Abstract
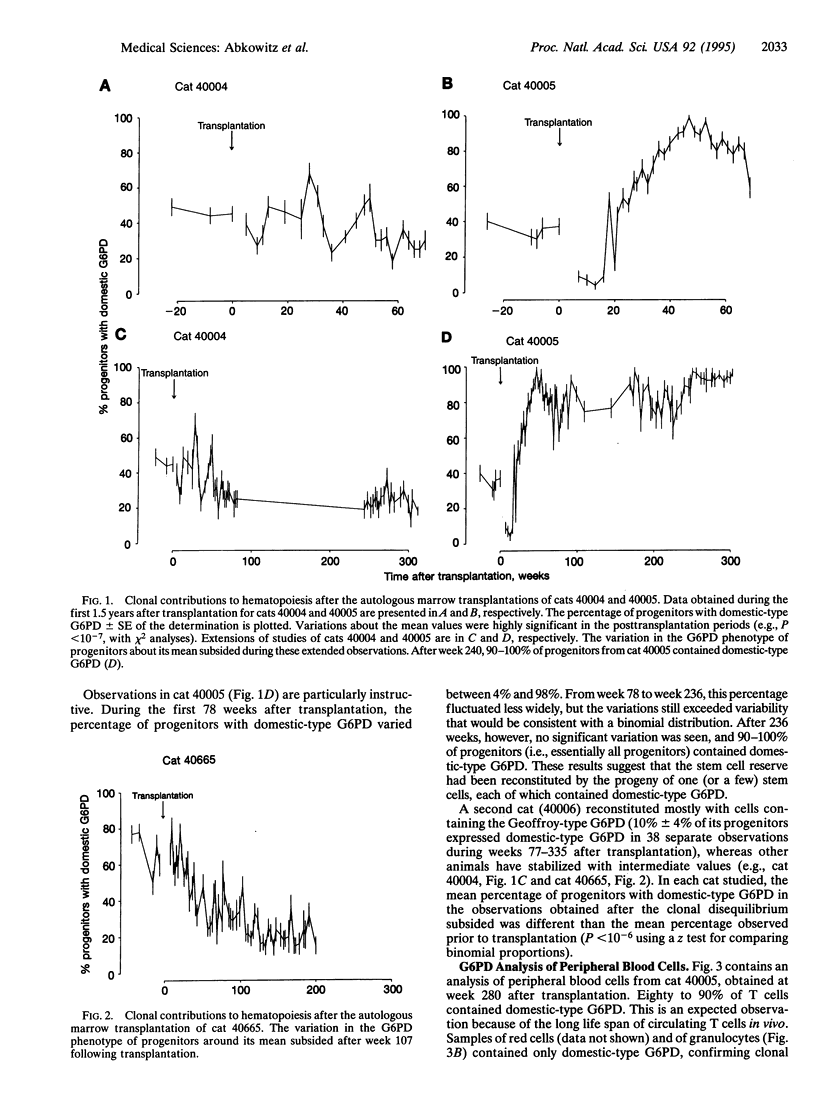
To study the behavior of hematopoietic stem cells in vivo, we transplanted glucose-6-phosphate dehydrogenase (G6PD) heterozygous (female Safari) cats with small amounts of autologous marrow. The G6PD phenotypes of erythroid burst-forming units and granulocyte/macrophage colony-forming units were repeatedly assayed for 3.5-6 years after transplantation to track contributions of stem cell clones to the progenitor cell compartment. Two phases of stem cell kinetics were observed, which were similar to the pattern reported in comparable murine studies. Initially there were significant fluctuations in contributions of stem cell clones. Later clonal contributions to hematopoiesis stabilized. The initial phase of clonal disequilibrium, however, extended for 1-4.5 years (and not 2-6 months as seen in murine experiments). After this subsided, all progenitor cells from some animals expressed a single parental G6PD phenotype, suggesting that blood cell production could be stably maintained by the progeny of one (or a few) cells. As the hematopoietic demand of a cat (i.e., number of blood cells produced per lifetime) is over 600 times that of a mouse, this provides evidence that an individual hematopoietic stem cell has a vast self-renewal and/or proliferative capacity. The long phase of clonal instability may reflect the time required for stem cells to replicate sufficiently to reconstitute a large stem cell reserve.
Full text
PDF



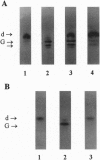
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abkowitz J. L., Linenberger M. L., Newton M. A., Shelton G. H., Ott R. L., Guttorp P. Evidence for the maintenance of hematopoiesis in a large animal by the sequential activation of stem-cell clones. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9062–9066. doi: 10.1073/pnas.87.22.9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abkowitz J. L., Ott R. L., Nakamura J. M., Steinmann L., Fialkow P. J., Adamson J. W. Feline glucose-6-phosphate dehydrogenase cellular mosaicism. Application to the study of retrovirus-induced pure red cell aplasia. J Clin Invest. 1985 Jan;75(1):133–140. doi: 10.1172/JCI111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecher G., Bookstein N., Redfearn W., Necas E., Pallavicini M. G., Cronkite E. P. Self-renewal of the long-term repopulating stem cell. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6028–6031. doi: 10.1073/pnas.90.13.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buescher E. S., Alling D. W., Gallin J. I. Use of an X-linked human neutrophil marker to estimate timing of lyonization and size of the dividing stem cell pool. J Clin Invest. 1985 Oct;76(4):1581–1584. doi: 10.1172/JCI112140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel B., Hawley R. G., Mintz B. Long- and short-lived murine hematopoietic stem cell clones individually identified with retroviral integration markers. Blood. 1990 Jun 15;75(12):2267–2270. [PubMed] [Google Scholar]
- Dai C. H., Krantz S. B., Dessypris E. N., Means R. T., Jr, Horn S. T., Gilbert H. S. Polycythemia vera. II. Hypersensitivity of bone marrow erythroid, granulocyte-macrophage, and megakaryocyte progenitor cells to interleukin-3 and granulocyte-macrophage colony-stimulating factor. Blood. 1992 Aug 15;80(4):891–899. [PubMed] [Google Scholar]
- Harrison D. E., Astle C. M., Lerner C. Number and continuous proliferative pattern of transplanted primitive immunohematopoietic stem cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):822–826. doi: 10.1073/pnas.85.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. E., Lerner C., Hoppe P. C., Carlson G. A., Alling D. Large numbers of primitive stem cells are active simultaneously in aggregated embryo chimeric mice. Blood. 1987 Mar;69(3):773–777. [PubMed] [Google Scholar]
- Harrison D. E., Zhong R. K. The same exhaustible multilineage precursor produces both myeloid and lymphoid cells as early as 3-4 weeks after marrow transplantation. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10134–10138. doi: 10.1073/pnas.89.21.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogge D. E., Coulombel L., Kalousek D. K., Eaves C. J., Eaves A. C. Nonclonal hemopoietic progenitors in a G6PD heterozygote with chronic myelogenous leukemia revealed after long-term marrow culture. Am J Hematol. 1987 Apr;24(4):389–394. doi: 10.1002/ajh.2830240408. [DOI] [PubMed] [Google Scholar]
- Jones R. J., Celano P., Sharkis S. J., Sensenbrenner L. L. Two phases of engraftment established by serial bone marrow transplantation in mice. Blood. 1989 Feb;73(2):397–401. [PubMed] [Google Scholar]
- Jordan C. T., Lemischka I. R. Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev. 1990 Feb;4(2):220–232. doi: 10.1101/gad.4.2.220. [DOI] [PubMed] [Google Scholar]
- Keller G., Snodgrass R. Life span of multipotential hematopoietic stem cells in vivo. J Exp Med. 1990 May 1;171(5):1407–1418. doi: 10.1084/jem.171.5.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine F., Najman A., Baillou C., Stachowiak J., Boffa G., Aegerter P., Douay L., Laporte J. P., Gorin N. C., Duhamel G. A prospective study of the value of bone marrow erythroid progenitor cultures in polycythemia. Blood. 1986 Nov;68(5):996–1002. [PubMed] [Google Scholar]
- Linenberger M. L., Abkowitz J. L. Studies in feline long-term marrow culture: hematopoiesis on normal and feline leukemia virus infected stromal cells. Blood. 1992 Aug 1;80(3):651–662. [PubMed] [Google Scholar]
- Nakao S., Yamaguchi M., Takamatsu H., Shiobara S., Matsuda T. Clonal hematopoiesis detected in an allogeneic marrow transplant recipient with poor engraftment. Transplantation. 1994 Apr 27;57(8):1266–1268. doi: 10.1097/00007890-199404270-00022. [DOI] [PubMed] [Google Scholar]
- Nash R., Storb R., Neiman P. Polyclonal reconstitution of human marrow after allogeneic bone marrow transplantation. Blood. 1988 Dec;72(6):2031–2037. [PubMed] [Google Scholar]
- Russell E. S. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- Smith L. G., Weissman I. L., Heimfeld S. Clonal analysis of hematopoietic stem-cell differentiation in vivo. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2788–2792. doi: 10.1073/pnas.88.7.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude G. J., Smith L., Uchida N., Ikuta K., Heimfeld S., Friedman J., Weissman I. L. Mouse hematopoietic stem cells. Blood. 1991 Sep 15;78(6):1395–1402. [PubMed] [Google Scholar]
- Turhan A. G., Humphries R. K., Phillips G. L., Eaves A. C., Eaves C. J. Clonal hematopoiesis demonstrated by X-linked DNA polymorphisms after allogeneic bone marrow transplantation. N Engl J Med. 1989 Jun 22;320(25):1655–1661. doi: 10.1056/NEJM198906223202504. [DOI] [PubMed] [Google Scholar]