Hyattsville, MD: National Center for Health Statistics; 2013. Births: Final data for 2011. [PubMed] [Google Scholar]
- 28.Young DR, He X, Genkinger J, Sapun M, Mabry I, Jehn M. Health status among urban African American women: Associations among well-being, perceived stress, and demographic factors. J Behav Med. 2004;27:63–76. doi: 10.1023/b:jobm.0000013644.74404.02. (2004). [DOI] [PubMed] [Google Scholar]
- 29.Nierop A, Bratsikas A, Klinkenberg A, Nater UM, Zimmermann R, Ehlert U. Prolonged salivary cortisol recovery in second-trimester pregnant women and attenuated salivary alpha-amylase responses to psychosocial stress in human pregnancy. J Clin Endocrin Metabol. 2006;91:1329–1335. doi: 10.1210/jc.2005-1816. (2006). [DOI] [PubMed] [Google Scholar]
- 30.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 31.Barnard KE, Johnson S, Booth CL, Bee H. Difficult Life Circumstances Scale. Seattle: NCAST Publications; 1989. [Google Scholar]
- 32.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Social Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 33.de Weerth C, Wied CC, Jansen LM, Buitelaar JK. Cardiovascular and cortisol responses to a psychological stressor during pregnancy. Acta Obstet Gynecol Scand. 2007:1–12. doi: 10.1080/00016340701547442. [DOI] [PubMed] [Google Scholar]
- 34.Kirschbaum C, Pirke KM, Hellhammer DH. The Trier Social Stress Test: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 35.Bolten M, Nast I, Skrundz M, Stadler C, Hellhammer DH, Meinlschmidt G. Prenatal programming of emotion regulation: neonatal reactivity as a differential susceptibility factor moderating the outcome of prenatal cortisol levels. J Psychosom Res. 2013;75:351–357. doi: 10.1016/j.jpsychores.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 36.Marangell LB, Martinez JM, Zboyan HA, Kertz B, Seung Kim HF, Puryear LJ. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Amer J Psychiatry. 2003;160:996–998. doi: 10.1176/appi.ajp.160.5.996. [DOI] [PubMed] [Google Scholar]
- 37.Makrides M, Gibson RA, McPhee AJ, Yelland L, Quinlivan J, Ryan P. DOMInO Investigative Team. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: A randomized controlled trial. JAMA. 2010;304:1675–1683. doi: 10.1001/jama.2010.1507. [DOI] [PubMed] [Google Scholar]
- 38.Deligiannidis KM, Freeman MP. Complementary and alternative medicine for the treatment of depressive disorders in women. Psychiatr Clin North Am. 2010;33:441–463. doi: 10.1016/j.psc.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki H, Belden AC, Spitznagel E, Dietrich R, Luby JL. Blunted stress cortisol reactivity and failure to acclimate to familiar stress in depressed and sub-syndromal children. Psychiatry Res. 2013;210:575–583. doi: 10.1016/j.psychres.2013.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lovallo WR. Early life adversity reduces stress reactivity and enhances impulsive behavior: implications for health behaviors. Int J Psychophysiol. 2013;90:8–16. doi: 10.1016/j.ijpsycho.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Werner E, Zhao Y, Evans L, Kinsella M, Kurzius L, Altincatal A, McDonough L, Monk C. Higher maternal prenatal cortisol and younger age predict greater infant reactivity to novelty at 4 months: an observation-based study. Dev Psychobiol. 2013;55:707–718. doi: 10.1002/dev.21066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81:131–148. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]