Abstract
Chronic wounds are rising in prevalence and creating significant socioeconomic burdens for patients and healthcare systems worldwide. Therefore, it is now more important than ever that clinicians follow evidence-based guidelines for wound care when developing personalized treatment plans for their patients with chronic wounds. Evidence-based guidelines for treating venous leg ulcers, diabetic foot ulcers, and pressure ulcers, the 3 main categories of chronic wounds, focus primarily on biologic therapies. However, there are also evidence-based guidelines for treating behavioral risks to poor healing, such as smoking, which should be incorporated into treatment plans when appropriate. The purpose of this article was to review the mechanisms through which smoking adversely impacts the wound healing process, and propose strategies for incorporating evidence-based guidelines for treating tobacco dependence into treatment plans for patients with chronic wounds who smoke.
Keywords: chronic wound healing, evidence-based practice, smoking, tobacco dependence treatment
Introduction
Chronic wounds create extensive economic and social burdens on our global society. It has been estimated that in the United States alone, chronic wounds affect 6.5 million people resulting in annual treatment costs up to $25 billion.1–4 Furthermore, in developed countries, approximately 1% to 2% of the population is expected to experience a chronic wound during their lives.5 Chronic wounds are associated with advancing age, obesity, and diabetes mellitus; their incidence and associated costs are expected to increase dramatically over time.6 Considering the vast financial, social, and clinical impact of chronic wounds, it is essential that clinicians treating patients with these conditions consider all modifiable factors that may delay wound healing including smoking.
Cigarette smoking negatively impacts wound healing on multiple levels.7–10 As a result, chronic wound patients who continue to smoke should be encouraged to quit and provided tools by healthcare providers to assist with that process. Existing evidence suggests that smokers are more successful with abstinence when presented with repeated personalized messages urging them to quit along with assistance in their attempts to quit smoking.11 The purpose of this article was to (1) review the mechanisms through which smoking negatively impacts the wound healing process, (2) present the current evidence-based guidelines for treating tobacco dependence, and (3) propose strategies for incorporating the clinical guidelines into chronic wound care treatment plans of patients who smoke.
Chronic Wounds
A chronic wound is defined as one that fails to progress through the timely, sequential steps of healing that end with wound closure; chronic wounds generally remain open for greater than 3 months.12 Nearly all chronic wounds can be grouped into 3 major categories: leg ulcers, diabetic ulcers, and pressure ulcers (PUs). Venous leg ulcers comprise the largest single group of leg ulcers treated in wound care clinics.13 Current statistics suggest approximately 15% of venous ulcers never heal or reoccur once or more times in up to 71% of affected patients.14,15 It has been estimated that venous ulcers incur treatment costs of approximately $3 billion annually in the United States and cause a loss of approximately 2 million working days.1,2,16–18 Diabetic foot ulcerations (DFUs) that fail to heal result from a combination of peripheral vascular disease and neuropathy.19 Approximately 1 in 4 individuals with diabetes mellitus will develop a foot ulcer during their lifetime and 15% of DFUs will lead to amputation.20 In the United States, DFUs account for 25% to 50% of the costs related to diabetic care.21 Pressure ulcers also pose significant problems, especially for those aged 65 years or older who suffer from impaired mobility, inadequate nutritional intake, or a critical illness. Total annual costs related to PU care in the United States have been estimated to be as high at $11 billion and predictions are that expenditures will continue to rise because of the growth in the aging population.22 In addition to the extensive financial costs, PUs are associated with impaired health-related quality of life.22
Although each of the major chronic wound types can be characterized by unique pathobiological processes, all have shared characteristics, including chronic inflammation, a proteolytic environment secondary to bacteria, underlying ischemia, and recurrent ischemia-reperfusion cycles.23 Cigarette smoking impairs the function of several cell types such as neutrophils and macrophages important to inflammatory and bactericidal activity24 and also compromises oxygen delivery to tissues. Thus, cigarette smoking has detrimental effects that compound the underlying pathobiology of chronic wounds.10,25
The harmful effects of cigarette smoking on wound healing have been reported in multiple studies. A higher incidence of wound complications associated with smoking include prolonged wound healing times,8,9 dehiscence,26,27 tissue flap necrosis,28–30 anastomotic leakage,31 decreased wound tensile strength,32 and infection.9,33,34 Conversely, smoking cessation has been associated with a reduction in incisional wound infections33,35 and other wound healing complication rates.7 In fact, many surgeons are hesitant to perform certain elective or aesthetic surgeries on individuals who reject smoking cessation advice. Although few studies have evaluated the effects of cigarette smoking specifically on chronic wound healing, the evidence of its negative impact on acute wound healing suggests that assessing for tobacco use and treating tobacco dependence in the chronic wound population should be a high priority for clinicians.
Smoking and Wound Healing
Wound healing is a complex process that can be divided into at least 3 continuous, but overlapping stages: an inflammatory stage, a proliferative stage leading to tissue restoration, and tissue remodeling stage.24,36 Wound healing is regulated by a synergistic interplay among multiple cell types, cytokines, and growth factors at the wound site. Normal tissue oxygen pressures are necessary for the entire reparative process including cell migration to wound sites, bacterial defense, and collagen synthesis.37 The healing trajectory can be interrupted at any stage by tissue hypoxia.38 Hypoxia has also been found to increase wound infection risk in surgical patients partially due to reduction of bactericidal actions of neutrophils.39–41 Tissue hypoxia is viewed as a fundamental mechanism through which cigarette smoking disrupts acute wound healing.42,43 Adverse effects of smoking on tissue oxygen concentrations have been demonstrated after smoking just 1 cigarette regardless of smoking history.42,44,45 Furthermore, Jensen and colleagues42 reported that “pack-per-day” smokers experience tissue hypoxia during a significant portion of each day.
It has been estimated that cigarette smoke contains more than 4000 toxic compounds. The primary toxins associated with impaired wound healing are nicotine and the gases carbon monoxide and hydrogen cyanide.46 The definitive mechanisms through delay healing are not understood, but all have been shown to impair oxygen supply to tissues.42,47,48
Perhaps the most frequently studied tobacco smoke constituent in relation to tissue hypoxia is nicotine. Nicotine is a colorless alkaloid that is rapidly absorbed during smoking; it is hypothesized to act as a major component of reduced blood flow owing to its vasoconstrictive actions.49 Some studies suggest that nicotine is harmful to skin and subcutaneous tissue because it stimulates the sympathetic nervous system to release catecholamines, which trigger peripheral vasoconstriction and diminish tissue perfusion rates.50–52 Tissue perfusion rates are also diminished by increased blood viscosity that results when catecholamines stimulate platelet aggregation via the adenylate cyclase system. Increased blood viscosity is also thought to be a causative component of nicotine-induced tissue hypoxia.47,53 Nevertheless, evidence concerning the impact of nicotine on tissue perfusion and wound healing remains mixed. Recent studies have challenged the belief that nicotine is the major agent in tobacco smoke causing reduced blood flow, tissue hypoxia, and subsequent wound complications.25 For example, Sorensen and colleagues25 reported a temporary, marginal increase in skin blood flow, a significant decrease in subcutaneous blood flow and no effect on the aerobic metabolism of tissue, when infusing 1.0 mg of nicotine intravenously, an amount similar to a nicotine replacement drug.25 These findings are supported by other studies showing that nicotine-replacement drugs had no adverse effects on acute wound healing33,54 unless given in toxic doses,55,56 suggesting that nicotine alone is not responsible for the total vasoactive effect of smoking or the association between smoking, local tissue hypoxia, and impaired wound healing.
Carbon monoxide and hydrogen cyanide are also hypothesized to negatively affect wound healing. Carbon monoxide leads to tissue hypoxia because it reduces the oxygen content of blood by binding to hemoglobin. Carbon monoxide has a 200 times greater affinity to bind hemoglobin than oxygen.46,47 As a result of a shift to the left in the oxygen dissociation curve, oxygenated hemoglobin in the bloodstream is reduced resulting in impaired tissue profusion and cellular hypoxia.52,57 Hydrogen cyanide also affects tissue oxygenation by impeding cellular oxygen metabolism via inhibition of the enzyme system necessary for this metabolic process.48,58
Although the exact mechanisms by which nicotine, carbon monoxide, and hydrogen cyanide lead to tissue hypoxia are not entirely understood, the negative impact of smoking on cellular function during the wound healing process has been reported by numerous studies. For example, cigarette smoke has been associated with a reduced proliferation of erythrocytes,47 white blood cells,47 and fibroblasts.47,59,60 Diminished numbers of erythrocytes lead to inadequate oxygen availability, which results in tissue hypoxia. White blood cells (macrophages) are essential for the phagocytosis of tissue debris, bacteria, and apoptotic neutrophils during the inflammatory stage of healing. They also generate a host of cytokines that signal subsequent healing processes such as angiogenesis. Smoking not only reduces white blood cell migration to the wound site, but also diminishes lymphocyte function,9,61 cytotoxicity of natural killer cells,9,61 macrophage sensing of gram-negative bacteria,62 and the bactericidal activity of neutrophils.63 Smoking is also associated with a reduction in fibroblast proliferation during the proliferative stage of wound healing. Fibroblasts produce essential structural proteins such as collagen fibronectin that are needed for granulation tissue formation and epithelialization.59,60,64 Collagen is the primary structural protein that affects a healing wound’s tensile strength and research has demonstrated that its production is diminished in smokers.65
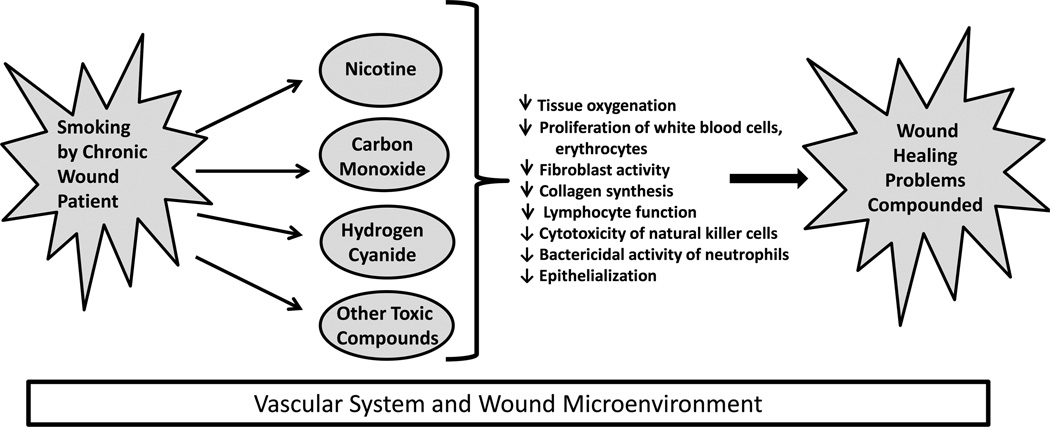
Considered collectively, these data provide compelling evidence that smoking impairs wound healing. However, most of these studies evaluated the effect of smoking on acute wound healing following surgery. Less is known about the influence of cigarette smoke on chronic wounds, although a relationship between smoking and an increased likelihood of PU formation has been observed in at least 1 study of patients with spinal cord injuries.66 Figure 1 illustrates a proposed model of how smoking and the toxic compounds contained in cigarette smoke may reduce healing in patients with chronic wound induced by venous dysfunction, prolonged exposure to pressure or shear, or diabetes. Additional studies are needed to elucidate the specific effects of smoking on chronic wound healing.
FIGURE 1.
Detrimental effects of smoking on healing of chronic wounds. Diagram illustrating how smoking in a patient with a chronic wound leads to inhalation of toxic components that have negative effects on tissue oxygenation and cellular activity in the vascular system and microenvironment of a chronic wound.
Several clinical practice guidelines provide evidence-based practice recommendations for clinicians who treat patients with chronic wounds.67–72 However, these guidelines do not provide specific recommendations to assist patients in quitting smoking or techniques for tobacco dependence treatment. Creating an appendix to the guidelines containing this information would provide clinicians with the resources that are needed to help smokers quit and thus potentially improve healing outcomes for these patients.
Evidence-Based Guidelines for Treating Tobacco Dependence
Smoking cessation is a dynamic process that typically requires smokers to make several serious attempts before achieving long-term success. Many smokers express the desire to quit smoking each year, but only one-third are actually successful.73 The US Public Health Service Treating Tobacco Use and Dependence guideline provides evidence-based recommendations for tobacco dependence treatment.11 Several studies have examined the implementation of the US Public Health Service guideline in the ambulatory setting and have found that approximately one-third of physician visits do not include measurement of tobacco use status73,74 despite guideline recommendations. When multiple clinicians (ie, physicians, nurses, pharmacists, social workers etc) advise a patient to quit smoking, it increases the patient’s motivation to quit and the number of serious attempts to quit (Table 1).11
TABLE 1.
The 5A’s Model for Treating Tobacco Use and Dependence and Suggestions for Implementing in Wound Care Clinics
| The 5A’s Model for Treating Tobacco Use and Dependencea | ||
|---|---|---|
| 5 A’s | 5A’s Definitions | Suggestions for Implementation in a Wound Care Clinic |
| Ask | Systematically, identify all tobacco users at every visit. | Obtain smoking status from each patient at each visit along with vital signs. |
| Advise | In a clear, strong, personalized message, urge all tobacco users to quit. |
Make connection between decreased wound healing and continued smoking. Use specific examples of how wound healing is affected by smoking such as:
|
| Assess | For current tobacco users, determine willingness to make a quit attempt. |
For patients willing to make a quit attempt, continue with 5 A’s. For patients not ready to make a quit attempt, refer to the 5 R’s: relevance, risk, rewards, roadblocks, and repetition (see Strategies to promote smoking cessation in wound management patients). |
| Assist |
|
|
| For patients unwilling to quit at this time, provide interventions to increase quit attempts in the future (see Strategies to promote smoking cessation in wound management patients). |
||
| For recent quitters, provide relapse prevention strategies | ||
| Arrange | All patients who received previous A’s should receive follow-up. | Follow up with each wound patient at each visit. Serial wound care visits represent a teachable moment for tobacco dependence treatment. |
Adapted from the US Public Health Service Treating Tobacco Use and Dependence Clinical Practice Guideline.11
Guidelines for smoking cessation follow 5 A’s: Ask, Advise, Assess, Assist, and Arrange (Table 1).11 Patients should be asked about their tobacco use and it should be documented at every visit. Patient who smoke are advised to quit smoking via a clear, strong personalized message delivered by a clinician. They should also be assessed for willingness to quit smoking. It is critical to assess patients who have recently quit smoking for challenges to remaining abstinent. Patients who are willing to make a quit attempt should be offered assistance with cessation with pharmacotherapy and either provided or referred to counseling or behavioral treatment. Subsequent contacts should be arranged for patients to follow up on the previous “A’s” discussions. Providing routine assistance to all patients who are interested in tobacco dependence treatment is the most important step that a clinician can provide.11
Smoking Cessation Counseling and Problem-Solving Skills
When counseling patients, teaching practical problem-solving skills and providing support and encouragement are important. Patients should be taught to recognize situations or smoking cues that may increase the risk of smoking or relapse such as being around other smokers, stress, or drinking alcohol. They need assistance in developing coping skills in order to anticipate and avoid temptation and trigger situations and cope with smoking urges. Some examples are learning distraction techniques and changing routines, assistance wtih accomplishing lifestyle changes that reduce stress and exposure to smoking cues, and learning basic information about smoking and successful quitting. Supportive counseling also may include encouragement for quit attempts, expression of concern and willingness to help, asking about fears and ambivalence regarding quitting, and encouraging patient discussion about the quitting process.11
Pharmacotherapy
Successful smoking cessation is a multicomponent strategy. Pharmacotherapy, along with behavioral counseling and problem-solving skills, offers the highest success for smoking cessation.11,75–77 The first-line agents discussed in this section have been found to be safe and effective for smoking cessation (Table 2). Pharmacotherapy includes nicotine replacement therapy (NRT), bupropion, and varenicline. Bupropion was the first nonnicotine medication to show efficacy with smoking cessation and was approved for use in smoking cessation in 1997.78 The possible mechanisms of action of bupropion include blockade of neuronal reuptake of dopamine and norepinephrine and blockade of nicotinic acetylcholinergic receptors. It can be used in combination with nicotine replacement medications. Bupropion is contraindicated for patients with seizure disorders and anorexia nervosa or patients taking monoamine oxidase inhibitors.
TABLE 2.
First-Line Pharmacotherapy (Most Effective When Used in Combination With Behavioral Counseling)a
| Pharmacotherapy | Dosage | Duration | Available | Warnings/Side Effects |
|---|---|---|---|---|
| Bupropion SR (Zyban, Wellbutrin) |
150 mg once daily (days 1–3); 150 mg twice daily |
Start 1–3 wk before quit date; duration 2–6 mo |
Prescription only: Generic available (may be combined with nicotine replacement therapy) |
Do not use with history of seizure or eating disorders, current monoamine oxidase inhibitor. Insomnia, dry mouth |
| Varenicline (Chantix) | 0.5 mg once daily (days 1–3); 0.5 mg twice daily (days 4–7); 1 mg twice daily |
Start 1 wk before quit date duration 3–6 mo |
Prescription only | Use with caution: significant renal impairment or on dialysis, serious psychiatric history. Nausea, insomnia, abnormal/ vivid dreams |
| Nicotine nasal spray (Nicotrol NS) |
1 dose = 1 squirt per nostril; 1–2 doses per hour; 8–40 doses per day |
duration 3–6 mo; taper at end |
Prescription only | Do not use with asthma. Nasal irritation |
| Nicotine inhaler (Nicotrol inhaler) |
6–16 cartridges per day Inhale 80 times per cartridge |
duration 6 mo; taper at end |
Prescription only | Local irritation of mouth and throat; may improve after use |
| Nicotine patch (Generic, Nicoderm CQ, Nicotrol) |
One patch per day; available strengths: 21 mg, 14 mg, 7 mg If ≥ 10 cigarettes per day: 21 mg If ≤ 10 cigarettes per day: may start with 14 mg |
duration 6 mo; may use longer |
Over the counter; Generic available |
Do not use if have severe eczema or psoriasis. Insomnia, local skin reaction |
| Nicotine Gum (Nicorette) |
1 piece q 1–2 hours; 6–15 pieces per day; available strengths: 2 mg, 4 mg If ≤ 24 cigarettes: 2 mg If ≥ 25 cigarettes: 4 mg |
duration 6 mo; may use longer |
Over the counter; Generic available |
Do not eat 15 min before or during use. Mouth soreness, stomachache |
| Nicotine lozenge (Commit) |
Weeks 1–6: 2–4 mg q 1–2 h Weeks 7–9: 2–4 mg q 2–4 h Weeks 10–12: 1–2 mg q 4–8 h available strengths: 2 mg, 4 mg |
duration 3–6 mo | Over the counter; Generic available |
Do not eat 15 min before or during use. Hiccups, cough, heartburn |
Adapted from the US Public Health Service Treating Tobacco Use and Dependence Clinical Practice Guideline.11
Varenicline is a nonnicotine medication that has been used for tobacco dependence treatment since 2006. It is a partial agonist of the α4β2 subtype of the nicotinic acetyl-choline receptor and therefore should not be used with nicotine replacement products. Varenicline has the highest 6-month abstinence rate compared to placebo of all the available pharmacotherapies. It is well tolerated and should be used with a reduced dose in renal dysfunction patients. Patients with a history of psychiatric illness should be monitored closely.75
Nicotine replacement agents are available in several forms: inhalers, patches, gums, nasal sprays, and lozenges. Some are available over the counter and some by prescription only (Table 2). The goal of NRT medications is to at least partially replace the nicotine obtained by cigarettes and to reduce the severity of nicotine withdrawal. The form of nicotine replacement medication offered can be based on patient preference and previous history when attempting to quit smoking. Other factors to consider may include whether the patient has dentures or skin sensitivities, in which cases the clinician may not want to recommend the gum or patch forms of NRT (Table 2).11
Smoking Cessation in Patients With Wounds
Patients with wound healing complications typically have recurring visits (daily, weekly, and monthly) in a wound care clinic or home care setting. These regularly recurring visits provide an excellent opportunity for clinicians to deliver evidence-based tobacco dependence treatment interventions to patients who continue to smoke. For wound care patients who continue to smoke or those who are having additional difficulty with successful cessation, the following 5R’s (relevance, risks, rewards, roadblocks, and repetition) intervention may be helpful.11 The clinician can advise a patient to quit smoking; however, it is important to allow patients to address each topic in their own words. The clinician can fill in gaps, point out discrepancies, and help refine the patient’s responses. The patient must first indicate why quitting smoking is personally relevant, being as specific as possible. Some reasons a wound patient may cite are personal health concerns, cost, and the health effects on children or grandchildren in the home. The patient should next identify potential risks associated with continuing to smoke such as decreased blood flow and oxygen to wound, increased risk of infection, slower healing, and decreased strength of scar tissue. It is important for the patient to identify potential rewards of smoking cessation such as faster healing of wound, increased mobility or activity following wound healing, alleviation of comorbid health conditions such as coronary heart disease and peripheral vascular disease, improved pulmonary symptoms, cough and dyspnea, and improved smell and taste that may lead to improved nutritional intake and thus faster wound healing.
Finally, the patient should identify barriers or roadblocks to quitting and the clinician should provide counseling or interventions that address these barriers such as problem-solving counseling, or medications discussed in the general recommendations section. Typical roadblocks to successfully quitting smoking may include withdrawal symptoms, fear of failure, weight gain, lack of support, depression, fear of losing the enjoyment of tobacco, being around other tobacco users, and limited knowledge of effective tobacco dependence treatment. Roadblocks specific to patients with chronic wounds frequently include immobility that prohibits working or regular daily activities. Lack of daily activities due to immobility may lead to boredom or anxiety, which may decrease the likelihood of success in quitting. We recommend repeating the 5 R’s every time the patient sees the clinician.
Conclusion
Chronic wounds are a significant health problem. Wound healing may be impaired by multiple factors, including smoking. Because cigarette smoking is a modifiable factor, WOC nurses and other wound care clinicians should incorporate personalized, evidence-based smoking cessation strategies in their care plans in order to improve healing outcomes in this population.
KEY POINTS.
-
✓
Although the exact mechanisms by which smoking compromises wound healing are not entirely understood, the negative impact of smoking has been reported by numerous studies.
-
✓
Wound care guidelines could be expanded to include the treatment of behavioral risks to poor healing, such as smoking.
-
✓
WOC nurses and other wound care clinicians should provide the resources to aid patients with chronic wounds to quit smoking.
ACKNOWLEDGMENTS
The authors are supported in part by funding from the following sources: National Institutes of Health Clinical and Translational Science Award to The Ohio State University UL1RR025755, NIH/NINR 1R21NR012803-01A1 (J.M.).
Footnotes
The authors declares no conflict of interests.
Contributor Information
Jodi C. McDaniel, College of Nursing, The Ohio State University, Columbus..
Kristine K. Browning, Clinical nursing College of Nursing, The Ohio State University, Columbus..
References
- 1.Abbade LP, Lastoria S. Venous ulcer: epidemiology, physiopathology, diagnosis and treatment. Int J Dermatol. 2005;44(6):449–456. doi: 10.1111/j.1365-4632.2004.02456.x. [DOI] [PubMed] [Google Scholar]
- 2.Valencia IC, Falabella A, Kirsner RS, Eaglstein WH. Chronic venous insufficiency and venous leg ulceration. J Am Acad Dermatol. 2001;44(3):401–421. doi: 10.1067/mjd.2001.111633. quiz 422–424. [DOI] [PubMed] [Google Scholar]
- 3.Gordon MD, Gottschlich MM, Helvig EI, Marvin JA, Richard RL. Review of evidenced-based practice for the prevention of pressure sores in burn patients. J Burn Care Rehabil. 2004;25(5):388–410. doi: 10.1097/01.bcr.0000138289.83335.f4. [DOI] [PubMed] [Google Scholar]
- 4.Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Am Podiatr Med Assoc. 2010;100(5):335–341. doi: 10.7547/1000335. [DOI] [PubMed] [Google Scholar]
- 5.Gottrup F. A specialized wound-healing center concept: importance of a multidisciplinary department structure and surgical treatment facilities in the treatment of chronic wounds. Am J Surg. 2004;187(5A):38S–43S. doi: 10.1016/S0002-9610(03)00303-9. [DOI] [PubMed] [Google Scholar]
- 6.Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan LK, Withey S, Butler PE. Smoking and wound healing problems in reduction mammaplasty: Is the introduction of urine nicotine testing justified? Ann Plast Surg. 2006;56(2):111–115. doi: 10.1097/01.sap.0000197635.26473.a2. [DOI] [PubMed] [Google Scholar]
- 8.Sorensen LT, Zillmer R, Agren M, Ladelund S, Karlsmark T, Gottrup F. Effect of smoking, abstention, and nicotine patch on epidermal healing and collagenase in skin transudate. Wound Repair Regen. 2009;17(3):347–353. doi: 10.1111/j.1524-475X.2009.00479.x. [DOI] [PubMed] [Google Scholar]
- 9.Sorensen LT, Horby J, Friis E, Pilsgaard B, Jorgensen T. Smoking as a risk factor for wound healing and infection in breast cancer surgery. Eur J Surg Oncol. 2002;28(8):815–820. doi: 10.1053/ejso.2002.1308. [DOI] [PubMed] [Google Scholar]
- 10.Whiteford L. Nicotine, CO and HCN: The detrimental effects of smoking on wound healing. Br J Community Nurs. 2003;8(12):S22–S26. doi: 10.12968/bjcn.2003.8.Sup6.12554. [DOI] [PubMed] [Google Scholar]
- 11.Fiore MC, Jaen CR, Baker TB, et al., editors. Treating Tobacco use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services. Public Health Service; 2008. [Google Scholar]
- 12.Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol. 1994;130(4):489–493. [PubMed] [Google Scholar]
- 13.Kistner RL, Shafritz R, Stark KR, Warriner RA., III Emerging treatment options for venous ulceration in today’s wound care practice. Ostomy Wound Manage. 2010;56(4):E1–E11. [PubMed] [Google Scholar]
- 14.Abenhaim L, Kurz X. The VEINES study (VEnous INsufficiency Epidemiologic and economic Study): an international cohort study on chronic venous disorders of the leg. VEINES group. Angiology. 1997;48(1):59–66. doi: 10.1177/000331979704800110. [DOI] [PubMed] [Google Scholar]
- 15.Nelzen O, Bergqvist D, Lindhagen A. Venous and non-venous leg ulcers: clinical history and appearance in a population study. Br J Surg. 1994;81(2):182–187. doi: 10.1002/bjs.1800810206. [DOI] [PubMed] [Google Scholar]
- 16.McGuckin M, Kerstein MD. Venous leg ulcers and the family physician. Adv Wound Care. 1998;11(7):344–346. [PubMed] [Google Scholar]
- 17.Heit JA, Rooke TW, Silverstein MD, et al. Trends in the incidence of venous stasis syndrome and venous ulcer: a 25-year population-based study. J Vasc Surg. 2001;33(5):1022–1027. doi: 10.1067/mva.2001.113308. [DOI] [PubMed] [Google Scholar]
- 18.Tran NT, Meissner MH. The epidemiology, pathophysiology, and natural history of chronic venous disease. Semin Vasc Surg. 2002;15(1):5–12. [PubMed] [Google Scholar]
- 19.Medina A, Scott P, Ghahary A, Tredget E. Pathophysiology of chronic nonhealing wounds. J Burn Care. 2005;26(4):306. doi: 10.1097/01.bcr.0000169887.04973.3a. [DOI] [PubMed] [Google Scholar]
- 20.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 21.Kantor J, Margolis DJ. Treatment options for diabetic neuropathic foot ulcers: a cost-effectiveness analysis. Dermatol Surg. 2001;27(4):347–351. doi: 10.1046/j.1524-4725.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- 22.Chou R, Dana T, Bougatsos C, et al. [Accessed July 6, 2013];Pressure ulcer risk assessment and prevention: comparative effectiveness AHRQ publication no. 12 (13)–EHC148-EF. Comparative Effectiveness Review - Report 87; Agency for Healthcare Research and Quality. 2013 http://effectivehealthcare.ahrq.gov/ehc/products/309/1489/pressure-ulcer-prevention-report-130528.pdf. Published. [PubMed]
- 23.Mustoe TA, O’Shaughnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Plast Reconstr Surg. 2006;117(7) suppl:35S–41S. doi: 10.1097/01.prs.0000225431.63010.1b. [DOI] [PubMed] [Google Scholar]
- 24.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorensen LT, Jorgensen S, Petersen LJ, et al. Acute effects of nicotine and smoking on blood flow, tissue oxygen, and aerobe metabolism of the skin and subcutis. J Surg Res. 2009;152(2):224–230. doi: 10.1016/j.jss.2008.02.066. [DOI] [PubMed] [Google Scholar]
- 26.Abbas SM, Hill AG. Smoking is a major risk factor for wound dehiscence after midline abdominal incision; case-control study. ANZ J Surg. 2009;79(4):247–250. doi: 10.1111/j.1445-2197.2009.04854.x. [DOI] [PubMed] [Google Scholar]
- 27.Manassa EH, Hertl CH, Olbrisch RR. Wound healing problems in smokers and nonsmokers after 132 abdominoplasties. Plast Reconstr Surg. 2003;111(6):2082–2087. doi: 10.1097/01.PRS.0000057144.62727.C8. discussion 2088–2089. [DOI] [PubMed] [Google Scholar]
- 28.Little SC, Hughley BB, Park SS. Complications with forehead flaps in nasal reconstruction. Laryngoscope. 2009;119(6):1093–1099. doi: 10.1002/lary.20243. [DOI] [PubMed] [Google Scholar]
- 29.Goldminz D, Bennett RG. Cigarette smoking and flap and full-thickness graft necrosis. Arch Dermatol. 1991;127(7):1012–1015. [PubMed] [Google Scholar]
- 30.Campos JH, Gomes HC, dos-Santos WL, Cardeal M, Ferreira LM. Effect of nicotine treatment and withdrawal on random-pattern skin flaps in rats. Exp Toxicol Pathol. 2008;60(6):449–452. doi: 10.1016/j.etp.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Sorensen LT, Jorgensen T, Kirkeby LT, Skovdal J, Vennits B, Wille-Jorgensen P. Smoking and alcohol abuse are major risk factors for anastomotic leakage in colorectal surgery. Br J Surg. 1999;86(7):927–931. doi: 10.1046/j.1365-2168.1999.01165.x. [DOI] [PubMed] [Google Scholar]
- 32.Sorensen LT. Effect of lifestyle, gender and age on collagen formation and degradation. Hernia. 2006;10(6):456–461. doi: 10.1007/s10029-006-0143-x. [DOI] [PubMed] [Google Scholar]
- 33.Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003;238(1):1–5. doi: 10.1097/01.SLA.0000074980.39700.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myles PS, Iacono GA, Hunt JO, et al. Risk of respiratory complications and wound infection in patients undergoing ambulatory surgery: smokers versus nonsmokers. Anesthesiology. 2002;97(4):842–847. doi: 10.1097/00000542-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Moller AM, Villebro N, Pedersen T, Tonnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359(9301):114–117. doi: 10.1016/S0140-6736(02)07369-5. [DOI] [PubMed] [Google Scholar]
- 36.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 37.Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P. Oxygen in acute and chronic wound healing. Br J Dermatol. 2010;163(2):257–268. doi: 10.1111/j.1365-2133.2010.09804.x. [DOI] [PubMed] [Google Scholar]
- 38.Hunt TK, Pai MP. The effect of varying ambient oxygen tensions on wound metabolism and collagen synthesis. Surg Gynecol Obstet. 1972;135(4):561–567. [PubMed] [Google Scholar]
- 39.Hopf HW, Hunt TK, West JM, et al. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg. 1997;132(9):997–1004. doi: 10.1001/archsurg.1997.01430330063010. discussion 1005. [DOI] [PubMed] [Google Scholar]
- 40.Allen DB, Maguire JJ, Mahdavian M, et al. Wound hypoxia and acidosis limit neutrophil bacterial killing mechanisms. Arch Surg. 1997;132(9):991–996. doi: 10.1001/archsurg.1997.01430330057009. [DOI] [PubMed] [Google Scholar]
- 41.Babior BM. Oxygen-dependent microbial killing by phagocytes (second of two parts) N Engl J Med. 1978;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- 42.Jensen JA, Goodson WH, Hopf HW, Hunt TK. Cigarette smoking decreases tissue oxygen. Arch Surg. 1991;126(9):1131–1134. doi: 10.1001/archsurg.1991.01410330093013. [DOI] [PubMed] [Google Scholar]
- 43.Morecraft R, Blair WF, Brown TD, Gable RH. Acute effects of smoking on digital artery blood flow in humans. J Hand Surg Am. 1994;19(1):1–7. doi: 10.1016/0363-5023(94)90216-X. [DOI] [PubMed] [Google Scholar]
- 44.Freiman A, Bird G, Metelitsa AI, Barankin B, Lauzon GJ. Cutaneous effects of smoking. J Cutan Med Surg. 2004;8(6):415–423. doi: 10.1007/s10227-005-0020-8. [DOI] [PubMed] [Google Scholar]
- 45.Benowitz NL, Porchet H, Sheiner L, Jacob P., III Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin Pharmacol Ther. 1988;44(1):23–28. doi: 10.1038/clpt.1988.107. [DOI] [PubMed] [Google Scholar]
- 46.Ahn C, Mulligan P, Salcido RS. Smoking—the bane of wound healing: biomedical interventions and social influences. Adv Skin Wound Care. 2008;21(5):227–236. doi: 10.1097/01.ASW.0000305440.62402.43. quiz 237–238. [DOI] [PubMed] [Google Scholar]
- 47.Sherwin MA, Gastwirth CM. Detrimental effects of cigarette smoking on lower extremity wound healing. J Foot Surg. 1990;29(1):84–87. [PubMed] [Google Scholar]
- 48.Mosely LH, Finseth F. Cigarette smoking: impairment of digital blood flow and wound healing in the hand. Hand. 1977;9(2):97–101. doi: 10.1016/s0072-968x(77)80001-6. [DOI] [PubMed] [Google Scholar]
- 49.Moreyra AE, Lacy CR, Wilson AC, Kumar A, Kostis JB. Arterial blood nicotine concentration and coronary vasoconstrictive effect of low-nicotine cigarette smoking. Am Heart J. 1992;124(2):392–397. doi: 10.1016/0002-8703(92)90603-s. [DOI] [PubMed] [Google Scholar]
- 50.Grassi G, Seravalle G, Calhoun DA, et al. Mechanisms responsible for sympathetic activation by cigarette smoking in humans. Circulation. 1994;90(1):248–253. doi: 10.1161/01.cir.90.1.248. [DOI] [PubMed] [Google Scholar]
- 51.Cryer PE, Haymond MW, Santiago JV, Shah SD. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemodynamic and metabolic events. N Engl J Med. 1976;295(11):573–577. doi: 10.1056/NEJM197609092951101. [DOI] [PubMed] [Google Scholar]
- 52.Nolan J, Jenkins RA, Kurihara K, Schultz RC. The acute effects of cigarette smoke exposure on experimental skin flaps. Plast Reconstr Surg. 1985;75(4):544–551. doi: 10.1097/00006534-198504000-00018. [DOI] [PubMed] [Google Scholar]
- 53.Renaud S, Blache D, Dumont E, Thevenon C, Wissendanger T. Platelet function after cigarette smoking in relation to nicotine and carbon monoxide. Clin Pharmacol Ther. 1984;36(3):389–395. doi: 10.1038/clpt.1984.193. [DOI] [PubMed] [Google Scholar]
- 54.Sorensen LT, Jorgensen LN, Zillmer R, Vange J, Hemmingsen U, Gottrup F. Transdermal nicotine patch enhances type I collagen synthesis in abstinent smokers. Wound Repair Regen. 2006;14(3):247–251. doi: 10.1111/j.1743-6109.2006.00118.x. [DOI] [PubMed] [Google Scholar]
- 55.Falcone RE, Ruberg RL. Pharmacologic manipulation of skin flaps: lack of effect of barbiturates or nicotine. Plast Reconstr Surg. 1980;66(1):102–104. doi: 10.1097/00006534-198007000-00019. [DOI] [PubMed] [Google Scholar]
- 56.Mosely LH, Finseth F, Goody M. Nicotine and its effect on wound healing. Plast Reconstr Surg. 1978;61(4):570–575. doi: 10.1097/00006534-197804000-00013. [DOI] [PubMed] [Google Scholar]
- 57.Richardson D. Effects of tobacco smoke inhalation on capillary blood flow in human skin. Arch Environ Health. 1987;42(1):19–25. doi: 10.1080/00039896.1987.9935790. [DOI] [PubMed] [Google Scholar]
- 58.Silverstein P. Smoking and wound healing. Am J Med. 1992;93(1A):22S–24S. doi: 10.1016/0002-9343(92)90623-j. [DOI] [PubMed] [Google Scholar]
- 59.Arredondo J, Hall LL, Ndoye A, et al. Central role of fibroblast alpha3 nicotinic acetylcholine receptor in mediating cutaneous effects of nicotine. Lab Invest. 2003;83(2):207–225. doi: 10.1097/01.lab.0000053917.46614.12. [DOI] [PubMed] [Google Scholar]
- 60.Snyder HB, Caughman G, Lewis J, Billman MA, Schuster G. Nicotine modulation of in vitro human gingival fibroblast beta1 integrin expression. J Periodontol. 2002;73(5):505–510. doi: 10.1902/jop.2002.73.5.505. [DOI] [PubMed] [Google Scholar]
- 61.Siana JE, Rex S, Gottrup F. The effect of cigarette smoking on wound healing. Scand J Plast Reconstr Surg Hand Surg. 1989;23(3):207–209. doi: 10.3109/02844318909075119. [DOI] [PubMed] [Google Scholar]
- 62.McMaster SK, Paul-Clark MJ, Walters M, et al. Cigarette smoke inhibits macrophage sensing of gram-negative bacteria and lipopolysaccharide: relative roles of nicotine and oxidant stress. Br J Pharmacol. 2008;153(3):536–543. doi: 10.1038/sj.bjp.0707595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sorensen LT, Nielsen HB, Kharazmi A, Gottrup F. Effect of smoking and abstention on oxidative burst and reactivity of neutrophils and monocytes. Surgery. 2004;136(5):1047–1053. doi: 10.1016/j.surg.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 64.Wong LS, Green HM, Feugate JE, Yadav M, Nothnagel EA, Martins-Green M. Effects of “second-hand” smoke on structure and function of fibroblasts, cells that are critical for tissue repair and remodeling. BMC Cell Biol. 2004;5(13) doi: 10.1186/1471-2121-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jorgensen LN, Kallehave F, Christensen E, Siana JE, Gottrup F. Less collagen production in smokers. Surgery. 1998;123(4):450–455. [PubMed] [Google Scholar]
- 66.Lamid S, El Ghatit AZ. Smoking, spasticity and pressure sores in spinal cord injured patients. Am J Phys Med. 1983;62(6):300–306. [PubMed] [Google Scholar]
- 67.Robson MC, Cooper DM, Aslam R, et al. Guidelines for the treatment of venous ulcers. Wound Repair Regen. 2006;14(6):649–662. doi: 10.1111/j.1524-475X.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 68.Steed DL, Attinger C, Colaizzi T, et al. Guidelines for the treatment of diabetic ulcers. Wound Repair Regen. 2006;14(6):680–692. doi: 10.1111/j.1524-475X.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- 69.Whitney J, Phillips L, Aslam R, et al. Guidelines for the treatment of pressure ulcers. Wound Repair Regen. 2006;14(6):663–679. doi: 10.1111/j.1524-475X.2006.00175.x. [DOI] [PubMed] [Google Scholar]
- 70.Kelechi TJ, Johnson JJ. Guideline for the management of wounds in patients with lower-extremity venous disease: an executive summary. J Wound Ostomy Continence Nurs. 2012;39(6):598–606. doi: 10.1097/WON.0b013e31827179e9. [DOI] [PubMed] [Google Scholar]
- 71.Bonham PA, Flemister BG, Goldberg M, Crawford PE, Johnson JJ, Varnado MF. What’s new in lower-extremity arterial disease? WOCN’s 2008 clinical practice guideline. J Wound Ostomy Continence Nurs. 2009;36(1):37–44. doi: 10.1097/01.WON.0000345174.12999.89. [DOI] [PubMed] [Google Scholar]
- 72.Crawford PE, Fields-Varnado M. Guideline for the management of wounds in patients with lower-extremity neuropathic disease: an executive summary. J Wound Ostomy Continence Nurs. 2013;40(1):34–45. doi: 10.1097/WON.0b013e3182750161. [DOI] [PubMed] [Google Scholar]
- 73.Thorndike AN, Regan S, Rigotti NA. The treatment of smoking by US physicians during ambulatory visits: 1994 2003. Am J Public Health. 2007;97(10):1878–1883. doi: 10.2105/AJPH.2006.092577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferketich AK, Khan Y, Wewers ME. Are physicians asking about tobacco use and assisting with cessation? Results from the 2001–2004 national ambulatory medical care survey (NAMCS) Prev Med. 2006;43(6):472–476. doi: 10.1016/j.ypmed.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 75.Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 76.Oncken C, Gonzales D, Nides M, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med. 2006;166(15):1571–1577. doi: 10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- 77.Ebbert JO, Wyatt KD, Hays JT, Klee EW, Hurt RD. Varenicline for smoking cessation: efficacy, safety, and treatment recommendations. Patient Prefer Adherence. 2010;4:355–362. doi: 10.2147/ppa.s10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jorenby D. Clinical efficacy of bupropion in the management of smoking cessation. Drugs. 2002;62(suppl 2):25–35. doi: 10.2165/00003495-200262002-00003. [DOI] [PubMed] [Google Scholar]