Summary
Newcastle Disease Virus (NDV) is an avian paramyxovirus, which has been demonstrated to possess significant oncolytic activity against mammalian cancers. This review summarizes the research leading to the elucidation of the mechanisms of NDV-mediated oncolysis as well as the development of novel oncolytic agents through the use of genetic engineering. Clinical trials utilizing NDV strains and NDV-based autologous tumor cell vaccines will expand our knowledge of these novel anti-cancer strategies and will ultimately result in the successful use of the virus in the clinical setting.
Keywords: NDV, oncolytic, immunotherapy, apoptosis, interferon, cancer
Introduction
“The most striking sign of leukemia, the excess of leukocytes, disappears, and sometimes the spleen and lymph glands return to their normal size. Yet that the change is not wholly favorable appears from the fact that no case has really recovered… Considering the hopelessness of the ordinary treatment of leukemia, it seems that carefully planned experiments, either with bacterial products or organ extracts, might show a more safe and permanent result” [1].
The idea of using bacteria and viruses for treatment of human malignancies initially stemmed from observations since the mid-1800’s of tumor regressions that were associated with natural infections[1]. Development of cell and virus culture techniques in the early 1950’s led to intensive exploration of virus therapy in small animal tumor models and eventually in humans[2]. Due to significant virulence associated with the use of some of the human pathogens, animal viruses were explored as an alternative, with Newcastle Disease Virus (NDV) becoming a promising oncolytic agent [3–13]. This review will summarize the developments in the field of NDV cancer therapy, including the delineation of the mechanism of its oncolytic specificity, clinical trials, and recent advancements with the advent of genetic engineering.
NDV Biology and Tropism
NDV derives its name from the site of the original outbreak in chickens at a farm near Newcastle-upon-Tyne in England in 1926 [14]. It is an economically important pathogen in multiple avian species and it is endemic in many countries. NDV is a member of the Avulavirus genus in the Paramyxoviridae family. Similar to other paramyxoviruses in its family, the 15,186 nucleotide negative single strand RNA genome of NDV encodes six genes including the nucleocapsid protein (NP), phosphoprotein (P), matrix protein (M), fusion protein (F), haemagglutinin-neuraminidase (HN), and RNA-dependent RNA polymerase (L) [15]. The genes are separated by junction sequences that consist of three elements, known as gene start (GS), intergenic (IG), and gene-end (GE) motifs, which regulate mRNA transcription. In the P gene, a unique RNA editing mechanism adds non-templated G residues resulting in the expression of V and (perhaps) W proteins that are collinear to P in the amino-terminal end [15–17]. The genomic RNA is bound in a ribonucleotide protein complex (RNP) consisting of NP, P, and L and is surrounded by a lipid envelope containing three virus glycoprotein spikes, HN, M and F [15].
NDV is categorized into three pathotypes depending on the severity of the disease that it causes in birds: lentogenic (avirulent), mesogenic (intermediate), or velogenic (virulent) (table 1)[14]. The cleavage site in the fusion (F) protein of the NDV has been shown to be a major determinant of virulence [18–22]. F protein is synthesized as an inactive precursor (F0) and becomes fusogenic after proteolytic cleavage into two disulfide-linked polypeptides by host cellular proteases. The F0 of lentogenic viruses have monobasic cleavage sites cleaved by trypsin-like proteases found only in respiratory and digestive tracts. In contrast, the polybasic cleavage site of the F0 protein of velogenic strains allows for cleavage by ubiquitous proteases such as furin, resulting in a more systemic infection [20,23,24]. In addition, highly-fusogenic F proteins expressed on the surface of infected cells allow for efficient formation of syncytia, facilitating the spread of the virus from cell to cell [15,25–30].
Table 1.
Pathogenic classification of NDV
| Pathotype | Virulence in birds | Oncolytic effect | F cleavage site |
|---|---|---|---|
| Lentogenic | Non-virulent, no overt clinical signs | Non-lytic | Monobasic |
| Mesogenic | Intermediate, mild respiratory and gastrointestinal disease |
Lytic | Polybasic |
| Velogenic | Highly-virulent, severe gastrointestinal and respiratory disease or neurotoxicity |
Lytic | Polybasic |
Pathogenic classification of NDV strains in birds correlates with their oncolytic properties in cancer cells. While velogenic strains can efficiently carry out multicycle replication in multiple tested human cancer cells with effective cell lysis, lentogenic strains tend to be more attenuated due to lack of activation of the F0 protein [31]. On the basis of this finding, in human cancers NDV strains have been classified as either lytic or non-lytic, with velogenic and mesogenic viruses being lytic and lentogenic viruses in general being non-lytic. As described further in this review, several studies demonstrated that the lytic abilities of lentogenic NDV strains could be enhanced by introduction of polybasic cleavage site into their F proteins [22,26,32]. For fusogenic NDV strains, syncytia formation has been shown to significantly augment NDV-mediated lysis of cancer cells, both in vitro and in vivo [27,33–36]. In fact, introduction of the fusogenic NDV F protein into vesicular stomatitis virus (VSV) enhanced syncytia formation and oncolytic properties of VSV [25].
Other NDV proteins including the NP, P, V, HN, and L have also been shown to be implicated in virulence [37–45]. Several studies demonstrated the type I interferon antagonist activity of NDV V protein with associated decrease in pathogenicity in recombinant viruses possessing attenuating mutations in the V protein or in viruses expressing lower V protein levels [37,38,44,46]. Recently, two studies demonstrated the role of the polymerase complex in viral pathogenesis, with L protein contributing to pathogenicity of the mesogenic Beaudette C strain, and NP, P, and L proteins contributing to pathogenicity of the velogenic Herts strain [42,43]. Finally, several studies noted the contribution of the HN protein to virulence, with specific amino acid mutations conferring a higher virulence phenotype [39–41,47].
Mechanisms of NDV-mediated oncolysis (figure 1)
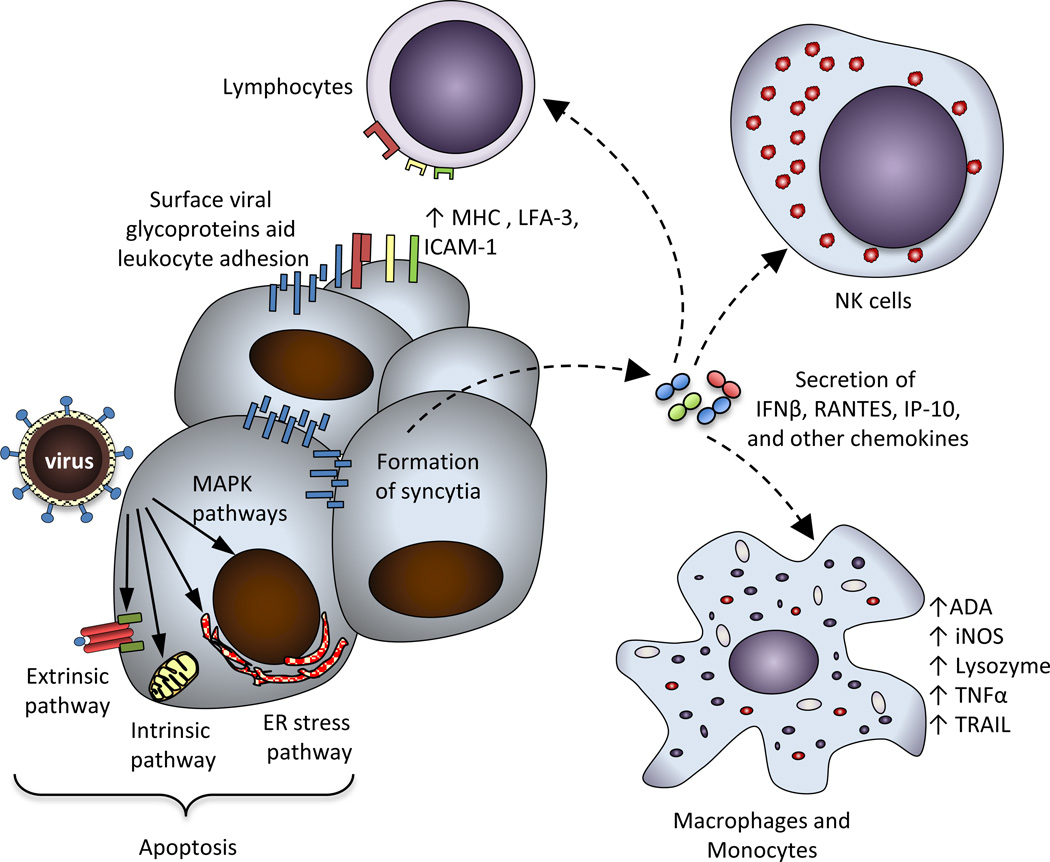
Figure 1. Mechanisms of NDV-mediated anti-tumor effects.
Cytolytic activity of NDV in cancer cells proceeds through direct and indirect mechanisms. The direct mechanisms include formation of multinucleated syncytia, activation of the extrinsic apoptotic pathway, activation of the intrinsic apoptotic pathway, activation of ER stress pathway, and involvement of MAPK pathways. The indirect mechanisms include secretion of proinflammatory cytokines and chemokines, which recruit mediators of both innate and adaptive immune responses such as NK cells, lymphocytes, and macrophages. Anti-tumor immune responses are further facilitated by the enhanced adhesion of leukocytes through binding to the viral glycoproteins expressed on the surface of infected cells, by upregulation of MHC and cell adhesion molecules, which serve to activate tumor-specific lymphocytes, and by activation of macrophages. MHC: major histocompatibility complex, LFA-3: lymphocyte function-associated antigen-3, ICAM-1: intercellular adhesion molecule-1, IFNβ: interferon β, RANTES: regulated upon activation, normal T cell expressed and secreted, IP-10: interferon gamma-induced protein-10, ADA: adenosine deaminase, iNOS: inducible nitric oxide synthase, TNFα: tumor necrosis factor α, TRAIL: TNF-related apoptosis-inducing ligand.
Direct mechanisms
Mechanisms underlying NDV-mediated cytotoxicity have been investigated in multiple studies. Experimentations on chicken embryos inoculated with NDV revealed evidence of apoptosis in embryonic tissues [48]. Multiple subsequent studies confirmed the dominant role of apoptosis in NDV-induced cell death [49–64]. Induction of apoptosis by NDV requires viral entry, replication, de-novo protein synthesis, and activation of caspases.
Delineation of apoptotic pathways activated in NDV-infected cells revealed common trends, though differences were noted depending on the system used [56,60–62,65]. Direct characterization of NDV strains Beaudette C and LaSota in cancer cell lines of ectodermal, endodermal, and mesodermal origin revealed that NDV can induce apoptosis by activation of both extrinsic and intrinsic apoptotic pathways [60]. NDV infection of tumor cells led to the production of TNFα and soluble TRAIL in tumor cell- and virus-specific manner, and resulted in activation of caspase 8, though in several cell lines apoptosis was induced without caspase 8 activation. In all tumor cell lines, NDV infection led to the loss of mitochondrial membrane potential and activation of caspase 9, highlighting the importance of the intrinsic pathway in activation of NDV-mediated apoptosis [60]. NDV-mediated induction of apoptosis was independent of IFN signaling, as apoptosis was also induced in tumor cell lines defective in the production of IFNα or defective in responses to IFNα [60]. The study thus suggested that activation of apoptosis likely proceeds through the mitochondrial pathway and activation of caspase 9, with subsequent activation of caspase 8 [60]. In line with these findings, a study by another group in several human cancer cell lines revealed that NDV MTH-68/H strain-induced cell death was independent of activation of initiator caspase-8 and caspase-9 or of cellular p53 status [61]. In the same study, viral replication in PC12 rat pheochromocytoma cells was shown to lead to the activation of the endoplasmic reticulum (ER) eIF2a kinase PERK with resultant phosphorylation of eIF2a and activation of caspase 12. The study suggested that ER stress-mediated mechanisms might be responsible for activation of apoptotic pathways in NDV-infected cancer cells [61]. In contrast to these findings, in NDV-infected Vero cells, activation of the extrinsic apoptotic pathway appeared to precede that of the intrinsic pathway [51]. Similarly, the extrinsic pathway was shown to be involved in A549 lung cancer cells after infection with NDV, while the previously shown phosphorylation of eIF2α or caspase 12 activation in PC12 cells could not be confirmed [62].
Other pathways were in addition implicated in NDV-induced apoptosis. Studies of NDV strain MTH/68 in PC12 rat pheochromocytoma cells revealed that induction of apoptosis was independent of the stress-inducible c-Jun N-terminal kinase pathway and the p38 pathway or of the mechanisms regulated by reactive oxygen species [56,59]. This was not confirmed in a subsequent study, where infection with NDV La Sota, Beaudette C, and FMW strains activated MAPK and downregulated Akt pathways in the infected A549 cells [62]. In that study, inhibition of p38 MAPK reduced cell death of NDV-infected A549 cells, though the mechanism for this is still unclear [62].
It is difficult to reconcile the discrepancies amongst these multiple studies, although many of the differences could possibly be explained by differences in the cell lines, viral strains, and detection assays used. In support of this, in the study by Elankumaran et al., different levels of soluble TRAIL production, and different levels of caspase 9 and 3 activation were noted amongst the different cell lines and viral strains tested [60]. Overall, based on multiple studies, activation of the intrinsic apoptosis pathway appears to play a key role in NDV-mediated cell death. Differential activation of the intrinsic pathway likely proceeds in a cell line- and virus-specific manner and appears to involve the extrinsic apoptotic pathway, ER stress pathway, receptor tyrosine kinase (RTK) pathways, and likely multiple other pathways that are yet to be characterized (figure 1).
Virus-specific factors have in addition been described to explain the mechanisms contributing to NDV-mediated apoptosis. Transfection of a plasmid encoding the HN glycoprotein into chicken embryonic fibroblasts (CEF) was shown to directly induce apoptosis by a yet uncharacterized mechanism [50]. Recently, the characterization of NDV protein sequences revealed pro-apoptotic Bcl-2 homology-3 (BH3) domain-like regions in NDV M, L, and F proteins [64]. Transfection of M and F proteins into HeLa cells promoted cell death, while deletion of the BH3-like domain from the M protein attenuated the induction of apoptosis by the protein. Co-immunoprecipitation experiments further revealed the interaction of the M protein with Bax and this interaction was abolished when the BH3-like deletion M protein was used [64]. Further similar analyses of NDV proteins will be important, as they may reveal additional mechanisms underlying the specificity of NDV for tumor cells.
Indirect mechanisms (immune-mediated)
In addition to its direct cytopathic effects, the anti-cancer activity of NDV is associated with the activation of both innate and adaptive immune responses.
Infection of murine macrophages with NDV strains LaSota, Ulster, and MTH-68/H has been shown to lead to the upregulation of macrophage enzymes such as ADA, iNOS, lysozyme, and acid phosphatase as well as the production of nitric oxide and TNF-alpha, resulting in the increase of in vitro and in vivo anti-tumor activity [66–69]. NDV Ulster-stimulated monocytes in addition were shown to mediate tumor cell killing via induction of TRAIL [58]. Activation of natural killer (NK) cells has also been implicated in NDV-mediated cytotoxicity, as direct incubation of NDV strain 73-T with PBMC’s from healthy donors enhanced their cytotoxicity against multiple tumor cell lines, with NK cells being the predominant mediator of lysis (figure 1) [70].
Studies also revealed that induction of innate immune responses in PBMC’s was not dependent on viral replication. In fact, transfection of a construct expressing NDV HN glycoprotein alone was sufficient to induce induction of IFN-alpha and TRAIL [57] and to induce apoptosis directly in transfected cells as discussed above [50]. Based on these findings, a DNA vaccine expressing cytoplasmic, secreted, and membrane-anchored forms of NDV HN glycoprotein was evaluated for anti-tumor effect. In this study, membrane-anchored HN was shown to have superior anti-tumor activity both in vitro and in vivo [71]. A study by another group revealed that prophylactic immunization with a plasmid encoding the NDV HN glycoprotein protected animals from subsequent challenge with DA3 mammary carcinoma cells [72], an effect that was shown to be associated with an increase in tumor NK cell infiltration and a decrease in myeloid-derived suppressor cells (MDSC) [72]. The mechanisms underlying those findings are unclear, though expression of NDV HN on the surface of antigen presenting cells has been shown to augment CTL responses against an unrelated antigen [73], which may be responsible for these observations.
Direct immunostimulatory effects on the tumor cells were also noted. Infection of tumor cells by NDV leads to expression of the viral HN and F glycoproteins on the surface, which has been shown to change tumor cell surface adhesiveness for erythrocytes and lymphocytes, leading to upregulation of T cell activation markers in mixed lymphocyte-tumor cell cultures (figure 1) [74]. Infection of human tumor cells with NDV Ulster was in addition shown to induce production of IFNβ, RANTES and IP-10, and to upregulate the expression of MHC and cell adhesion molecules ICAM-I and LFA-3 (figure 1) [75,76]. These studies imply that NDV infection may overcome the inhibitory effects of the tumor microenvironment and induce favorable inflammatory anti-tumor responses. Indeed, while tumor-infiltrating lymphocytes (TIL) isolated from freshly-resected melanomas lacked proliferation when stimulated with autologous melanoma cells, this proliferation was restored when the TILs were stimulated with the autologous melanoma cells infected with NDV Ulster [77]. Based on these studies, engineering of NDV armed with cytokines was undertaken as a strategy to improve the immunostimulatory activity of NDV (see below).
Overall, these studies suggest that NDV-mediated anti-tumor effects are dependent on the ability to induce apoptosis in cancer cells and the induction of inflammatory anti-tumor immune responses (figure 1). No less important, however, is the viral capacity to do so in a cancer-specific fashion, while sparing normal cells. Several studies were performed to delineate the mechanisms responsible for the apparent NDV specificity for tumor cells.
Mechanisms of NDV specificity for tumor cells (table 2)
Table 2.
Identified mechanisms of specificity of NDV for tumor cells
| Mechanism | Cellular defects | Ref. |
|---|---|---|
| Defects in activation of antiviral signaling pathways |
Defects in activation of RIG-I, IRF-3, IRF-7 | [46,85,86,88] |
| Defects in type I IFN signaling pathway | Deletion of IFNR1, decreased phosphorylation of STAT1 and STAT2, decreased expression of ISG’s |
[46,84,87] |
| Defects in apoptotic pathways |
Overexpression of Bcl-xL, overexpression of Livin | [63,89] |
| Activation of Ras signaling and expression of Rac1 protein |
Overexpression of oncogenic H-Ras and Rho GTPase Rac1 |
[90] |
IFN: interferon, RIG-I: retinoic acid-inducible gene I, IRF: interferon regulatory factor, IFNR1: interferon receptor 1, STAT: signal transducer and activator of transcription, ISG: interferon-stimulated gene
Specificity due to defects in the antiviral pathways
Viral infection of metazoan cells leads to the activation of multiple innate antiviral pathways. Cytokines of the type I IFN system, IFNα, IFNβ and IFNλ possess a wide range of biological activities, the most notable of which is their ability to block replication of certain viruses. Synthesis of type I IFN is induced by viral infection, in many cases, through production of viral nucleic acid that is recognized by different cellular sensors including the RIG-I-like helicase family (RLH), nucleotide binding oligomerization domain (NOD)-like receptors (NLR), and Toll-like receptors (TLR) [78,79]. These sensors mediate activation of several latent transcription factors, including the interferon regulatory factor (IRF)-3, IRF-7, NF-κB, and ATF2/c-jun, which are in turn involved in activation of the IFNβ promoter and synthesis of IFNβ[80–82].
Most if not all viruses have evolved strategies to evade the cellular type I interferon responses by synthesis of products antagonizing the pathway at different levels of signaling[83]. The NDV V protein antagonizes cellular innate immune responses through binding and degradation of STAT1 protein[38,44]. This function, however, was shown to be species-specific and is limited to avian cells, resulting in high sensitivity of the virus to anti-viral pathways induced in mammalian cells [44]. As such, NDV induces strong IFN responses in normal human cells.
Many cancer cells have been demonstrated to possess defects in anti-viral signaling pathways, which allows them to resist IFN-induced growth-inhibitory and apoptotic signals. It was therefore hypothesized that cancer cell-restricted replication of NDV is the result of the poor innate antiviral immune responses generated in these cells upon infection. Indeed, replication and spread of NDV were shown to be significantly reduced in primary human fibroblasts, when compared to the HT-1080 human fibrosarcoma cell line [84]. This effect was established to be due to the poor response of HT-1080 cells to IFNβ, with reduced phosphorylation of STAT1 and STAT2 proteins and reduced activation of IFN-regulated genes (table 2). Analysis of NDV-infected tumor cells revealed several defects in antiviral responses, which included delayed activation of antiviral proteins in response to UV-inactivated virus [85]. NDV infection of normal and tumor murine macrophage cell lines, revealed lower basal levels of expression of antiviral genes such as RIG-I, IRF-3, and IFN-beta in tumor cell lines and lower responsiveness to exogenously-added IFNα (table 2)[86]. Basal levels of expression of antiviral genes were inversely correlated with susceptibility to NDV infection [86,87]. Further analysis of NDV infection in macrophages deleted for IRF-3 or IRF-7 revealed a significant increase in viral replication in IRF-3 KO macrophages, highlighting the important role of IRF-3 in initiating the innate anti-viral response to NDV [88]. Additional studies in a panel of tumor cell lines by Elankumaran et al. demonstrated that most of the tested tumor cell lines in response to NDV infection failed to express IRF-7, while other cells were defective in induction of IFN-stimulated genes (ISG’s) such as RANTES, IP10, IRF-1, and ISG 6–16 (table 2) [46].
Specificity due to defects in apoptotic pathways
Despite these findings, several studies demonstrated effective oncolytic activity of NDV in cancer cell lines possessing robust induction of type I IFN and antiviral responses to NDV infection, suggesting other underlying mechanisms responsible for NDV specificity for cancer cells [27,89–91].
A study by Mansour et al. set out to investigate whether another common defect in cancer cells, namely, resistance to apoptosis, may be responsible for the observed selectivity of NDV for cancer cells [63]. When chemoresistant A549 or 293T cells stably overexpressing the anti-apoptotic Bcl-xL protein were infected with nonpathogenic NDV LaSota strain at low MOI’s, they showed dramatically enhanced sensitivity to NDV-mediated lysis with a two-log increase in viral replication. This observation was associated with a delay in early apoptosis because of the anti-apoptotic activity of Bcl-xL followed by a subsequent significant increase in apoptosis due to overwhelming virus replication and the production of type I IFN. Similar findings were seen with the use of the pan-caspase inhibitor Z-VAD-FMK. In support of these findings, knockdown of Bcl-xL in multiple cancer cell lines reduced viral replication and oncolytic activity [63]. The study suggested that the observed oncolytic specificity of NDV in IFN-competent cancer cells may be secondary to defects in apoptotic pathways or overexpression of anti-apoptotic proteins, which help to support productive viral infection and multicycle replication (table 2) [63].
A study by Lazar et al. identified that Livin, a member of the inhibitor of apoptosis (IAP) family of proteins overexpressed in advanced melanomas, conferred sensitivity of melanoma cells to the non-lytic NDV-HUJ strain [89]. While the majority of melanoma cells were resistant to the effect of multiple chemotherapeutic agents, they demonstrated sensitivity to NDV, which was particularly enhanced in the advanced melanoma cells lines overexpressing Livin. Despite the overexpression of the anti-apoptotic protein Livin, this NDV-mediated killing proceeded through activation of apoptosis. The authors further showed that activation of apoptosis was associated with cleavage of Livin to produce a proapoptotic cleavage product tLivin. This effect appeared to be independent of levels of expression of viral RNA and proteins and of IFN induction or signaling (table 2) [89].
Other pathways responsible for specificity
In a study performed by Lorence et al. in 1994, transformation of human fibroblast cell lines with N-Ras and H-Ras increased susceptibility to NDV infection by 1000-fold [92]. To evaluate the mechanism behind this increased sensitivity, a study by Puhlmann et al. used a multi-stage tumorigenesis model to identify pathways associated with increased susceptibility to NDV in transformed cells[90]. While immortal HaCaT cells were resistant to NDV, serial passage of HaCaT cells transformed with H-Ras resulted in the isolation of a highly malignant clone of cells that was highly susceptible to NDV infection. Detailed analysis of antiviral signaling pathways revealed no defects in activation of antiviral state or response to type I IFN in the transformed cells. After H-Ras protein was shown to be necessary, but not sufficient for tumor cell susceptibility to NDV, the authors performed a siRNA-based screening assay to identify NDV sensitizers. Of the identified genes, Rho GTPase Rac1 was shown to be necessary and sufficient for oncolytic NDV replication in HaCaT cells (table 2) [90]. This is a very interesting finding, since Rho family GTPases and interaction with actin filaments have been previously implicated in the replication cycle of several viruses, including NDV and other paramyxoviruses such as Sendai and measles viruses [93]. Exploring the involvement of Rac1 in more detail may provide further insights into the biology of NDV and perhaps other paramyxoviruses.
Genetically-engineered NDV (figure 2)
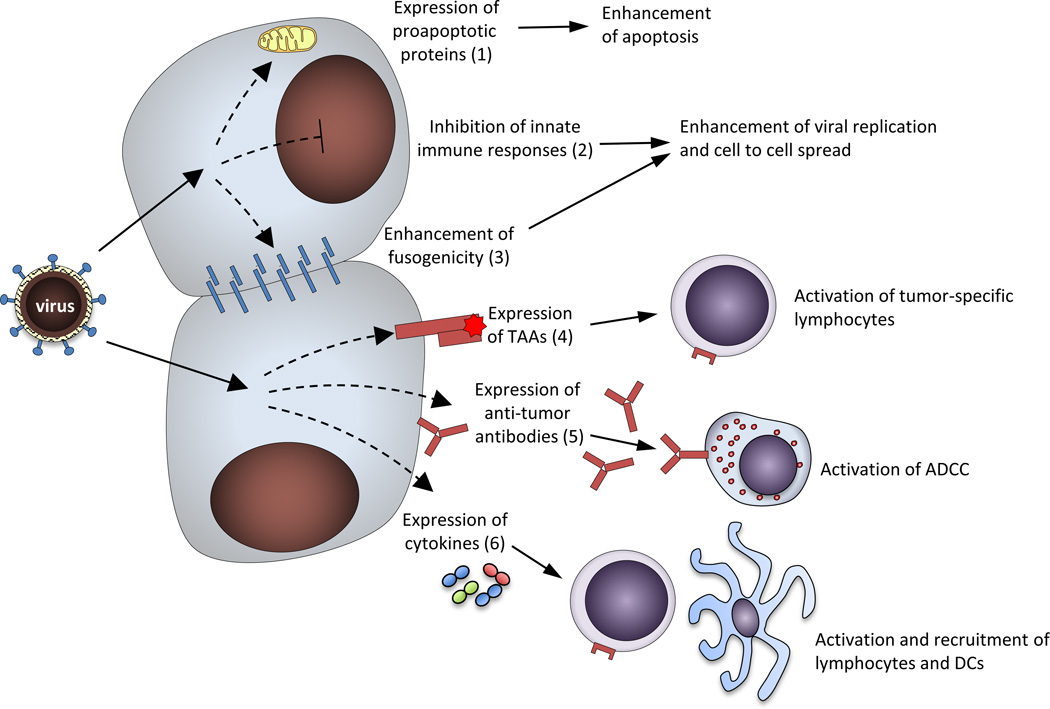
Figure 2. Strategies employed to enhance anti-tumor activity of NDV via genetic engineering.
Several approaches have been demonstrated to improve anti-tumor activity of NDV. Those include enhancement of intrinsic cytolytic properties of NDV via 1) increased fusogenicity; 2) inhibition of innate immune responses; and 3) expression of proapoptotic proteins; as well as enhancement of immune-mediated anti-tumor effects via 1) expression of tumor-associated antigens (TAA), leading to activation and expansion of tumor-specific lymphocytes; 2) expression of tumor-directed antibodies with activation of antibody-dependent cell-mediated cytotoxicity (ADCC); and 3) expression of immunostimulatory cytokines, leading to activation and recruitment of lymphocytes and dendritic cells (DC).
With the development of reverse-genetics for NDV, modification of the viral genome as well as introduction of foreign sequences became possible [94]. Using this system, recombinant NDV was demonstrated to serve as efficient vaccine vector against multiple pathogens in mammals and birds [32,95–106]. In addition, reverse genetics has been explored as an approach to improve the oncolytic activity of NDV.
Engineered fusogenic NDV
NDV F protein is responsible for viral fusion with the cell membrane and for viral spread from cell to cell via formation of syncytia. As discussed above, the presence of the multibasic cleavage site within the F protein allows for protein cleavage and activation by a broad range or proteases and is a determinant of virulence in velogenic viral strains [14]. To increase oncolytic potency of a highly attenuated lentogenic Hitchner B1 NDV strain, a polybasic cleavage site was introduced into the F protein to generate rNDV/F3aa. While the resultant virus exhibited only an intermediate virulence phenotype based on a mean death time in embryonated eggs, the virus formed large syncytia and was enhanced in its replication in cancer cells, leading to enhanced oncolytic effects in various animal tumor models (figure 2) [26,27,34–36,107]. Similar findings were shown when the F protein of the lentogenic NDV La Sota strain was modified in an analogous fashion [22,60]. The fusogenic and oncolytic activity of the rNDV/F3aa strain was further enhanced by a point mutation in the F protein at residue 289 from leucine to alanine, generating rNDV/F3aa (L289A). In an orthotopic immunocompetent liver tumor rat model, administration of the mutant virus via hepatic arterial infusion resulted in significant syncytia formation and necrosis, which translated to a significant 20% prolongation of survival over treatment with the original rNDV/F3aa virus[108].
A possible limitation of utilization of fusogenic NDV strains is their potential to become virulent in avian species. While retaining a good safety profile in humans, such viruses can theoretically cause outbreaks in poultry, in the unlikely event that they spread from treated patients to birds. In fact, while velogenic and mesogenic NDV strains have been previously shown to be the most effective oncolytic viruses, research on these strains became limited in the recent years due to classification of these viruses as select agents by USDA [109]. At present, all NDV strains, including recombinant, which possess polybasic F protein cleavage site, are considered to be select agents, which unfortunately complicates further clinical development of these strains [109]. An attenuated strain of a select agent, however, may be excluded from the requirements of the Select Agent Regulations. Recombinant fusogenic NDV of Hitchner B1 strain (NDV/F3aa) described above has been previously tested as a vaccine vector in chickens where it induced strong immune responses and was shown not to be pathogenic [32]. In addition, evaluation of this virus for mean death time (MDT) in embryonated eggs revealed a pathogenesis profile similar to that of mesogenic strains [32]. It appears that in adult birds the virus retains its safety profile, while having enhanced anti-cancer and vaccine vector properties. Further testing of this virus utilizing intracerebral pathogenicity index in day-old chicks will however be necessary to confirm its safety, prior to its further development as an anti-cancer agent.
Modulation of innate immune responses
As discussed above, the presumed specificity of NDV for cancer cells is thought to be in part due to defective innate immune responses in some cancer cells. While this allows for specificity of the attenuated and lentogenic NDV strains, many of the naturally occurring mesogenic and velogenic strains of NDV possess the ability to replicate in normal human cells. To avoid the potential toxicity of such strains, Elankumaran et al. generated an attenuated rNDV of the mesogenic Beaudette C strain with a mutation in the V protein. The resultant virus was attenuated in its ability to replicate in primary cells and tumor cell lines possessing intact IFN responses, but was equally effective to the parental virus in tumor cell lines and xenografts possessing defective components of the innate immune response [46]. The study established the mutation of the V protein as a means to attenuate NDV’s of highly pathogenic strains, while retaining oncolytic specificity. This approach, however, is limited to highly virulent velogenic and mesogenic NDV strains and is likely to be only effective in cancer cells that have defective innate immune responses.
To circumvent this problem, we sought to improve the oncolytic efficacy of the highly attenuated and interferon-sensitive Hitchner B1 strain by improving its capability to replicate in cancer cells with competent innate immune responses (figure 2). Introduction of the influenza A virus NS1 protein, a mammalian interferon antagonist, into the NDV genome resulted in enhanced viral replication in mammalian tumor cells [44]. NS1 is a multifunctional protein that inhibits induction of type I IFN and apoptosis through several mechanisms, including binding of double-stranded RNA, inhibition of PKR, and inhibition of RIG-I- and TLR3-mediated activation of IFN expression [110]. To explore the therapeutic implications of this finding, a fusogenic NDV of the attenuated Hitchner B1 strain expressing NS1 protein was generated [27]. The resultant virus was dramatically enhanced in its ability to form syncytia and lyse a variety of human and mouse tumor cell lines. Furthermore, the virus demonstrated stronger tumor clearance and a higher overall long-term survival in a mouse model of an aggressive malignant melanoma, when compared to the parental NDV/F3aa virus [27]. Administration of the virus intravenously or subcutaneously resulted in no toxicity, suggesting that the virus was still sufficiently attenuated in mammalian models not to cause disease [27]. This study demonstrated that by using nonpathogenic strains of NDV such as Hitchner B1, this approach could allow to target cancer cells with both defective and intact innate immune responses, without compromising therapeutic safety. Further testing would need to confirm whether the virus retains its attenuated phenotype in avian species.
NDV armed with pro-apoptotic protein
Regulation of apoptosis in cancer cells was recently explored as another strategy to enhance the oncolytic activity of NDV (figure 2). A study by Wu et al. explored a recombinant NDV FMW (rFMW) expressing the pro-apoptotic protein apoptin from chicken infectious anemia virus (rFMW/AP) [65]. When used at high multiplicities of infection (MOI) in tissue culture and xenograft models, the virus induced more apoptosis than the parental virus rFMW. Animals treated intratumorally with rFMW/AP and rFMW demonstrated significant tumor regressions, with the effects more pronounced in the rFMW/AP-treated animals[65]. While attractive, this approach may have some limitations in vivo by inducing early cell death, which would prevent multi-cycle viral replication and intratumoral spread.
Engineering NDV armed with cytokines
To enhance the immunostimulatory properties of NDV, several groups generated recombinant viruses expressing cytokines (figure 2). In a study by Janke et al., a recombinant NDV expressing GM-CSF was generated. The virus was shown to stimulate peripheral blood mononuclear cells (PBMC) to exert antitumor bystander effects in vitro in a tumor neutralization assay [111]. Vigil et al. generated recombinant fusogenic ND vectors expressing IFNγ, GM-CSF, IL-2, and TNFα [26]. In the syngeneic CT26 colon carcinoma mouse model, the viruses expressing IFNγ, GM-CSF, and TNFα demonstrated only a modest improvement in oncolytic activity over the parental virus. However, tumor-bearing mice treated intratumorally with IL-2-expressing virus had marked tumor regression and 60% long-term survival, when compared to the 20% survival of mice treated with parental virus. Furthermore, mice that underwent complete tumor regression were protected from further tumor challenge. Tumors isolated from treated animals demonstrated increased levels of CD4 and CD8 cell infiltration and lymphocytes isolated from draining lymph nodes produced significant levels of IFNγ when co-cultured with tumor cells [26]. In a subsequent study, NDV expressing IL-2 was shown to possess strong oncolytic activity against an aggressive syngeneic murine melanoma, leading to complete tumor clearance in 87% of animals with demonstrated establishment of protective anti-melanoma immune responses [107]. NDV expressing human IL-2 was further generated and demonstrated to express IL-2 upon infection of tumor cells in vitro with resultant T cell immunostimulatory activity with anti-tumor effects in a tumor neutralization assay [112,113]. Overall these studies demonstrate strong anti-tumor activity of NDV-IL-2 and prompt further investigation of IL-2-expressing NDVs as anti-cancer agents in humans.
NDV expressing immunoglobulins
In a study by Puhler et al., two transgenes encoding immunoglobulin heavy and light chains of antibody against ED-B fibronectin were inserted into the genome of the NDV strain MTH68 between the F and HN genes (figure 2) [114]. Expression of the transgenes did not affect viral growth or oncolytic properties in vitro and produced functional antibody that bound to tumor cell-derived EDB antigen and to tumor cells in infected fibrosarcoma xenografts. The study demonstrated the potential for expression of therapeutic antibodies from the genome of oncolytic viruses in order to enhance their therapeutic potency [114].
Viruses expressing tumor-associated antigens (TAA)
In addition to its use as a vaccine vector for mammalian and avian pathogens, NDV was explored in one study as a vaccine vector for expression of tumor-associated antigen (TAA) (figure 2). Intratumoral treatment of mice bearing CT26 tumors expressing beta-galactosidase with a virus expressing a beta-galactosidase epitope led to complete tumor regression in 60% of animals, compared with 20% of animals treated with control virus [33]. Addition of NDV expressing IL-2 to the treatment increased the complete regression rate to 90%. We recently reported another attractive strategy for potential NDV-based antigen presentation [100]. In that study, recombinant NDV expressing a fusion protein between the HIV Gag antigen and a single-chain antibody specific for the DC-restricted antigen uptake receptor DEC205 (scFV-Gag) was generated. The vaccination of mice with NDV expressing the DC-targeted Gag antigen resulted in enhanced Gag-specific CD8 and CD4 responses and protected the animals from the subsequent challenge with vaccinia virus expressing HIV Gag protein [100]. These studies highlighted the importance of the immunostimulatory component of NDV therapy and demonstrated the potential of recombinant NDV expressing a TAA to be used as a therapeutic cancer vaccine vector.
NDV modified with bispecific adapter proteins
The group of Schirrmacher et al. developed an additional unique approach to improve the targeting and immunostimulatory activity of NDV. By utilizing a recombinant fusion protein between the human IL-2 and a single-chain antibody directed against NDV HN protein (scHN-IL2), they were able to specifically retarget the virus to IL-2 receptor (IL-2R)- expressing human leukemia cells [115,116]. Further evaluation of the strategy in vivo revealed that the virus specifically was targeted to IL-2R positive tumor tissues, with decreased uptake in the liver, spleen, kidney, lung, and thymus, when compared to parental virus [117].
As an approach to enhance the immunostimulatory activity of NDV, the authors used bispecific single chain antibodies directed against NDV HN and the T cell co-stimulatory receptors CD3 or CD28 (bsHN-CD3 and bsHN-CD28). Application of the bispecific antibodies to the NDV-infected tumor cells showed enhanced activation and cytotoxicity of unstimulated primary human T cells added to the culture [118]. Furthermore, PBMC or purified T cells co-incubated with such a tumor vaccine were able to destroy new tumor cell monolayers after serial transfer [119]. The authors went on to further demonstrate that this approach was even more effective when scHN-IL2 fusion protein was used for T cell stimulation instead of bsHN-CD28 protein [120].
In their third approach, fusion proteins of single chain anti-F antibody and GM-CSF (bsF-GMCSF) and single chain anti-HN antibody and IL-2 and GM-CSF (tsHN-IL2-GM-CSF) were generated. Treatment of NDV-infected tumor cell vaccine with these fusion proteins and PBMCs led to PBMC activation and generation of anti-tumor responses [121]. Overall, these approaches present attractive strategies for enhancement of immunostimulatory activity of NDV and tumor-specific virus targeting and can likely be used in conjunction with other genetically-engineered NDVs described earlier.
Clinical trials with oncolytic NDV
Multiple preclinical studies demonstrated significant anti-cancer activity of naturally-occurring and recombinant NDV in pre-clinical models. Promising results were noted in preclinical models of leukemia, lymphoma, melanoma, neuroblastoma, fibrosarcoma, rat pheochromocytoma, colon carcinoma, lung carcinoma, prostate carcinoma, breast carcinoma, gastric carcinoma, mesothelioma, and head and neck carcinoma [7,9,12,13,26,27,31,34–36,56,59,68,70,77,92,107,108,122–128]. Clinical studies that followed consisted of NDV-based tumor vaccines and direct administration of naturally-occurring NDV to patients with advanced cancers and in post-operative setting.
NDV-based immunotherapeutic approaches (table 3)
Table 3.
Summary of clinical trials with NDV-based cellular vaccines and oncolysates
| Vaccine preparation |
Type of cancer | Type of study and patient number (n) |
Clinical outcome | Ref. |
|---|---|---|---|---|
| NDV 73T oncolysate |
Stage II and III melanoma | Phase II, n=83, historical controls |
Improved OS* | [129–131,134,142,148] |
| NDV Italien oncolysate |
Stage III melanoma | Phase II, n=24, historical controls |
No benefit | [149] |
| NDV 73T oncolysate |
Advanced renal cell carcinoma |
Phase II, n=208, historical controls |
Improved PFS* | [137] |
| NDV Ulster Whole-cell |
Colorectal cancer with liver metastases |
Phase III, n=51 Phase II, n=23 |
Improved PFS, improved OS for colon cancer subgroup |
[133,135,147] |
| NDV Ulster Whole-cell |
Resectable colorectal cancer |
Phase II, n=57, historical controls |
Improved OS | [139] |
| NDV La Sota Whole-cell |
Resectable colorectal cancer |
Phase III, n=567 | Improved OS | [144] |
| NDV Ulster Whole-cell |
Metastatic renal cell carcinoma |
Phase II, n=40, historical controls |
Improved OS | [138] |
| NDV Ulster Whole-cell |
Advanced ovarian cancer |
Phase II, n=82, historical controls |
Improved PFS | [156] |
| NDV Ulster Whole-cell |
Glioblastoma | Phase II, n=23, concurrent controls |
Improved PFS and OS | [146] |
| NDV Ulster Whole-cell |
Advanced head and neck |
Phase II, n=18, historical controls |
Improved OS | [157] |
| NDV Ulster Whole-cell |
Stage III melanoma | Phase II/III, n=29 | No benefit | [145] |
OS: overall survival,
PFS: progression-free survival
Recognizing the immunostimulatory properties of NDV, these viruses were employed for active tumor-specific immunization [129–147].
In the initial studies by Cassel et al. utilizing NDV 73T strain, patients with stage II and III melanoma were immunized with autologous or allogeneic NDV oncolysates [129,130,148]. Long-term follow-up of these patients demonstrated over 60% 10-year survival and 55% 15-year survival, a significant increase compared to historical controls [134,142]. These findings were not reproduced by a similar study subsequently performed at the MD Anderson Cancer Center, though a different method for the oncolysate preparation was used [149]. NDV 73T–generated oncolysates were subsequently used to prevent relapses of surgically-removed renal cell carcinomas in conjunction with recombinant IL-2 and IFN-alpha, similarly demonstrating improved survival when compared to historical controls [137,141].
A different strategy was developed by Schirrmacher and colleagues, utilizing whole-cell autologous irradiated tumor vaccines modified by infection with attenuated NDV Ulster strain (ATV-NDV), often with co-administration of immunostimulatory cytokines [150]. When compared to oncolysates, tumor cells retaining membrane integrity were shown to be more immunogenic [151]. The efficacy of this approach was initially demonstrated in preclinical models of ESb lymphoma [152], B16 melanoma [153], and 3LL Lewis lung carcinoma [154], with all animals showing protection against subsequent challenge with tumor cells.
Based on these findings, clinical trials utilizing immunization with ATV-NDV have been performed in patients with colorectal cancer, breast cancer, ovarian cancer, renal cell carcinoma, and malignant glioma.
Several clinical trials evaluated ATV-NDV for colorectal cancer, either in adjuvant (postoperative) setting or for treatment of unresectable disease [132,133,135,136,139,155]. Immune responses to the vaccine were monitored by skin delayed-type hypersensitivity (DTH) testing, and increased immune reactivity in 75% of patients was reported in one of the trials [155]. When tested in an adjuvant setting, 14 patients (61%) treated with autologous vaccine had relapses of their cancer, compared to relapses in 20 (87%) of 23 historical control subjects. The study achieved statistically significant improved disease free survival (DFS), but not overall survival (OS) [135]. In another adjuvant study involving 57 patients with resected locally advanced colorectal carcinomas or tumors with regional lymph node metastases, treatment of patients with ATV-NDV resulted in 97.9% 2 year survival in the treatment group, compared to 73.8% in a historical control group [139]. The overall survival in patients treated with vaccine was, however, comparable to that of the group of patients treated with adjuvant chemotherapy [139].
The largest study for treatment of gastrointestinal tumors with an autologous NDV-modified tumor cell vaccine was reported by Liang et al. [144]. Non-lytic NDV La Sota was used in this study. This was a phase III study comparing 310 patients with stage I-IV colorectal cancer treated with resection and immunotherapy, to 257 patients treated with resection alone. It is unclear whether the study was randomized or how allocation was performed. The manuscript also did not specify whether the patients on the study received any additional chemotherapy. Median overall survival was over 7 years in the vaccine group, compared to 4.46 years in the resection alone group and this was statistically significant. In addition, the same study included 25 patients with unresectable colorectal, stomach, liver, pancreatic, and gallbladder cancers who were all treated with autologous NDV-modified tumor cells[144]. At one-year follow-up, 88% of these patients showed some clinical benefit. While the study certainly looks promising, a more rigorous randomized clinical trial will be necessary to reach more definitive conclusions.
ATV-NDV was in addition evaluated in conjunction with low dose interleukin-2 and IFN-alpha in 40 patients with metastatic renal cell carcinoma. Of the 40 patients, 23 showed some clinical benefit, with median overall survival of 31 months, compared to 13 months for historical controls [138]. While these results are quite remarkable, they need to be interpreted with caution since it is unclear how much benefit can be attributed to the vaccine vs. the cytokine therapy alone.
A phase II study evaluated the use of ATV-NDV in ovarian cancer patients [156]. The trial enrolled 82 patients, but only 39 were available for final evaluation. Out of evaluable patients, clinical benefit was described for 23 patients with stage III disease with 15 complete responses (CR), 8 partial responses (PR), and one progressive disease (PD). Data for the remaining 15 patients was not presented. Patients in the study in addition received a course of platinum-based adjuvant chemotherapy, thus making it difficult to evaluate the degree of benefit from immunotherapy alone.
One study retrospectively evaluated the effect of vaccine quality on the survival of the patients with early stage breast cancer and metastatic breast and ovarian cancer [140]. In this study, patients who received sufficient numbers of tumor cells for at least two vaccinations demonstrated survival benefit, which was shown to be statistically significant in the early stage breast cancer group [140]. These encouraging findings certainly deserve evaluation in a larger prospective study.
A small pilot study was in addition performed in a group of 23 patients with glioblastoma multiforme, who were vaccinated postoperatively and compared to a cohort of 87 non-vaccinated patients [146]. The study demonstrated PFS of 40 weeks and OS of 100 weeks in the vaccinated group, compared to 26 weeks and 49 weeks in the control group, respectively. Both of the numbers were statistically significant. A similar post-operative vaccination was performed in a group of 20 patients with head and neck squamous cell carcinomas (HNSCC) [157]. Out of 18 vaccinated patients with stage III and IV disease, 61% demonstrated 5-year survival, an improvement over historical controls [157]. Finally, a randomized double-blind controlled study by Volt et al., utilizing autologous NDV-modified melanoma vaccine failed to demonstrate benefit from vaccine therapy, though the study was small in size [145].
Overall, these clinical studies demonstrate that adjuvant vaccination with autologous NDV-modified cancer cells is safe, and appears to have benefit in uncontrolled studies (table 3), though most of the studies had a variety of limitations. Thus, while no solid conclusions can be reached regarding the degree of clinical benefit from this approach, a randomized clinical trial would certainly be prudent to evaluate this therapeutic strategy more definitively.
Clinical trials with direct NDV administration (table 4)
Table 4.
Summary of clinical trials with systemically administered NDV
| Viral strain |
Route | Type of cancer | Type of study and patient number (n) |
Clinical outcome | Ref. |
|---|---|---|---|---|---|
| NDV MTH-68 |
*IV, *IH, *IC |
Various advanced | Case series (n=4) |
*PR or *CR, with long- term benefit |
[143] |
| NDV MTH-68 |
*IH | Various advanced | Phase II, n=59, placebo-controlled |
Improved OS | [159] |
| NDV MTH-68 |
IV | Glioblastoma | Case series (n=14) |
7/14 responses, long-term benefit in 4 patients |
[163] |
| NDV PV-701 |
IV | Various advanced | Phase I, n=79 |
1 CR, 1 PR, 7 SD | [165] |
| NDV PV-701 |
IV | Various advanced | Phase I, n=18 |
1 CR, 3 PR, 2 SD | [166] |
| NDV PV-701 |
IV | Various advanced | Phase I, n=16 |
Improved tolerability with desensitization |
[164] |
| NDV HUJ |
IV | Glioblastoma | Phase I/II, n=14 | One transient CR | [167] |
IV: intravenous,
IH: inhalational,
IC: intracolonic,
PR: partial response,
CR: complete response,
SD: stable disease
Several clinical trials have been conducted utilizing infectious NDV administered to cancer patients. The adverse effects associated with NDV infection have in general been demonstrated to be mild to moderate in severity, with the most common side effects being flu-like symptoms, conjunctivitis, and laryngitis [158–160]. The strains evaluated for direct human injection were PV-701, 73-T, MTH-68/H, and Hickman, which are lytic, and HUJ, which is non-lytic.
The first documented use of NDV for human cancer therapy was reported in 1964 by Wheelock and Dingle, where a patient with acute myelogenous leukemia was serially inoculated with multiple viruses in an attempt to induce remission [158]. Intravenous inoculation with NDV Hickman strain resulted in rapid reduction in leukemic blast count and improvement in symptoms, which lasted for almost 2 weeks.
NDV strain 73-T has been used for intratumoral injection in the treatment of a patient with advanced cervical cancer [10]. The patient achieved a partial response to injection. The same strain of virus was in addition administered to 17 healthy subjects intravenously and was noted to be a strong interferon inducer with a transient drop in blood counts and mild flu-like symptoms, but overall good tolerability [161]. Subsequent uses of this virus involved preparation of tumor oncolysates, as described above.
NDV strain MTH-68 was developed by Csatary and colleagues and has been tested in cancer patients via inhalational, intravenous, and intracolonic route. In a case report of a child with glioblastoma multiforme and a case series of four patients with colorectal cancer, Hodgkin disease, and melanoma, patients were treated with NDV for periods of time that ranged from 1 month to 7 years [143,162]. All of the patients had either partial or complete response to therapy. Four of the patients in addition were treated with conventional chemotherapy, making interpretation of the results difficult. In a separate randomized placebo-controlled phase II trial, 33 patients with various advanced cancers were treated with twice-weekly inhalation of NDV and 26 patients received placebo [159]. Two complete responses, six partial responses, and two patients with stable disease were reported in 10 patients in the treatment group, compared to no responses and 2 patients with stable disease in the placebo group. Overall one-year survival was 67% in the treatment group and 15% in the placebo group. While appearing positive, the trial was criticized for lack of randomization and a larger number of patients in the NDV treatment group receiving conventional therapy prior to initiation of treatment, compared to the placebo group [159]. In an additional series, 14 patients with glioblastoma were treated intravenously with NDV on various schedules. Seven of the patients achieved response to therapy with four of the patients surviving between 5 and 9 years at the time of the publication in 2004 [163].
Three phase I clinical trials with NDV strain PV-701 developed by Wellstat Biologics were conducted in patients with various advanced cancers [160,164–166]. In those studies, a total of 113 patients who had failed standard chemotherapy were treated with intravenous injection of the virus in four different treatment schedules. When the patients were desensitized with a lower initial dose, a higher subsequent dose could be administered with increased tolerability and efficacy [164]. In the initial trial of 79 patients, a complete response was observed for 1patient, a partial response in 1 patient, and 7 additional patients demonstrated minor responses. Overall, 14 patients demonstrated progression free survival that lasted from 4 months to over 30 months [160]. A subsequent trial utilizing a slower infusion rate with resultant administration of higher dose demonstrated improved tolerability and response rates, with demonstration of 4 major and 2 minor responses in 18 enrolled patients, with 6 patients surviving at least 2 years [165,166]. Nonlytic NDV strain HUJ has been evaluated in 14 patients with recurrent glioblastoma multiforme by intravenous administration [167]. Minimal toxicity was observed. One patient achieved complete response, while the rest of the patients had progressive disease [167].
Overall, direct administration of NDV to patients has been shown to result in minimal toxicity with suggestion of clinical benefit in some patients, warranting further evaluation (table 4).
Future perspectives
Our improved understanding of NDV biology, tropism, mechanisms of oncolysis and oncolytic specificity for cancer cells and the development of the reverse genetics system have paved the way for the intelligent design of effective oncolytic vectors and their clinical development as anti-cancer agents. Several avenues of research that will in particular be important to undertake in the future are discussed below.
Advantages of NDV
When compared to other oncolytic agents in development such as poxvirus, HSV-1, adenovirus, measles, and reovirus vectors, NDV as an oncolytic virus exhibits several advantages over other viruses, but in addition possesses several limitations [168].
First of all, NDV is an avian pathogen, which avoids the problem of preexisting immunity and pathogenicity of the virus in humans, which are potential problems with vaccinia, HSV-1, adenovirus, and measles vectors. Serologic studies indicated that approximately 96% of the human population is seronegative for NDV, which avoids the problem of pre-existing immunity seen with many human candidate oncolytic viruses [169,170]. The actual seropositivity in the modern day population may be even lower due to low human exposure to NDV, the outbreaks of which are primarily limited to farm settings. To attest for viral safety, administration of virulent strains to humans has been shown to result in only mild to moderate adverse effects, with mild conjunctivitis, laryngitis, and flu-like symptoms as the only reported infections. Natural human infections with highly virulent (avian) strains have been reported in the literature in farmers and laboratory workers and have been limited to mild symptoms such as conjunctivitis and laryngitis [14].
Secondly, similarly to all oncolytic viruses, NDV possesses strong immunostimulatory properties through induction of type I IFN and chemokines, upregulation of MHC and cell adhesion molecules, and facilitation of adhesion of lymphocytes and APC’s through expression of viral glycoproteins on the surface of infected cells [74,76,171]. These properties have been shown to generate effective anti-tumor immune responses, which may persist long after clearance of viral infection. Third, the NDV genome allows for incorporation and stable expression of foreign genes of relatively large size [94]. As these viruses replicate in the cytoplasm of the cell, unintended integration of the foreign gene into the host genome is avoided, which could be a problem with some of the DNA oncolytic vectors. Moreover, low to nonexistent rates of homologous RNA recombination ensure that the foreign genes expressed from viral genome are stable for many serial passages in cell culture [172].
Lastly, the ubiquitous nature of the NDV receptor allows for utilization of the virus against a wide variety of cancers. The specificity of the virus for cancer cells due to their defects in antiviral and apoptotic pathways ensures viral safety and may obviate the need for specific tumor targeting.
Improving tumor-specific targeting
Despite the advantages discussed above, utilization of NDV as an oncolytic agent presents several limitations. In particular, tumor-specific receptor targeting presents more of a challenge for NDV, when compared to some other vectors. First of all, the system used for generation of recombinant NDV, which relies on the use of embryonated eggs, presents constraints on modifications of the NDV HN protein for the purposes of its retargeting to other receptors, as was demonstrated with adenovirus and measles virus vectors [168]. Development of tissue culture-based systems for virus rescue and growth will be necessary in order to achieve this goal. Alternatively, utilization of bispecific antibodies as demonstrated by Schirrmacher and colleagues presents an attractive strategy and has been similarly used for retargeting of adenoviruses [115–117,168]. In addition, introduction of specific cleavage sites into the F protein may allow for utilization of tumor tissue-specific proteases, as was previously demonstrated with measles, Sendai, and retroviruses [173–175].
Tumor-specific targeting of NDV is further limited by the fact that it is an RNA virus, which prevents the utilization of tissue-specific promoters as was used with adenovirus and herpesvirus vectors [176]. As an alternative strategy, tumor-specific replication could be achieved by introduction of RNA destabilizing elements such as AU-rich elements (ARE) into viral proteins or virally-encoded transgene [177]. Activation of oncogenic pathways leads to overexpression of ARE-binding proteins such as tristetraprolin (TTP) and human antigen R (HuR), which stabilize mRNA of multiple tumor-promoting genes such as cytokines, chemokines, growth factors, and transcription factors [177]. Selective viral replication or expression of cytotoxic genes can thus be achieved in cancer cells overexpressing such proteins, while viral mRNA would be degraded in normal cells. Such strategy has already been successfully explored for tumor-specific replication of adenoviruses [178].
Avoiding the neutralizing immune response
Similar to other oncolytic viruses, NDV is very immunogenic and elicits strong neutralizing antibody responses, which may limit the viral therapeutic efficacy with repeated administrations. Despite the limited clinical data demonstrating continued benefit in patients treated with multiple administrations of the virus, the problem of viral neutralization will likely limit its therapeutic efficacy in the majority of patients. Potential solutions to this problem would rely on utilization of different NDV strains, use of viral carriers as discussed below, and utilization of different viral surface proteins through the use of reverse genetics. Induction of immune tolerance to viral administration via oral viral protein administration has also been suggested as a strategy [179].
Improving systemic delivery of NDV
While NDV and other oncolytic viruses have been shown to induce significant tumor regressions with intratumoral administration, poor systemic delivery secondary to immune clearance and off-target binding remains to be a significant challenge [126]. While phase I trials with intravenous administration of NDV demonstrated some clinical benefit, the benefit was only modest in most of the patients, which was possibly a reflection of poor intratumoral delivery of the virus [165]. Multiple groups have recently explored carrier cell systems for delivery of viruses with promising results in preclinical models[180]. With demonstration that mesenchymal and neural stem cells, immune cells, and cancer cells themselves possess tumor-homing characteristics, several groups demonstrated that such cells loaded with oncolytic viruses could enhance viral delivery to tumors, while avoiding systemic toxicity [180]. In particular, utilization of mesenchymal stem cell (MSC) carriers for paramyxovirus (measles) virotherapy has been shown to protect the virus from neutralizing antibodies and to effectively transfer the virus to tumors in an orthotopic xenograft model of ovarian cancer [181]. Exploration of similar methods for delivery of NDV to target tissues will certainly be important for its further development as a systemic agent.
Taking genetically-engineered NDV to the clinic
Despite the multiple studies demonstrating promising results of naturally-occurring NDV strains both in preclinical models and in clinical trials, the field has unfortunately remained relatively stagnant in the clinical arena. The majority of the studies have so far been relatively small and lacked adequate controls, though the number of studies reporting some degree of benefit certainly warrant further evaluation of NDV in larger trials. Progress in the field has also been hampered by demonstration of only marginal benefit in the majority of the studies, perhaps highlighting the shortcomings of the naturally-occurring NDV strains. With the advent of genetic engineering of NDV it has become possible to utilize the viral advantages discussed above in the design of agents with improved oncolytic activity and targeting in order to overcome those limitations. As demonstrated above, several studies utilizing genetically-engineered NDV have already demonstrated significant improvement of viral oncolytic activity and maintenance of long-term antitumor effects, when compared to parental wild-type strains. In particular, NDV expressing therapeutic antibodies, NDV engineered to modulate innate immune responses, and NDV expressing immunostimulatory cytokines appear to be highly attractive candidates for further evaluation in humans [26,27,33,107,108,111,113]. In the next 10 years we will hope to see the exploration of these viruses in clinical trials as well as further development of genetically-engineered NDV vectors and their translation to clinical setting.
Exploring the immunostimulatory properties of NDV
Even with efficient systemic viral delivery, it has become increasingly recognized that the oncolytic efficacy of viral therapy is highly dependent on the induction of immune responses, which at times can generate long-lasting anti-tumor immunity. These findings are further supported by the recent developments in the field of cancer immunotherapy, which highlight the key role of the immune system in eliminating and containing tumor growth. The discovery of the T cell co-stimulatory and co-inhibitory receptors has provided attractive targets for immune therapies aiming to enhance activation of anti-tumor immune responses or to reverse the immunosuppressive mechanisms governing tumor resistance to immune destruction. Significant anti-tumor activity has been demonstrated with stimulatory antibodies to 4–1BB, OX40, and GITR, as well as inhibitory antibodies to PD-1 and CTLA-4 [182]. In a phase III randomized clinical trial for advanced melanoma, treatment with anti-CTLA-4 antibody ipilimumab resulted in significant improvement in median overall survival, leading to its approval by the FDA [183]. In addition, a therapeutic autologous antigen-presenting cell vaccine (Sipuleucel-T) was recently approved by the FDA for metastatic prostate cancer, after demonstration of prolongation of overall survival in a randomized double-blind placebo-controlled IMPACT study [184].
In line with these findings, utilization of recombinant NDV and other oncolytic viruses such as vaccinia, reovirus, adenovirus, and HSV engineered to express immunostimulatory cytokines has already been demonstrated to have superior anti-tumor properties, when compared to the parental viruses[185]. Some of these viruses are far along in development and are currently being tested in phase II and III clinical trials [186,187]. Other strategies combining oncolytic virus therapy with immunotherapeutic approaches are already being explored by investigators, revealing significant improvement in antitumor activity [185]. It is obvious that both in preclinical and in clinical studies the NDV-induced activation of the immune system plays a major role in oncolysis and maintenance of long-lasting anti-tumor effects. Improvement of virus-induced systemic anti-tumor immune responses may thus eliminate the need for systemic delivery of the virus to all metastatic sites. The already known ability of the virus to activate both innate and adaptive arms of the immune response leaves us to speculate about the potential synergy between genetically-engineered NDV and the novel immunotherapeutic approaches. Further investigation of NDV as an anti-cancer agent over the next several years will build upon the already established powerful genetically-engineered platforms to develop treatment strategies designed to manipulate anti-tumor immune responses, leading to translation of these approaches to the clinical setting.
Executive summary.
NDV biology and tropism
NDV is an avian paramyxovirus, causing disease in a variety of avian species, while (rare) human infections with NDV have been mild and self-limited
NDV is classified into lentogenic (avirulent), mesogenic (intermediate), and velogenic (virulent) pathotypes, depending on severity of disease it causes in birds.
NDV fusion (F) glycoprotein cleavage site, inhibition of innate immune responses by the V protein, and specific mutations in the viral HN, L, NP, and P proteins are the known determinants of virulence.
Mechanisms of NDV-mediated oncolysis
Direct mechanisms of oncolysis include induction of apoptosis through activation of extrinsic and intrinsic apoptotic pathways as well as ER stress pathway.
Direct contribution of viral HN, M, F and possibly L proteins to apoptosis induction has also been described.
Indirect mechanisms include activation of macrophages and NK cells, and recruitment of adaptive immune responses through secretion of cytokines, and upregulation of MHC and cell adhesion molecules on the surface of infected cells
Mechanisms of NDV tumor specificity
NDV has been shown to replicate in cancer cells possessing various defects in antiviral and apoptotic signaling pathways.
Other proteins such as H-Ras and Rac1 have been shown to mediate oncolytic specificity in certain systems.
Genetically-engineered NDV
NDV genome allows for stable expression of foreign genes while avoiding the problem of recombination or integration into host genome.
Several genetically-engineered models have been developed and include fusogenic NDV, NDV modulating innate immune responses, NDV expressing immunostimulatory cytokines, NDV expressing TAAs, and NDV expressing anti-tumor antibodies.
Genetically-engineered NDV demonstrate significant improvement in anti-tumor activity over the parental strains.
Clinical trials with oncolytic NDV
Several trials demonstrated therapeutic efficacy of autologous NDV-modified cellular vaccines or oncolysates, but most were small or uncontrolled.
Direct administration of NDV to human subjects has been shown to be safe and resulted in clinical responses, but larger controlled clinical trials will be needed to confirm the degree of benefit.
Future perspectives
The nature of the virus, including its safety in humans, lack of pre-existing immunity in the human population, the ubiquitous nature of its receptor, specificity for cancer cells, and immunostimulatory properties make it an attractive vector for further development as anti-cancer agent.
Tumor-specific targeting of the virus presents a challenge and could be achieved through the development of tissue culture-based systems for viral engineering, the use of bispecific protein adaptors, utilization of tumor-specific protease F protein cleavage sites, and use of tissue-specific viral mRNA stabilization.
Neutralizing antibodies may limit repeated administration of the virus, but could be avoided through the use of cell carriers, different viral strains, and genetically-engineered viruses expressing different surface glycoproteins.
Enhancement of systemic delivery of NDV via the use of cell carriers may improve its anti-cancer activity.
Genetically-engineered NDV have shown significant promise in preclinical models and is likely to show improved efficacy in clinical setting
Harnessing the anti-tumor immune responses through the use of genetically-engineered NDV and novel immunotherapeutic approaches will prove crucial in development of treatment strategies with greatest anti-tumor potential.
References
- 1.Dock G. The influence of complicating diseases upon leukemia. Am J Med Sci. 1904;127:563–592. [Google Scholar]
- 2.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15(4):651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 3.Moore AE, Diamond LC, Mackay HH, Sabachewsky L. Influence of hemagglutinating viruses on tumor cell suspensions II Newcastle disease virus and Ehrlich carcinoma. Proc Soc Exp Biol Med. 1952;81(2):498–501. doi: 10.3181/00379727-81-19921. [DOI] [PubMed] [Google Scholar]
- 4.Prince AM, Ginsberg HS. Studies on the cytotoxic effect of Newcastle disease virus (NDV) on Ehrlich ascites tumor cells II The mechanism and significance of in vitro recovery from the effect of NDV. J Immunol. 1957;79(2):107–112. [PubMed] [Google Scholar]
- 5.Prince AM, Ginsberg HS. Studies on the cytotoxic effect of Newcastle disease virus (NDV) on Ehrlich ascites tumor cells ICharacteristics of the virus-cell interaction. J Immunol. 1957;79(2):94–106. [PubMed] [Google Scholar]
- 6.Prince AM, Ginsberg HS. Immunohistochemical studies on the interaction between Ehrlich ascites tumor cells and Newcastle disease virus. J Exp Med. 1957;105(2):177–188. doi: 10.1084/jem.105.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams WR, Prince AF. Cellular changes associated with infection of the Ehrlich ascites tumor with Newcastle disease virus. Ann N Y Acad Sci. 1959;81:89–100. doi: 10.1111/j.1749-6632.1959.tb49298.x. [DOI] [PubMed] [Google Scholar]
- 8.Goto M, Okazaki M, Yazaki H. Oncolytic effect of Newcastle disease virus on Yoshida sarcoma (1) Jpn J Microbiol. 1959;3:171–181. doi: 10.1111/j.1348-0421.1959.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 9.Mason EJ, Kaufman N. The toxic properties of massive inoculums of Newcastle disease virus and influenza virus (PR8) for cell strains derived from normal and neoplastic tissue. Am J Pathol. 1960;37:231–243. [PMC free article] [PubMed] [Google Scholar]
- 10.Cassel WA, Garrett RE. NEWCASTLE DISEASE VIRUS AS AN ANTINEOPLASTIC AGENT. Cancer. 1965;18:863–868. doi: 10.1002/1097-0142(196507)18:7<863::aid-cncr2820180714>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 11.Cassel WA, Garrett RE. Tumor immunity after viral oncolysis. J Bacteriol. 1966;92(3):792. doi: 10.1128/jb.92.3.792-.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaton MD, Levinthal JD, Scala AR. Contribution of antiviral immunity to oncolysis by Newcastle disease virus in a murine lymphoma. J Natl Cancer Inst. 1967;39(6):1089–1097. [PubMed] [Google Scholar]
- 13.Eaton MD, Scala AR. Further observations on the inhibitory effect of myxoviruses on a transplantable murine leukemia. Proc Soc Exp Biol Med. 1969;132(1):20–26. doi: 10.3181/00379727-132-34138. [DOI] [PubMed] [Google Scholar]
- 14.Alexander DJ. Newcastle disease, Newcastle disease virus -- an avian paramyxovirus. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1988. pp. 1–22. [Google Scholar]
- 15.Lamb RPGD. Paramyxoviridae: the viruses and their replication. In: Knipe D, editor. Fields Virology. Lippincott Williams & Wilkins; 2007. pp. 1449–1496. [Google Scholar]
- 16.Samson AC, Levesley I, Russell PH. The 36K polypeptide synthesized in Newcastle disease virus-infected cells possesses properties predicted for the hypothesized ‘V’ protein. J Gen Virol. 1991;72(Pt 7):1709–1713. doi: 10.1099/0022-1317-72-7-1709. [DOI] [PubMed] [Google Scholar]
- 17.Steward M, Vipond IB, Millar NS, Emmerson PT. RNA editing in Newcastle disease virus. J Gen Virol. 1993;74(Pt 12):2539–2547. doi: 10.1099/0022-1317-74-12-2539. [DOI] [PubMed] [Google Scholar]
- 18.Morrison T, McQuain C, Sergel T, McGinnes L, Reitter J. The role of the amino terminus of F1 of the Newcastle disease virus fusion protein in cleavage and fusion. Virology. 1993;193(2):997–1000. doi: 10.1006/viro.1993.1214. [DOI] [PubMed] [Google Scholar]
- 19.Nagai Y, Klenk HD, Rott R. Proteolytic cleavage of the viral glycoproteins and its significance for the virulence of Newcastle disease virus. Virology. 1976;72(2):494–508. doi: 10.1016/0042-6822(76)90178-1. [DOI] [PubMed] [Google Scholar]
- 20.Ogasawara T, Gotoh B, Suzuki H, et al. Expression of factor X and its significance for the determination of paramyxovirus tropism in the chick embryo. Embo J. 1992;11(2):467–472. doi: 10.1002/j.1460-2075.1992.tb05076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panda A, Huang Z, Elankumaran S, Rockemann DD, Samal SK. Role of fusion protein cleavage site in the virulence of Newcastle disease virus. Microb Pathog. 2004;36(1):1–10. doi: 10.1016/j.micpath.2003.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peeters BP, de Leeuw OS, Koch G, Gielkens AL. Rescue of Newcastle disease virus from cloned cDNA: evidence that cleavability of the fusion protein is a major determinant for virulence. J Virol. 1999;73(6):5001–5009. doi: 10.1128/jvi.73.6.5001-5009.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garten W, Berk W, Nagai Y, Rott R, Klenk HD. Mutational changes of the protease susceptibility of glycoprotein F of Newcastle disease virus: effects on pathogenicity. J Gen Virol. 1980;50(1):135–147. doi: 10.1099/0022-1317-50-1-135. [DOI] [PubMed] [Google Scholar]
- 24.Toyoda T, Sakaguchi T, Imai K, et al. Structural comparison of the cleavage-activation site of the fusion glycoprotein between virulent and avirulent strains of Newcastle disease virus. Virology. 1987;158(1):242–247. doi: 10.1016/0042-6822(87)90261-3. [DOI] [PubMed] [Google Scholar]
- 25.Ebert O, Shinozaki K, Kournioti C, Park MS, Garcia-Sastre A, Woo SL. Syncytia induction enhances the oncolytic potential of vesicular stomatitis virus in virotherapy for cancer. Cancer Res. 2004;64(9):3265–3270. doi: 10.1158/0008-5472.can-03-3753. [DOI] [PubMed] [Google Scholar]
- 26. Vigil A, Park MS, Martinez O, et al. Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus. Cancer Res. 2007;67(17):8285–8292. doi: 10.1158/0008-5472.CAN-07-1025. Demonstration of various uses of genetically-engineered NDV for anti-cancer therapy
- 27.Zamarin D, Martinez-Sobrido L, Kelly K, et al. Enhancement of oncolytic properties of recombinant newcastle disease virus through antagonism of cellular innate immune responses. Mol Ther. 2009;17(4):697–706. doi: 10.1038/mt.2008.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Leeuw OS, Hartog L, Koch G, Peeters BP. Effect of fusion protein cleavage site mutations on virulence of Newcastle disease virus: non-virulent cleavage site mutants revert to virulence after one passage in chicken brain. J Gen Virol. 2003;84(Pt 2):475–484. doi: 10.1099/vir.0.18714-0. [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Sergel T, Razvi E, Morrison T. Effect of cleavage mutants on syncytium formation directed by the wild-type fusion protein of Newcastle disease virus. J Virol. 1998;72(5):3789–3795. doi: 10.1128/jvi.72.5.3789-3795.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peeters BP, de Leeuw OS, Verstegen I, Koch G, Gielkens AL. Generation of a recombinant chimeric Newcastle disease virus vaccine that allows serological differentiation between vaccinated and infected animals. Vaccine. 2001;19(13–14):1616–1627. doi: 10.1016/s0264-410x(00)00419-9. [DOI] [PubMed] [Google Scholar]
- 31.Ahlert T, Schirrmacher V. Isolation of a human melanoma adapted Newcastle disease virus mutant with highly selective replication patterns. Cancer Res. 1990;50(18):5962–5968. [PubMed] [Google Scholar]
- 32.Park MS, Steel J, Garcia-Sastre A, Swayne D, Palese P. Engineered viral vaccine constructs with dual specificity: avian influenza and Newcastle disease. Proc Natl Acad Sci U S A. 2006;103(21):8203–8208. doi: 10.1073/pnas.0602566103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vigil A, Martinez O, Chua MA, Garcia-Sastre A. Recombinant Newcastle disease virus as a vaccine vector for cancer therapy. Mol Ther. 2008;16(11):1883–1890. doi: 10.1038/mt.2008.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li P, Chen CH, Li S, et al. Therapeutic effects of a fusogenic newcastle disease virus in treating head and neck cancer. Head Neck. 2010 doi: 10.1002/hed.21609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silberhumer GR, Brader P, Wong J, et al. Genetically engineered oncolytic Newcastle disease virus effectively induces sustained remission of malignant pleural mesothelioma. Mol Cancer Ther. 2010;9(10):2761–2769. doi: 10.1158/1535-7163.MCT-10-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song KY, Wong J, Gonzalez L, Sheng G, Zamarin D, Fong Y. Antitumor efficacy of viral therapy using genetically engineered Newcastle disease virus [NDV(F3aa)-GFP] for peritoneally disseminated gastric cancer. J Mol Med (Berl) 2010;88(6):589–596. doi: 10.1007/s00109-010-0605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mebatsion T, Verstegen S, De Vaan LT, Romer-Oberdorfer A, Schrier CC. A recombinant newcastle disease virus with low-level V protein expression is immunogenic and lacks pathogenicity for chicken embryos. J Virol. 2001;75(1):420–428. doi: 10.1128/JVI.75.1.420-428.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang Z, Krishnamurthy S, Panda A, Samal SK. Newcastle disease virus V protein is associated with viral pathogenesis and functions as an alpha interferon antagonist. J Virol. 2003;77(16):8676–8685. doi: 10.1128/JVI.77.16.8676-8685.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang Z, Panda A, Elankumaran S, Govindarajan D, Rockemann DD, Samal SK. The hemagglutinin-neuraminidase protein of Newcastle disease virus determines tropism and virulence. J Virol. 2004;78(8):4176–4184. doi: 10.1128/JVI.78.8.4176-4184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Leeuw OS, Koch G, Hartog L, Ravenshorst N, Peeters BP. Virulence of Newcastle disease virus is determined by the cleavage site of the fusion protein and by both the stem region and globular head of the haemagglutinin-neuraminidase protein. J Gen Virol. 2005;86(Pt 6):1759–1769. doi: 10.1099/vir.0.80822-0. [DOI] [PubMed] [Google Scholar]
- 41.Romer-Oberdorfer A, Veits J, Werner O, Mettenleiter TC. Enhancement of pathogenicity of Newcastle disease virus by alteration of specific amino acid residues in the surface glycoproteins F and HN. Avian Dis. 2006;50(2):259–263. doi: 10.1637/7471-111505R.1. [DOI] [PubMed] [Google Scholar]
- 42.Rout SN, Samal SK. The large polymerase protein is associated with the virulence of Newcastle disease virus. J Virol. 2008;82(16):7828–7836. doi: 10.1128/JVI.00578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dortmans JC, Rottier PJ, Koch G, Peeters BP. The viral replication complex is associated with the virulence of Newcastle disease virus. J Virol. 2010;84(19):10113–10120. doi: 10.1128/JVI.00097-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park MS, Garcia-Sastre A, Cros JF, Basler CF, Palese P. Newcastle disease virus V protein is a determinant of host range restriction. J Virol. 2003;77(17):9522–9532. doi: 10.1128/JVI.77.17.9522-9532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dortmans JC, Koch G, Rottier PJ, Peeters BP. Virulence of Newcastle disease virus: what is known so far? Vet Res. 2011;42(1):122. doi: 10.1186/1297-9716-42-122. Comprehensive review of virulence determinants of NDV
- 46.Elankumaran S, Chavan V, Qiao D, et al. Type I interferon-sensitive recombinant newcastle disease virus for oncolytic virotherapy. J Virol. 2010;84(8):3835–3844. doi: 10.1128/JVI.01553-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim SH, Subbiah M, Samuel AS, Collins PL, Samal SK. Roles of the fusion and hemagglutinin-neuraminidase proteins in replication, tropism, and pathogenicity of avian paramyxoviruses. J Virol. 2011;85(17):8582–8596. doi: 10.1128/JVI.00652-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lam KM, Vasconcelos AC, Bickford AA. Apoptosis as a cause of death in chicken embryos inoculated with Newcastle disease virus. Microb Pathog. 1995;19(3):169–174. doi: 10.1006/mpat.1995.0055. [DOI] [PubMed] [Google Scholar]
- 49.Ravindra PV, Tiwari AK, Ratta B, et al. Induction of apoptosis in Vero cells by Newcastle disease virus requires viral replication, de-novo protein synthesis and caspase activation. Virus Res. 2008;133(2):285–290. doi: 10.1016/j.virusres.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 50.Ravindra PV, Tiwari AK, Sharma B, et al. HN protein of Newcastle disease virus causes apoptosis in chicken embryo fibroblast cells. Arch Virol. 2008;153(4):749–754. doi: 10.1007/s00705-008-0057-2. [DOI] [PubMed] [Google Scholar]
- 51.Ravindra PV, Tiwari AK, Ratta B, et al. Time course of Newcastle disease virus-induced apoptotic pathways. Virus Res. 2009;144(1–2):350–354. doi: 10.1016/j.virusres.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 52.Ravindra PV, Tiwari AK, Ratta B, Chaturvedi U, Palia SK, Chauhan RS. Newcastle disease virus-induced cytopathic effect in infected cells is caused by apoptosis. Virus Res. 2009;141(1):13–20. doi: 10.1016/j.virusres.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 53.Zulkifli MM, Ibrahim R, Ali AM, et al. Newcastle diseases virus strain V4UPM displayed oncolytic ability against experimental human malignant glioma. Neurol Res. 2009;31(1):3–10. doi: 10.1179/174313208X325218. [DOI] [PubMed] [Google Scholar]
- 54.Molouki A, Hsu YT, Jahanshiri F, Rosli R, Yusoff K. Newcastle disease virus infection promotes Bax redistribution to mitochondria and cell death in HeLa cells. Intervirology. 2010;53(2):87–94. doi: 10.1159/000264198. [DOI] [PubMed] [Google Scholar]
- 55.Ali R, Alabsi AM, Ali AM, et al. Cytolytic Effects and Apoptosis Induction of Newcastle Disease Virus Strain AF2240 on Anaplastic Astrocytoma Brain Tumor Cell Line. Neurochem Res. 2011 doi: 10.1007/s11064-011-0529-8. [DOI] [PubMed] [Google Scholar]
- 56.Fabian Z, Torocsik B, Kiss K, et al. Induction of apoptosis by a Newcastle disease virus vaccine (MTH-68/H) in PC12 rat phaeochromocytoma cells. Anticancer Res. 2001;21(1A):125–135. [PubMed] [Google Scholar]
- 57.Zeng J, Fournier P, Schirrmacher V. Induction of interferon-alpha and tumor necrosis factor-related apoptosis-inducing ligand in human blood mononuclear cells by hemagglutinin-neuraminidase but not F protein of Newcastle disease virus. Virology. 2002;297(1):19–30. doi: 10.1006/viro.2002.1413. [DOI] [PubMed] [Google Scholar]
- 58.Washburn B, Weigand MA, Grosse-Wilde A, et al. TNF-related apoptosis-inducing ligand mediates tumoricidal activity of human monocytes stimulated by Newcastle disease virus. J Immunol. 2003;170(4):1814–1821. doi: 10.4049/jimmunol.170.4.1814. [DOI] [PubMed] [Google Scholar]
- 59.Szeberenyi J, Fabian Z, Torocsik B, Kiss K, Csatary LK. Newcastle disease virus-induced apoptosis in PC12 pheochromocytoma cells. Am J Ther. 2003;10(4):282–288. doi: 10.1097/00045391-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 60.Elankumaran S, Rockemann D, Samal SK. Newcastle disease virus exerts oncolysis by both intrinsic and extrinsic caspase-dependent pathways of cell death. J Virol. 2006;80(15):7522–7534. doi: 10.1128/JVI.00241-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fabian Z, Csatary CM, Szeberenyi J, Csatary LK. p53-independent endoplasmic reticulum stress-mediated cytotoxicity of a Newcastle disease virus strain in tumor cell lines. J Virol. 2007;81(6):2817–2830. doi: 10.1128/JVI.02490-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bian J, Wang K, Kong X, et al. Caspase- and p38-MAPK-dependent induction of apoptosis in A549 lung cancer cells by Newcastle disease virus. Arch Virol. 2011;156(8):1335–1344. doi: 10.1007/s00705-011-0987-y. [DOI] [PubMed] [Google Scholar]
- 63.Mansour M, Palese P, Zamarin D. Oncolytic specificity of Newcastle disease virus is mediated by selectivity for apoptosis-resistant cells. J Virol. 2011;85(12):6015–6023. doi: 10.1128/JVI.01537-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Molouki A, Hsu YT, Jahanshiri F, Abdullah S, Rosli R, Yusoff K. The matrix (M) protein of Newcastle disease virus binds to human Bax through its BH3 domain. Virol J. 2011;8(1):385. doi: 10.1186/1743-422X-8-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Y, Zhang X, Wang X, et al. Apoptin Enhances the Oncolytic Properties of Newcastle Disease Virus. Intervirology. 2011 doi: 10.1159/000328325. [DOI] [PubMed] [Google Scholar]
- 66.Schirrmacher V, Bai L, Umansky V, Yu L, Xing Y, Qian Z. Newcastle disease virus activates macrophages for anti-tumor activity. Int J Oncol. 2000;16(2):363–373. [PubMed] [Google Scholar]
- 67.Umansky V, Shatrov VA, Lehmann V, Schirrmacher V. Induction of NO synthesis in macrophages by Newcastle disease virus is associated with activation of nuclear factor-kappa B. Int Immunol. 1996;8(4):491–498. doi: 10.1093/intimm/8.4.491. [DOI] [PubMed] [Google Scholar]
- 68.Lorence RM, Rood PA, Kelley KW. Newcastle disease virus as an antineoplastic agent: induction of tumor necrosis factor-alpha and augmentation of its cytotoxicity. J Natl Cancer Inst. 1988;80(16):1305–1312. doi: 10.1093/jnci/80.16.1305. [DOI] [PubMed] [Google Scholar]
- 69.Hrabak A, Csuka I, Bajor T, Csatary LK. The cytotoxic anti-tumor effect of MTH-68/H, a live attenuated Newcastle disease virus is mediated by the induction of nitric oxide synthesis in rat peritoneal macrophages in vitro. Cancer Lett. 2006;231(2):279–289. doi: 10.1016/j.canlet.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 70.Zorn U, Dallmann I, Grosse J, Kirchner H, Poliwoda H, Atzpodien J. Induction of cytokines and cytotoxicity against tumor cells by Newcastle disease virus. Cancer Biother. 1994;9(3):225–235. doi: 10.1089/cbr.1994.9.225. [DOI] [PubMed] [Google Scholar]
- 71.Sui H, Bai Y, Wang K, et al. The anti-tumor effect of Newcastle disease virus HN protein is influenced by differential subcellular targeting. Cancer Immunol Immunother. 2010;59(7):989–999. doi: 10.1007/s00262-010-0821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ni J, Galani IE, Cerwenka A, Schirrmacher V, Fournier P. Antitumor vaccination by Newcastle Disease Virus Hemagglutinin-Neuraminidase plasmid DNA application: changes in tumor microenvironment and activation of innate anti-tumor immunity. Vaccine. 2011;29(6):1185–1193. doi: 10.1016/j.vaccine.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 73.Ertel C, Millar NS, Emmerson PT, Schirrmacher V, von Hoegen P. Viral hemagglutinin augments peptide-specific cytotoxic T cell responses. Eur J Immunol. 1993;23(10):2592–2596. doi: 10.1002/eji.1830231032. [DOI] [PubMed] [Google Scholar]
- 74. Haas C, Ertel C, Gerhards R, Schirrmacher V. Introduction of adhesive and costimulatory immune functions into tumor cells by infection with Newcastle Disease Virus. Int J Oncol. 1998;13(6):1105–1115. doi: 10.3892/ijo.13.6.1105. Demonstration of immunostimulatory properties of NDV in infected cells
- 75.Ten RM, Blank V, Le Bail O, Kourilsky P, Israel A. Two factors, IRF1 and KBF1/NF-kappa B, cooperate during induction of MHC class I gene expression by interferon alpha beta or Newcastle disease virus. C R Acad Sci III. 1993;316(5):496–501. [PubMed] [Google Scholar]
- 76.Washburn B, Schirrmacher V. Human tumor cell infection by Newcastle Disease Virus leads to upregulation of HLA and cell adhesion molecules and to induction of interferons, chemokines and finally apoptosis. Int J Oncol. 2002;21(1):85–93. doi: 10.3892/ijo.21.1.85. [DOI] [PubMed] [Google Scholar]
- 77.Termeer CC, Schirrmacher V, Brocker EB, Becker JC. Newcastle disease virus infection induces B7–1/B7–2-independent T-cell costimulatory activity in human melanoma cells. Cancer Gene Ther. 2000;7(2):316–323. doi: 10.1038/sj.cgt.7700109. [DOI] [PubMed] [Google Scholar]
- 78.Barber GN. Cytoplasmic DNA innate immune pathways. Immunol Rev. 2011;243(1):99–108. doi: 10.1111/j.1600-065X.2011.01051.x. [DOI] [PubMed] [Google Scholar]
- 79.O’Neill LA, Bowie AG. Sensing and signaling in antiviral innate immunity. Curr Biol. 2010;20(7):R328–R333. doi: 10.1016/j.cub.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 80.Wilkins C, Gale M., Jr Recognition of viruses by cytoplasmic sensors. Curr Opin Immunol. 2010;22(1):41–47. doi: 10.1016/j.coi.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Versteeg GA, Garcia-Sastre A. Viral tricks to grid-lock the type I interferon system. Curr Opin Microbiol. 2010;13(4):508–516. doi: 10.1016/j.mib.2010.05.009. Comprehensive review of innate immune responses and the immune evasion strategies employed by viruses
- 82.Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89(Pt 1):1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 83.Richards KH, Macdonald A. Putting the brakes on the anti-viral response: negative regulators of type I interferon (IFN) production. Microbes Infect. 2011;13(4):291–302. doi: 10.1016/j.micinf.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 84.Krishnamurthy S, Takimoto T, Scroggs RA, Portner A. Differentially regulated interferon response determines the outcome of Newcastle disease virus infection in normal and tumor cell lines. J Virol. 2006;80(11):5145–5155. doi: 10.1128/JVI.02618-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fiola C, Peeters B, Fournier P, Arnold A, Bucur M, Schirrmacher V. Tumor selective replication of Newcastle disease virus: association with defects of tumor cells in antiviral defence. Int J Cancer. 2006;119(2):328–338. doi: 10.1002/ijc.21821. [DOI] [PubMed] [Google Scholar]
- 86.Wilden H, Fournier P, Zawatzky R, Schirrmacher V. Expression of RIG-I, IRF3, IFN-beta and IRF7 determines resistance or susceptibility of cells to infection by Newcastle Disease Virus. Int J Oncol. 2009;34(4):971–982. doi: 10.3892/ijo_00000223. [DOI] [PubMed] [Google Scholar]
- 87.Fournier P, Wilden H, Schirrmacher V. Importance of retinoic acid-inducible gene I and of receptor for type disease virus. Int J Oncol. 2011 doi: 10.3892/ijo.2011.1222. [DOI] [PubMed] [Google Scholar]
- 88.Wilden H, Schirrmacher V, Fournier P. Important role of interferon regulatory factor (IRF)-3 in the interferon response of mouse macrophages upon infection by Newcastle disease virus. Int J Oncol. 2011;39(2):493–504. doi: 10.3892/ijo.2011.1033. [DOI] [PubMed] [Google Scholar]
- 89.Lazar I, Yaacov B, Shiloach T, et al. The oncolytic activity of Newcastle disease virus NDV-HUJ on chemoresistant primary melanoma cells is dependent on the proapoptotic activity of the inhibitor of apoptosis protein Livin. J Virol. 2010;84(1):639–646. doi: 10.1128/JVI.00401-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Puhlmann J, Puehler F, Mumberg D, Boukamp P, Beier R. Rac1 is required for oncolytic NDV replication in human cancer cells and establishes a link between tumorigenesis and sensitivity to oncolytic virus. Oncogene. 2010;29(15):2205–2216. doi: 10.1038/onc.2009.507. [DOI] [PubMed] [Google Scholar]
- 91.Yaacov B, Eliahoo E, Lazar I, et al. Selective oncolytic effect of an attenuated Newcastle disease virus (NDV-HUJ) in lung tumors. Cancer Gene Ther. 2008;15(12):795–807. doi: 10.1038/cgt.2008.31. [DOI] [PubMed] [Google Scholar]
- 92.Lorence RM, Katubig BB, Reichard KW, et al. Complete regression of human fibrosarcoma xenografts after local Newcastle disease virus therapy. Cancer Res. 1994;54(23):6017–6021. [PubMed] [Google Scholar]
- 93.Taylor MP, Koyuncu OO, Enquist LW. Subversion of the actin cytoskeleton during viral infection. Nat Rev Microbiol. 9(6):427–439. doi: 10.1038/nrmicro2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nakaya T, Cros J, Park MS, et al. Recombinant Newcastle disease virus as a vaccine vector. J Virol. 2001;75(23):11868–11873. doi: 10.1128/JVI.75.23.11868-11873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.DiNapoli JM, Yang L, Samal SK, Murphy BR, Collins PL, Bukreyev A. Respiratory tract immunization of non-human primates with a Newcastle disease virus-vectored vaccine candidate against Ebola virus elicits a neutralizing antibody response. Vaccine. 2010;29(1):17–25. doi: 10.1016/j.vaccine.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ge J, Tian G, Zeng X, Jiang Y, Chen H, Bua Z. Generation and evaluation of a Newcastle disease virus-based H9 avian influenza live vaccine. Avian Dis. 2010;54(1 Suppl):294–296. doi: 10.1637/8731-032509-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 97.Vagnozzi A, Garcia M, Riblet SM, Zavala G. Protection induced by infectious laryngotracheitis virus vaccines alone and combined with Newcastle disease virus and/or infectious bronchitis virus vaccines. Avian Dis. 2010;54(4):1210–1219. doi: 10.1637/9362-040710-Reg.1. [DOI] [PubMed] [Google Scholar]
- 98.Ge J, Wang X, Tao L, et al. Newcastle disease virus-Vectored rabies vaccine is safe, highly immunogenic, and provides long-lasting protection in dogs and cats. J Virol. 2011;85(16):8241–8252. doi: 10.1128/JVI.00519-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khattar SK, Samal S, Devico AL, Collins PL, Samal SK. Newcastle disease virus expressing human immunodeficiency virus type 1 envelope glycoprotein induces strong mucosal and serum antibody responses in Guinea pigs. J Virol. 2011;85(20):10529–10541. doi: 10.1128/JVI.05050-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100.Maamary J, Array F, Gao Q, et al. Newcastle disease virus expressing a dendritic cell-targeted HIV gag protein induces a potent gag-specific immune response in mice. J Virol. 2011;85(5):2235–2246. doi: 10.1128/JVI.02036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schroer D, Veits J, Keil G, Romer-Oberdorfer A, Weber S, Mettenleiter TC. Efficacy of Newcastle disease virus recombinant expressing avian influenza virus H6 hemagglutinin against Newcastle disease and low pathogenic avian influenza in chickens and turkeys. Avian Dis. 2011;55(2):201–211. doi: 10.1637/9539-092710-Reg.1. [DOI] [PubMed] [Google Scholar]
- 102.Xiao S, Kumar M, Yang X, et al. A host-restricted viral vector for antigen-specific immunization against Lyme disease pathogen. Vaccine. 2011;29(32):5294–5303. doi: 10.1016/j.vaccine.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim D, Martinez-Sobrido L, Choi C, et al. Induction of type I interferon secretion through recombinant Newcastle disease virus expressing measles virus hemagglutinin stimulates antibody secretion in the presence of maternal antibodies. J Virol. 2011;85(1):200–207. doi: 10.1128/JVI.01624-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Steel J, Burmakina SV, Thomas C, et al. A combination in-ovo vaccine for avian influenza virus and Newcastle disease virus. Vaccine. 2008;26(4):522–531. doi: 10.1016/j.vaccine.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martinez-Sobrido L, Gitiban N, Fernandez-Sesma A, et al. Protection against respiratory syncytial virus by a recombinant Newcastle disease virus vector. J Virol. 2006;80(3):1130–1139. doi: 10.1128/JVI.80.3.1130-1139.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nakaya Y, Nakaya T, Park MS, et al. Induction of cellular immune responses to simian immunodeficiency virus gag by two recombinant negative-strand RNA virus vectors. J Virol. 2004;78(17):9366–9375. doi: 10.1128/JVI.78.17.9366-9375.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zamarin D, Vigil A, Kelly K, Garcia-Sastre A, Fong Y. Genetically engineered Newcastle disease virus for malignant melanoma therapy. Gene Ther. 2009;16(6):796–804. doi: 10.1038/gt.2009.14. Genetically-engineered NDV expressing IL-2 possesses significant anti-cancer activity
- 108.Altomonte J, Marozin S, Schmid RM, Ebert O. Engineered newcastle disease virus as an improved oncolytic agent against hepatocellular carcinoma. Mol Ther. 2010;18(2):275–284. doi: 10.1038/mt.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Registry NSA. Select Agents and Toxins. 2011 [Google Scholar]
- 110.Kochs G, Garcia-Sastre A, Martinez-Sobrido L. Multiple anti-interferon actions of the influenza A virus NS1 protein. J Virol. 2007;81(13):7011–7021. doi: 10.1128/JVI.02581-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Janke M, Peeters B, de Leeuw O, et al. Recombinant Newcastle disease virus (NDV) with inserted gene coding for GM-CSF as a new vector for cancer immunogene therapy. Gene Ther. 2007;14(23):1639–1649. doi: 10.1038/sj.gt.3303026. [DOI] [PubMed] [Google Scholar]
- 112.Zhao H, Janke M, Fournier P, Schirrmacher V. Recombinant Newcastle disease virus expressing human interleukin-2 serves as a potential candidate for tumor therapy. Virus Res. 2008;136(1–2):75–80. doi: 10.1016/j.virusres.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 113.Janke M, Peeters B, Zhao H, et al. Activation of human T cells by a tumor vaccine infected with recombinant Newcastle disease virus producing IL-2. Int J Oncol. 2008;33(4):823–832. [PubMed] [Google Scholar]
- 114.Puhler F, Willuda J, Puhlmann J, Mumberg D, Romer-Oberdorfer A, Beier R. Generation of a recombinant oncolytic Newcastle disease virus and expression of a full IgG antibody from two transgenes. Gene Ther. 2008;15(5):371–383. doi: 10.1038/sj.gt.3303095. [DOI] [PubMed] [Google Scholar]
- 115.Bian H, Fournier P, Moormann R, Peeters B, Schirrmacher V. Selective gene transfer in vitro to tumor cells via recombinant Newcastle disease virus. Cancer Gene Ther. 2005;12(3):295–303. doi: 10.1038/sj.cgt.7700774. [DOI] [PubMed] [Google Scholar]
- 116.Bian H, Fournier P, Moormann R, Peeters B, Schirrmacher V. Selective gene transfer to tumor cells by recombinant Newcastle Disease Virus via a bispecific fusion protein. Int J Oncol. 2005;26(2):431–439. [PubMed] [Google Scholar]
- 117.Bian H, Fournier P, Peeters B, Schirrmacher V. Tumor-targeted gene transfer in vivo via recombinant Newcastle disease virus modified by a bispecific fusion protein. Int J Oncol. 2005;27(2):377–384. [PubMed] [Google Scholar]
- 118.Haas C, Lulei M, Fournier P, Arnold A, Schirrmacher V. T-cell triggering by CD3- and CD28-binding molecules linked to a human virus-modified tumor cell vaccine. Vaccine. 2005;23(19):2439–2453. doi: 10.1016/j.vaccine.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 119.Haas C, Lulei M, Fournier P, Arnold A, Schirrmacher V. A tumor vaccine containing anti-CD3 and anti-CD28 bispecific antibodies triggers strong and durable antitumor activity in human lymphocytes. Int J Cancer. 2006;118(3):658–667. doi: 10.1002/ijc.21390. [DOI] [PubMed] [Google Scholar]
- 120.Fournier P, Aigner M, Schirrmacher V. Transcriptome analysis and cytokine profiling of naive T cells stimulated by a tumor vaccine via CD3 and CD25. Int J Oncol. 2010;37(6):1439–1452. doi: 10.3892/ijo_00000796. [DOI] [PubMed] [Google Scholar]
- 121.Fournier P, Aigner M, Schirrmacher V. Targeting of IL-2 and GM-CSF immunocytokines to a tumor vaccine leads to increased anti-tumor activity. Int J Oncol. 2011;38(6):1719–1729. doi: 10.3892/ijo.2011.976. [DOI] [PubMed] [Google Scholar]
- 122.Reichard KW, Lorence RM, Katubig BB, Peeples ME, Reyes HM. Retinoic acid enhances killing of neuroblastoma cells by Newcastle disease virus. J Pediatr Surg. 1993;28(10):1221–1225. doi: 10.1016/s0022-3468(05)80302-1. discussion 1225–1226. [DOI] [PubMed] [Google Scholar]
- 123.Lorence RM, Reichard KW, Katubig BB, et al. Complete regression of human neuroblastoma xenografts in athymic mice after local Newcastle disease virus therapy. J Natl Cancer Inst. 1994;86(16):1228–1233. doi: 10.1093/jnci/86.16.1228. [DOI] [PubMed] [Google Scholar]
- 124.Tzadok-David Y, Metzkin-Eizenberg M, Zakay-Rones Z. The effect of a mesogenic and a lentogenic Newcastle disease virus strain on Burkitt lymphoma Daudi cells. J Cancer Res Clin Oncol. 1995;121(3):169–174. doi: 10.1007/BF01198099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bar-Eli N, Giloh H, Schlesinger M, Zakay-Rones Z. Preferential cytotoxic effect of Newcastle disease virus on lymphoma cells. J Cancer Res Clin Oncol. 1996;122(7):409–415. doi: 10.1007/BF01212880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schirrmacher V, Griesbach A, Ahlert T. Antitumor effects of Newcastle Disease Virus in vivo: local versus systemic effects. Int J Oncol. 2001;18(5):945–952. doi: 10.3892/ijo.18.5.945. [DOI] [PubMed] [Google Scholar]
- 127.Washburn B, Schirrmacher V. Human tumor cell infection by Newcastle Disease Virus leads to upregulation of HLA and cell adhesion molecules and to induction of interferons, chemokines and finally apoptosis. Int J Oncol. 2002;21(1):85–93. doi: 10.3892/ijo.21.1.85. [DOI] [PubMed] [Google Scholar]
- 128.Zulkifli MM, Ibrahim R, Ali AM, et al. Newcastle diseases virus strain V4UPM displayed oncolytic ability against experimental human malignant glioma. Neurol Res. 2009;31(1):3–10. doi: 10.1179/174313208X325218. [DOI] [PubMed] [Google Scholar]
- 129.Cassel WA, Murray DR, Torbin AH, Olkowski ZL, Moore ME. Viral oncolysate in the management of malignant melanoma I Preparation of the oncolysate and measurement of immunologic responses. Cancer. 1977;40(2):672–679. doi: 10.1002/1097-0142(197708)40:2<672::aid-cncr2820400213>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 130.Murray DR, Cassel WA, Torbin AH, Olkowski ZL, Moore ME. oncolysate in the management of malignant melanoma II Clinical studies. Cancer. 1977;40(2):680–686. doi: 10.1002/1097-0142(197708)40:2<680::aid-cncr2820400214>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 131.Cassel WA, Murray DR. Treatment of stage II malignant melanoma patients with a Newcastle disease virus oncolysate. Nat Immun Cell Growth Regul. 1988;7(5–6):351–352. [PubMed] [Google Scholar]
- 132.Lehner B, Schlag P, Liebrich W, Schirrmacher V. Postoperative active specific immunization in curatively resected colorectal cancer patients with a virus-modified autologous tumor cell vaccine. Cancer Immunol Immunother. 1990;32(3):173–178. doi: 10.1007/BF01771453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liebrich W, Schlag P, Manasterski M, et al. In vitro and clinical characterisation of a Newcastle disease virus-modified autologous tumour cell vaccine for treatment of colorectal cancer patients. Eur J Cancer. 1991;27(6):703–710. doi: 10.1016/0277-5379(91)90170-i. [DOI] [PubMed] [Google Scholar]
- 134.Cassel WA, Murray DR. A ten-year follow-up on stage II malignant melanoma patients treated postsurgically with Newcastle disease virus oncolysate. Med Oncol Tumor Pharmacother. 1992;9(4):169–171. doi: 10.1007/BF02987752. [DOI] [PubMed] [Google Scholar]
- 135.Schlag P, Manasterski M, Gerneth T, et al. Active specific immunotherapy with Newcastle-disease-virus-modified autologous tumor cells following resection of liver metastases in colorectal cancer First evaluation of clinical response of a phase II-trial. Cancer Immunol Immunother. 1992;35(5):325–330. doi: 10.1007/BF01741145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schirrmacher V, Schlag P, Liebrich W, Patel BT, Stoeck M. Specific immunotherapy of colorectal carcinoma with Newcastle-disease virus-modified autologous tumor cells prepared from resected liver metastasis. Ann N Y Acad Sci. 1993;690:364–366. doi: 10.1111/j.1749-6632.1993.tb44032.x. [DOI] [PubMed] [Google Scholar]
- 137.Kirchner HH, Anton P, Atzpodien J. Adjuvant treatment of locally advanced renal cancer with autologous virus-modified tumor vaccines. World J Urol. 1995;13(3):171–173. doi: 10.1007/BF00184874. [DOI] [PubMed] [Google Scholar]
- 138.Pomer S, Schirrmacher V, Thiele R, Lohrke H, Brkovic D, Staehler G. Tumor response and 4 year survival-data of patients with advanced renal-cell carcinoma treated with autologous tumor vaccine and subcutaneous R-IL-2 and IFN-alpha(2b) Int J Oncol. 1995;6(5):947–954. doi: 10.3892/ijo.6.5.947. [DOI] [PubMed] [Google Scholar]
- 139.Ockert D, Schirrmacher V, Beck N, et al. Newcastle disease virus-infected intact autologous tumor cell vaccine for adjuvant active specific immunotherapy of resected colorectal carcinoma. Clin Cancer Res. 1996;2(1):21–28. [PubMed] [Google Scholar]
- 140.Ahlert T, Sauerbrei W, Bastert G, et al. Tumor-cell number and viability as quality and efficacy parameters of autologous virus-modified cancer vaccines in patients with breast or ovarian cancer. J Clin Oncol. 1997;15(4):1354–1366. doi: 10.1200/JCO.1997.15.4.1354. [DOI] [PubMed] [Google Scholar]
- 141.Zorn U, Duensing S, Langkopf F, et al. Active specific immunotherapy of renal cell carcinoma: cellular and humoral immune responses. Cancer Biother Radiopharm. 1997;12(3):157–165. doi: 10.1089/cbr.1997.12.157. [DOI] [PubMed] [Google Scholar]
- 142.Batliwalla FM, Bateman BA, Serrano D, et al. A 15-year follow-up of AJCC stage III malignant melanoma patients treated postsurgically with Newcastle disease virus (NDV) oncolysate and determination of alterations in the CD8 T cell repertoire. Mol Med. 1998;4(12):783–794. [PMC free article] [PubMed] [Google Scholar]
- 143.Csatary LK, Moss RW, Beuth J, Torocsik B, Szeberenyi J, Bakacs T. Beneficial treatment of patients with advanced cancer using a Newcastle disease virus vaccine (MTH-68/H) Anticancer Res. 1999;19(1B):635–638. [PubMed] [Google Scholar]
- 144.Liang W, Wang H, Sun TM, et al. Application of autologous tumor cell vaccine and NDV vaccine in treatment of tumors of digestive tract. World J Gastroenterol. 2003;9(3):495–498. doi: 10.3748/wjg.v9.i3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Voit C, Kron M, Schwurzer-Voit M, Sterry W. Intradermal injection of Newcastle disease virus-modified autologous melanoma cell lysate and interleukin-2 for adjuvant treatment of melanoma patients with resectable stage III disease. J Dtsch Dermatol Ges. 2003;1(2):120–125. doi: 10.1046/j.1610-0387.2003.02014.x. [DOI] [PubMed] [Google Scholar]
- 146.Steiner HH, Bonsanto MM, Beckhove P, et al. Antitumor vaccination of patients with glioblastoma multiforme: a pilot study to assess feasibility, safety, and clinical benefit. J Clin Oncol. 2004;22(21):4272–4281. doi: 10.1200/JCO.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 147.Schulze T, Kemmner W, Weitz J, Wernecke KD, Schirrmacher V, Schlag PM. Efficiency of adjuvant active specific immunization with Newcastle disease virus modified tumor cells in colorectal cancer patients following resection of liver metastases: results of a prospective randomized trial. Cancer Immunol Immunother. 2009;58(1):61–69. doi: 10.1007/s00262-008-0526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cassel WA, Murray DR, Phillips HS. A phase II study on the postsurgical management of Stage II malignant melanoma with a Newcastle disease virus oncolysate. Cancer. 1983;52(5):856–860. doi: 10.1002/1097-0142(19830901)52:5<856::aid-cncr2820520519>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 149.Plager C, Bowen JM, Fenoglio C, Papadopoulos NEJ, Murray L, Chawla SP, Benjamin RS, Savage HE, Rosen RD, Hersh EM. Adjuvant immunotherapy with Newcastle disease virus oncolysate of M.D. Anderson (MDAH) stage III-B malignant melanoma. Proc Am Soc Clin Oncol. 1986;5(137a) [Google Scholar]
- 150.Schirrmacher V, Fournier P. Newcastle disease virus: a promising vector for viral therapy, immune therapy, and gene therapy of cancer. Methods Mol Biol. 2009;542:565–605. doi: 10.1007/978-1-59745-561-9_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schirrmacher V. Clinical trials of antitumor vaccination with an autologous tumor cell vaccine modified by virus infection: improvement of patient survival based on improved antitumor immune memory. Cancer Immunol Immunother. 2005;54(6):587–598. doi: 10.1007/s00262-004-0602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schirrmacher V, Heicappell R. Prevention of metastatic spread by postoperative immunotherapy with virally modified autologous tumor cells II Establishment of specific systemic anti-tumor immunity. Clin Exp Metastasis. 1987;5(2):147–156. doi: 10.1007/BF00058060. [DOI] [PubMed] [Google Scholar]
- 153.Plaksin D, Porgador A, Vadai E, Feldman M, Schirrmacher V, Eisenbach L. Effective anti-metastatic melanoma vaccination with tumor cells transfected with MHC genes and/or infected with Newcastle disease virus (NDV) Int J Cancer. 1994;59(6):796–801. doi: 10.1002/ijc.2910590615. [DOI] [PubMed] [Google Scholar]
- 154.Shoham J, Hirsch R, Zakay-Rones Z, Osband ME, Brennert HJ. Augmentation of tumor cell immunogenicity by viruses--an approach to specific immunotherapy of cancer. Nat Immun Cell Growth Regul. 1990;9(3):165–172. [PubMed] [Google Scholar]
- 155.Bohle W, Schlag P, Liebrich W, et al. Postoperative active specific immunization in colorectal cancer patients with virus-modified autologous tumor-cell vaccine First clinical results with tumor-cell vaccines modified with live but avirulent Newcastle disease virus. Cancer. 1990;66(7):1517–1523. doi: 10.1002/1097-0142(19901001)66:7<1517::aid-cncr2820660714>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 156.Mobus V, Horn S, Stock M, Schirrmacher V. Tumor cell vaccination for gynecological tumors. Hybridoma. 1993;12(5):543–547. doi: 10.1089/hyb.1993.12.543. [DOI] [PubMed] [Google Scholar]
- 157.Karcher J, Dyckhoff G, Beckhove P, et al. Antitumor vaccination in patients with head and neck squamous cell carcinomas with autologous virus-modified tumor cells. Cancer Res. 2004;64(21):8057–8061. doi: 10.1158/0008-5472.CAN-04-1545. [DOI] [PubMed] [Google Scholar]
- 158.Wheelock EF, Dingle JH. OBSERVATIONS ON THE REPEATED ADMINISTRATION OF VIRUSES TO A PATIENTWIT HACUTE LEUKEMIA A PRELIMINARY REPORT. N Engl J Med. 1964;271:645–651. doi: 10.1056/NEJM196409242711302. [DOI] [PubMed] [Google Scholar]
- 159.Csatary LK, Eckhardt S, Bukosza I, et al. Attenuated veterinary virus vaccine for the treatment of cancer. Cancer Detect Prev. 1993;17(6):619–627. [PubMed] [Google Scholar]
- 160.Pecora AL, Rizvi N, Cohen GI, et al. Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers. J Clin Oncol. 2002;20(9):2251–2266. doi: 10.1200/JCO.2002.08.042. [DOI] [PubMed] [Google Scholar]
- 161.Merigan TCDCE, Finkelstein MS, Clever L, Walker S, Waddell DJ. Clinical studies employing interferon inducers in man and animals. Ann N Y Acad Sci. 1970;173:746–759. [Google Scholar]
- 162.Csatary LK, Bakacs T. Use of Newcastle disease virus vaccine (MTH-68/H) in a patient with high-grade glioblastoma. JAMA. 1999;281(17):1588–1589. doi: 10.1001/jama.281.17.1588-a. [DOI] [PubMed] [Google Scholar]
- 163.Csatary LK, Gosztonyi G, Szeberenyi J, et al. MTH-68/H oncolytic viral treatment in human high-grade gliomas. J Neurooncol. 2004;67(1–2):83–93. doi: 10.1023/b:neon.0000021735.85511.05. [DOI] [PubMed] [Google Scholar]
- 164.Laurie SA, Bell JC, Atkins HL, et al. A phase 1 clinical study of intravenous administration of PV701, an oncolytic virus, using two-step desensitization. Clin Cancer Res. 2006;12(8):2555–2562. doi: 10.1158/1078-0432.CCR-05-2038. [DOI] [PubMed] [Google Scholar]
- 165.Lorence RM, Roberts MS, O’Neil JD, et al. Phase 1 clinical experience using intravenous administration of PV701, an oncolytic Newcastle disease virus. Curr Cancer Drug Targets. 2007;7(2):157–167. doi: 10.2174/156800907780058853. [DOI] [PubMed] [Google Scholar]
- 166.Hotte SJ, Lorence RM, Hirte HW, et al. An optimized clinical regimen for the oncolytic virus PV701. Clin Cancer Res. 2007;13(3):977–985. doi: 10.1158/1078-0432.CCR-06-1817. [DOI] [PubMed] [Google Scholar]
- 167.Freeman AI, Zakay-Rones Z, Gomori JM, et al. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol Ther. 2006;13(1):221–228. doi: 10.1016/j.ymthe.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 168.Cattaneo R, Miest T, Shashkova EV, Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol. 2008;6(7):529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Miller LT, Yates VJ. Reactions of human sera to avian adenoviruses and Newcastle disease virus. Avian Dis. 1971;15(4):781–788. [PubMed] [Google Scholar]
- 170.Charan S, Mahajan VM, Agarwal LP. Newcastle disease virus antibodies in human sera. Indian J Med Res. 1981;73:303–307. [PubMed] [Google Scholar]
- 171.Schirrmacher V, Haas C, Bonifer R, Ahlert T, Gerhards R, Ertel C. Human tumor cell modification by virus infection: an efficient and safe way to produce cancer vaccine with pleiotropic immune stimulatory properties when using Newcastle disease virus. Gene Ther. 1999;6(1):63–73. doi: 10.1038/sj.gt.3300787. [DOI] [PubMed] [Google Scholar]
- 172.Zhao H, Peeters BP. Recombinant Newcastle disease virus as a viral vector: effect of genomic location of foreign gene on gene expression and virus replication. J Gen Virol. 2003;84(Pt 4):781–788. doi: 10.1099/vir.0.18884-0. [DOI] [PubMed] [Google Scholar]
- 173.Peng KW, Vile R, Cosset FL, Russell S. Selective transduction of protease-rich tumors by matrix-metalloproteinase-targeted retroviral vectors. Gene Ther. 1999;6(9):1552–1557. doi: 10.1038/sj.gt.3300982. [DOI] [PubMed] [Google Scholar]
- 174.Kinoh H, Inoue M, Washizawa K, et al. Generation of a recombinant Sendai virus that is selectively activated and lyses human tumor cells expressing matrix metalloproteinases. Gene Ther. 2004;11(14):1137–1145. doi: 10.1038/sj.gt.3302272. [DOI] [PubMed] [Google Scholar]
- 175.Springfeld C, von Messling V, Frenzke M, Ungerechts G, Buchholz CJ, Cattaneo R. Oncolytic efficacy and enhanced safety of measles virus activated by tumor-secreted matrix metalloproteinases. Cancer Res. 2006;66(15):7694–7700. doi: 10.1158/0008-5472.CAN-06-0538. [DOI] [PubMed] [Google Scholar]
- 176.Dorer DE, Nettelbeck DM. Targeting cancer by transcriptional control in cancer gene therapy and viral oncolysis. Adv Drug Deliv Rev. 2009;61(7–8):554–571. doi: 10.1016/j.addr.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 177.Khabar KS. Post-transcriptional control during chronic inflammation and cancer: a focus on AU-rich elements. Cell Mol Life Sci. 2010;67(17):2937–2955. doi: 10.1007/s00018-010-0383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ahmed A, Thompson J, Emiliusen L, et al. A conditionally replicating adenovirus targeted to tumor cells through activated RAS/P-MAPK-selective mRNA stabilization. Nat Biotechnol. 2003;21(7):771–777. doi: 10.1038/nbt835. [DOI] [PubMed] [Google Scholar]
- 179.Ilan Y, Sauter B, Chowdhury NR, et al. Oral tolerization to adenoviral proteins permits repeated adenovirus-mediated gene therapy in rats with pre-existing immunity to adenoviruses. Hepatology. 1998;27(5):1368–1376. doi: 10.1002/hep.510270525. [DOI] [PubMed] [Google Scholar]
- 180.Nakashima H, Kaur B, Chiocca EA. Directing systemic oncolytic viral delivery to tumors via carrier cells. Cytokine Growth Factor Rev. 2010;21(2–3):119–126. doi: 10.1016/j.cytogfr.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mader EK, Maeyama Y, Lin Y, et al. Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model. Clin Cancer Res. 2009;15(23):7246–7255. doi: 10.1158/1078-0432.CCR-09-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Peggs KS, Quezada SA, Allison JP. Cancer immunotherapy: co-stimulatory agonists and co-inhibitory antagonists. Clin Exp Immunol. 2009;157(1):9–19. doi: 10.1111/j.1365-2249.2009.03912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 185. Melcher A, Parato K, Rooney CM, Bell JC. Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol Ther. 2011;19(6):1008–1016. doi: 10.1038/mt.2011.65. Comprehensive review of use of combination therapy between oncolytic viruses and immunotherapeutic approaches
- 186.Breitbach CJ, Burke J, Jonker D, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477(7362):99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- 187.Senzer NN, Kaufman HL, Amatruda T, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27(34):5763–5771. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- 188. Lech PJ, Russell SJ. Use of attenuated paramyxoviruses for cancer therapy. Expert Rev Vaccines. 2010;9(11):1275–1302. doi: 10.1586/erv.10.124. Comprehensive review of oncolytic paramyxoviruses