Abstract
Introduction:
The eye is an interesting organ. The tear flow and blinking reflex maintains a good environment and removes foreign material from the eye. Several polymeric systems have been used to fabricate ocular inserts for better ocular bioavailability and retention to drug of which gelling systems have shown advantages of convenient administration and increased contact time.
Aim:
The purpose of the present study was to develop a bioadhesive in-situ gelling ocular insert of Ketorolac tromethamine using polymeric system of sodium alginate as gelling and chitosan as bioadhesive agent.
Methods:
Various batches of ketorolac tromethamine bioadhesive in-situ gelling ocular inserts were prepared using sodium alginate and chitosan with glycerin as a plasticizer by solvent casting method. The formulated bioadhesive in-situ gelling ocular insert were then evaluated for physical appearance, thickness, weight variation, folding endurance, percentage moisture loss, percentage moisture absorption, tensile strength, percentage flatness, bioadhesive strength, force of adhesive, drug content, in vitro drug release, sterility test, in vitro antimicrobial efficacy, and stability study.
Results:
The formulation F4 was shown 98.62 % drug release at the end of 12 h. Hence that F4 formulation was maximum sustain drug release than other formulation and also optimum and better result of physicochemical properties than other formulation. This optimized formulation was subjected to sterility and stability test. There was no evidence of microbial growth and hence the ocular insert passed the sterility test and there was no significant change in the physicochemical properties from 0th to 30th day. Hence, the formulation was found to be stable.
Conclusion:
The said promising formulation (F4) would be able to offer benefits such as increase residence time, prolonged drug release, reduction in frequency of administration and thereby definitely prove to improve the patient compliance.
Keywords: Bioadhesive in-situ gelling ocular inserts, chitosan, in vitro drug release, ketorolac tromethamine, sodium alginate
INTRODUCTION
The eye is an interesting organ. The tear flow and blinking reflex maintains a good environment and removes foreign material from the eye. In ocular drug delivery, the physiological constraints imposed by protective mechanism of the eye lead to low absorption of drugs and sometimes short duration of therapeutics effect. One of the reasons for relatively low bioavailability of conventional eye drops is their short precorneal contact time. When drug solution is administered in the form of drops, effective tear drainage and blinking results in a 10-fold decrease in drug concentration in 4 to 20 min.[1] The drug absorption is also dependent upon the chemical nature of the drugs since the corneal permeability depends upon molecular size and hydrophobicity of drugs.[2] By tear drainage the main part of administered drug is transported via the naso-lachrymal duct to the gastrointestinal tract where it may be absorbed, sometimes causing the systemic side-effects. Rapid elimination of administered eye drops often results in a short duration of therapeutic effect making a frequent dosing regimen. In order to increase the effectiveness of the drug, a dosage form should be selected, which increases the contact time of the drug in the eye. This may increase bioavailability, reduce systemic absorption, and reduce the need for frequent administration leading to improved patient compliance. Ocular therapy would be significantly improved if precorneal residence time is increased and the most common way to achieve this is by increasing the viscosity of the solution. Gels and ointments moderately affect the contact time of the drug and have long residence time. They have a low patient compliance as they blur the vision and are recommended for bedtime use.[3,4]
Ophthalmic inserts are thin disks or small cylinders made with appropriate polymeric material and fitting into the lower or upper conjunctival sac. Ophthalmic inserts offer many advantages over conventional dosage forms, such as increased ocular residence, possibility of releasing drugs at a slow and constant rate, accurate dosing, and exclusion of preservatives, increased shelf life, and reduced systemic absorption.[5,6,7]
Ketorolac tromethamine is a nonsteroidal anti-inflammatory drug, used to treat seasonal allergic conjunctivitis. At present, it is available in the form of eye drops, which need to be administered 1 or 2 drops every 15 to 30 min. initially in acute infection and 1 or 2 drops administered 4 times daily or more in severe conditions. To overcome these limitations associated with dosage regimen, an attempt has been made to formulate bioadhesive in-situ gelling ocular inserts that may not only improve the efficiency of the therapy but also patient compliance. Several polymeric systems have been used to fabricate ocular inserts for better ocular bioavailability and retention to drug of which gelling systems have shown advantages of convenient administration and increased contact time. The purpose of this study was to develop a bioadhesive in-situ gelling ocular insert of ketorolac tromethamine using polymeric system of sodium alginate as gelling and chitosan as bioadhesive agent. The prepared dosage regimens provided ease in the application and capable to sustained drug release with reduced frequency of administration.[8,9,10,11]
MATERIALS AND METHODS
Materials
Ketorolac tromethamine was obtained as a gift sample from Symed Labs; Hyderabad. Chitosan and sodium alginate was obtained as a gift sample from Colorcon Laboratory, Mumbai.
Methods
The ketorolac tromethamine bioadhesive in-situ gelling ocular inserts were prepared based on sodium alginate and water soluble chitosan by using solvent casting technique. Polymeric solutions were prepared by dissolving sodium alginate and chitosan at distinct compositions along with ketorolac tromethamine and glycerin in distilled water.
Chitosan was added in aqueous solution of sodium alginate and with constant stirring. The plasticizer was added thereafter and the drug polymer solutions were stirred for 12 h and allowed to stand overnight to remove any entrapped air bubbles. The pH range of the solutions was found to be 5 to 8. The solutions were then poured into glass rings placed over mercury in the glass Petri dishes. Solvent was allowed to evaporate by placing the Petri dishes in oven (40 ± 2°C). Dried films were carefully removed from the Petri dish and then cut into oval shaped inserts with the help of a sharp edged die (13.2 mm in length and 5.4 mm in width). Each ocular insert contained 2 mg of the drug [Table 1].
Table 1.
Composition of different batches of ketorolac tromethamine bioadhesive in-situ gelling ocular inserts
EVALUATION TESTS[12,13,14,15,16,17]
Physical appearance
The visual appearance of the film was conducted. The color of the film as well as the texture was observed. Drug distribution within the film was also visualized.
Thickness
The films were evaluated for the thickness of each film using a micrometer of sensitivity of 0.001 mm. The average of 10 readings was taken. The mean thickness of standard deviation was calculated.
Folding endurance
The folding endurance was expressed as the number of folds number of times the insert was folded at the same place, either to break the specimen or to develop visible cracks. This test was important to check the ability of the sample to withstand folding. This also gives an indication of brittleness. The specimen was folded in the center, between the fingers and the thumb and then opened. This was termed as one folding. The process was repeated until the insert showed breakage or cracks in the center of insert. The total folding operations were named as folding endurance value.
Weight uniformity
The weight variation test was carried out by weighing three patches cut from different places of the same formulation and their individual weights were determine by using the digital balance. The mean value was calculated. The standard deviation of weight variation was compute from the mean value.
Surface pH determination
Inserts were left to swell for 5 h on an agar plate prepared by dissolving 2 % m/v agar in warm simulated tear fluid, sodium chloride: 0.670 g, sodium bicarbonate: 0.200 g, calcium chloride 0.008 g, and purified water quantity sufficient. 100 g of pH 7.4 under stirring and then pouring the solution into Petri dish until gelling at room temperature. The surface pH was measured by means of a pH paper placed on the surface of swollen patch.
Swelling index
Swelling of the polymer depends on the concentration of the polymer, ionic strength and the presence of water. To determine the swelling index of prepared ocular inserts, initial weight of the insert was taken, and then placed in freshly boiled and cooled artificial tear fluid pH 7.4 at 37°C. The insert was removed from plate after every 1 h and surface water was removed with the help of filter paper, and insert was reweighed. Swelling index was calculated.

Percentage moisture loss
The percentage moisture loss was carried out to check the integrity of the film at dry condition. The ocular inserts were preweighed accurately and kept in desiccators containing 100 ml of saturated solution of aluminum chloride. After 3 days, the films were taken out and weighed.
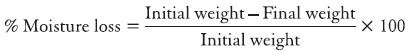
Percentage moisture absorption
The percentage moisture absorption was carried out to check the integrity of the film at dry condition. The ocular inserts were preweighed accurately and kept in desiccators containing 100 ml of saturated solution of aluminum chloride. After 3 days, the films were taken out and weighed.
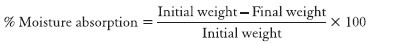
Tensile strength
Tensile strength was the maximum stress applied to a point at which the strip specimen breaks. It was calculated by the applied load at rupture divided by the cross-sectional area of the strip as given in the equation below.

Percentage elongation
When stress was applied, a strip sample stretches and this was referred to as strain. Strain was basically the deformation of strip divided by original dimension of the sample. In general, elongation of strip increases as the plasticizer content increases.
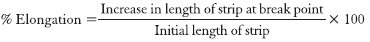
Bioadhesive strength
Bioadhesive strength of the insert was measured on a modified physical balance. Membrane was tied to open mouth of a glass vial filled with isotonic phosphate buffer. Vial was fitted in the center of a glass beaker filled with STF (pH 7.2, at 37 ± 1°C). Separately, insert was adhered to the lower side of a rubber stopper, which was attached to lever of physical balance. The mass (put on other limb of balance), which was required to detach the patch from the conjunctival surface was a measure of bioadhesive strength.
Drug content
Drug content was estimated by triturating ocular inserts in 20 ml of phosphate buffer pH 7.4 with the help of a mortar and pestle. The solution was filtered and 1 ml of the solution was withdrawn, diluted, and measured by a UV-Visible Spectrophotometer (Model-1700, Shimadzu, Japan).
In vitro drug release
In vitro drug release study was carried out by using biochemical donor-receptor compartment model. The commercial semi permeable egg membrane, presoaked overnight in the freshly prepared dissolution medium (STF pH 7.4) and was tied to one end of a cylinder (open at both the sides), which acted as donor compartment. The ocular insert was placed inside the donor compartment in contact with the semi-permeable membrane. The donor compartment was attached to a stand and suspended in 25 ml of the dissolution medium maintained at 37 ± 1°C in the way that touches the receptor medium surface. The dissolution medium was stirred at a low speed using magnetic stirrer. The aliquots of 5 ml were withdrawn at regular intervals for 12 h. and replaced by an equal volume of dissolution medium every time. The samples were analyzed on UV spectrophotometer.
Sterility test
Ultraviolet radiation was used to sterilize the ocular inserts and sterility testing was carried out under aseptic conditions. Alternate thioglycolate and soyabean casein digest media was used to check sterility of formulation.
Preparation of alternative thioglycolate medium
Dissolve 29.3 g alternative thioglycolate medium in 1000 ml distilled water by boiling and sterilized by autoclaving at 15 lbs pressure at 121°C for 15 min. According to IP 2007 procedures, two containers were selected for sterility test. In each test, three sterility test tubes were used in the study and labeled as “positive control”, “negative control”, and “test”.
Preparation of soyabean casein digest medium
About 40 g soyabean casein digest medium was suspended and boiled to dissolve the medium completely. It was sterilized by autoclaving at 15 lbs pressure at 121°C for 15 min. According to IP 2007 procedures, two containers were selected for sterility test. In each test, three sterility test tubes were used in the study and labeled as “positive control,” “negative control”, and “test”.
In vitro antimicrobial efficacy
The microbiological studies were carried out to ascertain the biological activity of ophthalmic inserts against microorganisms. Staphylococcus aureus and Escherichia coli were used as the test microorganisms. A layer of nutrient agar (20 ml) seeded with the test microorganism (0.2 ml) was allowed to solidify in the Petri plate. Cups were made on the solidified agar layer with the help of sterile borer of 8 mm diameter. Later, volume of the formulations (optimized formulation and marketed eye drops) containing equivalent amounts of drug was poured into the cups. After keeping Petri plates at room temperature for 4 h, the plates were incubated at 37°C for 24 h. The diameter of zone of inhibition was measured by using an antibiotic zone finder.
Stability study
A short term stability study of the optimized formulation is carried out as per International Conference on Harmonization guideline at temperature 40°C and relative humidity (RH) at 75 % for in stability chamber.
RESULT AND CONCLUSION
In this study, an attempt is made to design polymeric ocular drug delivery system of Ketorolac tromethamine bioadhesive in-situ gelling to overcome the disadvantages associated with the conventional Ophthalmic dosage forms (eye drops and suspensions), to achieve long duration of action and to improve ocular bioavailability. Ketorolac tromethamine bioadhesive in-situ gelling is a nonsteroidal anti-inflammatory drug, used to treat seasonal allergic conjunctivitis. The formulated ocular insert were then evaluated for physical appearance, thickness, weight variation, folding endurance, percentage moisture loss, percentage moisture absorption, tensile strength, percentage flatness, bioadhesive strength, force of adhesive, drug content, in vitro drug release, sterility test, in vitro antimicrobial efficacy, stability study.
Physical appearance
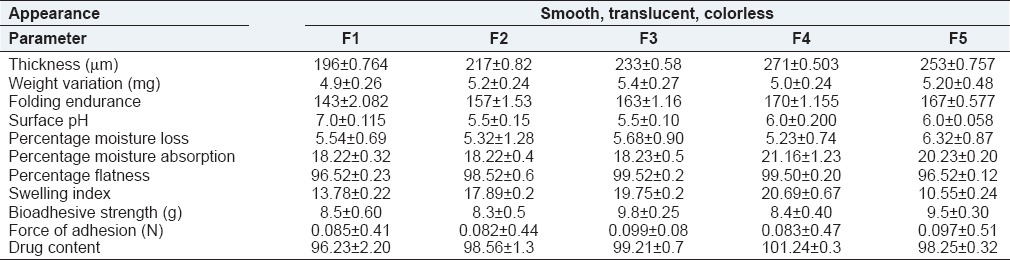
The prepared formulation F1-F5 batch ocular inserts were translucent, colorless, smooth in texture, uniform in appearance, and show no visible crack or imperfection.
Thickness
Thickness specifications may be set on an individual product basis. The thickness of the ocular inserts of all formulations was shown in Table 2. The range of thickness was observed from 196 ± 0.764 μm to 271 ± 0.503 μm. It was found that thickness of ocular inserts was increased with increase in polymer and plasticizer concentration. All polymers used for fabrication of ocular inserts showed good film forming properties and reproducibility.
Table 2.
Evaluation parameter of F1-F5 formulation
Weight variation
The weight uniformity test represents the effect of the concentration of polymers on the weight of the inserts. The weight of all formulations found to be uniform within a batch with low standard deviation value as shown in Table 2. The weights of formulations F1-F5 were found to be in the range of 4.9 ± 0.26 to 5.4 ± 0.27 mg. It was found that with the increase in the total concentration of the polymers the weight of the ocular inserts also increase. The uniformity of weight of the film indicates good distribution of the drug, polymer and plasticizer.
Folding endurance
Folding endurance represents the mechanical property of the property of the film to withstand the conditions during blinking of eye. The Folding Endurance of ocular insert of all formulations were shown in Table 2. The folding endurance of formulation F1-F5 ocular inserts were found to be in the range of 143 ± 2.082 to 170 ± 1.155. Use of less amount of plasticizer was observed to cause brittleness in the medicated discs, but use of greater amount of plasticizer displayed little opaqueness and good folding endurance. Folding endurance increased with the increased in the concentration of polymers. However, out of these five formulations F4 was shown a highest folding endurance for that concentration. The folding endurance was measured for all formulations manually.
Surface pH
Surface pH is a very important parameter to be evaluated to check the isotonic of ocular insert with tear fluid. The surface of formulations F1-F5 was measured using digital pH meter. The surface pH values were shown in Table 2. The surface pH of formulations F1-F5 ocular inserts were found to be in the range of 5.5 ± 0.10 to 7.0 ± 0.115, which were well within the pH of lachrymal secretion indicating no irritation.
Percentage moisture absorption and percentage moisture loss
The range of percentage moisture absorptions were observed from 18.22 ± 0.32 % to 21.16 ± 1.23 % and percentage moisture losses were observed from 5.23 ± 0.74 % to 6.32 ± 0.87 %. Though the percentage moisture absorption and percentage moisture loss were high, there was no change in integrity at high humid and dry conditions, which was observed by physical appearance. The values were calculated and shown in Table 2.
Swelling index
Swelling study was performed on all the batches of ocular insert for 1 h. The range of swelling index was observed from 10.55 ± 0.24 to 20.69 ± 0.67. From the results, it was concluded that swelling increases as the time passes because the polymer gradually absorb water due to hydrophilicity of polymer. In the present study, the higher swelling index was found for ocular insert of batch F4. Thus, the viscosity of the polymer had major influence on swelling process, matrix integrity.
Bioadhesive strength
The range of bioadhesive strength was observed 8.3 ± 0.50 to 9.8 ± 0.25. Formulation F3 showed maximum bioadhesive strength and hence maximum force of adhesion. It is evident from the results that inserts with higher chitosan content show better bioadhesive strength and force of adhesion. The results show the superiority of chitosan as promising bioadhesive material at neutral or slightly alkaline pH, which is found to be advantageous for adsorption on the ocular surface. It was suggested that at neutral and alkaline pH, chitosan has numerous amine and hydroxyl groups as well as a number of amino groups that may increase the interaction with the negatively charged group in biological membrane.
Drug content
The range of drug contents was observed from 96.23 ± 2.20 to 101.24 ± 0.28. Results of the content uniformity test complied with the IP 2007 requirements. These results showed that these values indicated homogeneous distribution of drug and the method for the preparation of inserts gave reproducible results.
Tensile strength
Tensile strength measures the ability of film to withstand rupture. Ocular insert with good tensile strength would resist tearing due to stress generated by blinking action of the eye. The tensile strength of formulation F1-F5 ocular inserts was shown in Table 3 and the graph was shown in Figure 1. The tensile strength of formulation F1-F5 was found to be a range of 1.82 ± 0.040 to 2.65 ± 0.028 g/mm2. Tensile strength of ketorolac tromethamine inserts increased as the total amount of polymer was increased. However, the tensile strength could be related to the sodium alginate content as the inserts with higher sodium alginate content showed greater tensile strength.
Table 3.
Tensile strength of F1-F5 formulation
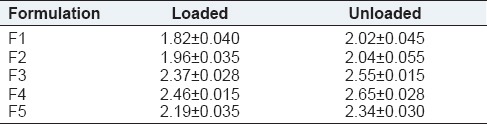
Figure 1.
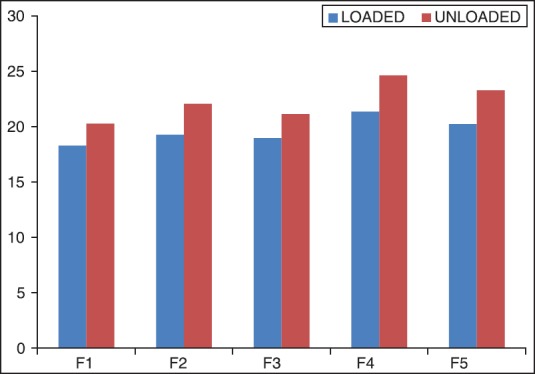
Column chart of tensile strength of F1-F5 formulations with STD
Percentage elongation
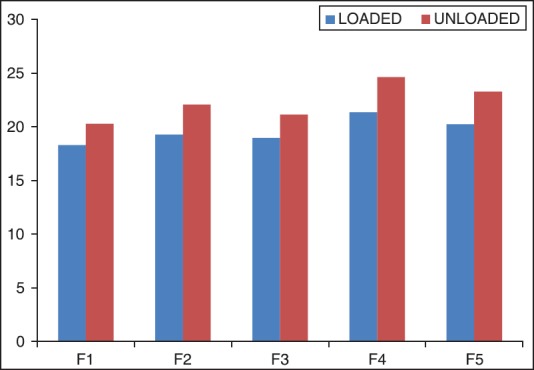
The percentage elongation of formulation F1-F5 ocular inserts was shown in Table 4 and the graph was shown in Figure 2. The percentage elongation of formulation F1-F5 ocular inserts was found to be a range of 18.29 ± 0.011 to 24.62 ± 0.028. The percentage elongation of ocular inserts reduced with increase a concentration of chitosan and the percentage elongation of ocular inserts increased with increased a concentration of sodium alginate. Percentage elongation was maximum for formulation F4 followed by F5, F3, F2, and F1 formulation.
Table 4.
Percentage elongation of F1-F5 formulation
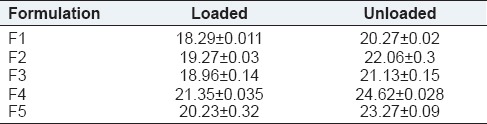
Figure 2.
Column chart of percentage elongation of F1-F5 formulations with STD
In vitro drug release
The effect of polymer concentration on drug release was studied for formulations F1-F5 prepared using as polymer sodium alginate and chitosan. The release profile of these formulations was shown in Figure 3.
Figure 3.
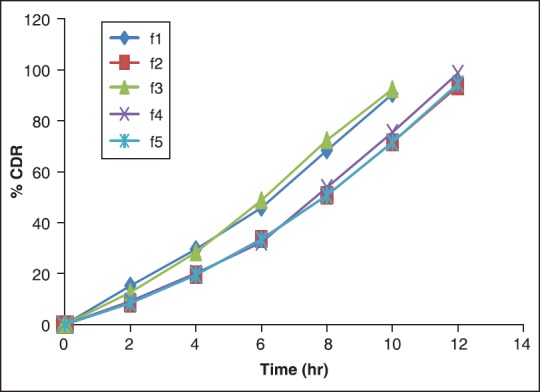
In vitro drug release profile of formulations F1-F5 with STD
Formulation F1 containing 1.5% sodium alginate and 1.5 % chitosan was prepared ocular inserts. The formulation F1 was shown 90.44 ± 0.98% drug release at the end of 10 h. Formulation F2 containing 2.0% sodium alginate and 1.5 % chitosan was prepared ocular inserts. The formulation F2 was shown 93.55 ± 0.72% drug release at the end of 12 h. Formulation F3 containing 1.5% sodium alginate and 2.5% chitosan was prepared ocular inserts. The formulation F3 was shown 92.38 ± 1.09% drug release at the end of 10 h. Formulation F4 containing 2.5% sodium alginate and 1.5% chitosan was prepared ocular inserts. The formulation F4 was shown 98.62 ± 1.32% drug release at the end of 12 h. Formulation F5 containing 2.0% sodium alginate and 2.0% chitosan was prepared ocular inserts. The formulation F4 was shown 94.52 ± 1.63% drug release at the end of 12 h.
The drug release study, however out of these five formulations, F4 batch was maximum sustain drug release and also having maximum folding endurance, swelling index, percentage drug content, optimized tensile strength, and percentage elongation.
Hence, on the basis of drug release profile and physicochemical parameters F4 formulation evolved as the best formulation.
Sterility test
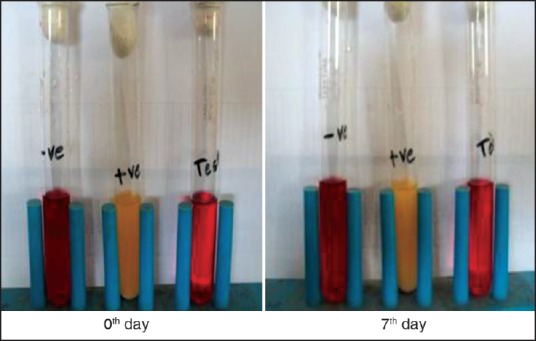
Positive (+ve) test tube (Medium and E. coli): Microorganism seen. So that media suitable for growth of microorganism.
Negative (−ve) test tube (Medium): No growth of microorganism seen. So that sterility maintained.
Control test tube (Medium and ocular insert): No growth of microorganism seen. So that product was sterilized [Figure 4].
Figure 4.
Test of sterility by fluid thioglycolate medium
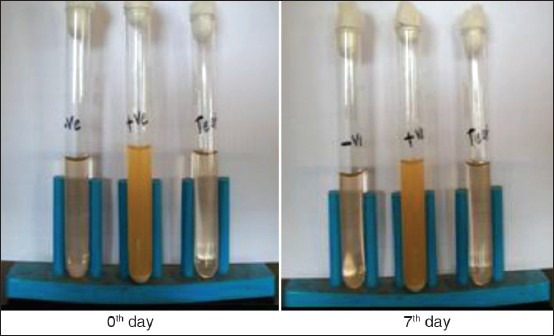
Positive (+ve) test tube (Medium and E. coli): Microorganism seen. So that media suitable for growth of microorganism.
Negative (−ve) test tube (Medium): No growth of microorganism seen. So that sterility maintained.
Control test tube (Medium and ocular insert): No growth of microorganism seen. So that product was sterilized [Figure 5].
Figure 5.
Test of sterility by soyabean casein digest medium
In vitro antimicrobial efficacy
The microbiological studies were carried out to ascertain the biological activity of ophthalmic inserts against microorganisms. Staphylococcus aureus and Escherichia coli were used as the test microorganisms.
Figure 6 shows that the disc containing ocular insert shown zone of inhibition by using Staphylococcus aureus and Escherichia coli.
Figure 6.
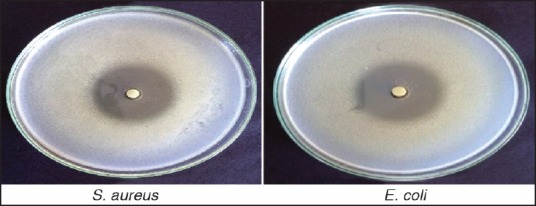
In vitro anti-microbial efficacy of optimized formula (F4)
Stability test
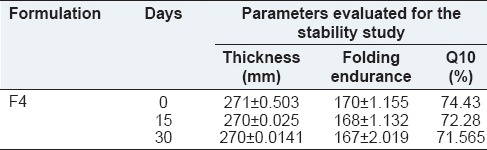
A short term stability study was carried out. A sufficient number of optimized ocular inserts (packed in aluminum foil) were stored in the stability chamber at temperature 40°C and 75 % RH for 1 month. After one month, the ocular inserts were taken out and were evaluated for thickness, folding endurance and in vitro drug release at 10th h. The evaluation parameters for stability studies was shown in Table 5 and it was found that there was no significant change in the physicochemical properties from 0th to 30th day. Hence, the formulation was found to be stable.
Table 5.
Evaluation parameters for stability study
CONCLUSION
Various batches of ketorolac tromethamine bioadhesive in-situ gelling ocular inserts were prepared using solvent casting method and evaluated. F4 was found to be better as it was smooth, translucent, and flexible. Physicochemical parameters like weight and thickness uniformity, folding endurance, tensile strength and percentage elongation was satisfactory and surface pH, swelling index, percentage flatness, percentage moisture absorption, percentage moisture loss, bioadhesive strength, force of adhesion showed optimum results and also better drug content uniformity in comparison with other formulations. The formulation F4 was shown 98.62 % drug release at the end of 12 h. So that F4 formulation was maximum sustain drug release than other formulation. This optimized formulation was subjected to sterility and stability test. There was no evidence of microbial growth and hence the ocular insert passed the sterility test and there was no significant change in the physicochemical properties from 0th to 30th day. Hence, the formulation was found to be stable.
From above results it can be concluded that ketorolac tromethamine bioadhesive in-situ gelling can be delivered in controlled manners for extended period of time in the form of ocular inserts. Release pattern of drug from these inserts can be altered by using different formulation variables. The said promising formulation (F4) would be able to offer benefits such as increase residence time, prolonged drug release, reduction in frequency of administration and thereby definitely prove to improve the patient compliance.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Grass GM, Robinson JR. Relationship of chemical structure to corneal penetration and influence of low-viscosity solution on ocular bioavailability. J Pharm Sci. 1984;73:1021–7. doi: 10.1002/jps.2600730803. [DOI] [PubMed] [Google Scholar]
- 2.Shahwal V. Ocular drug delivery: An overview. Inter J Biomed Adv Res. 2011;2:167–16. [Google Scholar]
- 3.Haders D. New controlled release technologies broaden opportunity for ophthalmic therapy. Drug Deliv Technol. 2008;8:48–5. [Google Scholar]
- 4.Nema RK, Rathore KS. Review on ocular inserts. Int J PharmTech Res. 2009;1:164–5. [Google Scholar]
- 5.Saettone MF, Giaccinni B, Ravecca S, La Marca F, Tota G. Polymers effect on ocular bioavailability the influence of different liquid vehicles on mydratic response of tropicamide in humans and in rabbits. Int J Pharm. 1984;20:187–15. [Google Scholar]
- 6.Shell JW. New ophthalmic drug delivery systems. In: Robinson JR, editor. Ophthalmic Drug Delivery Systems. Washington, DC: American Pharmaceutical Association; 1980. pp. 145–12. [Google Scholar]
- 7.Robinson JC. Ocular anatomy and physiology relevant to ocular drug delivery. In: Mitra AK, editor. Ophthalmic Drug Delivery. New York: Marcel Dekke Inc; 1993. p. 32. [Google Scholar]
- 8.Smadar C, Esther L, Amira T, Yale P. A novel in situ forming ophthalmic drug delivery system from alginates undergoing gelation in the eye. J Control Release. 1997;44:201–8. [Google Scholar]
- 9.Kas HS. Chitosan: properties, preparations and application to microparticulate systems. J Microencapsul. 1997;14:689–711. doi: 10.3109/02652049709006820. [DOI] [PubMed] [Google Scholar]
- 10.Janes KA, Calvo P, Alonso MJ. Polysaccharide colloidal particles as delivery systems for macromolecules. Adv Drug Deliv Rev. 2001;47:83–97. doi: 10.1016/s0169-409x(00)00123-x. [DOI] [PubMed] [Google Scholar]
- 11.Vodithala S, Khatry S, Khastri N, Sadanandam M. Formulation and evaluation of ion activated ocular gels of ketorolac tromethamine. Int J Curr Pharm Res. 2010;2:33–8. [Google Scholar]
- 12.Upadhyay N, Patidar A, Agrawal S. Development and evaluation of polymeric sustained release levofloxacin ocuserts. Res J Pharm Biol Chem Sci. 2011;2:411–21. [Google Scholar]
- 13.Gilhotra RM, Nagpal K, Mishra DN. Azithromycin novel drug delivery system for ocular application. Int J Pharm Investig. 2011;1:22–8. doi: 10.4103/2230-973X.76725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain D, Carvalho E, Banerjee R. Biodegradable hybrid polymeric membranes for ocular drug delivery. Acta Biomater. 2010;6:1370–9. doi: 10.1016/j.actbio.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Makadia M. Formulation and evaluation of ocular insert of diclofenac sodium. SJ Thakkar Pharm Coll. 2013;4:47–6. [Google Scholar]
- 16.Parmar R, Tank H. Design formulation and evaluation of reservoir type controlled released moxifloxacin hydrochloride ocular insert. Asian J Pharm Clin Res Sci. 2013;3:19–5. [Google Scholar]
- 17.Mortazavi SA, Jaffariazar Z, Damercheli E. Formulation and in-vitro evaluation of ocular ciprofloxacin-containing minitablets prepared with different combinations of carbopol 974P and various cellulose derivatives. Iran J Pharm Res. 2010;9:107–14. [PMC free article] [PubMed] [Google Scholar]


