Abstract
Background:
Cryosurgery is one of the simplest, most effective gingival depigmentation methods. Repigmentation may happen after a while in each method. The aim of this study is to compare the recurrence rate after treatment by liquid nitrogen swap and a cryoprob in 18 months.
Materials and Methods:
A total of 26 patients with physiologic gingival pigmentation were selected. The anterior sextant was divided into left and right segments; each segment was treated randomly by swap technique or cryoprob. Standard photos were evaluated with photoshop software (Red, Green, Blue, Cyan, Magenta, Yellow, Black [RGB, CMYK]) before and at 2 week, 1, 3, 6, 9, 12, 15, 18 months after the treatment. The results were compared, by the independent t-test and repeated measure ANOVA thereafter least significant difference post-hoc. The viewpoints of patients and physicians regarding the treatment outcomes were obtained by a questionnaire and consequently evaluated qualitatively by McNemar test (P < 0.05 was considered as significant level).
Results:
The statistical analysis showed a significant difference in color change after the treatment during 18 months, in each group (P < 0.001). The mean values of RGB had significantly increased after 2 weeks in both methods (P < 0.001), but the differences from 2 weeks to 18 months after treatment were not significant (P > 0.05). The mean values of CMYK significantly decreased after 2 weeks in both methods (P < 0.001), but the differences from 2 weeks to 18 months after treatment were not significant (P > 0.05). Qualitative evaluation showed the preference of the cryosurgery with swap method (P < 0.001). No significant recurrence was observed during 18 months follow-up.
Conclusion:
Both methods of cryosurgery are appropriate in treatment of gingival depigmentation because no significant recurrence was observed during 18 months follow-up.
Keywords: Cryosurgery, gingiva, nitrogen, nitrous oxide, pigmentation
INTRODUCTION
A beautiful smile surely enhances the individuals self-confidence. The harmony of the smile is attributable to the shape, position and color of the teeth in conjunction with the gingival tissue.[1]
Gingival color is generated by several factors such as the number and size of the blood vessels, epithelial thickness, quantity of keratinization and quality and magnitude of the pigments within the gingival epithelium.[2] Physiologic pigmentation of the oral mucosa is clinically manifested as multifocal or diffuse melanin pigmentation in different ethnic groups world-wide[3,4] and it occurs in all races.[3,5] A high amount of melanin granules is found in individuals of African and East Asian ethnicity.[6] The frequency of oral pigmentation in Iranian population is 43.47%, indicating that in Iranian population the degree of pigmentation is moderate. This value was higher than Europeans, whereas lower than East Asian populations.[7] It appears to be a positive correlation between the occurrence of gingival and dermal pigmentation.[8] However, melanin pigmentation of the gingiva is symmetric and does not alter the normal gingival architecture.[3]
Physiologic gingival pigmentation is the most common type of gingival pigmentation and results from excessive melanin. It is not in connection with increase in melanocyte number, but rather enhanced melanocytic activity.[9] The color of melanin-pigmented gingiva varies from light to dark brown or black.[10] This phenomenon is usually physiologic, although pathologic disorders (e.g., Albright syndrome, malignant melanoma, antimalarial therapy, Peutz Jeghers syndrome, trauma, hemochromatosis, chronic pulmonary disease and racial pigmentations) also serve as etiologic factors for oral melanin pigmentation.[11] The attached gingiva is the most popular location, however, physiological pigmentation can be noted anywhere in the oral cavity, including the tips of the fungiform papillae on the dorsal tongue.[9]
The patients with black/brown discoloration of gingival tissue combined with excess gingival display (gummy smile) often demand for pigmentation removal.[12] The aim of gingival depigmentation is removing pigment layer or masking pigmented gingiva.[13] Gingival depigmentation is a periodontal plastic surgical procedure whereby the gingival hyperpigmentation is removed or reduced by various techniques,[14] such as scalpel surgical technique,[15] surgical abrasion,[16] cryosurgery,[17,18] electrosurgery,[19] lasers,[11,20,21,22] chemical methods,[16] free gingival grafts,[19,21,23] and acellular dermal matrix allografts.[24] Advantages of cryotherapy include being a bloodless treatment, very low incidence of secondary infection and with a relative lack of scarring and pain.[25] It provides a very good esthetic result and may be either the first choice or an alternative option to conventional surgery.[26] Cryotherapy is the deliberate destruction of tissue by application of extreme cold that leads to freezing. The commonly used cryogens include liquid nitrogen (−196°C), nitrous oxide (−80°C), solidified CO2 (−780°C) (dry ice CO2 snow), chlorodifluromethane (−410°C), dimethyl ether and propane (−240°C, −420°C). The oral mucosa, due to humidity and smoothness, is a suitable site for applying this technique.[27] Three methods are used for cryosurgery: Probe system, liquid nitrogen sprays and cotton swabs.
The probe system follows the principles of Joule-Thompson expansion which enable substances to undergo a drop in temperature when moved from a high pressure area to a low pressure area. For instance, when nitrous oxide is released from the high pressure inside the cryoprob to the lower pressure cryotip, the drop in temperature allows freezing of the tissues.[27] Cotton-swab cryotherapy technique was performed by direct application of liquid nitrogen on the lesion with a cotton swab.[25]
Gingival repigmentation refers to the reappearance of melanin pigmentation following a period of time after depigmentation.[28] It is a common concern in the treatment of gingival hyperpigmentation and starts with migration of melanocytes from the adjacent free gingiva. The extent and time interval of recurrence varies depending upon the treatment modalities performed, in addition to the length of follow-up.[29,30] The mechanism of repigmentation is not well-known; although according to the migration theory, active melanocytes from the adjacent pigmented tissues migrate to the treated areas.[31] The aim of this study was to compare the recurrence rate of gingival pigmentation after treatment by liquid nitrogen swap and a cryoprob (with nitrous oxide as a cryogen). There was no available study about the comparison of recurrence rate after gingival pigmentation removal by applying these two techniques.
MATERIALS AND METHODS
The study comprised of 26 patients (16 females, 10 males) with mean age 25 years (16-40 years), who had been referred to the Khorasgan branch, Islamic Azad University, Dental School with the chief complaint of melanin pigmentation were selected based on inclusion and exclusion criteria. Inclusion criteria were gingival physiologic melanin pigmentation of the anterior segment and the exclusion criteria based on the patient's medical history included gingival pigmentation due to any systemic disease such as Peutz Jeghers syndrome, -myxoma-endocrine activity and, pigmentation that resulted from other origin except melanin (pigmentation caused by certain medications such as anti-malarial drugs, anti-bacterial drugs), -smoking, -gingivitis, and-known hypersensitivity to the liquid nitrogen and N2O. The ethical code number was 90021007904, Registration ID in IRCT: IRCT2013022512594N1.
Treatment procedure
Each patient was given a number from 1 to 26. The anterior sextant was divided into left and right segments; each segment was treated randomly by swap technique (Liquid nitrogen with −196°C) or cryoprob (Nitrous oxide with −80°C) based on even or odd number of the patients. Both the maxilla and mandible were isolated by using suction and cotton rolls. Liquid nitrogen was applied to pigmented areas by a cotton swab (4 mm in diameter) with a forward and backward rolling technique. A freezing appearance was observed after approximately 15-20 s after application of liquid nitrogen. This procedure was repeated 4 or 5 times for each area.
The other segment was treated with cryoprob (Danesh company, Tehran, Iran, model C505T). The prob was applied to pigmented areas (4 mm in diameter) with a forward and backward rolling technique. A freezing appearance was observed after application for approximately 15-20 s. This procedure was repeated 4 or 5 times for each segment.
Image acquisition and analysis
Standard digital photographs were taken pre-operatively and at 2 weeks, 1, 3, 6, 9, 12, 15 and 18 months post-operatively. For each photograph, the patient sat on a unique vertically positioned dental seat with the head positioned upright in a natural head position and the lips and cheeks opened using a mouth opener (Kerr Company, West Collins, United States). A digital camera (Canon, IXY 31s, Japan) (macro and flash set to off) was used to take all photographs with the same resolution (12 megapixels) and distance (10 cm from the sites). After taking all pre and post-operative photographs, an examiner expert in using photoshop software traced and measured the pigmented areas of all images with photoshop software (CS3)[32] (Adobe system, United States). Four equal sized round areas in each segment of an image were selected and calculated regard to the mean of Red, Green, Blue (RGB) and Cyan, Magenta, Yellow, Black (CMYK) [Figures 1 and 2]. The mean pigmented values of each of the 9 visits were compared.
Figure 1.
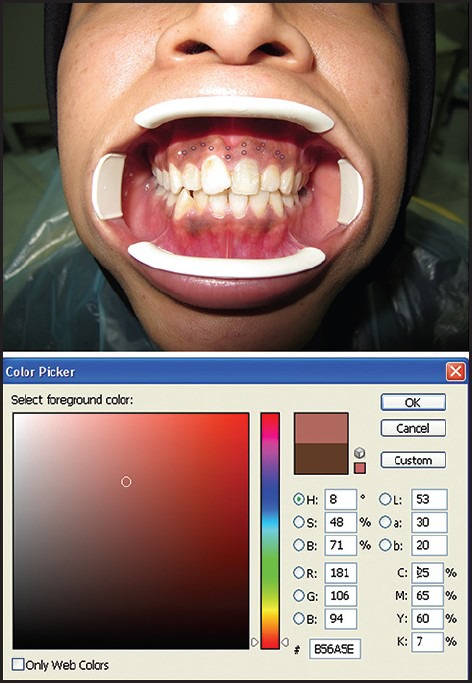
Photoshop analyzing of each point
Figure 2.
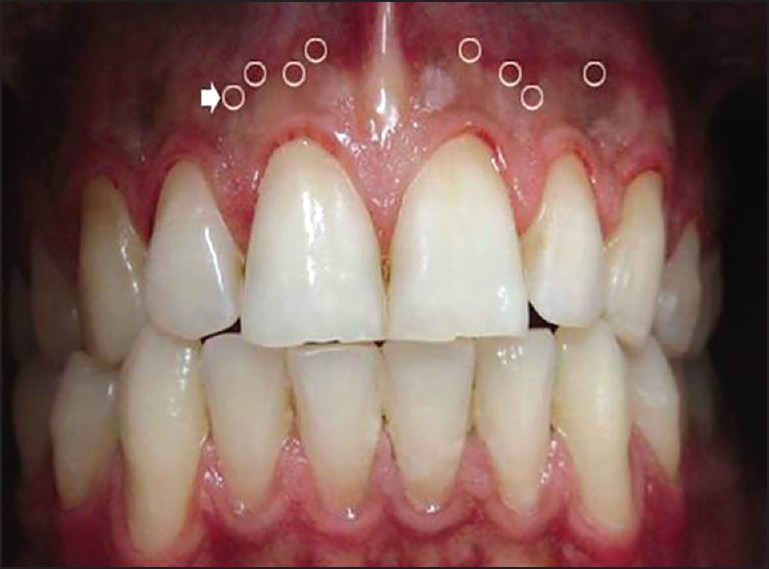
Selected points
Statistical analysis
The results were analyzed using Statistical Package for the Social Sciences 20 for Windows (IBM Corporation, United States). Independent t-test, repeated measure ANOVA and least significant difference (LSD) were used. The pre-treatment and post-treatment photos of the patients were evaluated in quantitative and qualitative form. In quantitative evaluation, the pre-treatment and post-treatment photos were evaluated by the use of color analyzing system of RGB and CMYK in photoshop. The results were compared by the independent t-test and repeated measure ANOVA and also LSD post-hoc. The viewpoints of patients and physicians were obtained by a questionnaire and each cryosurgery method was evaluated qualitatively by McNemar test (P < 0.05 was considered as significant level).
RESULTS
Hyperemia and edema of the treated area took place immediately after cryotherapy. Local swelling and bullous formation became apparent during the following 2-3 days. Subsequently, superficial necrosis occurred and the treated area was covered by a thin layer of yellowish pseudomembrane. Epithelization of each treated area was complete after 10-14 days. The healing process was uneventful without any complications.
All 26 patients were followed for 18 months. The statistical analysis showed significant difference in color change after the treatment during 18 months, in each group (P < 0.001). The results of repeated measure ANOVA test are detailed in Tables 1, 2 and Figures 3, 4.
Table 1.
Mean value of RGB in observation times in both groups
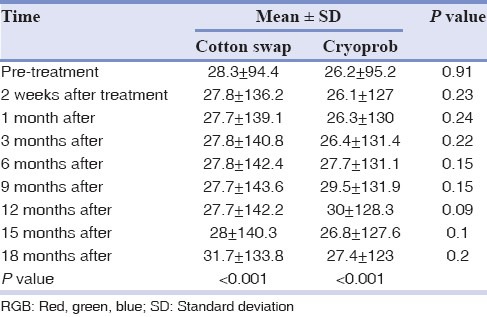
Table 2.
Mean value of CMYK in observation times in both groups
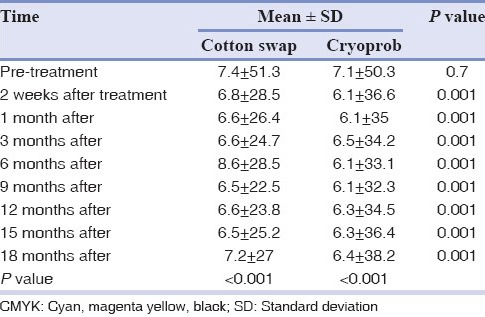
Figure 3.
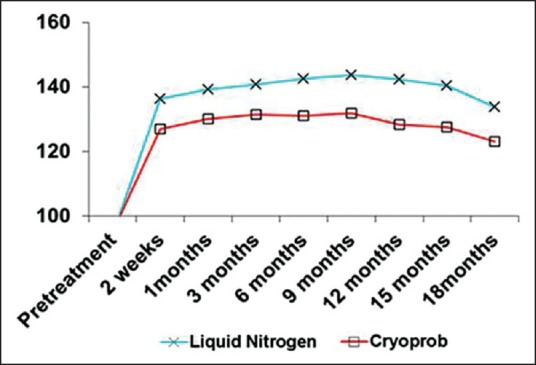
Mean value of Red, Green, Blue in observation times in both groups
Figure 4.
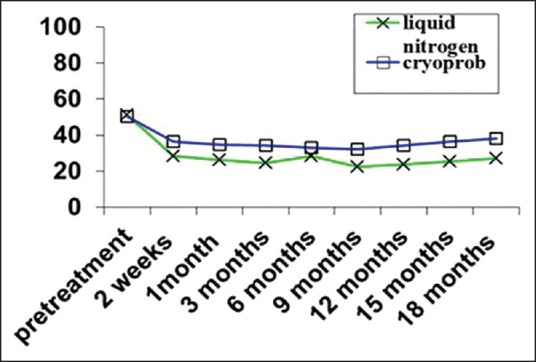
Mean value of Cyan, Magenta, Yellow, Black in observation times in both groups
The mean values of RGB had significantly increased after 2 weeks in both methods (P < 0.001), but the difference from 2 weeks to 9 months after treatment was not significant (P > 0.05). From 12th to 18th month after treatment the mean values of RGB decreased, however, the differences were not significant. Despite the fact that the independent t-test showed no significant differences between two methods in observation times (P > 0.05), the mean value of RGB in liquid nitrogen group was a little more than the cryoprob group.
The LSD post-hoc showed a significant differences in mean of RGB after treatment compared to pretreatment in each group (P < 0.001), but no significant difference was found between 2 weeks, 1, 3, 6, 9, 12, 15, 18 months after treatment (P > 0.05).
The mean values of CMYK significantly decreased after 2 weeks in both methods (P < 0.001), but the difference between 1st, 3rd, 6th, 9th months after the treatment was not signification. From 12th to 18th month after treatment the mean values of CMYK increased in both groups, however, the differences were not significant (P > 0.05). The independent t-test revealed that the mean values of CMYK in liquid nitrogen group were significantly less than the cryoprob group (P < 0.001).
The LSD post-hoc showed a significant differences in mean values of CMYK after treatment compared to pretreatment (P < 0.001), whereas there was no significant difference between 2 weeks, 1, 3, 6, 9, 12, 15, 18 months after treatment in both groups (P > 0.05).
The McNemar test was used for qualitative evaluation. It was found that cryosurgery with liquid nitrogen was significantly better than cryosurgery with cryoprob method (P < 0.01).
Slight recurrence was observed after 12 months; however, it was not statistically significant (P > 0.05). Qualitative evaluation showed that cryosurgery with liquid nitrogen had a better result and lower recurrence (P < 0.01) [Figures 5–10].
Figure 5.
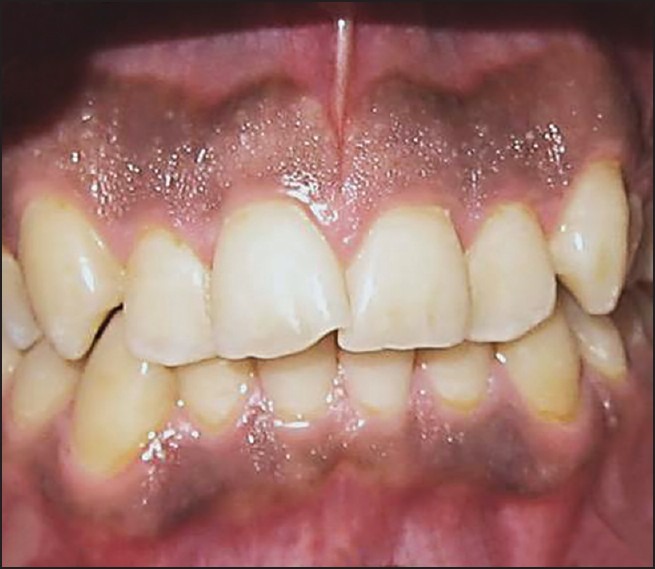
Frontal view of a patient before treatment
Figure 10.
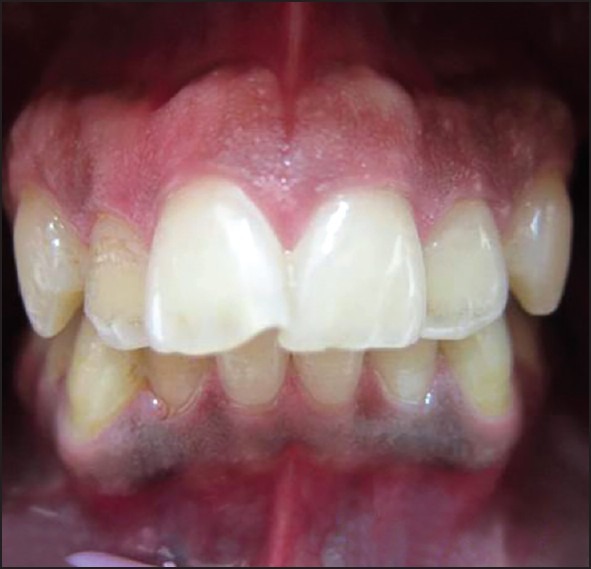
Frontal view of a patient 18 months after treatment
Figure 6.
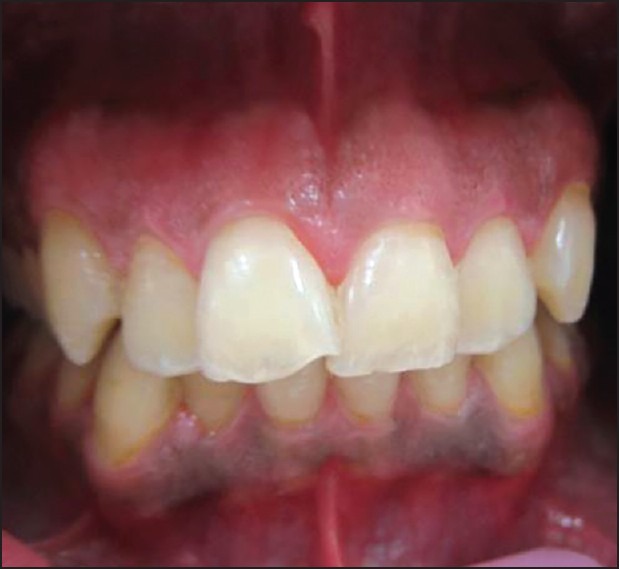
Frontal view of a patient 2 weeks after treatment
Figure 7.
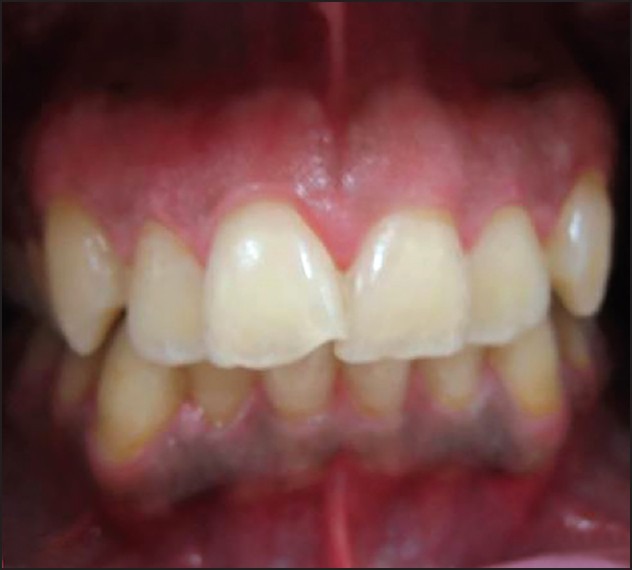
Frontal view of a patient 1 month after treatment
Figure 8.
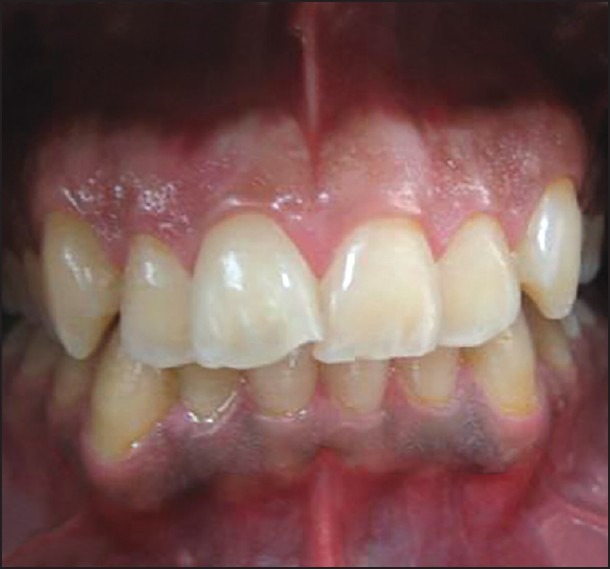
Frontal view of a patient 6 months after treatment
Figure 9.
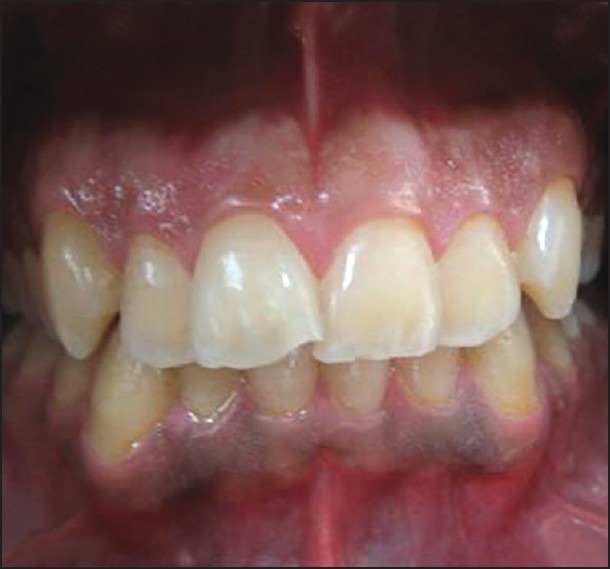
Frontal view of a patient 12 months after treatment
DISCUSSION
Despite the fact that gingival melanin pigmentation is completely benign but cosmetic concerns are common,[33] particularly in patients suffering from a very high smile line.[1] Different treatment methods are implied to remove the pigmentation. An ideal treatment technique should be painless, safe and cost benefit. In the present study, each group demonstrated significant differences in color change after the treatment during 18 months. The mean values of RGB did not designate significant difference between two methods even though in liquid nitrogen group it was a little more than the cryoprob. In both groups, significant differences in mean values of CMYK after treatment compared to pretreatment was observed. The mean values of CMYK in liquid nitrogen group were significantly less than the cryoprob group. No statistical significant recurrence was observed 9 months after treatment, but a slight recurrence began from 12th to 18 months in both techniques. Qualitative evaluations showed that cryosurgery with swap method had a better result and lower recurrence. Among the reasons of preference of cryosurgery with liquid nitrogen are lower temperature of the cryogen, direct contact of cryogen with gingival tissue and suitable conformity of the swap with tissue.
Contrary to the other methods, gingival cryosurgery does not require local anesthesia or periodontal dressing, it is relatively painless[17] and it is safe and causes no bleeding. The wound heals by complete regeneration and sterile inflammatory reactions.[17] Recurrence was observed after 1.5 years by utilizing gingivectomy technique by investigating on 5 patients.[30] Unlike cryosurgery, gingivectomy need periodontal dressing and the operation processes are painful with bleeding and long healing period.[29] In the present study, there was no significant recurrence during the same time in both cryosurgery methods. Bone Denudation and Abrasion were also used to remove gingival pigmentation. Recurrence was not observed until 6 months in Bone Denudation technique, but there was a mild relapse in Slicing and Abrasion techniques after 24-56 days.[34] In the present study, no repigmentation was observed until 9 months after treatment in none of the methods, although a slight recurrence in 12th month after treatment in each technique was found qualitatively, despite the fact that it was not statistically significant. Using high speed handpiece with diamond bur, after 20 months, Farnoosh found slight repigmentation.[35] However, no relapse was observed 18 months after treatment with the same technique by Mokeem.[16] In the present study, the patients were recalled at 3 months intervals and a slight, but not significant relapse was found only 12 months after the treatment. Using low speed handpiece for treatment, repigmentation in 10 of 54 patients in 90 days follow-up was reported.[36] Despite this fact that by using free gingival graft recurrence was not observed even after 4 and 5 years,[23] free gingival graft technique requires the use of additional surgical sites and is uncomfortable and healing was reported to be slow and painful. Moreover, the esthetic result is not always satisfactory due to poor tissue color matching at the recipient site.[37,38] Recently, acellular dermal matrix allograft is used for elimination of the gingival pigmentation. Even though, this method reduced surgical time, decreased post-operative complications and provided unlimited amount of graft material and a predictable and satisfactory esthetic result compared with free gingival graft, it is expensive and requires clinical expertise.[24,39] Laser therapy was also utilized for depigmentation with good and satisfactory results.[2,11,21,40,41,42,43] Relapse was reported 24 months after CO2 laser therapy in 2 of 10 patients.[11] Good and esthetic results from applying erbium-doped yttrium aluminium garnet laser was also reported in 6 month follow-up for 10 patients.[2] Laser surgery has some disadvantages such as delayed type of inflammatory reaction that may happen with mild post-operative discomfort lasting up to 1-2 weeks. Epithelial regeneration is delayed compared with conventional surgery. Moreover, expensive and sophisticated equipment makes the treatment very expensive. Another disadvantage is loss of tactile feedback while using lasers.[14,42]
CONCLUSION
According to the results of this study, both methods of cryosurgery are appropriate in treatment of gingival depigmentation because no significant recurrence was observed during 18 months follow-up.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Bhusari BM, Kasat S. Comparison between scalpel technique and electrosurgery for depigmentation: A case series. J Indian Soc Periodontol. 2011;15:402–5. doi: 10.4103/0972-124X.92580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tal H, Oegiesser D, Tal M. Gingival depigmentation by erbium: YAG laser: Clinical observations and patient responses. J Periodontol. 2003;74:1660–7. doi: 10.1902/jop.2003.74.11.1660. [DOI] [PubMed] [Google Scholar]
- 3.Dummett CO. Oral pigmentation: First symposium of oral pigmentation. J Periodontol. 1960;31:345–85. [Google Scholar]
- 4.Dummett CO, Barens G. Pigmentation of the oral tissues: A review of the literature. J Periodontol. 1967;38:369–78. doi: 10.1902/jop.1967.38.5.369. [DOI] [PubMed] [Google Scholar]
- 5.Page LR, Corio RL, Crawford BE, Giansanti JS, Weathers DR. The oral melanotic macule. Oral Surg Oral Med Oral Pathol. 1977;44:219–26. doi: 10.1016/0030-4220(77)90272-9. [DOI] [PubMed] [Google Scholar]
- 6.Fry L, Almeyda JR. The incidence of buccal pigmentation in caucasoids and negroids in Britain. Br J Dermatol. 1968;80:244–7. doi: 10.1111/j.1365-2133.1968.tb11966.x. [DOI] [PubMed] [Google Scholar]
- 7.Rabiei M, Nasrin R. Frequency of oral mucosal pigmentation among patients referred to the faculty of dentistry, Guilan University of Medical Sciences. J Indian Dent Assoc. 2005;17:95–100. [Google Scholar]
- 8.Barrett AW, Scully C. Human oral mucosal melanocytes: A review. J Oral Pathol Med. 1994;23:97–103. doi: 10.1111/j.1600-0714.1994.tb01095.x. [DOI] [PubMed] [Google Scholar]
- 9.Müller S. Melanin-associated pigmented lesions of the oral mucosa: Presentation, differential diagnosis, and treatment. Dermatol Ther. 2010;23:220–9. doi: 10.1111/j.1529-8019.2010.01319.x. [DOI] [PubMed] [Google Scholar]
- 10.Arikan F, Gürkan A. Cryosurgical treatment of gingival melanin pigmentation with tetrafluoroethane. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:452–7. doi: 10.1016/j.tripleo.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Esen E, Haytac MC, Oz IA, Erdoğan O, Karsli ED. Gingival melanin pigmentation and its treatment with the CO2 laser. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:522–7. doi: 10.1016/j.tripleo.2004.02.059. [DOI] [PubMed] [Google Scholar]
- 12.Mahesh HV, Harish MR, Shashikumar BM, Ramya KS. Gingival pigmentation reduction: A novel therapeutic modality. J Cutan Aesthet Surg. 2012;5:137–40. doi: 10.4103/0974-2077.99458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasagani SK, Nutalapati R, Mutthineni RB. Esthetic depigmentation of anterior gingiva. A case series. N Y State Dent J. 2012;78:26–31. [PubMed] [Google Scholar]
- 14.Kathariya R, Pradeep AR. Split mouth de-epithelization techniques for gingival depigmentation: A case series and review of literature. J Indian Soc Periodontol. 2011;15:161–8. doi: 10.4103/0972-124X.84387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almas K, Sadig W. Surgical treatment of melanin-pigmented gingiva; an esthetic approach. Indian J Dent Res. 2002;13:70–3. [PubMed] [Google Scholar]
- 16.Mokeem SA. Management of gingival hyperpigmentation by surgical abrasion: Report of three cases. Saudi Dent J. 2006;18:162–6. [Google Scholar]
- 17.Tal H, Landsberg J, Kozlovsky A. Cryosurgical depigmentation of the gingiva. A case report. J Clin Periodontol. 1987;14:614–7. doi: 10.1111/j.1600-051x.1987.tb01525.x. [DOI] [PubMed] [Google Scholar]
- 18.Yeh CJ. Cryosurgical treatment of melanin-pigmented gingiva. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:660–3. doi: 10.1016/s1079-2104(98)90199-8. [DOI] [PubMed] [Google Scholar]
- 19.Deepak P, Sunil S, Mishra R, Sheshadri Treatment of gingival pigmentation: A case series. Indian J Dent Res. 2005;16:171–6. doi: 10.4103/0970-9290.29901. [DOI] [PubMed] [Google Scholar]
- 20.Ozbayrak S, Dumlu A, Ercalik-Yalcinkaya S. Treatment of melanin-pigmented gingiva and oral mucosa by CO2 laser. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:14–5. doi: 10.1067/moe.2000.106396. [DOI] [PubMed] [Google Scholar]
- 21.Sharon E, Azaz B, Ulmansky M. Vaporization of melanin in oral tissues and skin with a carbon dioxide laser: A canine study. J Oral Maxillofac Surg. 2000;58:1387–93. doi: 10.1053/joms.2000.18272. [DOI] [PubMed] [Google Scholar]
- 22.Tamizi M, Taheri M. Treatment of severe physiologic gingival pigmentation with free gingival autograft. Quintessence Int. 1996;27:555–8. [PubMed] [Google Scholar]
- 23.Phillips GE, John V. Use of a subepithelial connective tissue graft to treat an area pigmented with graphite. J Periodontol. 2005;76:1572–5. doi: 10.1902/jop.2005.76.9.1572. [DOI] [PubMed] [Google Scholar]
- 24.Pontes AE, Pontes CC, Souza SL, Novaes AB, Jr, Grisi MF, Taba M., Jr Evaluation of the efficacy of the acellular dermal matrix allograft with partial thickness flap in the elimination of gingival melanin pigmentation. A comparative clinical study with 12 months of follow-up. J Esthet Restor Dent. 2006;18:135–43. doi: 10.1111/j.1708-8240.2006.00004_1.x. [DOI] [PubMed] [Google Scholar]
- 25.Yu CH, Chen HM, Chang CC, Hung HY, Hsiao CK, Chiang CP. Cotton-swab cryotherapy for oral leukoplakia. Head Neck. 2009;31:983–8. doi: 10.1002/hed.21055. [DOI] [PubMed] [Google Scholar]
- 26.Bansal A, Jain S, Gupta S. Cryosurgery in the treatment of Oro-facial lesions. Indian J Dent Res. 2012;23:297. doi: 10.4103/0970-9290.100468. [DOI] [PubMed] [Google Scholar]
- 27.Sunitha J. Cryotherapy – A review. J Clin Diagn Res. 2010;4:2325–9. [Google Scholar]
- 28.Holtzclaw D, Toscano NJ, Tal H. Spontaneous pigmentation of non-pigmented palatal tissue after periodontal surgery. J Periodontol. 2010;81:172–6. doi: 10.1902/jop.2009.090289. [DOI] [PubMed] [Google Scholar]
- 29.Dummett CO, Bolden TE. Postsurgical clinical repigmentation of the gingiva. Oral Surg Oral Med Oral Pathol. 1963;16:353–65. [Google Scholar]
- 30.Bergamaschi O, Kon S, Doine AI, Ruben MP. Melanin repigmentation after gingivectomy: A 5-year clinical and transmission electron microscopic study in humans. Int J Periodontics Restorative Dent. 1993;13:85–92. [PubMed] [Google Scholar]
- 31.Kaur H, Jain S, Sharma RL. Duration of reappearance of gingival melanin pigmentation after surgical removal-A clinical study. J Indian Soc Periodontol. 2010;14:101–5. doi: 10.4103/0972-124X.70828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mogharehabed A, Rabiei F. Evaluation of the treatment for gingival pigmentation by cryotherapy (n2 liquid) J Dent Sch. 2006;23:656–62. [Google Scholar]
- 33.Dummett CO, Sakumura JS, Barens G. The relationship of facial skin complexion to oral mucosa pigmentation and tooth color. J Prosthet Dent. 1980;43:392–6. doi: 10.1016/0022-3913(80)90207-3. [DOI] [PubMed] [Google Scholar]
- 34.Ginwalla TM, Gomes BC, Varma BR. Surgical removal of gingival pigmentation.(A preliminary study) J Indian Dent Assoc. 1966;38:147–50. [PubMed] [Google Scholar]
- 35.Farnoosh AA. Treatment of gingival pigmentation and discoloration for esthetic purposes. Int J Periodontics Restorative Dent. 1990;10:312–9. [PubMed] [Google Scholar]
- 36.Pal TK, Kapoor KK, Parel CC, Mukherjee K. Gingival melanin pigmentation-A study on its removal for esthetic. Indian Soc Periodontol. 1994;3:52–4. [Google Scholar]
- 37.Shulman J. Clinical evaluation of an acellular dermal allograft for increasing the zone of attached gingiva. Pract Periodontics Aesthet Dent. 1996;8:201–8. [PubMed] [Google Scholar]
- 38.Fowler EB, Breault LG, Galvin BG. Enhancing physiologic pigmentation utilizing a free gingival graft. Pract Periodontics Aesthet Dent. 2000;12:193–6. [PubMed] [Google Scholar]
- 39.Novaes AB, Jr, Pontes CC, Souza SL, Grisi MF, Taba M., Jr The use of acellular dermal matrix allograft for the elimination of gingival melanin pigmentation: Case presentation with 2 years of follow-up. Pract Proced Aesthet Dent. 2002;14:619–23. [PubMed] [Google Scholar]
- 40.Nakamura Y, Hossain M, Hirayama K, Matsumoto K. A clinical study on the removal of gingival melanin pigmentation with the CO(2) laser. Lasers Surg Med. 1999;25:140–7. doi: 10.1002/(sici)1096-9101(1999)25:2<140::aid-lsm7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 41.Trelles MA, Verkruysse W, Seguí JM, Udaeta A. Treatment of melanotic spots in the gingiva by argon laser. J Oral Maxillofac Surg. 1993;51:759–61. doi: 10.1016/s0278-2391(10)80416-1. [DOI] [PubMed] [Google Scholar]
- 42.Atsawasuwan P, Greethong K, Nimmanon V. Treatment of gingival hyperpigmentation for esthetic purposes by Nd: YAG laser: Report of 4 cases. J Periodontol. 2000;71:315–21. doi: 10.1902/jop.2000.71.2.315. [DOI] [PubMed] [Google Scholar]
- 43.Berk G, Atici K, Berk N. Treatment of gingival pigmentation with Er, Gr: YSGG laser. J Oral Laser Appl. 2005;5:249–53. [Google Scholar]


