Abstract
Introduction:
Silymarin is one of the best known hepatoprotective drugs, which is obtained from the seeds of Silybum marianum L., Family: Asteraceae or Compositae. The plant has traditionally been used for centuries as a natural remedy for liver and biliary tract diseases. The aim of the present investigation was to enhance the hepatoprotective activity of silymarin by incorporating it in chitosan (Ch) nanoparticles (NPs) for passive targeted delivery, thereby prolonging its retention time.
Materials and Methods:
Silymarin loaded NPs were prepared by ionic gelation technique, which were then optimized using a central composite design in order to minimize the particle size and maximize the drug entrapment efficiency. The optimized formulation was evaluated for in vitro drug release study and in vitro study on Swiss Albino mice using carbon tetrachloride (CCL4) induced hepatotoxicity model.
Results:
In vitro dissolution studies illustrated sustained, zero order drug release from optimized formulation; also its therapeutic potential was amplified during in vitro studies on Swiss Albino mice using CCL4 induced hepatotoxicity model.
Conclusion:
The results suggested that NPs of silymarin could successfully enhance its hepatoprotective effect by passive targeting and sustained release.
Keywords: Carbon tetrachloride, chitosan, hepatotoxicity, optimized nanoparticles, sustained release
INTRODUCTION
Liver, the major detoxifying organ of the body, plays a vital role in transforming and clearing chemicals from systemic circulation and is, therefore, highly susceptible to the toxicity caused by chemotherapeutic agents. Certain medicinal agents, when taken in quantity exceeding its maximum tolerated dose, and sometimes even within the therapeutic ranges, may aggravate liver injury. Various chemicals that elicit liver injury are called hepatotoxic agents. Antibiotics, chemotherapeutics, peroxidized oils, acetaminophen, aflatoxin, carbon tetrachloride (CCL4), chlorinated hydrocarbons, alcohol etc., are few chemicals which might lead to infections of liver and autoimmune liver disorders.[1]
Silymarin is one of the best known hepatoprotective drugs obtained from the seeds of Silybum marianum L., Family: Asteraceae or Compositae.[2] Since ages, this plant, also known as milk thistle, is being used as a herbal cure for liver and biliary tract diseases. This plant has been known to safeguard and also regenerate the liver cells in various diseases affecting liver such as cirrhosis, jaundice and hepatitis. It exhibits strong anti-oxidant action via free radical scavenging activity and inhibits lipid peroxidation. It also prevents the entry of harmful toxicants such as heavy metals, pesticides, alcohols, medicines, CCL4 etc. in liver, thereby protecting the liver cells from further damage.[3,4]
Silymarin is a mixture of flavonolignans, which comprises of silybin, isosilybin, silydianin and silychristine.[5,6,7] It is poorly soluble in water and exerts its hepatoprotective action at an oral dose of 240-800 mg/day in two or three divided dose. When administered orally, peak plasma concentration reaches in 2-4 h, with t1/2 = 6 h. Its poor bioavailability is mainly due to extensive metabolism, poor aqueous solubility and rapid excretion through urine and bile as well as low permeability across intestinal epithelial cells.[8,9]
The short half-life, poor bioavailability and high frequency of administration of silymarin presented immense scope for the development of nanoparticlulate drug delivery systems.
Thus, the aim of the present investigation was to formulate and optimize nanoparticulate drug delivery system using chitosan (Ch) containing silymarin by ionotropic gelation technique, followed by evaluation of its therapeutic potential in terms of hepatoprotective activity using suitable animal model.
MATERIALS AND METHODS
Materials
Silymarin and Ch were generously gifted by Micro Labs, Bengaluru (India) and Central Institute of Fisheries and Technology, Kochi (India) respectively. Sodium tripolyphosphate (TPP) and mannitol were procured from Himedia laboratories Pvt. Ltd., Mumbai (India). All other chemicals were of analytical grade and were used as and when supplied.
Animals
Swiss Albino mice were procured from the disease-Free Small Animal House, Lala Lajpat Rai University of Veterinary Sciences, Hisar, Haryana (India). The experimental protocol was approved by the Institutional Animals Ethics Committee (IAEC) and proper care of laboratory animals was taken as per the guidelines of Committee for the purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Forests and Environment, Government of India (Registration number 0436). The “Principles of Laboratory Animal Care” were adhered to, during the entire study.
Methods
Preparation of silymarin-chitosan nanoparticles
Nanoparticles (NPs) were prepared by ionotropic gelation method as was described by Lay Chuah et al. and Nagpal et al.[10,11] Different concentration of Ch, depending upon the experimental design, was prepared in 2% acetic acid solution. pH of this solution was adjusted to a value of five using 1M sodium hydroxide. Drug solution was prepared by dissolving silymarin (10 mg) in 1 mL ethanol and this solution was added dropwise into the solution of Ch with continuous stirring on a magnetic stirrer for 30 min. Different concentrations of sodium TPP solution, adjusted to pH 5 using 0.1M HCl, were prepared as per the requirements of experimental design. This solution was dropwise added to the above drug-polymer solution with continuous stirring for 1 h at 800 rpm. The formulated NPs were separated by centrifugation at 11,000 rpm at 10°C for 45 min using cooling centrifuge (C-24, Remi Cool Equipments, Mumbai, India). The pellet obtained after centrifugation was redispersed in 10 mL distilled water and was sonicated for 30 min using bath sonicator (Powersonic 410, Cyberlab HWASHIN Technology). The prepared NPs were subjected to lyophilisation after addition of 5% D-mannitol as a cryoprotectant and were sealed immediately after freeze drying to prevent particle agglomeration.
Central composite design and optimization
Based on the results obtained in preliminary experiments, concentration of Ch (X1) and ratio of Ch:TPP (X2) were found to be the independent variables majorly affecting the entrapment efficiency (Y1) and particle size (Y2), which were selected as the dependent variables. Hence, a 2-factor 3-level (3)2 central composite experimental design including −α and +α values was selected for optimizing the preparation of silymarin loaded Ch NPs.
The experimental design with formulation parameters have been depicted in Table 1. The test factors were coded as −1, 0 and +1 which respectively corresponded to 30, 40, and 50 mg of Ch and 3:1, 3.5:1 and 4:1 ratio of Ch:TPP. Trial experiments were run before selecting these values of the independent variables in which it was found that varying the amount of Ch from 30 to 50 mg and Ch:TPP ratio from 3:1 to 4:1, affected the formulation of the NPs to a great extent.
Table 1.
Experimental design with dependent and independent variables

Characterization
Determination of particle size and size distribution
The particle size and polydispersity index (PDI) of drug loaded NPs were analyzed by dynamic light scattering technique using ZetaSizer Nano ZS90 (Malvern Instruments Limited, UK). The sonicated dispersion (1 mL) of the sample, diluted 10-folds with double distilled water was utilized for the determination of particle size (hydrodynamic diameter) and PDI.
Determination of encapsulation efficiency
Suspension of NPs (10 mL) was centrifuged at 11,000 rpm for 45 min at 10°C using cooling centrifuge (C-24, Remi Cool Equipment's, Mumbai, India). Clear supernatant procured after centrifugation was diluted 10 times with double distilled water to quantify the amount of unbound (free) silymarin using UV-visible spectrophotometer (Varian Holdings Pvt Ltd, Australia) at λmax = 287 nm. Encapsulation efficiency (EE) of NPs was determined using the following equation:

Morphological study
The morphological study of optimized formulation was carried out using transmission electron microscope (TEM). A suspension of lyophilized NPs was prepared in double distilled water and few drops of the suspension were deposited onto carbon-coated copper grid and immobilized for 1-2 min. After immobilization the excess solution was wicked off with filter paper and was stained with uranyl acetate and lead acetate. The stained NPs were examined using Phillips CM-10 TEM (FEI, USA) at an acceleration voltage of 200 kV.
Differential scanning calorimetry
The physical state of drug inside the NPs was investigated by differential scanning calorimetry (DSC). The thermogram of the drug loaded NPs were obtained using DSC (TA instruments, Model no. Q10). A small amount (2-7 mg) of sample was sealed in the aluminum pan and the temperature was raised at 10°C min from 40°C to 300°C.
Fourier transform infrared spectroscopy spectral analysis
Infrared spectroscopy of the different formulations was studied to confirm the drug loading and drug-excipient interaction by KBr pellet method at moderate scanning speed between 4000 and 400 cm−1 was carried out using Fourier transform infrared spectroscopy (FT-IR) (Perkin-Elmer Life and Analytical Sciences).
In vitro drug release study
The drug release rate was studied using USP XXII dissolution apparatus II (Paddle type). NPs containing drug equivalent to 5 mg were taken in a dialysis membrane bag (Himedia, MWCO, and molecular mass cut off 12,000-14,000, pore size 2.4 nm) and was placed into a flask containing 250 ml phosphate buffer (pH 7.4) with 0.1% tween 20. Tween 20 had been used in dissolution media for poorly soluble drugs.[12] The paddle was rotated at 50 rpm and the temperature of dissolution media was maintained at 37°C ± 1°C. 5 ml aliquots were withdrawn at specific time intervals (0.25, 0.50, 1, 2, 3, 4, 5, 6, 8, 9, 11 and 24 h) and were subsequently replaced by fresh dissolution medium.
The aliquots were analyzed by measuring absorbance at 287 nm using UV spectrophotometer (UV-Visible Double beam Spectrophotometer 2203, Systronics India Ltd.). All dissolution studies were carried out in triplicate.
In vivo study
The efficacy of Silymarin loaded NPs were compared with pure drug Silymarin, by evaluating in vivo hepatoprotective activity in mice against CCL4 induced hepatotoxicity.
Experimental animals
Albino mice of either sex (weighing 25-30 g) were procured from LLRUVAS, Hisar (India). The mice were placed in a group of six mice/cage (cage size = 29 × 22 × 14) under standard environmental conditions (25°C ± 2°C and relative humidity 50% ± 5%) with alternating dark and light cycle of 12 h each. The animals were maintained on standard pellet food and water ad libitum for 2 weeks, in order to acclimatize them to laboratory environment before the experimentation. The experimental protocol was approved by IAEC and animal care was taken as per guidelines of CPCSEA, Ministry of Environment and Forests, Government of India (Registration No. 0436). Attempts were made throughout the study to attenuate animal misery.
Animal model for evaluation of hepatoprotective activity (mice)
Carbon tetrachloride induced hepatotoxicity
The mice weighing 25-30 g were used for CCl4 induced hepatotoxicity studies.[13,14] Mice were randomly divided into four groups of six mice each:
Group I: Control group
Group II: 1 mL/kg CCl4 and olive oil [1:1] (Toxic Control group).
Group III: 50 mg/kg body weight Silymarin (Standard group).
Group IV: 50 mg/kg body weight Silymarin loaded NPs (Test group).
The doses were administered to test animals through gavage using gastric tube. The animals of group I received distilled water for 4 days and served as normal control. The second group animals received distilled water for all 4 days and a single dose of CCl4 that is, 1 mL/kg intraperitoneally (i.p.) on 4th day, and served as the toxic control. The third group served as the standard and animals were treated with an oral suspension of standard drug silymarin in Tween 20 (1%, v/v) at a dose of 50 mg/kg/day on all 4 days and CCl4 (1 mL/kg, i.p.) on day 4, 1 h after the administration of the standard drug. Group four served as the test group for treatment with reconstituted silymarin NPs in distilled water at a dose of 50 mg/kg/day on all 4 days and was also administered CCl4 (1 mL/kg, i.p.) on day 4, 1 h after the administration of formulation. The blood was collected after 24 h of CCl4-intoxication treatment via cardiac puncture in glass tubes. The blood and liver samples were assessed for their biochemical changes including levels of serum glutamic pyruvic transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT) and alkaline phosphatase (ALP).
Statistical analysis
The values are expressed as mean ± standard deviation. The statistical analysis was carried out by One-way analysis of variance followed by Dunnett's t-test. P < 0.01 were considered to be significant.
RESULTS
Data analyses and validation of optimization model for nanoparticles (using design expert v 7.1.6)
Design Expert version 7.1.6 (Stat Ease, Inc.) was used for the formulation optimization of NPs using response surface methodology (RSM). The goal of optimization was to maximize entrapment efficiency and to achieve the particle size around 100 nm by varying Ch and Ch:TPP ratio for the prepared formulation. Quadratic model was selected for given response variables particle size (PS and EE), polynomial equation using multiple linear regression analyses were generated. Model was found to be significant (P < 0.05) with non-significant lack of fit (P > 0.05). Response variable particle size (Y1) and entrapment efficiency (Y2) both fitted best into the quadratic model without transformation.
Response surface analyses
Three-dimensional response surface graphs were obtained using Design Expert software as shown below in Figures 1 and 2.
Figure 1.

Three-dimensional response surface curve depicting the influence of chitosan (Ch) and Ch:Tripolyphosphate on encapsulation efficiency of given formulation
Figure 2.
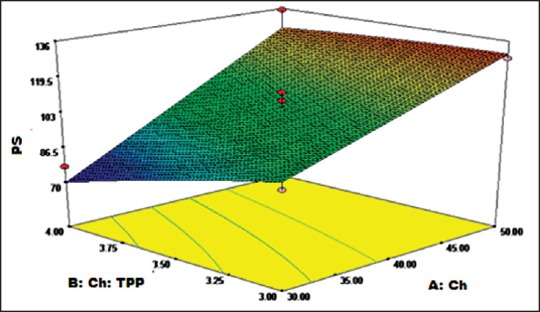
Three-dimensional response surface curve depicting the influence of chitosan (Ch) and Ch:Tripolyphosphate on particle size of given formulation
It is evident from the above three-dimensional curve [Figure 1] that EE almost is linearly dependent on ratio of Ch:TPP, that is, its value increases on increasing the ratio from 3:1 to 4:1 while, it is nonlinearly dependent on Ch concentration, that is, it initially increases which is then followed by a decrease from its maximum value as the concentration of Ch is increased because optimum rigidization of NPs takes place at in the midrange of Ch concentration, that is, 40.0 mg.
From three-dimensional response curve for particle size [Figure 2], it is evident that particle size is inversely proportional to Ch:TPP and directly proportional to Ch concentration. Minimum particle size can be achieved at low Ch concentration and high Ch:TPP ratio.
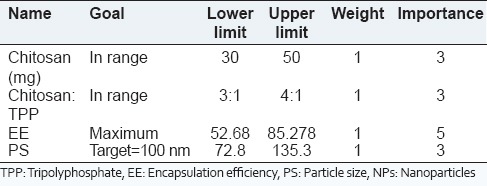
The optimization criteria with goals, limits and importance used for optimization of silymarin NPs are shown in Table 2. The criteria for obtaining optimized solution of NPs were set so as to obtain maximum entrapment efficiency and particle size was targeted to around 100 nm.
Table 2.
Criteria used for formulation optimization of silymarin NPs
Based on desirability approach for optimization one solution was obtained, which suggested Ch level at 42.08 mg and Ch:TPP ratio at 4:1 should yield entrapment efficiency of 83.23 and particle size at 104.05 nm with higher level of desirability (0.920) as indicated in Table 3 and represented in Figure 3. Table 3 highlights that the predicted values of entrapment efficiency and particle size, as suggested by the software, were similar to their observed values obtained upon preparation and characterization of the optimized formulation. This negligible error between the predicted and observed values certainly approve the success of optimization tool and technique and the relationship thus generated between formulation variables and responses in the optimization technique was relevant and can be successfully utilized for designing other dosage forms.
Table 3.
Solution of numerical optimization

Figure 3.

Three-dimensional response surface curve depicting the influence of chitosan (Ch) and Ch:Tripolyphosphate on desirability of given formulation
Above graph [Figure 3] clearly depicts that maximum desirability (0.920) can be achieved at highest level of Ch:TPP (4:1) and around mid-value of Ch concentration (42.08).
Evaluation of nanoparticles
Particles size and polydispersity index of nanoparticles
Particle size of the samples prepared as per experimental design along with final optimized batch is shown in Table 1. The PS of NPs varied between 72.8 nm and 135.3 nm and the PDI of NPs were in the range 0.077-0.373. In case of optimized batch formulation, the PDI was observed as 0.206 indicating monodispersed phase.
Entrapment efficiency of nanoparticles
Drug EE of the prepared NPs was observed in the range of 52.68-85.28%, while for optimized batch, it was 81.52%. It was observed that entrapment efficiency increases with increase in ch:TPP ratio and it initially increases and then decreases with increase in Ch concentration from 30.0 to 50.0.
Morphological study of nanoparticles by transmission electron microscope
Transmission electron microscope image of silymarin loaded NPs is shown in Figure 4 which reveals spherical NPs with rough surface. It was observed that mean particle size of silymarin loaded NPs (92.8 nm) which was lower than that obtained by zetasizer (103.7 nm), may be attributed to the dehydration of NPs during sample preparation for TEM analysis. Photon correlation spectroscopy measures the hydrodynamic diameter of NPs which includes the hydrodynamic layers that form around the hydrophilic particles leading to amplification of particle size.[12]
Figure 4.
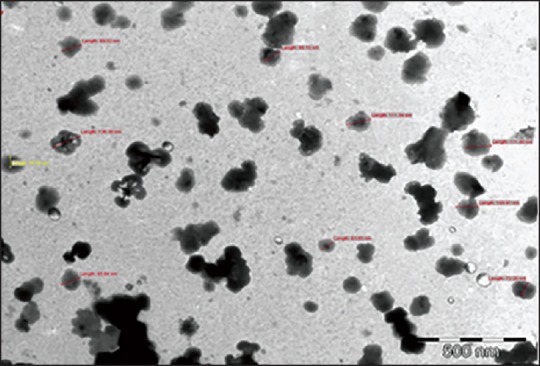
Transmission electron microscope image of silymarin nanoparticles
Differential scanning calorimetry
The DSC thermograms provide information about physical and chemical changes that involve endothermic or exothermic processes or change in heat capacity. An overlay was prepared using the thermograms of polymer (Ch), drug (silymarin), and optimized silymarin-loaded NPs, as shown in Figure 5. The peaks observed in DSC thermograms of Ch and silymarin were obtained in that of NPs formulation, indicating that there was no chemical interaction between the drug and the polymer in the NPs formulation. However, the broad peak at 166.39°C exhibited by silymarin was not visible in silymarin loaded NPs due to overlapping of mannitol endotherm which also has melting point in this range.
Figure 5.
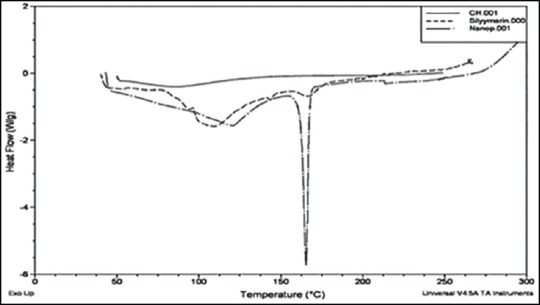
Overlay of differential scanning thermogram of polymer, drug and nanoparticles of silymarin
Fourier transform infrared spectroscopic analysis
The FT-IR spectra of Ch, sodium TPP, silymarin, and drug loaded NPs are shown in Figure 6. Optimized batch of NPs exhibited all characteristic peaks of silymarin. Thus, the drug exhibited no chemical interaction with the excipients in the formulation. Thus, the processing technique did not affect the chemical stability of the drug.
Figure 6.

Comparative Fourier transform infrared spectroscopy spectra of silymarin, chitosan, tripolyphosphate and nanoparticles
In vitro drug release
Optimized formulation
The in vitro drug release behavior of optimized batch as per the experimental design and pure drug (silymarin) was performed [Figure 7]. Only 52.6% of drug was released from Ch NPs in 24 h while 99.94% of drug was released from pure silymarin thereby, showing sustained release behavior of formulated NPs. The release data were fitted into various models. It was found that zero order was most suitable for describing drug release kinetics from optimized formulation. Hence drug release rate is independent of amount of drug present in sustained release formulation which is an ideal drug delivery for sustained release dosage form.
Figure 7.
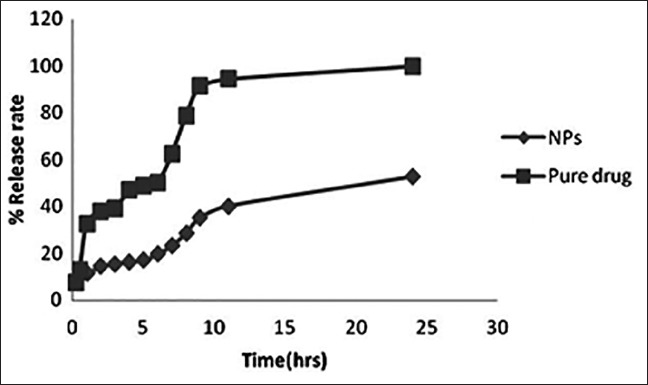
Dissolution profile of chitosan nanoparticles and pure drug
In vivo study
The efficacy of Silymarin loaded NPs compared to pure drug silymarin, were evaluated by in vivo study in mice for hepatoprotective activity by measuring SGOP, SGPT, ALP, etc.
Biochemical estimations
The results of hepatoprotective activity of silymarin NPs on CCl4 treated mice are shown in Table 4.
Table 4.
Effect of silymarin and its formulations on CCL4 induced liver damage
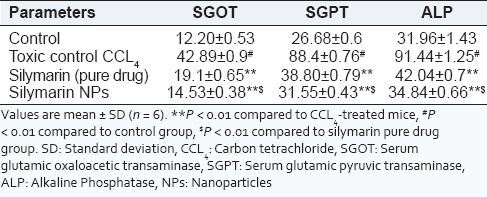
The results in Table 4 indicate that with the animals treated with pure drug silymarin and silymarin loaded NPs, there was a significant lowering of SGOT, SGPT and ALP levels (P < 0.01) in experimental animals after 24 h of the CCl4 administration in all the groups. The results have also exhibited in Figure 8. The decrease in levels of SGPT, SGOT and ALP indicated the hepatoprotective activity of drug and formulation from CCL4 induced hepatotoxicity. Moreover, the hepatoprotective potential of nanoparticulate formulation was higher than the hepatoprotective action shown by pure drug dispersion of silymarin, which may be attributed to passive drug targeting to liver due to entrapment of silymarin NPs by reticulo-endothelial cells of liver.
Figure 8.
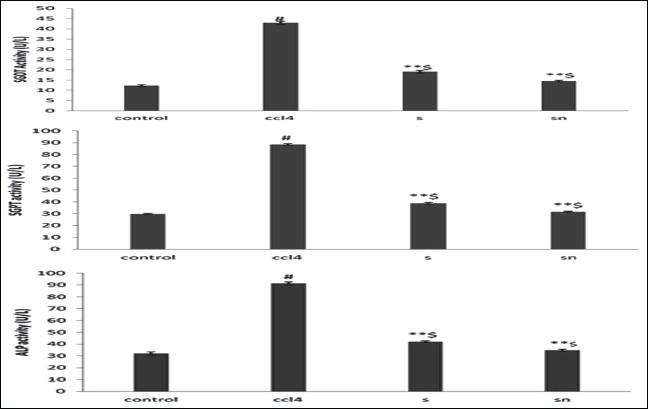
Effect of silymarin and silymarin loaded Nanoparticles on levels of liver markers (serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase and alkaline phosphatas) after 24 h of carbon tetrachloride administration
DISCUSSION
The present investigation was an effort to increase the solubility associated bioavailability of silymarin by formulating nano formulation, and also to control its release of silymarin from the dosage form, so as to achieve a prolonged duration of hepatoprotective action. A plethora of literature have reported that NPs can be used in vivo to protect the drug entity in the systemic circulation and to target the drug at specific sites or to deliver the drug at a controlled and sustained rate at the site of action, which may reduce the dosing frequency.
Particle size of formulated NPs plays a pivotal role during passive drug targeting as it has been demonstrated that NPs in the size range of 100-200 nm are rapidly taken up by the phagocytes of the reticulo-endothelial cells and are preferentially distributed in liver, as has been justified by Li et al.[15] TEM study suggested that the entrapped drug was uniformly distributed within the core shell. The particles of all the formulated NPs were found to be spherical and non-aggregated with relatively narrow size distribution around 100 nm. DSC studies of drug and formulations confirmed no polymorphic changes in the drug loaded NPs. FT-IR also confirmed successful formulation of colloidal particles without any significant chemical interaction. The in vitro dissolution studies of the optimized formulation was carried out in phosphate buffer (pH = 7.4). It exhibited only 52.6% drug release in 24 h from NPs owing to sustained release formulation. Zero order drug release was found to be the best fit model for the optimized formulation. The value of dependent variables was close to predicted values of solution suggested by software which indicated success of optimization technique.
The therapeutic potency of Silymarin loaded NPs was evaluated through in vivo studies on Albino mice using CCL4 induced hepatotoxicity as an experimental model. The levels of serum SGPT, SGOT and ALP activities, generally considered as good markers of hepatic injury and hepatocellular integrity, were determined. An augmented activity of marker transaminases in the serum indicates liver damage. Aggravated potential of silymarin loaded NPs was observed. This was also evidenced from the downturn in levels of marker enzymes of test groups compared with the toxic control group and pure drug. The improvement in the enzyme activity was due to sustained and targeted action of NPs on the hepatocytes which due to their pronounced antioxidant effect may have diminished the release of SGOT, SGPT and ALP enzymes from the liver cells, thereby eliciting hepatoprotective activity.
CONCLUSION
The NPs of hepatoprotective drug silymarin were successfully prepared by ionotropic gelation technique using Ch as a polymer which was cross-linked with sodium TPP. The effect of various formulation variables like concentration of polymer Ch and ratio of Ch:TPP on particle size, release rate and entrapment efficiency was performed through RSM using central composite design. The optimized solution, as deduced from the software, suggested that 40 mg Ch and 4:1 ratio of Ch:TPP was the best so far as the ionic gelation technique is used for the formulation of Ch NPs. This was also confirmed by preparing the optimized formulation and characterizing it for particle size and entrapment efficiency. The formulations were found to exhibit sustained release of drug, as studied through the in vitro dissolution techniques. The in vivo studies on Albino mice using CCL4 induced hepatotoxicity as an experimental model suggested that the silymarin loaded NPs significantly enhanced the hepatoprotective activity of the drug in the formulation.
It can thus be concluded that the nanoparticulate approach developed for silymarin can help to navigate and target the silymarin molecules in vivo in an effective way for hepatoprotection. Furthermore, it was successful in enhancing the passive liver targeting of antihepatotoxic drugs via oral route of administration, which may be attributed to its small size, larger surface area and coating the formulation with biodegradable and biocompatible polymer Ch (matrix reservoir), with sustained hepatoprotective action. However, further clinical studies are cardinal to brace the verdict of pharmacodynamic investigations and to certify the performance of formulated NPs in vivo.
ACKNOWLEDGEMENTS
The authors express gratitude to Micro Labs, Bangalore (India) and Central institute of fisheries and technology, Kochi (India) for providing gift sample of silymarin and Ch respectively. The authors also acknowledge their sincere thanks to All India Council of Technical Education, New Delhi for financial support.
Footnotes
Source of Support: The present work was financially supported by All India Council of Technical Education, New Delhi.
Conflict of Interest: None declared.
REFERENCES
- 1.Han DW. Intestinal endotoxemia as a pathogenetic mechanism in liver failure. World J Gastroenterol. 2002;8:961–5. doi: 10.3748/wjg.v8.i6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flora K, Hahn M, Rosen H, Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93:139–43. doi: 10.1111/j.1572-0241.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- 3.Negi AS, Kumar JK, Luqman S, Shanker K, Gupta MM, Khanuja SP. Recent advances in plant hepatoprotectives: A chemical and biological profile of some important leads. Med Res Rev. 2008;28:746–72. doi: 10.1002/med.20115. [DOI] [PubMed] [Google Scholar]
- 4.Pei YP, Chen J, Li WL. Progress in research and application of silymarin. Med Aromat Plant Sci Biotechnol. 2009;3:1–8. [Google Scholar]
- 5.Mourelle M, Muriel P, Favari L, Franco T. Prevention of CCL4-induced liver cirrhosis by silymarin. Fundam Clin Pharmacol. 1989;3:183–91. doi: 10.1111/j.1472-8206.1989.tb00449.x. [DOI] [PubMed] [Google Scholar]
- 6.Ding T, Tian S, Zhang Z, Gu D, Chen Y, Shi Y, et al. Determination of active component in silymarin by RP-LC and LC/MS. J Pharm Biomed Anal. 2001;26:155–61. doi: 10.1016/s0731-7085(01)00364-8. [DOI] [PubMed] [Google Scholar]
- 7.Kvasnicka F, Bíba B, Sevcík R, Voldrich M, Krátká J. Analysis of the active components of silymarin. J Chromatogr A. 2003;990:239–45. doi: 10.1016/s0021-9673(02)01971-4. [DOI] [PubMed] [Google Scholar]
- 8.Wagner H, Seligmann O. Liver therapeutic drugs from Silybum marianum. In: Chang HM, Yeung HW, Tso WW, Koo A, editors. Advances in Chinese Medicinal Materials Research. Singapore: World Scientific Publ. Co; 1985. [Google Scholar]
- 9.Javed S, Kohli K, Ali M. Reassessing bioavailability of silymarin. Altern Med Rev. 2011;16:239–49. [PubMed] [Google Scholar]
- 10.Chuah LH, Billa N, Roberts CJ, Burley JC, Manickam S. Curcumin-containing chitosan nanoparticles as a potential mucoadhesive delivery system to the colon. Pharm Dev Technol. 2013;18:591–9. doi: 10.3109/10837450.2011.640688. [DOI] [PubMed] [Google Scholar]
- 11.Nagpal K, Singh SK, Mishra DN. Optimization of brain targeted gallic acid nanoparticles for improved antianxiety-like activity. Int J Biol Macromol. 2013;57:83–91. doi: 10.1016/j.ijbiomac.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Shah VP, Konecny JJ, Everett RL, McCullough B, Noorizadeh AC, Skelly JP. In vitro dissolution profile of water-insoluble drug dosage forms in the presence of surfactants. Pharm Res. 1989;6:612–8. doi: 10.1023/a:1015909716312. [DOI] [PubMed] [Google Scholar]
- 13.Yen FL, Wu TH, Lin LT, Cham TM, Lin CC. Naringenin-loaded nanoparticles improve the physicochemical properties and the hepatoprotective effects of naringenin in orally-administered rats with CCl(4)-induced acute liver failure. Pharm Res. 2009;26:893–902. doi: 10.1007/s11095-008-9791-0. [DOI] [PubMed] [Google Scholar]
- 14.Girish C, Koner BC, Jayanthi S, Rao KR, Rajesh B, Pradhan SC. Hepatoprotective activity of six polyherbal formulations in CCl4-induced liver toxicity in mice. Indian J Exp Biol. 2009;47:257–63. [PubMed] [Google Scholar]
- 15.Li FQ, Su H, Chen X, Qin XJ, Liu JY, Zhu QG, et al. Mannose 6-phosphate-modified bovine serum albumin nanoparticles for controlled and targeted delivery of sodium ferulate for treatment of hepatic fibrosis. J Pharm Pharmacol. 2009;61:1155–61. doi: 10.1211/jpp/61.09.0004. [DOI] [PubMed] [Google Scholar]


