Abstract
Using low molecular weight chitosan nanoparticles (CNPs) prepared by an ionic gelation method, the authors report the effect of low-intensity pulsed ultrasound (US) on cell viability and nanoparticle uptake in cultured murine preosteoblasts. Particle size and zeta potential are measured using dynamic light scattering, and cell viability is evaluated using the of [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS] assay. Results show that 30 min delivery of CNPs at 0.5 mg/mL is able to prevent loss of cell viability due to either serum starvation or subsequent exposure to US (1 W/cm2 or 2 W/cm2, up to 1 min). Additionally, flow cytometry data suggest that there is a close association between cellular membrane integrity and the presence of CNPs when US at 2 W/cm2 is administered.
I. INTRODUCTION
Application of pulsed ultrasound has been widely used in clinical diagnostics as well as for wound healing and cancer therapy.1–3 Ultrasound (US) has been shown to promote bone fracture healing in animals and humans,4,5 as well as enhance the proliferation of osteoblasts in vitro.5–7 There has also been interest in utilizing US in triggerable drug delivery systems.8 Low-intensity and low-frequency US in particular can enhance the delivery efficiency of drug carriers such as polyethylene glycol, liposomes, and micelles, thereby increasing the therapeutic efficacy of the cargo.8–12 US is composed of a propagating pressure wave or sound wave, which can help transfer mechanical energy into various tissues of the body.13 The frequency, duty cycle, and duration all contribute toward the total absorbance of energy.
Various natural or synthetic polymer-based hydrogels have been used in drug and gene delivery applications. Synthetic water soluble polymers are biodegradable but have the potential to be extremely toxic.14,15 Natural polymer-based hydrogels such as chitosan and chitosan derivatives are biodegradable and nontoxic.14,16 A linear polysaccharide derived from the exoskeleton of crustaceans, cell walls of fungi, and cocoons of insects,17 chitosan is a low-immunogenic cationic polymer that can form solid colloidal nanoparticles in the range of 1 nm–1000 nm.18 Chitosan is highly biocompatible19–22 and has antibacterial, anti-inflammatory, and neuroprotective properties.23
Although low-intensity US has been shown to be effective in assisting drug release from polymer-based vehicles, very few studies have documented the viability of the healthy tissue at the irradiation site. Despite the wealth of information on chitosan-based nanotherapeutics, there has been limited knowledge concerning their effect on cell viability when used in combination with US. The primary objective of this work therefore is to examine the effect of ultrasound-assisted delivery of chitosan nanoparticles (CNPs) in a mammalian cell line by investigating cell viability and uptake. A more complete understanding of noninvasive sonodynamic therapy can help evaluate its efficacy as a potential adjuvant to conventional drug delivery.
II. EXPERIMENT
A. Materials
Low molecular weight chitosan (deacetylation degree 75%–85%), sodium tripolyphosphate (TPP, technical grade, 85%), 2% ninhydrin reagent solution (ninhydrin and hydrindantin in dimethyl sulfoxide and lithium acetate buffer, pH = 5.2), and fluorescein 5(6)-isothiocyanate (FITC) were purchased from Sigma-Aldrich (St. Louis, MO). Sodium hydroxide pellets (reagent grade, ≈98.8%), formaldehyde solution (Reagent A.C.S, ≈37%), and glacial acetic acid (analytical grade, ≈99.9%) were obtained from J.T. Baker (Center Valley, PA). Methanol (Reagent A.C.S, ≈99.9%) and water (high-performance liquid chromatography grade) were purchased from Pharmco-AAPER (Brookfield, CT). d–(+)-Trehalose dihydrate (≈99%) was obtained from Acros Organics (NJ, USA) and dialysis cassettes (3500 MWCO) were purchased from Thermo Scientific (Rockford, IL).
B. Synthesis
1. Chitosan-TPP nanoparticles
An ionic gelation method was used to synthesize the chitosan nanoparticles, similar to previous reports.18,24 Low molecular weight chitosan was dissolved in acetic acid (1% v/v, in water) for 30 min under constant stirring to make a 0.5% (w/v) chitosan stock solution, which was filtrated with a 0.8 μm syringe filter before gelation. A few drops of sodium hydroxide were added to the chitosan stock solution to adjust the pH value to 4.6–4.8. TPP solution (0.25% w/v, in water) was added dropwise into the chitosan solution until opalescent droplets could be seen. After 5 min of reaction, the nanoparticle solution was centrifuged at 2700 RPM for 30 min and rinsed extensively with deionized (DI) water. d-(+)-Trehalose was added to nanoparticle solution to a final concentration of 5% (w/v). The nanoparticles were stored in a −80 °C freezer for at least 24 h and lyophilized. To prepare FITC-labeled CNP (FITC-CNP), FITC-conjugated chitosan was first prepared using published protocols.25 The same ionic gelation method was conducted in the dark.
C. Characterization
1. FITC labeling efficiency
In order to calculate the labeling efficiency of FITC, a standard curve plotting absorbance versus FITC concentration was used. FITC standard solutions ranging from 0.01 μg/mL to 0.08 μg/mL were prepared by diluting 100 μg/mL FITC stock solutions (in methanol) with 1× phosphate-buffered saline (PBS). An FITC–chitosan test sample was prepared by dissolving FITC-chitosan in 0.1 M acetic acid followed by dilution with PBS, until a final concentration of 1.0 μg/mL was achieved. All solutions were evaluated in triplicate by ultraviolet–visible spectroscopy (Perkin Elmer Lambda 950). FITC labeling efficiency was calculated using
| (1) |
2. Particle size and zeta potential
Measurements of particle size and surface charge (characterized as zeta potential) were performed by dynamic light scattering, using a ZEN3600 Nano Zetasizer (Malvern Instruments, UK) located at the Center for Functional Nanomaterials of Brookhaven National Laboratory (Upton, NY). Freeze-dried chitosan nanoparticles prepared at 6:1 chitosan:TPP mass ratio were dissolved in DI water to make a 1 mg/mL CNP suspension. In order to remove large aggregates, the suspension was then passed through a 0.8 μm syringe filter, and the filtrate was collected and stored at 4 °C prior to analysis. The particle size measurements were conducted at room temperature and each measurement lasted for 120 s. For zeta potential measurement, a specific zeta dip cell was used and 30 measurements were collected for each sample. All measurements were performed at 25 °C in triplicate.
D. Nanoparticle delivery and ultrasound irradiation
MC3T3-E1 murine preosteoblasts (subclone 4; American Type Culture Collection, Manassas, VA) were maintained in α-modified minimum essential medium (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (pen-strep) solution. The cells were maintained at 37 °C in an atmosphere of 5% carbon dioxide and 95% relative humidity.
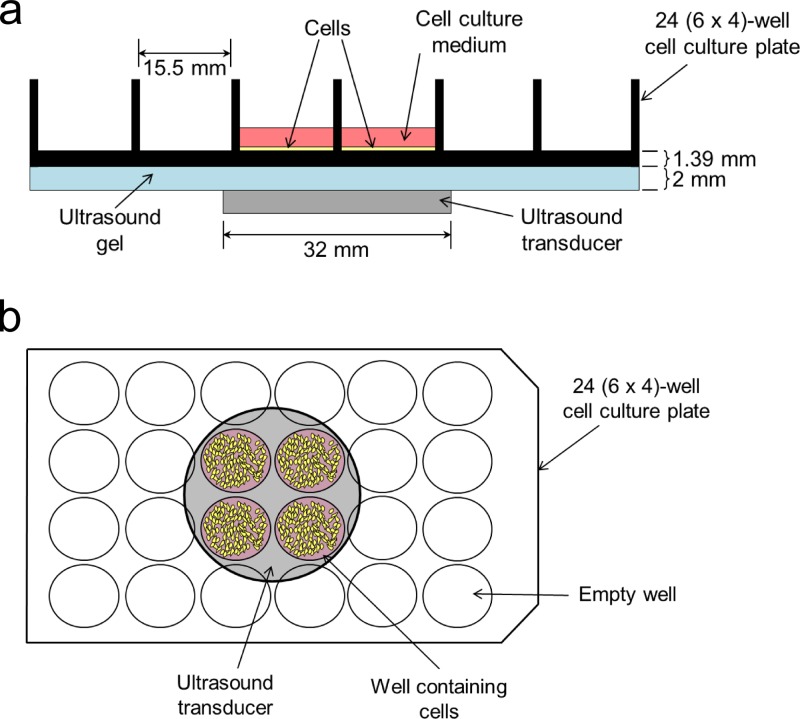
Twenty-four hours prior to the delivery of chitosan-TPP nanoparticles, MC3T3-E1 cells were seeded onto a 24-well tissue culture plate at 10,000 cells/cm2 (for viability assay) or 5000 cells/cm2 (for confocal microscopy), or a 35 mm (diam.) tissue culture plate at 50,000 cells/cm2 (for flow cytometry). Cells were incubated for 24 h in a humidified incubator (5% CO2) to attach completely. The supernatant was aspirated, and the cells were washed with PBS. Fresh complete medium (supplemented with FBS) or serum-free medium containing either 0 or 0.5 mg/mL CNPs was added. For flow cytometry experiments, the cells were exposed to FITC-labeled CNPs. The dose of 0.5 mg/mL was chosen based on preliminary data, showing the minimum concentration needed to produce a strong intracellular fluorescence signal (data not shown). The plates were gently swirled before being returned to the incubator. After 30 min, cells that were to receive ultrasound (US) treatment were removed from the incubator and immediately sonicated by an acoustic device (Sonicator 740; Mettler Electronic Corp. Anaheim, CA) with a 10-cm2 transducer (32 mm in diameter) using a frequency of 1 MHz and a pulse duration of 0.1 μs at an intensity of either 1 W/cm2 or 2 W/cm2, for a total exposure time of either 30 s or 60 s. All stimulations targeted the same region of each 24-well tissue culture plate (Fig. 1). The 2 mm distance between the transducer and the bottom of the tissue culture plate was filled with ultrasound gel (Aquasonic 100 Ultrasound Transmission Gel; Parker Laboratories, Fairfield, NJ). Cells that were not treated with US were kept on the lab bench for the same amount of time. All cells were then returned to the incubator for an additional 90 min. The 2-h duration for CNP exposure was chosen based on previous uptake studies showing strong presence of chitosan nanoparticles with minimal lysosomal degradation.26,27
Fig. 1.
Schematic representation of side (a) and top (b) views of experimental setup.
E. Cell viability
Cytotoxic effect of ultrasound and CNP was examined using a commercially available proliferation assay kit (CellTiter 96® One Solution Cell Proliferation; Promega Corporation, Madison, WI). Aliquots of [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] (MTS) and phenazine methosulfate (PMS) solutions were added to the supernatant in each cell culture well, and the tissue culture plate was swirled gently before being incubated for 1 h at 37 °C. A 200 μL volume of the supernatant was transferred to a 96-well tissue culture plate and the absorbance was immediately read at 490 nm using a microplate reader (BioTek FLx800; BioTek, Winooski, VT).
F. Confocal microscopy
Cells were fixed with 3.7% formaldehyde (in PBS) for 15 min, followed by extensive rinsing with PBS and immersion in 2.5 μg/mL 4′,6-diamidino-2′-phenylindole dihydrochloride (DAPI) solution for 5 min. After removal of the DAPI solution, the cells were rinsed two more time with PBS. Fluorescence micrographs were then captured using a disk scanning unit (DSU) confocal microscope (Olympus IX2-DSU) and Z-scans were obtained with a total scan depth of 5.8 μm, with a 0.2 μm step size.
G. Flow cytometry
Untreated cells, cells exposed only to 2 W/cm2 US for 60 s, cells exposed only to FITC-CNPs, and cells exposed to both FITC-CNPs and 2 W/cm2 US for 60 s were detached from the tissue culture plate with 0.05% Trypsin-EDTA and resuspended in 1 mL complete medium. Propidium iodide (10 μg/mL in PBS) was applied for 1 min at 4 °C in the dark. Samples were immediately read by a FACS Calibur flow cytometer (BD Biosciences) using a 488 nm laser.
III. RESULTS AND DISCUSSION
A. Physicochemical properties of chitosan-TPP nanoparticles
The FITC labeling efficiency of FITC-chitosan calculated using Eq. (1) was 2.4%, which is similar to previously reported values ranging from 1.78% to 3.1%.28–31 Average particle size of freeze dried CNPs and FITC-CNPs resuspended in water was 288.4 ± 1.2 nm and 294.0 ± 10.8 nm, respectively. Average zeta potential of CNP and FITC-CNP in water was 36.87 0.27 mV and 32.90 ± 0.64 mV, respectively.
B. In vitro uptake of FITC-labeled chitosan-TPP nanoparticles
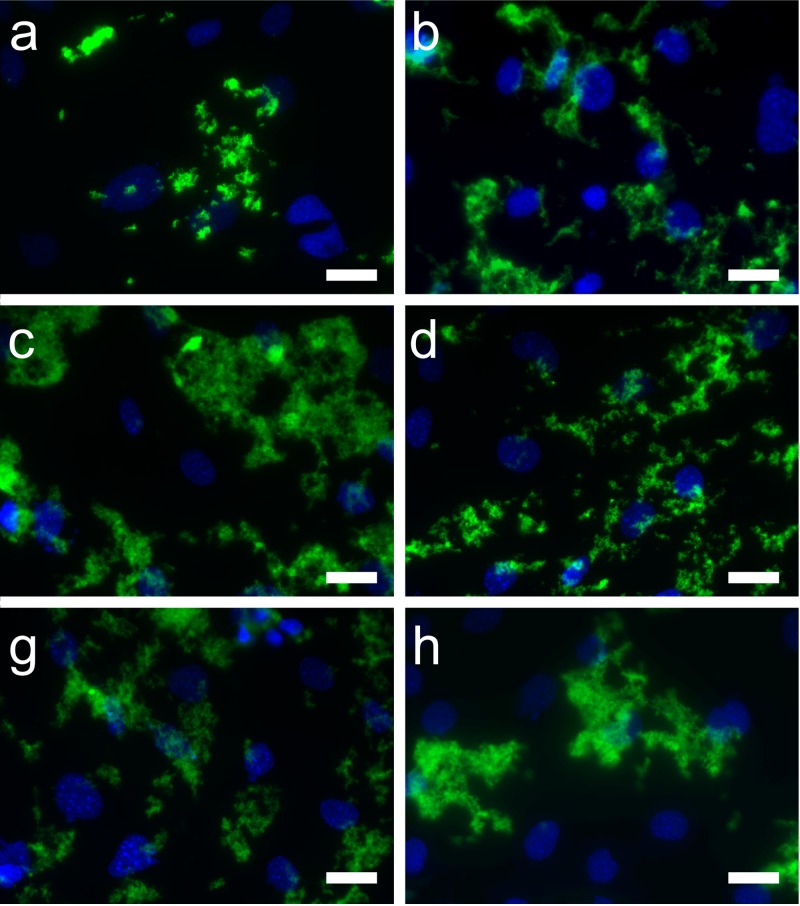
Confocal micrographs showed greater internalization of the FITC-CNP by the MC3T3-E1 cells under US exposure compared to the nonirradiated cells (Fig. 2). Aggregates of FITC-CNP appeared larger in cells that were treated for either 30 s at 1 W/cm2 or 60 s at 2 W/cm2.
Fig. 2.
Fluorescence micrographs showing FITC-labeled chitosan nanoparticles (visualized in the green channel) after being internalized by MC3T3-E1 preosteoblasts (nuclei visualized in the blue channel). Cells were exposed to nanoparticles in the absence (a), (b) or presence (c)–(f) of ultrasound and incubated for a total of 120 min. Ultrasound was applied at 1 W/cm2 for 30 s (c) or 60 s (d), and at 2 W/cm2 for 30 s (e) or 60 s (f). Images are representative of two independent experiments. Scale, 25 μm.
C. Cell viability
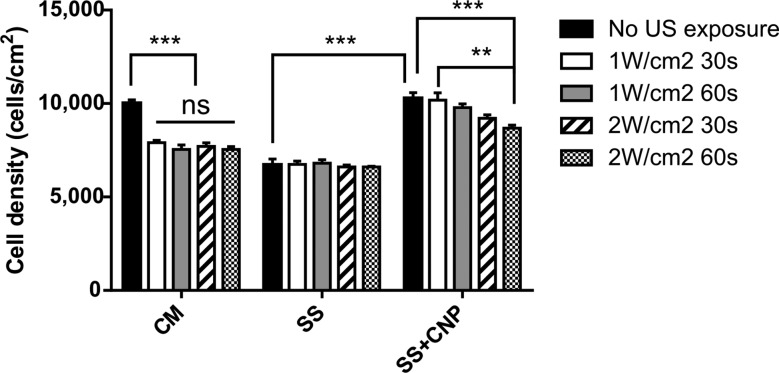
Results from the MTS viability assay showed that when the untreated control MC3T3-E1 cells were cultured in complete medium (“CM” group, Fig. 3) without exposure to either US or CNP, cell density after 26 h of attachment reached the seeding density of 10,000 cells/cm2 (Fig. 3). This is reasonable, as the doubling time for the MC3T3-E1 cell line is 2–3 days.32 Application of US at either 1 or 2 W/cm2 for a minimum of 30 s to cells maintained in complete medium significantly decreased cell density by ∼20% (p < 0.001; Fig. 3). There was no significant difference among the US treatments. When cells were temporarily serum-starved but not exposed to CNP (“SS” group, Fig. 3), cell density at 2 h dropped to 6600–6800 cells/cm2 for all samples irrespective of US treatment, corresponding to a 32–34% reduction relative to untreated controls (p < 0.001). Interestingly, when CNPs were added during the 2 h serum starvation period (“SS + CNP” group, Fig. 3), cells appeared to be more viable compared to those that were serum-starved for the same duration in the absence of CNP. More specifically, cell survival did not appear to be significantly different between the untreated controls and those exposed either to CNP alone (p > 0.2) or simultaneously to CNP and low-dose US (1 W/cm2) (Fig. 3). At the higher US intensity of 2 W/cm2, cell density decreased significantly by 8% after 30 s of exposure (p < 0.001) and 13% after 60 s of exposure (p < 0.01).
Fig. 3.
Effect of ultrasound on viability of MC3T3-E1 cells cultured in complete medium (CM), serum-starved medium (SS), or serum-starved medium containing chitosan nanoparticles (SS + CNP) 24 h after CNP delivery and US stimulation. All values were expressed as mean ± SE, **p < 0.01, ***p < 0.001 (n = 3).
These results show that whereas chitosan enhances cell survival, US appears to diminish viability, particularly at higher doses. In contrast, many previous studies report an increase in osteoblast viability due to US stimulation. For example, Doan et al. reported that stimulation of 1 MHz pulsed US at 0.7 W/cm2 increased cell proliferation in human osteoblasts by more than 40%.5 Hayton et al.6 observed a 10% increase in the proliferation of human osteoblastlike Saos-2 cells after 40 min of US stimulation (1.5 MHz at 30 mW/cm2). Recently Katiyar et al. reported that 2.5 MHz US at 18–74 mW/cm2 increased the proliferation of murine osteoblastlike MC3T3-E1 cells by 40–50%.7 These differences likely result from the use of a higher intensity US (2 W/cm2) in our study.
Additionally, our study showed that when CNPs were present in the cells, US had very little effect on viability, which suggests that the CNPs have cytoprotective properties. Recently, Yang et al.33 reported considerable loss in viability (∼50%) when human embryonic kidney epithelial cells were exposed simultaneously to both 1 MHz US at 2.75 W/cm2 and chitosan-alginate nanoparticles. We find these comparisons to be quite interesting. Although the US parameters used in our study are very similar to those used in the Yang et al. study, our pulses were much shorter and the US stimulation was less likely to cause heating, thus contributing to better cell survival.
D. Flow cytometry
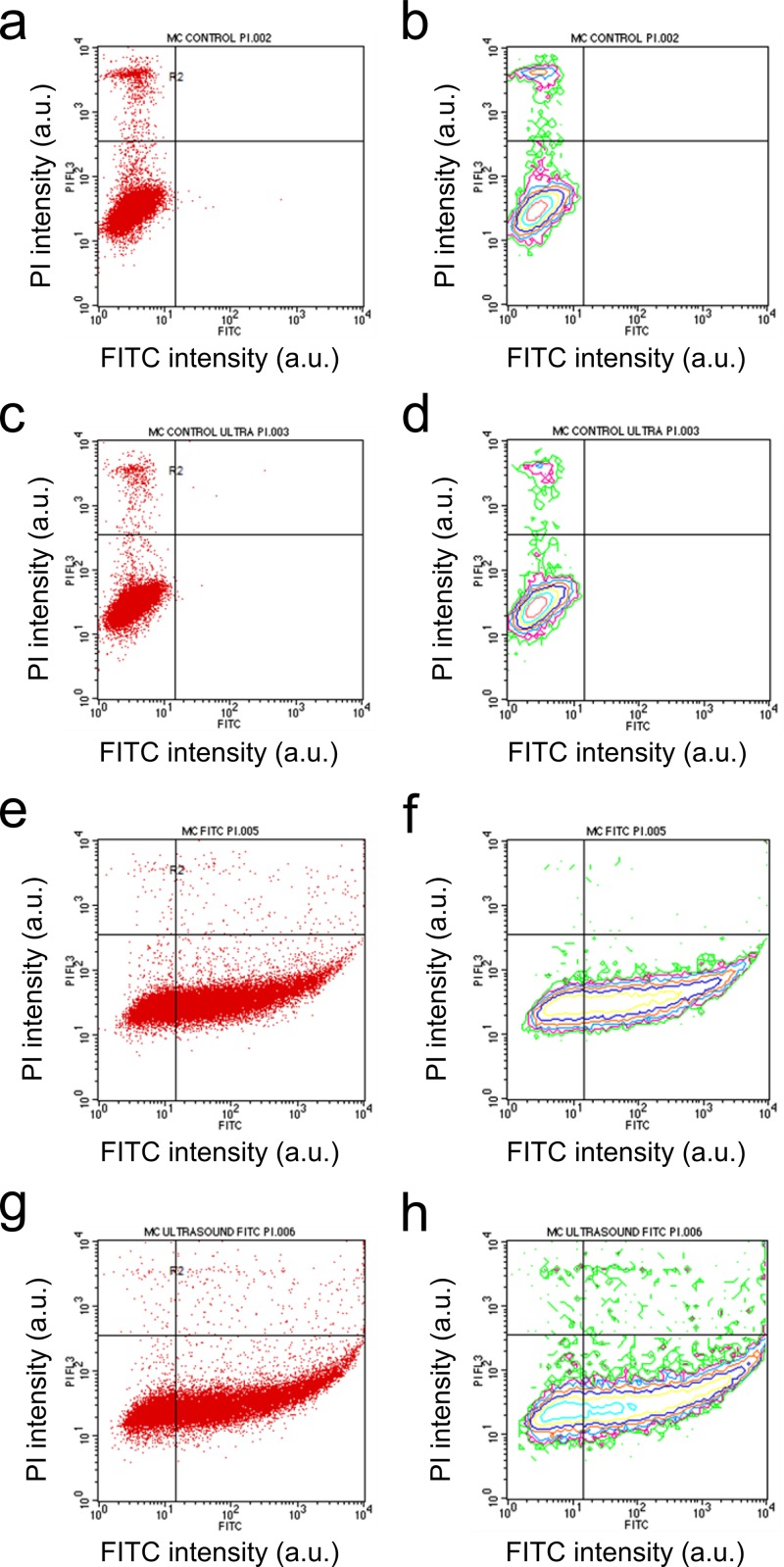
To further investigate the significant decrease in cell viability after the MC3T3-E1 cells were exposed to 60 s of US at 2 W/cm2, we conducted flow cytometry measurements following the addition of propidium iodide (PI) to the treated cells. Scatter plots indicated that US alone did not have an effect on the cells' ability to uptake PI [Figs. 4(a) and 4(c)], which suggests that cellular membrane was not damaged by US, even though metabolic activity was decreased as seen from the MTS data (Fig. 3). It is possible that although US at 2 W/cm2 may not have altered membrane integrity, it could have caused low level heating and, as a result, changed the viability of the cells.
Fig. 4.
Scatter (a), (c), (e), (g) and contour (b), (d), (f), (h) plots of flow cytometry data for untreated cells (a), (b), cells exposed only to 2 W/cm2 US for 60 s (c), (d), cells exposed only to FTIC-labeled chitosan nanoparticles (e), (f), and cells exposed both to chitosan nanoparticles and 2 W/cm2 US for 60 s (g), (h). Fluorescence intensities of propidium iodide (PI) and FITC are displayed on the x- and y-axes, respectively.
When FITC-labeled CNPs were delivered alone in the absence of US stimulation, FITC fluorescence was notably stronger, as shown by the dense scatter in the lower-right quadrant [Figs. 4(e) and 4(f)]. This suggests that CNPs had a great affinity for the MC3T3-E1 cells. We also observed that uptake of PI was diminished in this case, as seen by an absence of dense clusters in the upper-left quadrant of the scatter plot [Figs. 4(e) and 4(f)] compared to either untreated cells [Figs. 4(a) and 4(b)] or those exposed to US alone [Figs. 4(c) and 4(d)]. This indicates that CNPs may have protected the cell membrane from being damaged during sample preparation. When US was applied immediately after CNP delivery, FITC fluorescence was even more greatly enhanced [lower-right quadrant, Figs. 4(g) and 4(h)], indicating that more CNPs were associated with, and possibly internalized by, the MC3T3-E1 cells. In addition, PI fluorescence was also enhanced [upper-right quadrant, Figs. 4(g) and 4(h)], which suggests that cellular membrane may have become less intact. Moreover, because the population of cells with strong PI fluorescence overlapped those with strong FITC fluorescence [Figs. 4(g) and 4(h)], we believe that membrane leakiness may be associated with the presence of CNPs.
The cytoprotective effect of chitosan has been reported for serum starved human astrocytes34 and hydrogen peroxide stressed human umbilical vein endothelial cells,35 possibly via inactivation of the p53 tumor suppressor or direct inhibition of apoptosis. Other naturally derived polymers have also demonstrated cytoprotective effects;36,37 however, to our knowledge, this is the first study to investigate the synergistic effect of coadministered chitosan and US on cell viability.
IV. SUMMARY AND CONCLUSIONS
In this study, chitosan/TPP (CNP) and FITC-labeled chitosan/TPP nanoparticles (FITC-CNP) were successfully formulated by a modified ionic gelation method. FITC-CNPs were delivered to murine preosteoblasts (MC3T3-E1 cell line, subclone 4), and the uptake was shown to be enhanced by ultrasound (US) irradiation. Serum starvation for 2 h resulted in a 32–34% reduction in cell viability, which was not observed when CNPs were administered simultaneously. The same phenomenon was observed when low-intensity US treatment (1 W/cm2) was applied during serum starvation, but at higher intensity (2 W/cm2) cell viability was notably decreased. Additionally, there seems to be a close association between ultrasound-assisted CNP delivery and membrane integrity.
Taken together, these results suggest that CNPs may alter the sensitivity of preosteoblasts to therapeutic levels of low-intensity pulsed ultrasound.
ACKNOWLEDGMENTS
This work is also kindly supported by the National Institute of Health (R01 AR52379 and R01AR61821), and the National Space Biomedical Research Institute through NASA contract NCC 9-58. Research was carried out in part at the Center for Functional Nanomaterials, Brookhaven National Laboratory, which is supported by the U.S. Department of Energy, Office of Basic Energy Sciences, under Contract No. DE-AC02-98CH10886.
References
- 1.Kane D., Grassi W., Sturrock R., and Balint P. V., Rheumatology 43, 931 (2004). 10.1093/rheumatology/keh004 [DOI] [PubMed] [Google Scholar]
- 2.Maeda T., Masaki C., Kanao M., Kondo Y., Ohta A., Nakamoto T., and Hosokawa R., J. Prosthodontic Res. 57, 93 (2013). 10.1016/j.jpor.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 3.Wu F., Chen W. Z., Bai J., Zou J. Z., Wang Z. L., Zhu H., and Wang Z. B., Ultrasound Med. Biol. 27, 1099 (2001). 10.1016/S0301-5629(01)00389-1 [DOI] [PubMed] [Google Scholar]
- 4.Duarte L. R., Arch. Orthop. Trauma Surg. 101, 153 (1983). 10.1007/BF00436764 [DOI] [PubMed] [Google Scholar]
- 5.Doan N., Reher P., Meghji S., and Harris M., J. Oral Maxillofac. Surg. 57, 409 (1999). 10.1016/S0278-2391(99)90281-1 [DOI] [PubMed] [Google Scholar]
- 6.Hayton M. J., Dillon J. P., Glynn D., Curran J. M., Gallagher J. A., and Buckley K. A., Ultrasound Med. Biol. 31, 1131 (2005). 10.1016/j.ultrasmedbio.2005.04.017 [DOI] [PubMed] [Google Scholar]
- 7.Katiyar A., Duncan R., and Kausik S., J. Ther. Ultrasound 2, 1 (2014). 10.1186/2050-5736-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timko B. P., Dvir T., and Kohane D. S., Adv. Mater. 22, 4925 (2010). 10.1002/adma.201002072 [DOI] [PubMed] [Google Scholar]
- 9.Johnson M. E., Mitragotri S., Patel A., Blankschtein D., and Langer R., J. Pharm. Sci. 85, 670 (1996). 10.1021/js960079z [DOI] [PubMed] [Google Scholar]
- 10.Pitt W. G., Husseini G. A., and Staples B. J., Expert Opin. Drug Delivery 1, 37 (2004). 10.1517/17425247.1.1.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rapoport N. Y., Herron J. N., Pitt W. G., and Pitina L., J. Controlled Release 58, 153 (1999). 10.1016/S0168-3659(98)00149-7 [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z., Xu K., Bi Y., Yu G., Wang S., Qi X., and Zhong H., Plos One 8, e70685 (2013). 10.1371/journal.pone.0070685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosseinkhani H., Aoyama T., Ogawa O., and Tabata Y., Curr. Pharm. Biotechnol. 4, 109 (2003). 10.2174/1389201033489883 [DOI] [PubMed] [Google Scholar]
- 14.Kadajji V. G. and Betageri G. V., Polymers 3, 1972 (2011). 10.3390/polym3041972 [DOI] [Google Scholar]
- 15.Raemdonck K., Martens T. F., Braeckmans K., Demeester J., and Smedt S. C. De, Adv. Drug Delivery Rev. 65, 1123 (2013). 10.1016/j.addr.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 16.Kim J. K., Kim H. J., Chung J. Y., Lee J. H., Young S. B., and Kim Y. H., Arch. Pharmacal Res. 37, 60 (2014). 10.1007/s12272-013-0280-6 [DOI] [PubMed] [Google Scholar]
- 17.Kumar M., Muzzarelli R. A. A., Muzzarelli C., Sashiwa H., and Domb A. J., Chem. Rev. 104, 6017 (2004). 10.1021/cr030441b [DOI] [PubMed] [Google Scholar]
- 18.Calvo P., RemunanLopez C., VilaJato J. L., and Alonso M. J., J. Appl. Polym. Sci. 63, 125 (1997). [DOI] [Google Scholar]
- 19.Boucard N., Viton C., Agay D., Mari E., Roger T., Chancerelle Y., and Domard A., Biomaterials 28, 3478 (2007). 10.1016/j.biomaterials.2007.04.021 [DOI] [PubMed] [Google Scholar]
- 20.Helander I. M., Nurmiaho-Lassila E. L., Ahvenainen R., Rhoades J., and Roller S., Int. J. Food Microbiol. 71, 235 (2001). 10.1016/S0168-1605(01)00609-2 [DOI] [PubMed] [Google Scholar]
- 21.Suh J. K. F. and Matthew H. W. T., Biomaterials 21, 2589 (2000). 10.1016/S0142-9612(00)00126-5 [DOI] [PubMed] [Google Scholar]
- 22.Roy K., Mao H. Q., Huang S. K., and Leong K. W., Nat. Med. 5, 387 (1999). 10.1038/7385 [DOI] [PubMed] [Google Scholar]
- 23.Pangestuti R. and Kim S. K., Mar. Drugs 8, 2117 (2010). 10.3390/md8072117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi L. F., Xu Z. R., Jiang X., Hu C. H., and Zou X. F., Carbohydr. Res. 339, 2693 (2004). 10.1016/j.carres.2004.09.007 [DOI] [PubMed] [Google Scholar]
- 25.Huang M., Khor E., and Lim L. Y., Pharm. Res. 21, 344 (2004). 10.1023/B:PHAM.0000016249.52831.a5 [DOI] [PubMed] [Google Scholar]
- 26.Huang M., Fong C. W., Khor E., and Lim L. Y., J. Controlled Release 106, 391 (2005). 10.1016/j.jconrel.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 27.Nam H. Y.et al. , J. Controlled Release 135, 259 (2009). 10.1016/j.jconrel.2009.01.018 [DOI] [PubMed] [Google Scholar]
- 28.Huang M., Ma Z. S., Khor E., and Lim L. Y., Pharm. Res. 19, 1488 (2002). 10.1023/A:1020404615898 [DOI] [PubMed] [Google Scholar]
- 29.Tallury P., Kar S., Bamrungsap S., Huang Y.-F., Tand W., and Santra S., Chem. Commun. 2347 (2009). 10.1039/b901729a [DOI] [PubMed] [Google Scholar]
- 30.Malatesta M., Giagnacovo M., Costanzo M., Conti B., Genta I., Dorati R., Galimberti V., Biggiogera M., and Zancanaro C., Eur. J. Histochem. 56, e20 (2012). 10.4081/ejh.2012.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia X., Chen X., Xu Y., Han X., and Xu Z., Carbohydr. Polym. 78, 323 (2009). 10.1016/j.carbpol.2009.04.020 [DOI] [Google Scholar]
- 32.Byskov K., Jensen N., Kongsfelt I. B., Wielsoe M., Pedersen L. E., Haldrup C., and Pedersen L., Cell Div. 7, 7 (2012). 10.1186/1747-1028-7-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang S., Chang S., Tsai K., Chen W., Lin F., and Shieh M., J. Gene Med. 12, 168 (2010). 10.1002/jgm.1418 [DOI] [PubMed] [Google Scholar]
- 34.Koo H. N., Jeong H. J., Hong S. H., Choi J. H., An N. H., and Kim H. M., J. Nutr. Biochem. 13, 245 (2002). 10.1016/S0955-2863(01)00218-2 [DOI] [PubMed] [Google Scholar]
- 35.Liu H. T., He J. L., Li W. M., Yang Z., Wang Y. X., Bai X. F., Yu C., and Du Y. G., Carbohydr. Polym. 80, 1062 (2010). 10.1016/j.carbpol.2010.01.025 [DOI] [Google Scholar]
- 36.Marcia G., Messing J., Menchicchi B., Goycoolea F. M., Faller G., Graciela F. D., and Hensel A., Int. J. Biol. Macromol. 62, 217 (2013). 10.1016/j.ijbiomac.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 37.Chen S. G., Siu K. C., Wang W. Q., Liu X. X., and Wu J. Y., Carbohydr. Polym. 94, 332 (2013). 10.1016/j.carbpol.2012.12.067 [DOI] [PubMed] [Google Scholar]