Abstract
Both hydrophilic and lipophilic therapeutics can be delivered successfully into deep and peripheral tissues such as cerebrospinal fluid and central nervous system by encapsulating them with crystalline lipids as lipospheres. The advent of lipospheres was meant to deliver both therapeutic moieties with enhanced efficacy and added stability to reach out intended tissue areas. Although extensive information is available on physicochemical, analytical and biopharmaceutical aspects of lipospheres, there was no specific order pertaining to critical composition and rationale of component selection available for academic and pilot scale processing of lipospheres. With the interest of compiling key points in a typical formulation of lipid lipospheres, this article was intrigued to discuss melt method, co-solvent, microemulsion, super critical fluid, spray drying and spray congealing techniques that were employed to scale up lipospheres. The selection criteria for both the drugs and lipids in liposphere formulations were demonstrated here. The quality assessment with variables like loading capacity and entrapment efficiency was explained. A note on preliminary screening factors to determine the liposphere formation such as liposphere dimensions with morphological scenario was detailed in this article. This article also includes the stability and storage issues with reference to photolysis. The marked differential in enhancing solubility and permeability characteristics of Class II and IV drug candidates by liposphere delivery systems with an evident of in vivo outcomes were emphasized.
Keywords: Critical composition, entrapment efficiency, lipids, loading capacity, morphology, stability
INTRODUCTION
Multiple-unit drug delivery systems such as nanoparticles, microparticles, microemulsions and liposomes offer more advantages than the single-unit systems with respect to their uniform distribution in the gastrointestinal tract resulting in uniform absorption of the drug. The drawback of these particulate systems, being the degradation of the polymer,[1] organic solvent residues present in the delivery system that could result in severe acceptability and toxicity problems.[2] To resolve these issues, lipid microspheres, often called lipospheres, have been proposed as a new type of fat-based encapsulation system for drug delivery of bioactive compounds. Lipospheres are lipid based water dispersible solid particles bearing particle size between 0.01 and 100 μm in diameter, composed of a solid hydrophobic lipid core containing active drug moiety dissolved or dispersed in a solid fat matrix, which is stabilized by a layer of phospholipid molecules as external coat.[3,4,5] Improved stability of the drug in the formulation, freeze dry and reconstitution properties, controlled particle size, high-drug load, well controlled drug release and no carrier toxicity made liposphere systems superior over other particulate delivery systems such as emulsions, liposomes and microspheres.[6,7] In addition, lipospheres protect the drug candidates from hydrolysis, corroborate the shelf life facilitating high bioavailability and prolonged plasma levels.[8] The lipospheres offer well controlled delivery to a variety of drug candidates like antiinflammatory compounds, local anesthetics, antibiotics and anticancer agents. The lipid entrapped vaccines were administered with an adjuvant effect through liposphere systems.[9,10,11,12]
CRITICAL COMPOSITION OF LIPOSPHERES
Lipospheres are composed of solid lipid core surrounded by a single unit phospholipid layer that may entrap the drug or enrich its coat with the drug. The emulsifier or stabilizer is used to form uniform coat around the core material and to facilitate partitioning of the drug between the lipid and aqueous phases.[13,14] Low molecular polyethylene glycols (PEGs), as plasticizers could impart tensile strength to the external lipid coat. The strong affinity between progesterone, a lipophilic drug and lipid was observed and evidenced with high-entrapment efficiency (EE) of 70% which resulted in sustained release.[15] On the other hand, w/o/w emulsion system was employed for a better entrapment of a hydrophilic drug. Sodium cromoglycate release was found to be dependent on the principle of the stabilizer. Different stabilizers such as gelatin and poloxamer 407 used in hydrophilic drug containing liposphere formulation have exhibited sigmoid release and biphasic release sequentially.
Morphology and characteristics of lipospheres are affected of excipients. This was exemplified with a highly lipophilic drug allopurinol containing lipid systems. The lipids such as beeswax, stearic acid, cetyl alcohol, stearyl alcohol and cetostearyl alcohol as excipients were used in combination of pluronic-68 as dispersant at different ratios. The size of allopurinol with bees wax lipid particles found to be small followed by stearyl alcohol.[16]
The lipid compounds such as tristearin, tripalmitin, trilaurin and tricaprin were being used as major lipid composition in the development of liposphere formulations to establish lipid core and allows interaction of the drug moiety. The phospholipids were being employed to establish the external coat of lipospheres which are found to biosimilar to cell components. The important phospholipids used in liposphere formulations are of purified ones from natural origin, such as soyabean phosphotidyl choline and its derivative hydrogenated soyabean phosphotidyl choline. The synthesized phospholipids are viz. dimyristoyl phosphatidylcholine, dipalmitoyl phosphatidylcholine and distearoyl phosphatidylcholine were proved effective as coat materials.[17] The integrity and physical stability of lipospheres were contributed by the size of lipid particle and its surface charge. Affect of the nonionic surfactants such as Tween 20 and Tween 80 on controlling particle size and reducing the surface charges of lipid crystal particle formulations led to prolonged stability period.[18]
SELECTION CRITERIA OF DRUGS AND EXCIPIENTS
Delivery of lipophilic drugs to the target site was the main theme of liposphere formulation, where the phospholipid coat causes increased permeability by minimizing the solubility problem of the lipophilic moiety. In the case of hydrophilic drug moieties, the permeability through the biomembrane is limited; this can be successfully overcome by incorporating the active moiety in the lipid core. Hence both types of drugs can be incorporated in the lipospheres, whereas till date the lipophilic drug encapsulation was reported to be higher. The effective delivery of peptides was achieved by lipospheres with enhanced stability of peptides by reducing their exposure to different pH environmental conditions. Thus, the demerits of other site specific/targeted drug delivery systems could be minimized by proper selection of liposphere carrier, which enables the delivery of the drug moiety effectively at the specific tissue/organs.[19]
The key factors to be considered for selection of the carrier are physicochemical properties, compatibility between drug and carrier and drug distribution in solid lipid matrix (SLM).[20] Among the physical characteristics, the selection priority belongs to the melting point of the carrier. The melting point of carrier should be >45°C to minimize the stability problems.[21] The hydrophilic lipophilic balance value of core materials should be <2, since they are more lipophilic and have high chances to form solid matrices over the hydrophilic materials which form colloidal dispersions. The carrier should have the capability to solubilize the drug and to form particles of optimum size and strength enabling the drug release at desired site.[22]
CORE PRINCIPLES FOR FORMATION OF LIPOSPHERES
Distribution of drug in the SLMs was found to be in three ways, such as homogeneous matrix, drug-enriched shell and drug-enriched core [Figure 1]. Cold and hot homogenization processes led to homogeneous matrix in which the active moiety was dispersed in SLM either in molecular form or amorphous cluster. In drug-enriched shell, lipids get precipitated without drug and then drug filled shell got crystallizes on the lipid core and led to burst release of drug from lipospheres. Drug-enriched core formed by precipitating the drug followed by lipid shell containing less amount of drug. This model obeys Fick's law of diffusion and releases the drug in a controlled manner. Drug distribution patterns in SLM were depended on the structure of matrix, chemical properties of the drug, excipients and their magnitude of interaction and also on production conditions. The drug distribution in SLM cannot be determined by lysis owing to a smaller size of particles and low melting points of lipids.[23] Simulation methods were employed to analyse the drug distribution in SLMs and thereby release characteristics of the drug were assessed.[24] In the compritol SLM, ibuprofen molecules were distributed in outer matrix formed by the polar hydroxyl groups of compritol, which interact with carboxyl groups of ibuprofen. Hence, the hydrophobic groups of the ibuprofen molecules remain in the body of the carrier with their carboxyl groups at the oil/water interface along with the hydroxyl groups of compritol. By this distribution of drug molecules in the lipid, matrix was demonstrated.
Figure 1.
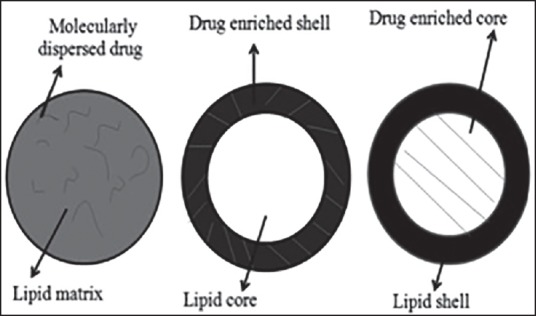
Drug incorporation models in solid lipid matrix
TECHNIQUES WIDELY EMPLOYED IN PREPARATION OF LIPOSPHERES
Melt method
The lipophilic drug was melted along with the lipid material, and the temperature was maintained at slightly higher than the melting point of the lipid or lipid mixture to maintain in the molten state. Phospholipids such as soya lecithins were added to the phosphate buffer or the aqueous phase, which was maintained at a temperature nearly or slightly higher than the lipid phase. The lipid mixture and the aqueous phase containing phospholipids were mixed together. In addition, emulsifier at required concentration can be added to form uniform sized spheres at range of 38-50 μm.[25] Melatonin lipospheres of melt method for topical application was proved to be effective compared to that of gels or lotions.[26] Several drugs like bupivacaine, glipizide, aceclofenac, retinyl acetate, progesterone, sodium cromglycate, diclofenac, carbamazepine, C14-diazepam, proteins like somatostatin, casein,[27] bovine serum albumin, R32NS1 malaria antigen, tripalmitin based lipospheres for lab-on-chip applications have been prepared by melt dispersion methods.
Co-solvent method
Use of co-solvent in liposphere development facilitates the enhanced solubility of lipids and lipophilic drugs and to obtain clear homogenous solutions. This was evident when chloroform was used as co-solvent to prepare peptide loaded lipospheres and to solubilize the polylactic acid, hydrogenated soyabean phosphotidyl choline and thereby to obtain a clear solution of N-methyl pyrollidone containing peptide.[28] Selection of solvents and co-solvents was the result of miscibility which affects the output. The highly hydrophobic drugs viz. somatostatin, triptorelin, leuprolide were formulated as lipid systems in the presence of co-solvents such as dichloromethane, ethyl acetate, acetone, methyl ethyl ketone, tetrahydrofurane and acetonitrile and made them to solubilize along with phospholipids and polymers.[29]
Multiple microemulsion method
The uniform size about 300 nm with 90% EE was reported with multiple microemulsion method. In this approach, the hydrophilic drugs were dissolved in an aqueous phase, and this solution was added to the lipid phase to yield primary emulsion at high temperatures. Then, the solution was added to the oil phase containing hydrophobic emulsifier to yield uniform size lipospheres.[30] The same technique was employed on thymopentin encapsulation using sodium hexadecyl phosphate, a lipophilic counter ion.[31]
Super critical fluid method
The lipid and drug dissolved in a suitable organic solvent to form a solution which was emulsified in an aqueous phase to form an emulsion containing a discontinuous phase of micelles comprised of organic solvent, drug and lipid. Finally, the emulsion was treated with a super critical fluid (SCF) under suitable conditions, which results in the extraction of the organic solvent from the micelles and precipitation of solid composite lipospheres in the aqueous dispersion.[32] Rapid removal of pressure causes the supersaturation of particles leading to enhanced stability. Use of CO2 as a SCF was favored due its low cost, nontoxicity, a critical point at 31°C and 74 bars of pressure.[33] Insulin SLM was followed SCF method for pulmonary delivery.[34]
Spray drying method
Spray drying provides smaller size particles with homogeneous distribution compared to other methods. The shape of particles was affected by drying rate, viscosity and surface tension of the drying liquid.[35] The key parameters involved with this method were inlet and outlet temperatures, feeding rate, drying gas medium, gas flow rate, gas humidity and residence time. The rate of particle formation was controlled by these key parameters. As complete removal of solvent was observed, the chance of toxicity was also minimized. This technique was highly applicable n food industry in producing peptide loaded lipospheres.[36]
Spray congealing method
Spray congealing was successfully employed for preparing lipid microparticles loaded with therapeutics such as clarithomycin, theophylline, verapamil and indomethacin.[37,38,39,40] The molten lipid-containing dispersed drug at 70°C was made to flow into the spray congealer specifically into cyclone which was maintained at −20°C, which lead to separation of solid particles that were again made to atomize to remove adhered condensed water. The atomization pressure and spraying temperature affect the particles size distribution and also product yield. It was noticed that the increased spraying temperature and pressure in spray congealing cause the result of reduced particle size.[41] This method can be recommended for scale-up process of sensitive drugs like peptides to favor the stability of the active moiety and to sustain the release.
Lipospheres are differing from other nano lipid carrier systems in terms of preparation itself. The solid lipid nanoparticles can be prepared by double emulsification method whereas nanostructured liquid crystals can be obtained by emulsification of cubic lipid (e.g., glyceryl monooleate) phases in aqueous buffers.[42]
FACTORS AFFECTING THE MORPHOLOGY OF LIPOSPHERES
The solvent(s) used in solvent evaporation or co-solvent technique affect the particle morphology, and the solvent remnant were highly toxic. Solvent traces on the particle surface made them aggregate whereas, in melt method, particle aggregation was absent. In the case of oxytetracycline lipospheres prepared by melt method, mean particle size of 15-40 μm was obtained whereas blank lipospheres showed an average size of 10 μm where the drug moiety remained dispersed as particles of 5 μm. Thus, the average size of loaded particles raised compared to blank lipospheres.[43]
The production of lipospheres by spray congeal and spray drying, involved with atomization of the drug and lipids that form coat, which favors microencapsulation. Avobenzene lipospheres morphology was found to follow the preparation methods. The avobenzene lipid particles composed of caranuaba wax and phosphotidyl choline were yielded with uniform and spherical particles by spray drying compared to that of melt method. The morphology of lipospheres also affects the drug release patterns which were noticed with 26% of avobenzene release in spray dried particles whereas 60% release in melt method within given time.
Size analysis
Particle size and size distribution studies can be performed using Coulter counter method to correlate the size and drug release from lipospheres. The lipid particles showed burst release profile for compritol lipospheres loaded with tetracaine, which was due to more surface area of exposure.[44] In spray congealing of insulin loaded SLM, the temperature, pressure and type of lipid determines the size of the particle. As the concentration of insulin increases, the particle size get increased, which was revealed in scanning electron microscopy (SEM) images. The smaller sized particles of about 182 μm were best suited for parenteral application.[41] The mean particle diameter of 20 μm was obtained by melt method using bupivacaine, glyceryl palmitostearate and Miglyol 812, Tween 80 and stearic acid as observed with photon correlation spectroscopy (PCS).[45] In pilocarpine loaded lipospheres of size range between 75 and 85 nm have accomplished the drug release as required.[46]
The morphology of lipospheres was characterized by transmission electron microscopy where interaction of the electrons with lipid surface produces the images and SEM where electronic transitions with particle surface produce the images.[47,48] On the other hand, PCS and laser diffractometry were also able to provide information on particle shape and size. Field emission SEM (FESEM) can be effective in case of particles that were not recognized by SEM where sample preparation may damage the particle morphology. Cryogenic FESEM, where liquid nitrogen was used to freeze the liquid dispersion, produced microscopic images in the frozen state. The above methods were of two-dimensional analyses of the particles and three-dimensional profiles which include structural, mechanical, functional and topographical information of lipospheres were given by atomic field microscopy.[49] Nuclear magnetic resonance spectroscopy being widely used for analyzing the nature of the lipids and the lamillarity of the lipid particles formed, using paramagnetic ions like manganese (Mn2+) and preseodimium (Pr3+) that form complexes with the lipids based on the polar groups available. Liposphere formulation containing phosphatidylethanolamine were identified using the trinitrobenzenesulfonic acid labeling with evidence of 70-90% of the phospholipid polar heads in the particle surfaces.[50,51]
IN VITRO EVALUATION TECHNIQUES
The entrapment efficiency is defined as the drug entrapped in the lipid based particles, relative to the total amount of drug added, that is percent of drug included in the particles versus percent of drug remaining in the dispersion medium, which can be calculated from Equation 1. The EE increases with drug concentration. The EE depends on the polymer concentration as well. This was evident with that of EE of gentamycin, which was depended on PEG and EE and subsequent microencapsulation were increased gradually with PEG concentration.[52] The EE was also affected by the lipid composition/ratio used in formulating the lipospheres. The reason behind it may be due to the presence of small amounts of fat in the inner core of the lipospheres which lead to saturation of the fat core of the lipospheres by the drug incorporated in dispersion.[53] The EE also depends upon the drug solubility in the solvent system used for processing. Various co-solvents such as ethanol, dimethyl sulfoxide and dimethyl formamide been often used in the formulation of lipospheres since they aid in a higher drug entrapment.[54] Ultrafiltration and microdialysis were considered as the most reliable techniques for EE quantification, while result obtained by ultracentrifugation, the fastest and easiest technique, but not always accurate.[55] Loading capacity (LC) was the percentage of drug incorporated into the lipid particles, relative to the total weight of the lipid phase (drug + lipid) and it would be computed from the Equation 2. LC being an important parameter for characterization and optimization of lipid-based drug carrier, depends mainly on the solubility of the drug under investigation in the core lipid/lipids blend, miscibility of drug melt and lipid melt, chemical and physical stature of the SLM and the polymorphic state of the lipid. The reported LC values range between 1% for prednisolone, 20-25% for cyclosporine A (CsA) and up to 50% for extremely lipophilic compound Vitamin E.[56]
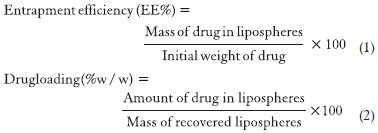
SIGNS OF INCOMPATIBILITY
The incompatibility between the drug and solid lipid core that cause the escape of drug from lipospheres was observed in bupivacaine lipospheres. Core formed by ethyl stearate could not incorporate bupivacaine due to incompatibility. Bupivacaine migrated out of the particles and got needle-like crystals which were due to a gradual dissolution of the drug by aqueous medium to saturation. The migration was occurred due to the presence of water in the liposphere dispersion, which could be avoided by lyophilization using cryo-protectant like sucrose and kept dry until reconstituted before use.[9]
SUITABLE FORMULATIONS OF LIPOSPHERES
Lipospheres being possible alternative to avoid the side effects resulting from the oral administration. The aceclofenac was formulated into lipospheres successfully to sustain the release topically.[57] The antigen or immunogen, alone or in combination with a phospholipid carrier were able to form lipospheres with aid of melt method and also with solvent preparation.[58] One of the most promising approaches for the delivery of poor water-soluble drugs is the use of layer-by-layer assembly technology for the encapsulation of the lipid based drugs. This technique permits the step-wise adsorption of the various components as the layer growth is governed by their electrostatic attraction and allows the formation of multi-layer shells with nanometer-scale precision. The application of layer-by-layer assembly for emulsions, nanoparticles and capsule based delivery systems for lipid based drugs were extensively developed.[59] The lipid microparticles as a parenteral controlled release device for peptides were also established.[6]
SUITABLE STORAGE CONDITION
As the storage conditions are important for lipid dispersion, the formulated lipospheres can be stored at 4°C in order to prevent the degradation of the coat and core material and thereby maintaining the structural integrity. Lipospheres are very stable after 3 months storage at 2-8°C manifested by low leakage rate (<7%) and no major changes in particle size.[57] Oxytetracycline injectable lipospheres meant for veterinary use were analyzed for the injectability when stored at 4°C showed stability irrespective of the lipid used in liposphere formulations.[60] If proper storage conditions were not maintained the problems of stability could be aroused leading to failure and may cause toxicity due to degradation of lipids.
STABILITY STUDIES
Many studies have conducted on liposphere stability at various stress conditions. Among them, the photolysis based stability testing was proven to be a benchmark. The photolysis based stability studies were explained with an example of butyl methoxydibenzoylmethane (BMDBM), a sunscreen agent complexed with hydroxypropyl-β-cyclodextrin (HP-β-CD). The lipospheres of BMDBM were developed with tristearin and hydrogenated soybean phosphotidylcholine. The resulting cream was undertaken for photo degradation study about 3 months in which permeability of lipospheres and also the release of drug were evaluated. It was learnt that the lipospheres are able to provide further superior protection to the drug in formulation, apart from the protection provided by HP-β-CD inclusion complexation.[43]
IN VIVO EVALUATION
The efficiency of target/site specific delivery of liposphere systems was supported by in vivo studies. Out of these evidences, the curcumin loaded lipospheres targeted to colon for treating intestinal bowel disorder were evaluated based on the degree of inflammation and the presence of edema or ulceration, diarrheal score and visible fecal blood.[61] Lipospheres of bupivacaine was pharmacodynamically assessed for its nerve blockade. Both sensory and motor blockade lasted for 11 h on greater side and the least being 1.8 h.[62] The CsA loaded lipospheres were developed by spray drying along with hyaluronic acid and sodium lauryl sulfate and had increased in vitro and in vivo parameters compared with CsA powder. Such research findings provide strong support to lipospheres as effective oral dosage forms for poorly water-soluble drugs.[63]
APPLICATIONS
Griseofulvin, a Biopharmaceutics Classification System Class IV has 50% efficacy in clearing the lesions of Tinea corpis and 75% of mycological cure rate by liposphere formulation.[64] Oral particulate CsA liposphere dispersion was found to have improved bioavailability about 60%, while treating transplant rejection and autoimmune diseases.[65] Donepezil lipospheres were found to be efficacious in Alzheimer's treatment when it was administered as subcutaneous and intramuscular depot.[66] A formulation of buoyant lipospheres with lercanidipine, an antihypertensive agent was successfully proved as controlled delivery formulation.[5] On other hand, sunscreen antioxidants were successfully formulated into lipospheres for topical and cosmetic purpose with vobenzene and BMDBM. Bupivacaine, a local anesthetic liposphere, had also followed in similar lines.
LIMITATIONS
High pressure induced drug degradation, lipid crystallization, gelation phenomena and co-existence of several colloidal species were found as drawbacks associated with liposphere processing. These drawbacks were the results of manufacturing process controls such as high pressure and rapid temperature changes.[19] Cytotoxic effects after phagocytosis, toxic effects of organic residues after the production of polymers and lack of industrial-scale up made their practice restricted.[67]
CONCLUSION
Lipospheres as crystal lipid particles composed of lipids, which are typically selected on the basis stability and physicochemical parameters. Though the lipids are prone to degradation, the formulations can be stable and firm with lipids and stabilizers. The sensitive and potent drugs can be formulated as lipospheres to enhance stability and therapeutic efficacy at low doses. The highly lipophilic or hydrophilic therapeutics and also drug candidates having narrow therapeutic indices can be delivered effectively by prolonging the circulation time and facilitates to reach the desired site of action. Moreover, the liposphere formulations are well suitable to administer by the most common routes such as oral, topical and intravenous in order to enable therapeutics to reach deep/peripheral tissues such as cerebrospinal fluid and central nervous system (CNS). This approach is very useful in treating chronic CNS ailments like Alzheimer and neurodegenerative disorders. The lipospheres are viable for commercial scale production of life saving drugs and cosmetics.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Ravi Kumar MN. Nano and microparticles as controlled drug delivery devices. J Pharm Pharm Sci. 2000;3:234–58. [PubMed] [Google Scholar]
- 2.Jenning V, Thünemann AF, Gohla SH. Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int J Pharm. 2000;199:167–77. doi: 10.1016/s0378-5173(00)00378-1. [DOI] [PubMed] [Google Scholar]
- 3.Domb AJ, Bergelson L, Amselem S. Lipospheres for controlled delivery of substances. In: Benita S, editor. Microencapsulation, Methods and Industrial Applications. New York: Marcel Dekker; 1996. pp. 377–410. [Google Scholar]
- 4.Maniar MH, Hannibal D, Amselem S, Xie X, Burch R, Domb A. Characterization of lipospheres: Effect of carrier and phospholipid on the loading of drug into the lipospheres. Pharm Res. 1991;8:175–185. [Google Scholar]
- 5.Bekerman T, Golenser J, Domb A. Cyclosporin nanoparticulate lipospheres for oral administration. J Pharm Sci. 2004;93:1264–70. doi: 10.1002/jps.20057. [DOI] [PubMed] [Google Scholar]
- 6.Reithmeier H, Herrmann J, Göpferich A. Lipid microparticles as a parenteral controlled release device for peptides. J Control Release. 2001;73:339–50. doi: 10.1016/s0168-3659(01)00354-6. [DOI] [PubMed] [Google Scholar]
- 7.Almeida AJ, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev. 2007;59:478–90. doi: 10.1016/j.addr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Souto EB, Muller RH. Lipid nanoparticles (SLN and NLC) for drug delivery. In: Domb AJ, Tabata Y, Ravi Kumar MN, Farber S, editors. Nanoparticles for Pharmaceutical Applications. California: American Scientific Publishers; 2007. pp. 103–22. [Google Scholar]
- 9.Domb AJ. Lipospheres for controlled delivery of substances. In: Benita S, editor. Microencapsulation, Methods and Industrial Applications. Boca Raton: Taylor and Francis; 2006. pp. 297–314. [Google Scholar]
- 10.Amselem S, Domb AJ. Liposphere delivery systems for vaccines. In: Bernstein H, Cohen S, editors. Microparticulate Systems for Drug Delivery. New York: Marcel Dekker; 1996. pp. 149–68. [Google Scholar]
- 11.Amselem S, Alving CR, Domb AJ. Polymeric biodegradable lipospheres as vaccine delivery systems. Polym Adv Technol. 1992;3:351–57. [Google Scholar]
- 12.Amselem S, Domb AJ, Alving CR. Lipospheres as a vaccine carrier systems: Effects of size, charge, and phospholipid composition. Vaccine Res. 1992;1:383–95. [Google Scholar]
- 13.Zhang L, Long C, Qian Y, Liu L. Stabilizer distribution on the surface of solid lipid microparticles and SLM stability: Mesoscale simulation and experiments. J Chem Ind Eng. 2007;58:181–89. [Google Scholar]
- 14.Lewis L, Boni R, Adeyeye CM. Effect of emulsifier blend on the characteristics of sustained release diclofenac microspheres. J Microencapsul. 1998;15:283–98. doi: 10.3109/02652049809006858. [DOI] [PubMed] [Google Scholar]
- 15.Cortesi R, Esposjto E, Luca G, Nastruzzi C. Production of lipospheres as carriers for bioactive compounds. Biomaterials. 2002;23:2283–94. doi: 10.1016/s0142-9612(01)00362-3. [DOI] [PubMed] [Google Scholar]
- 16.El-Gibaly I, Abdel-Ghaffar SK. Effect of hexacosanol on the characteristics of novel sustained-release allopurinol solid lipospheres (SLS): Factorial design application and product evaluation. Int J Pharm. 2005;294:33–51. doi: 10.1016/j.ijpharm.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 17.Toongsuwan S, Li LC, Erickson BK, Chang HC. Formulation and characterization of bupivacaine lipospheres. Int J Pharm. 2004;280:57–65. doi: 10.1016/j.ijpharm.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann E, Müller RH. Electrolyte- and pH-stabilities of aqueous solid lipid nanoparticle (SLN) dispersions in artificial gastrointestinal media. Eur J Pharm Biopharm. 2001;52:203–10. doi: 10.1016/s0939-6411(01)00167-9. [DOI] [PubMed] [Google Scholar]
- 19.Mehnert W, Mäder K. Solid lipid nanoparticles: Production, characterization and applications. Adv Drug Deliv Rev. 2001;47:165–96. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 20.Gasco MR, Inventor . United States Patent US 5250236A; 1993. Method for producing solid lipid microspheres having a narrow size distribution. [Google Scholar]
- 21.van Krevelen DW, Hoftyzer PJ. 3rd ed. New York: Elsevier; Properties of Polymers. [Google Scholar]
- 22.Zhang LJ, Qian Y, Long CX, Chen Y. Systematic procedures for formulation design of drug-loaded solid lipid microparticles: Selection of carrier material and stabilizer. Ind Eng Chem Res. 2008;47:6091–100. [Google Scholar]
- 23.Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;(54 Suppl 1):S131–55. doi: 10.1016/s0169-409x(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 24.Long CX, Zhang LJ, Qian Y. Mesoscale simulation of drug molecules distribution in the matrix of solid lipid microparticles (SLM) Chem Eng J. 2006;119:99–106. [Google Scholar]
- 25.Domb AJ, Maniar M, Inventor Nova Pharmaceutical Corp. United States Patent 90-US6519(9107171); 1990. Lipospheres for controlled delivery of pharmaceuticals, pesticides, and fertilizers. [Google Scholar]
- 26.Tursilli R, Casolari A, Iannuccelli V, Scalia S. Enhancement of melatonin photostability by encapsulation in lipospheres. J Pharm Biomed Anal. 2006;40:910–4. doi: 10.1016/j.jpba.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 27.Favaro-Trindade CS, Santana AS, Monterrey-Quintero ES, Trindade MA, Netto FM. The use of spray drying technology to reduce bitter taste of casein hydrolysate. Food Hydrocoll. 2010;24:336–40. [Google Scholar]
- 28.Rasiel A, Sheskin T, Bergelson L, Domb AJ. Phospholipid coated poly (lactic acid) microspheres for the delivery of LHRH analogue. Polym Adv Technol. 2002;13:127–136. [Google Scholar]
- 29.Eldridge JH, Staas JK, Meulbroek JA, McGhee JR, Tice TR, Gilley RM. Biodegradable microspheres as a vaccine delivery system. Mol Immunol. 1991;28:287–94. doi: 10.1016/0161-5890(91)90076-v. [DOI] [PubMed] [Google Scholar]
- 30.Morel S, Gasco MR, Cavalli R. Incorporation in lipospheres of [D-Trp-6] LHRH. Int J Pharm. 1995;119:126. [Google Scholar]
- 31.Morel S, Ugazio E, Cavalli R, Gasco MR. Thymopentin in solid lipid nanoparticles. Int J Pharm. 1996;132:259–61. [Google Scholar]
- 32.Shukla D, Chakraborty S, Singh S, Mishra B. Lipid-based oral multiparticulate formulations - advantages, technological advances and industrial applications. Expert Opin Drug Deliv. 2011;8:207–24. doi: 10.1517/17425247.2011.547469. [DOI] [PubMed] [Google Scholar]
- 33.Bahrami M, Ranjibarian S. Production of micro and nano composites by super critical carbon dioxide. J Supercrit Fluids. 2007;40:263–83. [Google Scholar]
- 34.Amidi M, Pellikaan HC, de Boer AH, Crommelin DJ, Hennink WE, Jiskoot W. Preparation and physicochemical characterization of supercritically dried insulin-loaded microparticles for pulmonary delivery. Eur J Pharm Biopharm. 2008;68:191–200. doi: 10.1016/j.ejpb.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Johnson KA. Preparation of peptide and protein powders for inhalation. Adv Drug Deliv Rev. 1997;26:3–15. doi: 10.1016/s0169-409x(97)00506-1. [DOI] [PubMed] [Google Scholar]
- 36.Barbosa CM, Morais HA, Delvivo FM, Mansur HS, Oliveira MC, Silvestre MP. Papain hydrolysates of casein: Molecular weight profile and encapsulation in lipospheres. J Sci Food Agric. 2004;84:1891–900. [Google Scholar]
- 37.Yajima T, Umeki N, Itai S. Optimum spray congealing conditions for masking the bitter taste of clarithromycin in wax matrix. Chem Pharm Bull (Tokyo) 1999;47:220–5. doi: 10.1248/cpb.47.220. [DOI] [PubMed] [Google Scholar]
- 38.Albertini B, Passerini N, González-Rodríguez ML, Perissutti B, Rodriguez L. Effect of aerosil on the properties of lipid controlled release microparticles. J Control Release. 2004;100:233–46. doi: 10.1016/j.jconrel.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 39.Passerini N, Perissutti B, Albertini B, Voinovich D, Moneghini M, Rodriguez L. Controlled release of verapamil hydrochloride from waxy microparticles prepared by spray congealing. J Control Release. 2003;88:263–75. doi: 10.1016/s0168-3659(03)00009-9. [DOI] [PubMed] [Google Scholar]
- 40.Fini A, Rodriguez L, Cavallari C, Albertini B, Passerini N. Ultrasound-compacted and spray-congealed indomethacin/polyethyleneglycol systems. Int J Pharm. 2002;247:11–22. doi: 10.1016/s0378-5173(02)00327-7. [DOI] [PubMed] [Google Scholar]
- 41.Maschke A, Becker C, Eyrich D, Kiermaier J, Blunk T, Göpferich A. Development of a spray congealing process for the preparation of insulin-loaded lipid microparticles and characterization thereof. Eur J Pharm Biopharm. 2007;65:175–87. doi: 10.1016/j.ejpb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Jin X, Zhang ZH, Li SL, Sun E, Tan XB, Song J, et al. A nanostructured liquid crystalline formulation of 20(S)-protopanaxadiol with improved oral absorption. Fitoterapia. 2013;84:64–71. doi: 10.1016/j.fitote.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Domb AJ. Long acting injectable oxytetracycline-liposphere formulations. Int J Pharm. 1995;124:271–8. [Google Scholar]
- 44.zur Mühlen A, Schwarz C, Mehnert W. Solid lipid nanoparticles (SLN) for controlled drug delivery — Drug release and release mechanism. Eur J Pharm Biopharm. 1998;45:149–55. doi: 10.1016/s0939-6411(97)00150-1. [DOI] [PubMed] [Google Scholar]
- 45.Pietkiewicz J, Sznitowska M, Placzek M. The expulsion of lipophilic drugs from the cores of solid lipid microspheres in diluted suspensions and in concentrates. Int J Pharm. 2006;310:64–71. doi: 10.1016/j.ijpharm.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 46.Cavalli R, Morel S, Gasco MR, Chetoni P, Saettone MF. Preparation and evaluation in vitro of colloidal lipospheres containing pilocarpine as ion pair. Int J Pharm. 1995;117:243–46. [Google Scholar]
- 47.Sawant KK, Dodiya SS. Recent advances and patents on solid lipid nanoparticles. Recent Pat Drug Deliv Formul. 2008;2:120–35. doi: 10.2174/187221108784534081. [DOI] [PubMed] [Google Scholar]
- 48.Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12:62–76. doi: 10.1208/s12249-010-9563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drake B, Prater CB, Weisenhorn AL, Gould SA, Albrecht TR, Quate CF, et al. Imaging crystals, polymers, and processes in water with the atomic force microscope. Science. 1989;243:1586–9. doi: 10.1126/science.2928794. [DOI] [PubMed] [Google Scholar]
- 50.Fenske DB. Structural and motional properties of vesicles as revealed by nuclear magnetic resonance. Chem Phys Lipids. 1993;64:143–62. doi: 10.1016/0009-3084(93)90063-9. [DOI] [PubMed] [Google Scholar]
- 51.Mukherjee S, Ray S, Thakur RS. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J Pharm Sci. 2009;71:349–58. doi: 10.4103/0250-474X.57282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gander B, johansen P, Nam-Tran H, Merkle HP. Thermodynamic approach to protein microencapsulation into poly (D,L-lactide) by spray drying. Int J Pharm. 1996;129:51–61. [Google Scholar]
- 53.Brown SA, Chime SA, Attama AA, Agu CI, Onunkwo GC. In-vito and In-vivo characterization of piroxicam loaded Dika wax lipospheres. Trop J Pharm Res. 2013;12:33–8. [Google Scholar]
- 54.Naeem M, Bhise KS. Formulation and development of Fenofibrate loaded liposphere system. J Drug Deliv Ther. 2013;3:1–10. [Google Scholar]
- 55.Liu X, Zhang Y, Tang X, Zhang H. Determination of entrapment efficiency and drug phase distribution of submicron emulsions loaded silybin. J Microencapsul. 2009;26:180–6. doi: 10.1080/02652040802211741. [DOI] [PubMed] [Google Scholar]
- 56.Muchow M, Maincent P, Muller RH. Lipid nanoparticles with a solid matrix (SLN, NLC, LDC) for oral drug delivery. Drug Dev Ind Pharm. 2008;34:1394–405. doi: 10.1080/03639040802130061. [DOI] [PubMed] [Google Scholar]
- 57.Nasr M, Mansour S, Mortada ND, El Shamy AA. Lipospheres as carriers for topical delivery of aceclofenac: Preparation, characterization and in vivo evaluation. AAPS PharmSciTech. 2008;9:154–62. doi: 10.1208/s12249-007-9028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Domb AJ. Baltimore, MD: Patent; Liposphere carriers of vaccines. 5340588. [Google Scholar]
- 59.Shchukina EM, Shchukin DG. LbL coated microcapsules for delivering lipid-based drugs. Adv Drug Deliv Rev. 2011;63:837–46. doi: 10.1016/j.addr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 60.Scalia S, Tursilli R, Sala N, Iannuccelli V. Encapsulation in lipospheres of the complex between butyl methoxydibenzoylmethane and hydroxypropyl-beta-cyclodextrin. Int J Pharm. 2006;320:79–85. doi: 10.1016/j.ijpharm.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 61.Yadav VR, Suresh S, Devi K, Yadav S. Novel formulation of solid lipid microparticles of curcumin for anti-angiogenic and anti-inflammatory activity for optimization of therapy of inflammatory bowel disease. J Pharm Pharmacol. 2009;61:311–21. doi: 10.1211/jpp/61.03.0005. [DOI] [PubMed] [Google Scholar]
- 62.Söderberg L, Dyhre H, Roth B, Björkman S. In vitro release of bupivacaine from injectable lipid formulations investigated by a single drop technique — Relation to duration of action in-vivo. J Pharm Pharmacol. 2002;54:747–55. doi: 10.1211/0022357021779087. [DOI] [PubMed] [Google Scholar]
- 63.Woo JS, Piao MG, Li DX, Ryu DS, Choi JY, Kim JA, et al. Development of cyclosporin A-loaded hyaluronic microsphere with enhanced oral bioavailability. Int J Pharm. 2007;345:134–41. doi: 10.1016/j.ijpharm.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 64.Kassem MA, Esmat S, Bendas ER, El-Komy MH. Efficacy of topical griseofulvin in treatment of tinea corporis. Mycoses. 2006;49:232–5. doi: 10.1111/j.1439-0507.2006.01221.x. [DOI] [PubMed] [Google Scholar]
- 65.Yehia SA, Elshafeey AH, Elsayed I. Biodegradable donepezil lipospheres for depot injection: Optimization and in-vivo evaluation. J Pharm Pharmacol. 2012;64:1425–37. doi: 10.1111/j.2042-7158.2012.01530.x. [DOI] [PubMed] [Google Scholar]
- 66.Pandit SS, Patil AT. Formulation and in vitro evaluation of buoyant controlled release lercanidipine lipospheres. J Microencapsul. 2009;26:635–41. doi: 10.3109/02652040802593908. [DOI] [PubMed] [Google Scholar]
- 67.Toshi A, Mazzitelli S, Capretto, Guerrieri R, Nastruzzi C. Optimization of lipospheres production by factorial design and their performances on a dielectrophoretic lab-on-a-chip platform. Colloids surf A Physicochem Eng Asp. 2009;340:77–85. [Google Scholar]


