Abstract
The clinical success and US FDA approval of two immunotherapies (sipuleucel-T and ipilimumab) have brought tumor immunology to the forefront of cancer research. It has been long recognized that the immune system can infiltrate and survey the tumor microenvironment. The field of tumor immunology has been actively examining this phenomenon since the 1890s when William Coley first treated patients with live pathogenic bacteria and observed occasional regressions leading to long term survival. Recent progress in understanding mechanisms of immune activation and tolerance has led to the development of novel therapies that aim to either overcome inhibitory pathways (i.e. checkpoint blockade such as anti-CTLA-4 and anti-PD-1) or stimulate immune cell activation (i.e. co-stimulation such as anti-GITR and anti-OX40). A major part of the success of immunotherapy has been the development of appropriate mouse models. This review will outline the history and the major findings leading to the accomplishments of modern day immunology with specific attention to the usefulness of animal models.
The larger scientific and medical community perceived cancer immunotherapy with speculation and considered it a non-realistic and unconventional venue for cancer treatment until recently. Radiation therapy, chemotherapy and, more recently, targeted therapies had overshadowed immunotherapy as an effective mean to treat cancer. It is only in the last 2–3 years that the use of the immune system has emerged as a transformative approach to cancer therapeutics [1]. The US FDA approvals of sipuleucel-T for the treatment of prostate cancer and ipilimumab for the treatment of melanoma have generated a great deal of enthusiasm and restored confidence in the idea that manipulating the immune system is a realistic mean to treat cancer [2]. Further, recent data showing that blockade of the PD-1 axis can benefit patients with prevalent diseases, such as non-small cell lung cancer, has demonstrated that immunotherapy can have meaningful activity outside of the ‘usual’ target diseases of melanoma and renal cell carcinoma[1]. The central dogma of the field is that the immune system prevents the occurrence of cancer by discriminating between normal and transformed cells. In addition, enhancement of long-lasting memory response by cells in the repertoire allows for durability of clinical benefit. If changes in the tumor cells make them invisible to the immune system (ex; loss of MHC expression) or if the immune system is inhibited, the cancer will progress. This phenomenon is termed immune evasion. The manipulation of the immune system to overcome these barriers will restore immune recognition and lead to elimination of cancer cells and eventually regression/ complete eradication of tumors. Here we will give an overview on the steps that led to the development of modern immunotherapy and the contribution of mouse model systems in this discipline.
History of Tumor Immunology
The recorded history of cancer immunology is acknowledged to have started in the late 19th century when William Coley made observations regarding occurrence of post-surgical infections and clinical outcomes in patients with cancer (Figure 1). He conducted hypothesis-driven clinical experiments that laid the foundation for the field, injecting live Streptococcus pyogenes organisms in tumors of a patient with inoperable cancer in the neck and tonsils [3]. As a result, the patient developed high fever due to the severity of the infection. In addition, the tumors regressed and the patient was tumor free for at least 10 years. Based on this initial observation, he treated hundreds of cancer patients with a safer cell-free mixture of bacteria cultures (also referred to as Coley’s Toxins). This seminal observation evoked the notion that the immune system controls tumor progression and can regress existing tumors. His work was followed by a series of studies by other groups using transplantable tumor models, which regrettably measured allograft tumor rejection rather than tumor immunity [4]. These studies were inconclusive due to lack of appropriate mouse models and instilled a great deal of skepticism in the field of tumor immunology. It wasn’t until the first half of the 20th century when the development of inbred (syngeneic) mice enabled researchers to methodically examine immune mediated rejection of “syngeneic” tumors derived from mice with the same genetic background [5,6]. These experiments showed that protective immunity was specific for each tumor type and suggested the existence of tumor-specific antigens. These and similar studies that followed led to the realization that immunization with tumor or tumor-derived products could stimulate the immune system to recognize and reject implanted tumors in a manner very similar to vaccination strategies employed against pathogens. More importantly these studies led to the inception of a theory of immune surveillance of cancer [7,8] and subsequent verification and mechanistic insights into the “3 E’s” of cancer immune surveillance [9], which will be discussed in more detail below.
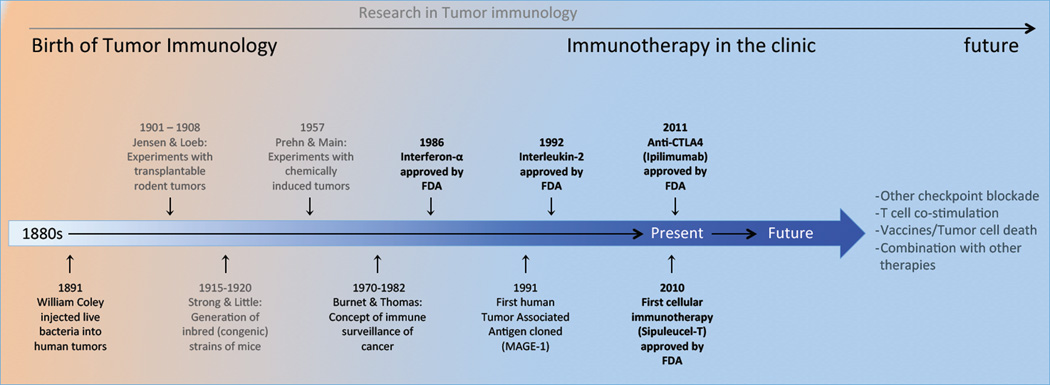
Figure 1. Research in Tumor Immunology.
Timeline of the history of tumor immunology from its inception in the late 1900s with William Coley's experiments to modern day tumor immunology with the FDA approval of 2 immunotherapies for treatment of cancer. Based on current clinical outcomes and laboratory based research using animal models future promising therapies will be combining checkpoint blockade, co-stimulation alone or in combination with conventional approved treatments.
Cancer Immunoediting
MacFarlane Burnet and Lewis Thomas first proposed the idea that the immune system recognizes and monitors tumors during their development [7,8]. Osias Stutman [10] subsequently tested this hypothesis by comparing the growth of chemically induced tumors in nude (T cell deficient) mice and control mice. His results clearly showed that immune deficient mice did not grow tumors faster thereby temporarily refuting the validity of cancer immune surveillance. Robert Schreiber and others further investigated this using mice which were more profoundly immune deficient and showed, using chemically induced, transplantable and spontaneous tumor models, that there was indeed a role for immune surveillance in preventing the emergence of malignancy in mice. Schreiber adapted and transformed this concept into what is now referred to as cancer immunoediting [9]. Cancer immunoediting is a dynamic process tumors undergo during their course of development. In its most complex form, it consists of three distinct phases: elimination, equilibrium, and escape (the 3 E’s of cancer immunoediting). During the early phase of tumor development, the immune system is able to recognize and eliminate the most highly immunogenic tumor cells (Figure 2). In this elimination phase, the innate and adaptive immune systems cooperate to eliminate the evolving tumor it before it becomes detectable in size. Given the heterogeneous nature of tumors, the immune system usually eliminates the more immunogenic tumor cells leaving the poorly immunogenic tumor cells behind. This leads to an equilibrium phase where the immune system keeps the tumors at bay; therefore, the tumors do not grow. In rare occasions (where progressive cancer occurs), tumors are able to finally escape control by immune system and continue to become symptomatic. In some cases, tumors may directly enter into either the equilibrium or escape phases without passing through an earlier phase. Tumor immune escape can be due to epigenetic changes that make tumors invisible to the immune system (such as loss of antigen or MHC molecules). In addition, tumors can recruit immune suppressor cells (regulatory T cells, myeloid derived suppressor cells) that are capable of inhibiting immune responses to tumors [11,12].
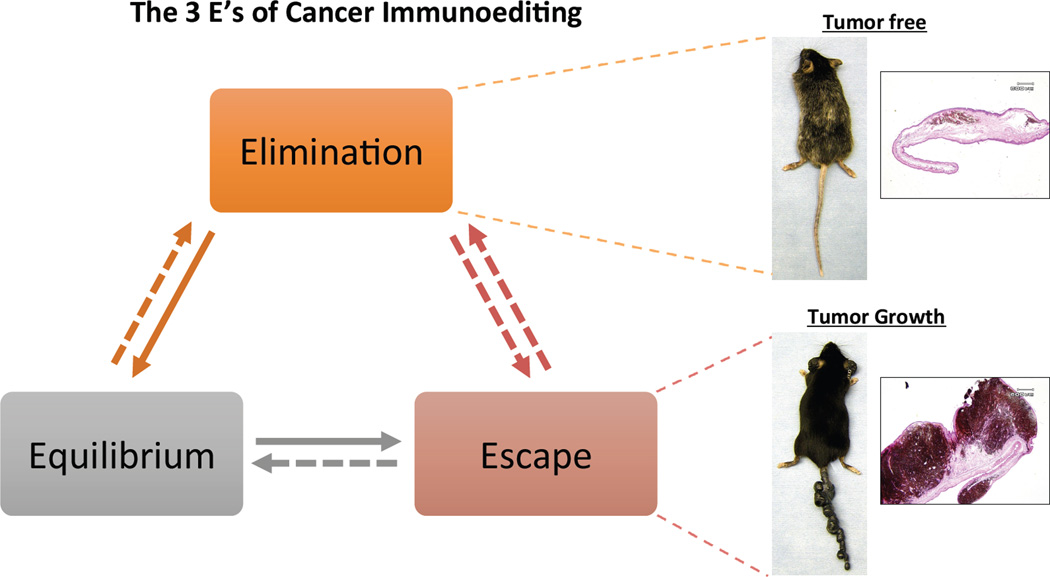
Figure 2. The 3 E's of Cancer Immunoediting.
The concept of cancer immunoediting during the course of tumor development consists of three phases Elimination, Equilibrium and Escape. Elimination: during the early phase of tumorigenesis, when tumors are microscopic, the immune system eliminates the highly immunogenic cells leaving the poorly immunogenic ones behind. Equilibrium: as the immunogenic cells are eliminated, tumors enter an equilibrium (or dormant) phase where the immune system prevents tumor growth. Escape: changes in the tumor cells or stroma within the microenvironment can facilitate uncontrolled tumor growth and metastasis. Shown on the right are representative photos of melanoma bearing Grm1 (Glutamate metabotropic receptor) transgenic mice, where tumors regress when mice are treated with immunotherapy (top). Both melanoma cells and melanocytes are eliminated as shown by the absence of tumor and depigmentation of the fur. Histology staining (H and E) of paraffin sections of ear shows no tumor burden with residual melanin. In some instances, tumors are non-responsive to immunotherapy and continue to progress (bottom). Histology staining of these ears show heavy tumor burden.
Current approaches to tumor immunology
The studies outlined above led to the current period of tumor immunology. It is now apparent that tumor progression does not depend solely on the cancer cell itself but also its interaction with the complex network of stromal cells including immune infiltrates within the tumor microenvironment. Functionally, we can consider that there are two categories of immune cells in the tumor: “effector” immune cells capable of destroying the tumor and “suppressor” immune cells that disable and regulate the effector immune response to tumors. Of the effector cells, cytotoxic T lymphocytes (CTLs), natural killer cells (NK), and macrophages constitute the major effector populations of the immune system and are generally the ones responsible for rejecting tumors. These cells exert their cytolytic activity mainly by secreting lytic products, such as cytokines, granzymes and perforins into target cells [13]. CTLs are part of the adaptive immune system and are tumor antigen specific. NK cells and macrophages are part of the innate system and are not antigen specific. There are two main subsets of suppressor immune cells: myeloid derived suppressor cells (MDSCs) and regulatory T cells (Tregs) [11,12].
The immune suppression conferred by these cells can lead to tumor immune evasion describe in the section above. These cells suppress mainly by secreting anti-inflammatory cytokines such as transforming growth factor beta (TGF-β) and interleukin 10 (IL-10). However, in certain instances, they employ other mechanisms to suppress the immune response such as CTLA-4, indoleamine dioxygenase (IDO) and IL-35 [11,12].
The mechanisms explained above describe cellular immunity to tumors. However, the presence of tumors elicits the generation of antibodies directed to antigens on tumors as well. Such tumor specific antibodies can neutralize cell growth directly or aid in activating innate immune cells. This is referred to as humoral immunity and can typically involve the generation of antibodies in the affected patient or the passive infusion of monoclonal antibodies, which have been previously generated. Immunotherapies have generally focus on the active immunization of the cellular arm of the immune system at expense of the humoral response. The design of strategies that optimally activate both arms of the immune system and reduce immune suppression concurrently has the potential to produce effective anti-tumor immunity.
Immunologic studies of tumors in mouse models
Several murine models have been developed to study cancer. The models used in the cancer research field are generally established to study the etiology and mechanisms of carcinogenesis and to examine responses to conventional therapies. These experimental mouse models can be subdivided in three main categories:- transplantable tumors, genetically engineered/transgenic models and humanized mouse models of cancer. We will describe each of these briefly and delineate their potential use to study cancer therapy taking into account the role of the immune system in tumor progression. We will also describe their potential use as tools to develop and test novel immunotherapies.
Transplantable tumors
One of the most commonly used models is based on the implantation of human tumor cell lines into immune compromised mice (xenograft model). Immune deficient mice will not reject non-autologous tumors. While these studies have provided a great deal of insight in tumor biology, they do not take into account the contribution of the adaptive immune system in tumorigenesis. In contrast, tumor immunologists utilize immune competent mice to fully appreciate the role of the immune system in tumor development. In fact, the vast majority of the milestones in tumor immunology, and more broadly in immunology, have been established in mouse models. The basic tenets of cancer immunology arose from studies of tumor rejection in genetically identical (syngeneic) mice. Following the development of inbred mouse strains in the 1940–1950s, tumor transplantation studies showed that mice could be immunized with transplantable tumors that arose in the same strain [14]. These initial experiments established that immunity to cancer was real and that immune-mediated tumor rejection was not an artifact due to the genetic origin of the tumor and recipient mice. These studies have also uncovered several principles, including the existence of tumor antigens, the immunogenicity of dying tumors, and the generation of anti-tumor responses by the host. The knowledge gained from these basic mechanisms has enabled us to understand how to modify the host and/or the tumor cells to induce tumor rejection.
Mouse cancer cell lines have been established for a variety of cancer types and derived from multiple genetic backgrounds. As examples, we can list the most common ones such as the B16 cell lines for melanoma, TRAMP for prostate cancer, MC38 for colon cancer and EL4 for lymphoma were all generated in the C57BL/6 background. The 4T1 line for breast cancer and CT26 for colon cancer were generated in BALB/c mice. The immunogenicity of these cell lines is variable and is taken into account when evaluating immune responses to therapies. As an example, B16 cells are considered poorly immunogenic and are generally more difficult to regress. These cell lines are generally injected subcutaneously to evaluate tumor growth and response to treatment. They are also injected intravenously to evaluate dissemination to lungs, liver or spleen. In this case, tumor progression is followed using imaging techniques that will allow the evaluation of tumor progression. The background of the mouse strain is also a variable that is important to consider. For example, BALB/c mice favor the production of stronger humoral responses when compared to C57BL/6 mice.
The main advantage of transplantable models is the fact that they permit experiments to be done in a timely manner. Moreover, pathways and mechanisms can be explored more readily as many genetically modified mouse strains are available and the cancer cell lines can be modified as needed. They also allow for the ease of tumor isolation and studies of immune cell infiltrates and are ideal for rapid screening of new agents. However, they also present several disadvantages as they do not recapitulate the tumor microenvironment and the multistep processes occurring during spontaneous tumor development. The concept of immunoediting is applicable to spontaneous tumors; however, transplantable tumor cell lines do not undergo all three stages of immunoediting.
Genetically engineered/transgenic mouse models
The recent advances in the characterization of the molecular basis of cancer in several tumor types has generated a wealth of information [14]. This led to the design and derivation of mice that more faithfully recapitulate the genetic lesions in human cancers. These models utilize the recent advances in mouse genetic engineering, allowing for the expression of oncogenes and/or inactivation of suppressor genes, in a given tissue under very specific conditions. As an example the Braf mutated oncogene (V600E) was expressed in an inducible manner in melanocytes (using the tyrosinase promoter) with conditional deletion of Pten using the CreER/Loxp system [15]. The resulting mice developed melanoma after tamoxifen induction. The ability to allow for the expression of an oncogene or inactivate a suppressor gene in an inducible manner has allowed for the introduction of ‘driver’ genetic lesions in a subset of cells (somatic mutation) in mice at the adult stage. The ability to induce oncogenic mutations in a subset of cells in the adult mice allows for recapitulating the multiple steps of tumorigenesis and the reciprocal interaction between tumor and stroma, including immune cells more faithfully. However, the use of transgenic mouse models requires longer follow up and the expansion of unique and limited mouse colonies in order to perform tumor treatment experiments. The length of time to conduct a single experiment could take up 6 to 12 months. There is also greater variability in the phenotype observed in these models when compared to transplantable tumor models. The other caveat is that the mutations are continuously present due to the presence of the transgene, which is different from cancer in human patients. There is also generally a decrease in the efficacy of immunotherapies when compared to transplantable tumors. This is probably due to use of a more stringent and aggressive model and tolerance induced by the presence of the mutation(s) early during mouse development. Another caveat is that these genetically engineered mouse strains have been generated in mixed genetics backgrounds and need to back-crossed into a pure background for multiple generations before they can be used [14] . A systematic approach is for the initial preclinical immune studies to be conducted in transplantable tumor models and the findings then confirmed in genetically engineered mice.
Humanized cancer models
As mentioned above, xenograft experiments have used mice deficient in adaptive immunity. Nude mice and severe combined immunodeficiency (SCID) mice have been used commonly. The in vivo engraftment of human cells in these mice was greatly increased by genetically inactivating the IL-2 receptor gamma chain (IL2rynull). Growth of human melanoma lung metastases in NOD-scid IL2rynull mice was greatly increased compared to NOD-scid, NOD-scid β2mnull for example [16]. NSG also known as NOG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) have become the most commonly used immunodeficient mouse strain for human xenograft studies [17,18]. Attempts are now being made to reconstitute the immune system of these animals with human immune cells and thus far, the NSG mice have shown greater efficiency in hematopoietic cell engraftment [19]. Although the use of humanized mice remains a bit limited, it has provided insights in human disease and preclinical data. Further modifications will continue to improve the ability of these mice to recapitulate the human disease.
Status of the human immune therapy clinical trials
The recent unprecedented success of cancer immunotherapy has triggered a substantial growth of current immunotherapy clinical trials for a wide variety of cancers. These therapies are currently based on several approaches such as cell transfer, immune modulating antibodies, cytokines and vaccines. Some target tumor cells directly (adoptive T-cell transfer, targeted antibodies) while others act indirectly by enhancing pre-existing tumor immunity (cytokines, immune-modulating antibodies). It is now clear that further improvement of these therapies will be achieved by carefully designing innovative trials, focusing on the optimal means to combine two or more treatment modalities. As mentioned above there are several immune modulating antibodies that either block a negative checkpoint (CTLA-4 or PD-1) of T cells or activate co-stimulatory receptors on T cells. In 2011, the monoclonal antibody ipilimumab (blocks the inhibitory receptor CTLA-4) was approved for treatment of patients with metastatic melanoma. Based on this, agents that target a second inhibitory receptor, PD-1, or its ligand, PD-L1, are in clinical development. More recently a phase one clinical study combining PD-1 blockade (nivolumab) and ipilimumab revealed the feasibility of combination checkpoint blockade and suggested that the clinical efficacy of the combination could be greater than either therapy alone [20]. New innovative combinations will also use active immunization approaches in combination with immune modulation. As an example there is an ongoing clinical trial that combines sipuleucel-T (prostate specific cellular vaccine) with ipilimumab (NCT01832870). Another approach is based on the hypothesis that a combination of treatments that are aimed at killing tumors directly (thereby releasing antigens and altering the microenvironment) in combination with immune modulation. The combination of Braf inhibition and anti-CTLA-4 in pre-clinical models has shown promising results [21]; however, a combination of vemurafenib and ipilimumab has shown increased hepatotoxicity when used in patients with advanced melanoma [22]. An ongoing trial (NCT01767454) is evaluating the safety of a different BRAF inhibitor (dabrafenib) with a MEK inhibitor (trametinib) in combination with ipilimumab. This example highlights the importance of careful timing when combining such therapeutic modalities.
The flurry of clinical trials involving immunotherapy cannot be recapitulated in this short review, attesting to the field’s significant recent progress. An important concept is that combination of novel immunotherapies with conventional therapies, targeted therapies or other immunotherapies is the next focus in clinical development of innovative treatment programs for cancer.
Conclusion and perspectives
Progress in understanding the mechanisms underlying immune regulation, with immune modulation as a focus (i.e. CTLA-4 checkpoint blockade), have led to the development of immunotherapeutic treatments for a subset of patients with advanced cancer. Despite the recent great success of cancer immunotherapy, a fraction of patients remain refractory to these treatments indicating a need for improvement. Several other immune modulatory molecules and adoptive cell therapies using tumor-infiltrating lymphocytes are showing promise in the treatment of metastatic melanoma and other cancers [23]. It is well documented that radiation therapy and chemotherapies by themselves have immune modulatory properties. Therefore, the combination of immunotherapy with existing therapies such as radiation therapy and chemotherapy are the logical next step in improving cancer treatment.
The rapid evaluation and improvement of these combinations will need to appropriate pre-clinical models. It is widely accepted that mouse models are able to provide useful pre-clinical and mechanistic information about novel immunotherapies and cancer therapies. However, an argument that is very often brought up is that animal studies are uninformative because they are not predictive of results in humans. Inadequacies in experimental designs may account for some of these failures. As tumor biologists select models to evaluate an immunotherapy or ask a specific question, it is vital to insure that the proposed models recapitulate and mimic the human disease as closely as possible, ensuring that pathology, metastatic potential, stage of disease, extent of tumor burden, hormone responsiveness and immune suppression are adequately and faithfully recapitulated in the animal models corresponding to each studied cancer type. It is also important to take into account the predictability and limits of each model when translating mouse experimental data into clinic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sadna Budhu, Ludwig Collaborative Laboratory, Memorial Sloan-Kettering Cancer Center, New York, NY 10065.
Jedd Wolchok, Weill Cornell Medical College, New York, NY 10065.
Taha Merghoub, Ludwig Collaborative Laboratory, Memorial Sloan-Kettering Cancer Center, New York, NY 10065.
References
- 1. Callahan MK, Postow MA, Wolchok JD. Immunomodulatory therapy for melanoma: ipilimumab and beyond. Clin Dermatol. 2013;31:191–199. doi: 10.1016/j.clindermatol.2012.08.006.. A review of the success and future prospects of ipilimumab, the first FDA approved immunotherapy for the treatment of melanoma.
- 2.Zhou G, Levitsky H. Towards curative cancer immunotherapy: overcoming posttherapy tumor escape. Clin Dev Immunol. 2012;2012:124187. doi: 10.1155/2012/124187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res. 1991:3–11. [PubMed] [Google Scholar]
- 4.Foley EJ. Antigenic properties of methylcholanthrene-induced tumors in mice of the strain of origin. Cancer Res. 1953;13:835–837. [PubMed] [Google Scholar]
- 5.Little CC. A Possible Mendelian Explanation for a Type of Inheritance Apparently Non-Mendelian in Nature. Science. 1914;40:904–906. doi: 10.1126/science.40.1042.904. [DOI] [PubMed] [Google Scholar]
- 6.Prehn RT, Main JM. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst. 1957;18:769–778. [PubMed] [Google Scholar]
- 7.Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- 8.Thomas L. On immunosurveillance in human cancer. Yale J Biol Med. 1982;55:329–333. [PMC free article] [PubMed] [Google Scholar]
- 9. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486.. Schreiber et al. desribes the unifying conceptual framework of cancer immunoediting, which integrates the host-protective and tumor-promoting roles of the immune system.
- 10.Stutman O. Chemical carcinogenesis in nude mice: comparison between nude mice from homozygous matings and heterozygous matings and effect of age and carcinogen dose. J Natl Cancer Inst. 1979;62:353–358. [PubMed] [Google Scholar]
- 11.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 12.Solito S, Bronte V, Mandruzzato S. Antigen specificity of immune suppression by myeloid-derived suppressor cells. J Leukoc Biol. 2011;90:31–36. doi: 10.1189/jlb.0111021. [DOI] [PubMed] [Google Scholar]
- 13.Andersen MH, Schrama D, Thor Straten P, Becker JC. Cytotoxic T cells. J Invest Dermatol. 2006;126:32–41. doi: 10.1038/sj.jid.5700001. [DOI] [PubMed] [Google Scholar]
- 14.Holland EC. Mouse models of human cancer. Hoboken, N.J.: Wiley-Liss; 2004. [Google Scholar]
- 15.McMahon M, Dankort D, Curley D, Filenova E, Bosenber M. A New Mouse Model of BRafV600E-induced Metastatic Melanoma. SMR emtting. Pigment Cell Res. 2007;20 [Google Scholar]
- 16.Carreno BM, Garbow JR, Kolar GR, Jackson EN, Engelbach JA, Becker-Hapak M, Carayannopoulos LN, Piwnica-Worms D, Linette GP. Immunodeficient mouse strains display marked variability in growth of human melanoma lung metastases. Clin Cancer Res. 2009;15:3277–3286. doi: 10.1158/1078-0432.CCR-08-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 18.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, et al. Human lymphoid and myeloid cell development in NOD/LtSzscid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 19.McDermott SP, Eppert K, Lechman ER, Doedens M, Dick JE. Comparison of human cord blood engraftment between immunocompromised mouse strains. Blood. 2010;116:193–200. doi: 10.1182/blood-2010-02-271841. [DOI] [PubMed] [Google Scholar]
- 20. Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. The New England journal of medicine. 2013;369:122–133. doi: 10.1056/NEJMoa1302369.. This study hightlights the importance and success of combining one or more immunotherapies.
- 21.Callahan MK, Masters G, Pratilas CA, Ariyan CE, Katz J, Kitano S, Russell V, Gordon RA, Vyas S, Yuan J, et al. Paradoxical activation of T cells via augmented ERK signaling mediated by a RAF inhibitor. Cancer Immunology Research. 2013 doi: 10.1158/2326-6066.CIR-13-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. The New England journal of medicine. 2013;368:1365–1366. doi: 10.1056/NEJMc1302338. [DOI] [PubMed] [Google Scholar]
- 23.Grupp SA, June CH. Adoptive cellular therapy. Curr Top Microbiol Immunol. 2011;344:149–172. doi: 10.1007/82_2010_94. [DOI] [PubMed] [Google Scholar]