Abstract
Ameloblastoma has intrigued clinicians as well as pathologists due to its diverse clinical behavior and histomorphologic presentations. Keratoameloblastoma is a rare histologic sub type, characterized by extensive keratin formation within ameloblastic epithelium, with only a handful number of cases described in the literature. Here, we report a case of this uncommon sub type of ameloblastoma in a young female patient presenting as an extensive lesion in mandibular ramus area. The radiological and fine needle aspiration findings suggested of a keratinizing cystic lesion and incisional biopsy showed features of ameloblastoma. Patient underwent segmental mandibulectomy and histological examination of excisional specimen revealed features of ameloblastoma with abundant keratinization leading to a diagnosis of keratoameloblastoma. The diagnostic pitfalls related with the lesion have been discussed along with a short review of the literature.
Keywords: Ameloblastoma, fine needle aspiration, keratoameloblastoma, odontogenic keratocyst, papilliferous keratoameloblastoma
INTRODUCTION
Ameloblastoma is a true neoplasm of odontogenic epithelial origin, which does not undergo differentiation to the point of hard tissue formation. It is slow-growing but locally invasive, with a high rate of recurrence if not treated adequately. Its incidence, combined with its clinical behavior, makes ameloblastoma the most significant odontogenic neoplasm. Furthermore, the histomorphological diversity exhibited by the tumor and its possible clinical implications make it even more intriguing. The follicular and plexiform are the most common histological subtypes while the other patterns are relatively rare.[1] Ameloblastomas showing extensive keratinization are extremely rare and have been termed as keratoameloblastomas.[2] Although a small amount of keratinization is accepted within the histological spectrum of acanthomatous variant of ameloblastoma, those cases described in literature as keratoameloblastoma are characterized by distinctively large amounts of keratin production within the neoplastic odontogenic epithelium. First report of such a lesion was by Pindborg in 1970, who observed a jaw tumor composed of keratinizing cysts and partly of islands with papilliferous appearance and suggested the term papilliferous keratoameloblastoma.[2] Review of English literature has revealed that subsequently fifteen more cases of ameloblastomas showing extensive keratinization have been reported, some exhibiting papilliferous component as described by Pindborg while others lacking this feature.[3,4,5,6,7,8,9,10,11,12,13,14] These have all been included in the group named keratoameloblastoma; though, it has been suggested that despite the similarity of the names, keratoameloblastoma and papilliferrous keratoameloblastoma should be considered as distinct morphological entities.[3] This article report a case of this rare variant of ameloblastoma presenting as an extensive mandibular lesion along with a short review of the literature.
CASE REPORT
A 22-year-old female patient of Indian origin reported to the out-patient department with a chief complaint of a painful swelling in the right posterior mandible of 6 months duration. The swelling was insidious in onset and had progressed slowly to cause facial asymmetry along with mild parasthesia. On examination, there was a diffuse hard swelling on the right side of face with smooth margins extending anteroposteriorly from anterior border of the masseter muscle to the posterior border of the ramus and superoinferiorly from tragus to the inferior border of the mandible [Figure 1]. The skin overlying the swelling was normal in appearance with no rise in local temperature and there was no associated cervical lymphadenopathy. Intraorally there was bicortical expansion in the posterior region of the right mandible with a firm, tender soft-tissue mass posterior to the second molar. The mucosa overlying the lesion was erythematous and the third molar was clinically absent. There was no other apparent abnormality in the oral cavity except for missing maxillary third molar on the right side. The hematological parameters of the patient were within normal range. An orthopantomograph was advised and it revealed a large unilocular lesion in the ramus of the right mandible extending supero-inferiorly from the superior border of the ramus, involving the coronoid process, to the inferior border of the mandible, leaving a thin rim of bone inferiorly. Postero-anteriorly the lesion extended from posterior border of the ramus up to the body of the mandible distal to the second molar and involving the entire width and the anterior border of the ramus [Figure 2]. Based on the clinical and radiographic findings a provisional diagnosis of ameloblastoma was made with other benign/malignant odontogenic neoplasm, keratocystic odontogenic tumor, calcifying cystic odontogenic tumor or any other intraosseous neoplasm being kept as differential diagnosis. Fine needle aspiration (FNA) was performed, which yielded fluid with some cheesy white material. Papanicolau and H and E stained smears prepared from the aspirate showed abundant poorly preserved superficial epithelial squames along with numerous nucleated and anucleate keratin flakes in a background of necrosis, debris and a moderate amount of leukocytes [Figure 3]. The cytological findings were suggestive of a keratinizing cystic lesion leading to a tentative diagnosis of odontogenic keratocyst (OKC). An incisional biopsy was done and referred to a general Pathologist and was reported as ameloblastoma. Following the diagnosis of ameloblastoma and the extensive nature of the lesion, wide surgical excision was planned. Segmental mandibulectomy up to the distal of right second premolar was performed with preservation of condyle followed by reconstruction using titanium reconstruction plate and iliac crest graft.
Figure 1.

Clinical photograph showing diffuse swelling of the right side of the face causing facial asymmetry
Figure 2.

Orthopantomograph showing large unilocular radiolucent lesion on the right mandibular ramus involving the coronoid process. Well-defined sclerotic margins and sparing of the condylar process can be noted
Figure 3.

Photomicrograph showing fine needle aspiration cytology from the lesion revealing epithelial squames along with numerous nucleated and anucleate keratin flakes in a background of necrosis and debris (PAP, ×400)
The resected specimen showed a creamy white, brittle soft-tissue mass involving the ramus with complete destruction of the lingual cortical plate. The histological sections from the tumor mass revealed proliferation of odontogenic epithelium in the form of sheets, broad interconnected plexiform ribbons [Figure 4] and few follicles with peripheral layer of tall columnar cells having hyperchromatic palisaded nuclei showing reverse polarity in focal areas. The central cells were ovoid to stellate reticulum like in appearance. Large areas of squamous metaplasia with extensive keratinization were evident within sheets of odontogenic epithelium [Figure 5]. In few places “keratin filled cystic spaces” were also evident [Figure 6], some showing Pacinian corpuscle like stacks of lamellated parakeratin [Figure 7]. The background stroma was scanty, loose and edematous with areas of cystic degeneration but relatively free of inflammation. Based on the histological findings a final diagnosis of keratoameloblastoma was made. Patient is currently kept under close follow-up and so far has not shown any signs of persistent or recurrent disease 24 months post-surgery.
Figure 4.

Photomicrograph showing proliferation of odontogenic epithelium in sheets and broad ribbons. Note the scanty loose stroma with cystic degeneration (H and E, ×100)
Figure 5.
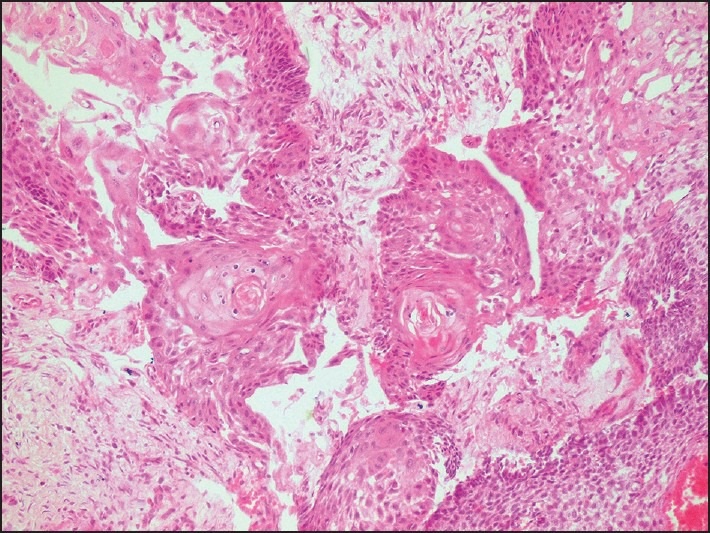
Photomicrograph showing areas of squamous metaplasia and keratin pearl formation (H and E, ×200)
Figure 6.

Photomicrograph showing central cystic degeneration in ameloblastic follicle leading to the formation of keratin filled cyst (H and E, ×200)
Figure 7.

Photomicrograph showing keratin arranged in concentric lamellated flakes resembling Pacinian corpuscles (H and E, ×400)
DISCUSSION
The term keratoameloblastoma has been used to describe a histologically heterogeneous group of ameloblastoma variants, which have in common the formation of keratin, in a considerable amount, by the ameloblastic epithelium.[3] Review of the available literature on keratoameloblastoma shows a wide variation in the histomorphological features of this neoplasm even though only few cases have been reported. Whitt et al.[3] have classified these lesions histologically into four groups:
Papilliferrous histology,
Simple histology,
Simple histology with OKC like features and
Complex histology.
Parakeratinization is most commonly observed, but orthokeratinization may also be seen. The feature most consistently described is the proliferation of ameloblastic epithelium in the form of follicles, the center of which shows keratinization with or without cystic areas. The case initially reported by Pindborg consisted partly of keratinizing cysts and partly of tumor islands with papilliferous appearance.[2] Later other cases showing keratinizing cysts lined by papilliferous epithelium were reported,[5,7,12] but a larger subset of cases lacked such papillary epithelium.[3,4,6,9,10,11,13] Some of the initial descriptions of the lesion also lacked convincing histological evidence of typical ameloblastoma like a reversal of polarity thus making it difficult to assess the true nature of these lesions.[5] Another variation has been a presence of OKC like features in the cystic elements of the tumor. This was first reported by Siar and Ng[6] who suggested that their cases may in fact represent a hybrid lesion, but others have advocated the inclusion of tumors with such histology into the spectrum of keratoameloblastoma.[9] The cases reported by Siar and Ng[6] also did not show the characteristic reversal of polarity of basal cells and the histopathological description resembled that of a case described by Ide et al.[15] who considered the lesion as solid cystic tumor variant of OKC. Whether these cases represent true ameloblastomas or fall in spectrum of neoplastic OKC needs to be explored. Few lesions have been described as having a complex histomorphology with parakeratin packed epithelial follicles along with ribbons of epithelium showing lamellar arrangement of keratin forming hair like structures[11] or Pacinian like stacks.[3,13] Extrusion of keratin into the fibrous connective tissue stroma with or without associated foreign body reaction has been reported.[3,7,11,13] It is suggested that packing of keratin within the follicle may cause its rupture leading to extrusion of keratin into the connective tissue,[3] but the lack of any inflammatory response against this extruded keratin in most of the cases makes this assumption equivocal. On the other hand, lamellar stacks of keratin filling up the entire follicle and lying in the close proximity of surrounding connective tissue fibers may give an erroneous impression of extrusion though they may actually be separated by a delicate basement membrane. The eosinophilia demonstrated by all three tissue components (i.e., keratin, collagen and basement membrane) in H and E stain may further compound this problem and even trichrome staining may fail to resolve the issue. Possibly use of immunohistochemical marker for basement membrane components may help in shedding some light on this problem. Other reported features include cribriform or solid areas with squamous metaplasia, tubular structures, focal granular cell change, cementum or bone like calcifications and necrotic debris within cystic lumina.[3,11,12] The connective tissue stroma is usually mature fibrocollagenous, but rarely may show myxoid changes.[13] The present case showed few unique previously unreported or rarely reported features like proliferation of odontogenic epithelium in sheets and broad interconnected ribbons rather than the typical keratin packed follicles. The stroma also was scanty, loose and myxoid with areas of cystic degeneration, features reminiscent of the plexiform variety of ameloblastoma. Even though we found Pacinian like stacks of lamellated keratin, none of them were seen extruding into the connective tissue as has been seen in few previous cases.[3,11,13] At this point, we would also like to emphasize on the utility of FNA in diagnosis of this lesion. Though FNA has been reported to be a valuable tool in the pre-operative diagnosis of ameloblastoma by few authors,[16,17] its accuracy in certain histological variants such as acanthomatous ameloblasoma and keratoameloblastoma needs to be examined carefully. Presence of abundant keratinous debris in absence of typical epithelial component, as seen in our case, could make it difficult to differentiate from other keratinizing lesions, such as OKC and intraosseous keratinizing squamous cell carcinoma.
Clinically, a diverse age distribution has been reported ranging from 26 to 76 years with peak incidence in the fourth decade making the present case youngest of the reported cases. Three fourth of the cases were seen in males, which is in contrast to conventional ameloblastoma, which tends to occur in almost equal frequency among the genders.[2] The lesion has been reported from a diverse population groups including Caucasian,[3] African[13] and East Asian[11] but to the best of our knowledge it has not been reported in a native Indian subcontinent population. Similar to conventional ameloblastoma, mandibular cases outnumber maxillary ones by almost 3:1 with mandibular molar-ramus area being the most common site. On the other hand, a greater number of maxillary lesions (50%) have been found to occur in the anterior region when compared with conventional ameloblastoma.[2] Radiographically, majority of lesions presented as multilocular radiolucencies, a minority being unilocular as seen in the present case. Two of the lesions have also been reported exhibiting mixed radiolucency and opacity giving a ground glass appearance.[6,14] Curettage, enucleation, segmental resection and hemimandibulectomy are the various modalities which have been used for treating cases of keratoameloblastoma. Though there is very limited post-treatment follow-up data available for this rare variant of ameloblastoma, recurrences have been reported in two of the four reported follow-ups. In one case recurrence was seen within 6 months following curettage.[9] In another case, exhibiting papilliferous histopathology, which was treated by hemimandibulectomy, there were two local recurrences in the 4th and 5th year respectively following the initial surgery. This prompted the authors to consider the papilliferous variant as a malignancy and suggest renaming of the lesion to papillary ameloblastic carcinoma.[12] Based on the available data, it would be prudent to follow a similar treatment protocol for these lesions as that of a conventional solid multicystic ameloblastoma with a close and extended follow-up of the patients.
CONCLUSION
Although ameloblastoma is the most common occurring odontogenic neoplasm, a considerable clinical, radiological and histomorphological diversity may sometimes make the diagnosis difficult especially in the absence of classical pathological characteristics. The problem may be compounded in cases where a small incisional biopsy sample or FNA findings might show features overlapping with other lesions. Keratoameloblastoma, though rare, is one such variant where tendency for extensive keratinization may mask the pathognomonic features and may mislead toward the diagnosis of other keratinizing lesions. Since there is limited literature on this variant of ameloblastoma, we suggest that a close follow-up of the patients and reporting of recurrences, if encountered, would help in further understanding the nature of these lesions and formulating appropriate treatment modalities for the patients.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Adebiyi KE, Ugboko VI, Omoniyi-Esan GO, Ndukwe KC, Oginni FO. Clinicopathological analysis of histological variants of ameloblastoma in a suburban Nigerian population. Head Face Med. 2006;2:42. doi: 10.1186/1746-160X-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reichart PA, Philipsen HP. London: Quintessence Publishing Co. Ltd; 2004. Odontogenic Tumors and Allied Lesions; pp. 43–58. [Google Scholar]
- 3.Whitt JC, Dunlap CL, Sheets JL, Thompson ML. Keratoameloblastoma: A tumor sui generis or a chimera? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:368–76. doi: 10.1016/j.tripleo.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 4.Altini M, Lurie R, Shear M. A case report of keratoameloblastoma. Int J Oral Surg. 1976;5:245–9. doi: 10.1016/s0300-9785(76)80020-8. [DOI] [PubMed] [Google Scholar]
- 5.Altini M, Slabbert HD, Johnston T. Papilliferous keratoameloblastoma. J Oral Pathol Med. 1991;20:46–8. doi: 10.1111/j.1600-0714.1991.tb00887.x. [DOI] [PubMed] [Google Scholar]
- 6.Siar CH, Ng KH. ‘Combined ameloblastoma and odontogenic keratocyst’ or ‘keratinising ameloblastoma’. Br J Oral Maxillofac Surg. 1993;31:183–6. doi: 10.1016/0266-4356(93)90122-d. [DOI] [PubMed] [Google Scholar]
- 7.Norval EJ, Thompson IO, van Wyk CW. An unusual variant of keratoameloblastoma. J Oral Pathol Med. 1994;23:465–7. doi: 10.1111/j.1600-0714.1994.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 8.Raubenheimer EJ, van Heerden WF, Noffke CE. Infrequent clinicopathological findings in 108 ameloblastomas. J Oral Pathol Med. 1995;24:227–32. doi: 10.1111/j.1600-0714.1995.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 9.Said-al-Naief NA, Lumerman H, Ramer M, Kopp W, Kringstein GJ, Persenchino F, et al. Keratoameloblastoma of the maxilla. A case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:535–9. doi: 10.1016/s1079-2104(97)90270-5. [DOI] [PubMed] [Google Scholar]
- 10.Kaku T, Ohuchi T, Hattori Y, Nishimura M, Nakade O, Abiko Y, et al. Keratoameloblastoma of the mandible. J Oral Pathol Med. 2000;29:350. [Google Scholar]
- 11.Takeda Y, Satoh M, Nakamura S, Ohya T. Keratoameloblastoma with unique histological architecture: An undescribed variation of ameloblastoma. Virchows Arch. 2001;439:593–6. doi: 10.1007/s004280100489. [DOI] [PubMed] [Google Scholar]
- 12.Collini P, Zucchini N, Vessecchia G, Guzzo M. Papilliferous keratoameloblastoma of mandible: A papillary ameloblastic carcinoma: Report of a case with a 6-year follow-up and review of the literature. Int J Surg Pathol. 2002;10:149–55. doi: 10.1177/106689690201000210. [DOI] [PubMed] [Google Scholar]
- 13.Adeyemi B, Adisa A, Fasola A, Akang E. Keratoameloblastoma of the mandible. J Oral Maxillofac Pathol. 2010;14:77–9. doi: 10.4103/0973-029X.72507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sisto JM, Olsen GG. Keratoameloblastoma: Complex histologic variant of ameloblastoma. J Oral Maxillofac Surg. 2012;70:860–4. doi: 10.1016/j.joms.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 15.Ide F, Mishima K, Saito I. Solid-cystic tumor variant of odontogenic keratocyst: An aggressive but benign lesion simulating keratoameloblastoma. Virchows Arch. 2003;442:501–3. doi: 10.1007/s00428-003-0764-8. [DOI] [PubMed] [Google Scholar]
- 16.Günhan O. Fine needle aspiration cytology of ameloblastoma. A report of 10 cases. Acta Cytol. 1996;40:967–9. doi: 10.1159/000334010. [DOI] [PubMed] [Google Scholar]
- 17.Mathew S, Rappaport K, Ali SZ, Busseniers AE, Rosenthal DL. Ameloblastoma. Cytologic findings and literature review. Acta Cytol. 1997;41:955–60. doi: 10.1159/000332767. [DOI] [PubMed] [Google Scholar]


