Abstract
Objective
Universal HIV screening is recommended but challenging to implement. Selectively targeting those at risk is thought to miss cases, but prior studies are limited by narrow risk criteria, incomplete implementation, and absence of direct comparisons. We hypothesized that targeted HIV screening, when fully implemented and using maximally broad risk criteria, could detect nearly as many cases as universal screening with many fewer tests.
Methods
This single-center, cluster-randomized trial compared universal and targeted patient selection for HIV screening in a lower prevalence urban emergency department. Patients were excluded for age (<18, >64), known HIV infection, or prior approach for HIV testing that day. Targeted screening was offered for any risk indicator identified from charts, staff referral, or self-disclosure. Universal screening was offered regardless of risk. Baseline seroprevalence was estimated from consecutive de-identified blood samples.
Results
There were 9,572 eligible visits during which the patient was approached. For universal screening, 40.8% (1,915/4,692) consented with six newly diagnosed (0.31%, CI95 0.13%–0.65%). For targeted screening, 37% (1,813/4,880) had no testing indication. Of the 3,067 remaining, 1,454 (47.4%) consented with 3 newly diagnosed (0.22%, CI95 0.06%–0.55%). Estimated seroprevalence was 0.36% (CI95 0.16%–0.70%). Targeted screening had a higher proportion consenting (47.4% v. 40.8%, p<0.002), but a lower proportion of ED encounters with testing (29.7% v. 40.7%, p<0.002).
Conclusions
Targeted screening, even when fully implemented with maximally permissive selection, offered no important increase in positivity rate or decrease in tests performed. Universal screening diagnosed more cases, because more were tested, despite a modestly lower consent rate.
Keywords: HIV, Mass Screening, Emergency Medicine, Informed Consent, Disease Prevalence, Epidemiology
INTRODUCTION
Early diagnosis of HIV is critical.1 Once diagnosed, infected individuals can engage in behavior change2,3 and treatment4–7 which combine to decrease infectivity and reduce cost. Emergency departments (EDs) have been emphasized as a partner in HIV screening because of high patient volume, access to vulnerable populations, and utilization by a broad spectrum of society.1,8–20 Unfortunately, EDs are already overburdened,21–23 and separate, dedicated funds to scale-up testing are not widely available. Identifying the most efficient approaches to screening will facilitate implementation.
Deciding which patients should be tested is a major challenge. Public health advocates have called for universal or non-targeted screening.1,18,24 This requires many tests, and concerns persist about feasibility and effectiveness,15,25–42 particularly when prevalence is low or presumed low. Selectively targeting increased likelihood of undiagnosed infection requires fewer tests, but failure to identify all cases is frequently cited.1,18,43–48 However, targeted screening may have failed because of incomplete implementation rather than inadequately sensitive criteria.35 If so, expanding targeting criteria beyond conventional risk criteria should increase sensitivity. There has been little comparative study about the trade-offs of such an approach or which precise targeting criteria would be most advantageous.
A growing number of studies have reported yield from non-targeted screening.17,20,36,37,39,49–59 Direct comparisons between non-targeted and targeted approaches are scarce 17,20,54 and confounded by many other factors such as consent method, assay type, targeting criteria and assessment method, who conducts screening, and incomplete implementation. We compared the efficacy of universal and targeted screening in the context of an opt-in ED HIV screening program, hypothesizing that given full implementation and maximally permissive selection criteria, targeted screening would detect nearly as many cases while requiring many fewer tests.
METHODS
Design Overview
This single-center, cluster-randomized trial compared the yield of universal and targeted screening for HIV in the ED, in the context of two concurrent seroprevalence studies. Seroprevalence estimates were obtained either by approaching patients consecutively (patient-based) or from de-identified, discarded blood samples, originally obtained for clinical purposes (remnant-based).
The study enrolled patients from January 2008 through December 2010. Blocks of patients were randomized to be approached for HIV screening on a universal or targeted basis or, for the first two years of enrollment, in the patient-based seroprevalence study. The remnant-based seroprevalence study was conducted over the third year of the screening study. The study was approved by the Institutional Review Board and overseen by a Data and Safety Monitoring Board (DSMB). No harms or unintended study effects were recorded.
Setting and Participants
This study was conducted in the ED of a Midwestern, urban, 450-bed, teaching hospital with 90,000 annual ED patient encounters. Of ED patients, about 50% were black, 0.5% were Hispanic, and 40% were uninsured. Pediatric patients were rarely seen, since a large pediatric ED is located nearby. A publicly funded HIV counseling and testing program has operated in this ED since 1998.60–62 The cumulative diagnosis rate of HIV/AIDS in the surrounding county is 229 per 100,000 persons.63
For the screening arms, patients were ineligible for age <18 or >64, known HIV infection, or previous approach for ED HIV testing that day. Post-exposure testing (e.g. occupational, assault,) was not provided. For the seroprevalence arms, patients were ineligible for age <18 or >64.
Randomization and Interventions
Randomization
Patients were approached for universal screening, targeted screening, or the patient-based seroprevalence estimate according to cluster randomization. Clusters were defined by combinations of four 6 hour periods spanning the full 24 hours and four separated ED patient care areas. The probability of each combination being a study period was set to achieve about 4 study periods per week. Study periods were then randomly assigned to being one of the three study arms.
Study periods were cancelled without replacement if: 1) the assigned ED area was closed during that time, 2) two study periods were adjacent in time and space, 3) staffing was insufficient. Patients were allocated to periods based on their physical presence and not their time of arrival to the ED.
ED HIV Counseling and Testing Program
The screening study used the same procedures as the existing clinical screening program,60–62 but staffing was augmented to include every eligible patient. Additional study intervention was limited to randomizing patients between different selection criteria, both of which were used regularly by the program prior to the study.
HIV testing was offered at no cost with signed, opt-in consent, detailed risk-assessment, and formal prevention counseling. Ohio statutes and hospital policy mandated opt-in consent for HIV testing until 2011. There was no separate research consent; the clinical consent for HIV testing included IRB approved language advising that information could be “analyzed to monitor the quality of the testing program and combined and reported to improve our understanding of issues related to the spread of HIV”.
Services were provided by cadre of 10 to 12 counselors dedicated counselors operating in parallel with usual ED care. Counselors worked between 16 and 40 hours per week for about a year or more. They were trained in basic information regarding HIV illness, testing, and transmission, formal prevention counseling, and ED and study operations. Training included 12 hours of didactic instruction, followed by 4 hours of role-play and observation or initial patient encounters. This was followed with periodic observation of counselor performance, feedback based on quality assurance review of data forms and enrollment logs, monthly meetings, and remediation if needed. Counselor work schedules were created entirely separately from activity randomization, so there would be no systematic difference in the way individual counselors were allocated between study arms. Risk assessment and counseling used a questionnaire driven interview to promote an individualized plan for risk reduction. The questionnaire, recommendations, and HIV testing information were entered into the program’s electronic record system.
Before October 2008, testing used conventional HIV ELISA, with confirmatory Western blot. In 2008, testing switched to rapid assay (OraQuick ADVANCE® Rapid HIV-1/2 Antibody Test using oral fluid). If reactive, the patient underwent repeat rapid assay but with a whole blood sample and also had blood drawn for confirmatory testing by Western blot. Negative results were reported to patients by telephone (conventional assay) or in person (rapid assay); positive results were provided in person.
Selection and Recruitment for HIV Screening
Universal Arm
Counselors approached every patient not known to meet exclusion criteria. Counselors could encourage participation by discussing the importance of testing generally, but did not use individualized risk information to motivate testing.
Targeted Arm
Counselors reviewed triage notations and electronic medical records to target patients, or acted on staff referral. Indications for targeting contained over fifty items (supplemental electronic appendix), including: 1) clinician identified signs and symptoms of HIV, and 2) clinician or counselor identified risk behaviors, homelessness, mental illness, STD exposure or infection, violence, substance use, pregnancy, and incarceration. Counselors did not systematically assess patients for every possible risk, but were well familiar with criteria and approached patients whenever at least one risk was apparent. Patients for whom no risk was readily apparent were asked directly if they had 1) ever injected drugs, exchanged sex for drugs or money, had sex with a man (if male), or had sex with a partner with or at-risk for HIV, or 2) in the past two years used cocaine or methamphetamine, had sex while using drugs or alcohol, been diagnosed with a STD, or had more than one sex partner. Counselors could use risk information to encourage testing.
In all cases, counselors recorded the reasons prompting the test offer. Inability to complete the testing offer was counted as a failed approach, separate from declined offers. Counselors were necessarily unblinded, though separation of study arms was enforced through training, oversight, and color-coding of study forms. Patients may have been aware of indications for testing but not that these varied systematically.
Recruitment for Seroprevalence Estimation
Patient-Based Seroprevalence
Study personnel consecutively approached every eligible patient, to invite participation in a “study of diseases of public health importance”. Patients received $10 for a blood sample and $5 a health history. The consent process emphasized that data would be stripped of all identifiers before any analysis, and disclosed HIV as one disease among others for which samples might be tested.
Remnant-Based Seroprevalence
Discarded blood samples were obtained from the hospital laboratory for ED patients one week after receiving care during one of seventeen 24-hour periods. Periods were purposively selected to provide data for one or two days each month and all days of the week. Research consent requirements were waived by the IRB.
Data collection
HIV counseling and testing data were extracted from screening program records. For the patient-based seroprevalence study, health questionnaires included information about HIV risk integrated within a broad health history. For the remnant-based seroprevalence estimate, trained abstractors conducted a structured chart review to collect analogous information. HIV status for both seroprevalence components was determined by a sequential method. If a clinical HIV test was performed and negative on or after the date of enrollment, the seroprevalence sample was presumed antibody negative without assay. Remaining samples were assayed in pools of 100 µL serum samples from ten subjects, created from constituent pools of five.64–66 Pools were tested for HIV antibodies by standard immunoassay (Abbott HIVAB™ HIV-1/HIV-2 (rDNA) EIA) and OraQuick ADVANCE® Rapid HIV-1/2 Antibody Test. Individual positive samples were tested a second time and then confirmed by standard Western blot.
Outcomes and Follow-up
The primary outcome was the proportion of new HIV diagnoses in the targeted and universal study arm. A positive HIV test was assumed to be a new diagnosis when there was no indication of prior HIV diagnosis from other sources such as the patient, medical record, other treatment providers, or health department.
Secondary outcomes included: proportion of eligible and approachable ED patients who were tested and who were newly diagnosed; proportion offered testing who consented; risk-profile of tested patients; proportion tested who were notified; the number of positive patients linked to care; reasons for declining testing; and initial CD4 count of newly diagnosed patients. Seroprevalence estimates are provided to contextualize the screening comparison.
Statistical Analysis
Comparison between Screening Arms
The proportion positive, or testing yield, was compared by estimating the difference between proportions and the 95% CI of the differences. Secondary comparisons between groups used the Chi-square test, Fisher’s Exact test, or the Mann Whitney U test. All statistical analyses were conducted using SPSS 20.0 (IBM Corporation, Armonk, NY). We did not adjust for clustering in our analysis; there was no reason to expect that patients within a cluster were more similar than patients in different clusters.
Individual patients often visit an ED more than once.67 We used encounters rather than patients as our primary unit of analysis.68 Because we have previously shown some differences in HIV screening program outcomes depending on unit of analysis,69 we conducted a secondary analysis to determine whether using only the first or the last test for patients with multiple encounters would influence the results.
Seroprevalence Estimation
The patient-based and remnant-based seroprevalence studies were combined for analysis, although proportion positive was also calculated for each separately. Only the final enrollment was included in cases of duplicate enrollment. Patients were excluded from analysis if already diagnosed with HIV, either by self-report or medical record, or if HIV status could not be determined (e.g. sample insufficient).
Sample size Considerations
The positivity rate using targeted screening was expected to be approximately 1%. We selected 0.5% as the clinically important absolute difference in positivity that would be sufficient to influence program planning and design. A sample size of 2000 completed tests in each group was required for 80% power to detect a two-sided 95% CI for the difference in proportion positive to be ±0.5%. The study was stopped before reaching the enrollment goal because of exhausted resources.
RESULTS
Study Sample
Comparison of Patient Selection Methods
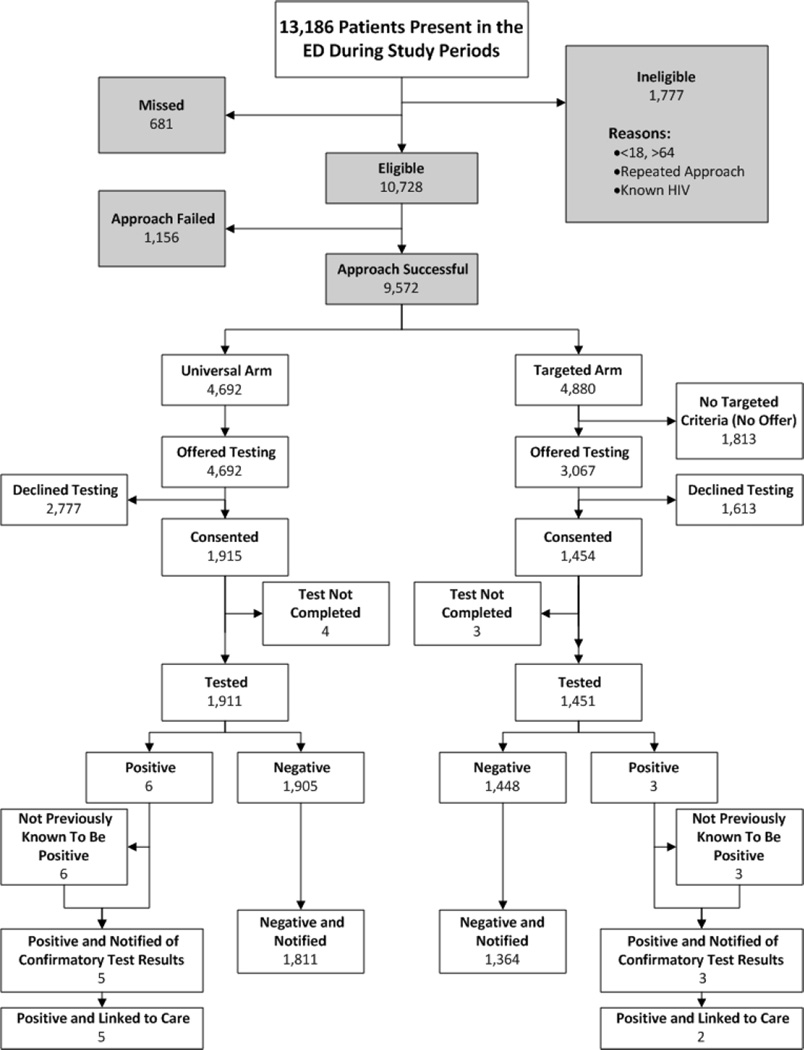
Of the 666 study periods specified by randomization, 600 were completed. In 58 cases the ED care area was closed or study periods were adjacent; staffing was insufficient in 8 cases. Figure 1 illustrates study flow. Of the 13,186 patient encounters, 681 (5%) were missed by counselors, 1,777 were ineligible, and 1,156 could not be approached due to patient or clinical care reasons, such as impaired cognition or critical illness. Missed patients were evenly distributed between study arms, and 63% were missed because the patient left the ED before counselors had a chance to interact with them. In the universal screening arm, 1,915/ 4,692 (40.8%, CI95 39.4%–42.2%) consented. When targeting, 1,813/4,880 (37.2%, CI95 35.8%–38.5%) had no apparent testing indication. Table 1 lists reasons for targeting the remaining 3,067 who were offered testing. There were 1,454/3,067 targeted patients who consented to testing (47.4%, CI95 45.6%–49.2%).
Figure 1.
Flow of Patients Through the Study
Table 1.
Reason for Offer of Targeted Testing*
| Total | Refused | Consented | ||||
|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | |
| Counselor identified risks | 2,091 | (68.2) | 1,149 | (71.2) | 942 | (64.8) |
| Systematic high-risk screen | 818 | (26.7) | 415 | (25.7) | 403 | (27.7) |
| Not documented | 61 | (2.0) | 37 | (2.3) | 24 | (1.7) |
| Self - request | 60 | (2.0) | 0 | (0.0) | 60 | (4.1) |
| Clinical staff identified risks | 23 | (0.7) | 9 | (0.6) | 14 | (1.0) |
| Clinical staff identified symptoms | 14 | (0.5) | 3 | (0.2) | 11 | (0.8) |
The reason for offering testing in the universal arm was, by definition, patient presence in the ED.
Seroprevalence Estimate
For the patient-based seroprevalence estimate, 1,934 were eligible for enrollment. The initial approach could not be completed for 334 (17.3%), 112 (5.8%) were missed, and 454 (23.5%) declined. Of the 1,034 who consented, 24 (2.3%) were already diagnosed with HIV and 37 (3.3%) would have been duplicate enrollments. There was insufficient sample to determine serostatus in 45 (4.6%) who did not have a subsequent negative test documented in the medical record. Two were inadvertently assigned the same sample identification number and were excluded. For the sample-based seroprevalence estimate, there were 1,083 samples collected for patients aged 18 to 64 years. Of these, 22 (2.0%) were previously diagnosed with HIV, 29 (2.7%) were duplicate enrollments, and 10 (0.9%) had insufficient sample and no subsequent record of negative HIV test result in the clinical record.
Primary Outcome
Among the 1,911 patients tested on a universal basis, 6 were newly diagnosed (0.31%, CI95 0.13%–0.65%). For the 1,451 tested on a targeted basis, 3 were newly diagnosed (0.22%, CI95 0.06%–0.55%). In the corresponding combined seroprevalence study, 7/1,948 (0.36%, CI95 0.16%–0.70%) were found to be HIV antibody positive and not previously known to be diagnosed with HIV (4/926 in the patient-based component and 3/1,022 in the remnant-based component).
Secondary Outcomes
Characteristics of Tested Patients (Table 2)
Table 2.
Patient Characteristics Stratified by Study Arm
|
Targeted N=1,451 |
Universal N=1,911 |
Seroprevalence* N=1,948 |
|||||
|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | ||
| Age | 18–29 | 559 | (38.5) | 685 | (35.8) | 497 | (25.5) |
| 30–39 | 300 | (20.7) | 384 | (20.1) | 360 | (18.5) | |
| 40–49 | 339 | (23.4) | 444 | (23.2) | 447 | (22.9) | |
| 50–64 | 256 | (17.6) | 402 | (21.0) | 608 | (31.2) | |
| Not documented | -- | -- | -- | -- | 36 | (1.8) | |
| Sex^ | Male | 736 | (50.7) | 951 | (49.8) | 955 | (49.9) |
| Female | 713 | (49.1) | 955 | (50.0) | 963 | (49.4) | |
| Not documented | 4 | (0.3) | 9 | (0.5) | 30 | (1.5) | |
| Transgendered | 1 | (0.1) | 0 | (0.0) | 0 | (0.0) | |
| Race | White | 530 | (36.5) | 716 | (37.5) | 820 | (42.1) |
| Black | 886 | (61.1) | 1131 | (59.2) | 990 | (50.8) | |
| Hispanic | 23 | (1.6) | 46 | (2.4) | 0 | (0.0) | |
| Other/Not documented | 15 | (1.0) | 22 | (1.2) | 138 | (7.1) | |
| Last HIV test | Never | 362 | (24.9) | 548 | (28.7) | 199 | (10.2) |
| Last year | 364 | (25.1) | 446 | (23.3) | 323 | (16.6) | |
| Over one year ago | 676 | (46.6) | 868 | (45.4) | 397 | (20.4) | |
| Not documented | 52 | (3.6) | 53 | (2.8) | 1029 | (52.8) | |
| Multiple partners, not always using condoms# | 618 | (42.6) | 647 | (33.9) | 243 | (12.5) | |
| Person ever | |||||||
| having vaginal sex | 1,253 | (86.4) | 1,622 | (84.9) | 899 | (46.1) | |
| having any anal sex | 323 | (22.3) | 351 | (18.4) | 175 | (9.0) | |
| receiving anal sex | 167 | (11.5) | 193 | (10.1) | 108 | (5.5) | |
| giving anal sex | 188 | (13.0) | 187 | (9.8) | 83 | (4.3) | |
| having sex with an at-risk partner | 509 | (35.1) | 544 | (28.5) | 291 | (14.9) | |
| man having sex with men | 49 | (6.7) | 50 | (2.6) | 20 | (2.1) | |
| having sex with a prisoner | 377 | (26.0) | 397 | (20.8) | 201 | (10.3) | |
| having sex with an iv drug user | 137 | (9.4) | 146 | (7.6) | 72 | (3.7) | |
| having sex with a std positive partner | 89 | (6.1) | 90 | (4.7) | 70 | (3.6) | |
| having sex with an HIV positive partner | 20 | (1.4) | 16 | (0.8) | 2 | (0.1) | |
| having sex under the influence | 841 | (57.8) | 953 | (49.9) | 558 | (28.6) | |
| trading drugs or money for sex | 146 | (10.1) | 147 | (7.7) | 77 | (4.0) | |
| had a prior std | 741 | (51.1) | 852 | (44.6) | 446 | (22.9) | |
| used injection drugs | 131 | (9.0) | 134 | (7.0) | 121 | (6.2) | |
| ever used crack& | 311 | (21.4) | 287 | (15.0) | 329 | (16.9) | |
Missing data was coded as “none reported”. Approximately 8% of risk data (sexual and other risk) for the targeted and universal arms was missing and in the seroprevalence arm approximately 1% of the risk data was missing.
Combined seroprevalence estimate (prospective consecutive enrollment + remnant sample collection)
Sex as perceived by patient
Within the past year
defined as non-injected cocaine use in seroprevolence study
Screening groups were of similar demographics and self-reported prior testing history. Patients in the targeted arm self-reported risk behavior with greater frequency. CD4 was available for 5 patients of the 9 new diagnoses identified by screening (154, 256, 493, 512, and 882 cells/mm3); four of these were obtained within six months of diagnosis.
Consent and Reasons for Declining Testing (Table 3)
Table 3.
Reasons for Declining Offered Testing by Study Arm
| Targeted | Universal | ||||
|---|---|---|---|---|---|
| N | (%) | N | (%) | ||
| Confidentiality | |||||
| Prefers anonymous testing | 8 | 0.5 | 21 | (0.8) | |
| Setting | |||||
| Doesn't want to interview/counseling | 414 | (25.7) | 697 | (25.1) | |
| Doesn't want to wait for ED testing | 67 | (4.2) | 115 | (4.1) | |
| Problems with followup notification* | 0 | (0.0) | 1 | (0.0) | |
| Doesn't want venipuncture* | 15 | (0.9) | 20 | (0.7) | |
| Wants rapid testing* | 11 | (0.7) | 8 | (0.3) | |
| Privacy of environment insufficient | 3 | (0.2) | 5 | (0.2) | |
| Prior testing | |||||
| Negative test < 3 months prior | 528 | (32.7) | 758 | (27.3) | |
| Negative test > 3 months prior | 382 | (23.7) | 536 | (19.3) | |
| Test in last month & results pending | 17 | (1.1) | 28 | (1.0) | |
| Blood donor < 3 months prior | 10 | (0.6) | 16 | (0.6) | |
| Blood donor > 3 months prior | 2 | (0.1) | 6 | (0.2) | |
| Deny risk& | |||||
| abstains from sex | 120 | (7.4) | 278 | (10.0) | |
| abstains from IDU | 60 | (3.7) | 115 | (4.1) | |
| Monogamous | 235 | (14.6) | 491 | (17.7) | |
| uses barrier methods | 17 | (1.1) | 28 | (1.0) | |
| uses clean needles | 2 | (0.1) | 2 | (0.1) | |
| Other reasons | |||||
| Too much pain/nausea/illness | 255 | (15.8) | 432 | (15.6) | |
| Alternative test planned | 44 | (2.7) | 58 | (2.1) | |
| Afraid/doesn’t want to know results | 28 | (1.7) | 37 | (1.3) | |
| HIV positive | |||||
| Already diagnosed HIV positive | 7 | (0.4) | 16 | 0.6) | |
Patients could indicate more than one reason for declining testing. Reasons were coded prospectively according to these a priori categories..
Relevant for early portion of testing program operating with conventional, non-rapid, testing
Reasons why the patient thought themselves not to be at risk
More patients consented when approached on a targeted basis than a universal basis (1,454/3,067 (47.4%) v. 1,915/4,692 (40.8%); p<0.002). However, the proportion of all ED patients who were approached and tested was greater for the universal than the targeted arm (1,911/4,692 (40.7%) v. 1,454/3,067 (29.7%); p<0.002). When compared with patients who declined testing in the universal arm, patients who declined testing in the targeted arm more often reported their reason as prior negative testing (59.8% v. 48.9%; p<0.001) and less often that they were not at risk (23.1% v. 28.8%; p<0.001).
Repeat Testing
Of the 3,369 HIV tests conducted, 142 (4%) were for patients that had been previously tested in the study (83 universal; 59 targeted). The median duration of time between repeat tests was 267 days (range 7–1,024 days). Overall, 3,107 (96%) were tested once, 103 (3%) were tested twice, and 17 (0.5%) were tested 3 or more times. Sensitivity analysis including only the first or the last encounter had no effect on results.
DISCUSSION
The CDC’s rationale for recommending universal screening is strong,1 but implementation remains a challenge. We tested the hypothesis that a broadly permissive and fully implemented targeted screening strategy would require considerably fewer tests than a universal approach yet achieve a similar number of new diagnoses. We found that this targeted screening strategy was unlikely to be different from universal screening in any clinically significant way. Reduction in testing volume when targeting was matched proportionally by a reduction in the detection of new cases.
Our study offers two primary advances. First, we applied sufficient resources to achieve comprehensive implementation. Prior studies have not delineated whether targeting was inadequately sensitive or inadequately applied. Second, we used a broad set of targeting criteria, whereby any indication more specific than simple presence in the ED led to an offer. Combined, these factors 1) gave targeting the best chance of detecting as many cases as universal screening, while still avoiding testing where no indication of any risk was apparent, and 2) mimicked our existing screening program, which tries to balance the need for manageable testing volumes with the need to detect all cases.
We propose several explanations for the unexpected similarity between targeted and universal screening in our study. Although our environment has low HIV prevalence, risk for HIV was endemic throughout both study arms. We also posit that the lower risk population expected in the universal arm may have declined testing. In addition, targeting criteria were so broad that when fully implemented, the targeted group was not very different from the universal group. Of note, partial implementation of the same targeting criteria by our clinical screening program appears much more selective. The positivity rate of the clinical program was close to 1% both immediately before and immediately after this study (data not shown). This suggests counselors naturally select higher risk patients when resources are insufficient to test all those with some identified risk, even when eligibility criteria are the same. If true, this finding highlights the potential for differences between efficacy and effectiveness studies.
Consent rate was higher in the targeted arm than the universal arm, despite the use of signed, opt-in consent in both arms. This is most likely because targeted patients had higher behavioral risk,70,71 but it is possible that a lesser emphasis on the need for testing in the universal arm contributed to the lower consent rate. We think this is unlikely, counselors were encouraged to emphasize the need for testing in both arms, and using risk information to motivate testing has not been highly efficacious.72,73
The finding that consent varies by patient selection strategy demonstrates the importance of controlled comparisons. Outcomes from studies that alter or fail to control for such operational features will be difficult to interpret. Not only do multiple variables influence program outcomes, but these variables interact with one another in unknown ways. Interactions between different aspects of a screening likely extend beyond consent and patient selection to include myriad factors such as assay method, which staff offer testing, and when in the course of care testing is offered.
Recommendations to expand HIV testing have encouraged changes in methods for both patient selection and consent. Our study provides some insight into the relative impact and interaction of those factors. The proportion who declined universal screening (59%) was greater than the proportion that were not targeted (37%). Still, non-targeted approaches may result in greater increases in testing than changes in consent strategy. The number without perceived risk would be much greater than in our study if using only conventional behavioral risk factors, as is common. Also, improvements in consent are likely to be suboptimal in most settings. Nonetheless, research to improve consent rates should remain a high priority, particularly given the widely ranging rates observed with “opt-out” strategies (e.g. 24%–99.7%).17,59 Our data also indicate that strategies to increase consent are particularly important for non-targeted selection, since consent was less frequent in the universal arm.
Consent may be even more important when going beyond the number tested to consider the number of new infections detected. Extrapolation from our seroprevalence estimate of undiagnosed HIV (0.4%) would suggest 17 total cases among those eligible and approachable in the universal arm. Only six were identified by screening. Moreover, this extrapolation assumes an identical proportion with undiagnosed HIV among those who were and were not tested, while others have suggested positivity rates to be greater among those who decline testing than among those who consent.74,75
Our findings inform but do not resolve the tension between universal and targeted patient selection strategies. Methods to optimize the trade-off between missed cases and fewer tests when resources are insufficient remain largely unexplored. Recent reports have suggested the Denver HIV Risk Score to be a promising instrument to enhance targeting.76,77 This and other targeting approaches, including our baseline clinical program, should be compared individually with universal screening.
Our study should be considered in light of several limitations. Our results may not be generalizable to centers with different epidemiology; EDs differ in terms of disease prevalence even in the same region,78,79 and EDs are certain to differ from other healthcare venues.10,19 Our study was strengthened by controlling overall screening methodology to isolate differences resulting from patient selection strategy. Nonetheless, overall results were necessarily influenced by our screening model, which is only one of many.18,80 A tiny proportion of those tested in the targeted screening arm were actually referred for diagnostic testing18 (i.e. testing for signs and symptoms of HIV). The number of screened patients who may also have met criteria for diagnostic testing is unknown. Our targeting criteria were not used as an instrument to systematically assess risk. Our analysis does not consider the relative costs of the two patient selection strategies. Prospective seroprevalence sampling was biased by the consent requirement, but included patients who would not have had blood remnants available and used survey for health history. The remnant study was not biased by consent, but only included individuals with adequate remnant samples, and health history was obtained by chart review. The study was stopped before planned sample size was reached, although it is inconceivable that further testing could have led to a difference in proportion positive greater than ±0.5%.
When using opt-in consent in low prevalence areas, universal HIV screening results in more testing with a similar proportion positive compared to a fully implemented targeting strategy using broad eligibility. More specific criteria would be needed whenever a higher positivity rate or reduced testing volumes are desired. Increasing consent rate may be as important as patient selection strategy to increase testing and is particularly necessary for non-targeted approaches.
Supplementary Material
Acknowledgement
We would like to thank Josette Robinson-Eaton, Research Assistant and Virology Laboratory Manager of the University of Cincinnati Retrovirology Reference Laboratory for her assistance in processing the samples for the seroprevalence study component. We also express our appreciation to the patients who participated as subjects in the study.
Financial Disclosures: Dr. Lyons received investigator-initiated research funding from Gilead Sciences, Inc.
Funding/Support and Role of the Sponsor: The counseling and testing program described in this report was supported by the Ohio Department of Health via the Cincinnati health Department and also by Ryan White funding provided by the Cincinnati Health Network. The research component was supported in part by NIAID K23 AI068453, in part by an investigator-initiated research award from Gilead Sciences, Inc., and in part by an Institutional Clinical and Translational Science Award, NIH/NCRR Grant Number 5UL1RR026314-03. These funding organizations had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented: CROI, Atlanta, GA, March 5, 2013
Conflicts of Interest
There are no other potential conflicts of interest to disclose.
Author Contributions: Dr. Lyons had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Lyons, Lindsell, Ruffner, Trott, Fichtenbaum.
Acquisition of data: Ruffner, Wayne, Sperling.
Analysis and interpretation of data: Lyons, Hart, Lindsell, Trott, Fichtenbaum.
Drafting of the manuscript: Lyons, Lindsell, Hart, Fichtenbaum.
Critical revision of the manuscript for important intellectual content: Lyons, Lindsell, Ruffner, Wayne, Hart, Sperling, Trott, Fichtenbaum.
Statistical analysis: Hart, Lindsell.
Obtained funding: Lyons, Lindsell, Trott, Fichtenbaum.
Administrative, technical, or material support: Ruffner, Wayne, Sperling, Hart.
Study supervision: Lyons, Lindsell, Trott, Fichtenbaum.
REFERENCES
- 1.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006 Sep 22;55:1–17. [PubMed] [Google Scholar]
- 2.Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. Aids. 2006 Jun 26;20(10):1447–1450. doi: 10.1097/01.aids.0000233579.79714.8d. [DOI] [PubMed] [Google Scholar]
- 3.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005 Aug 1;39(4):446–453. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 4.Dieffenbach CW, Fauci AS. Universal voluntary testing and treatment for prevention of HIV transmission. Jama. 2009 Jun 10;301(22):2380–2382. doi: 10.1001/jama.2009.828. [DOI] [PubMed] [Google Scholar]
- 5.Folkers GK, Fauci AS. Controlling and ultimately ending the HIV/AIDS pandemic: a feasible goal. Jama. Jul 21;304(3):350–351. doi: 10.1001/jama.2010.957. [DOI] [PubMed] [Google Scholar]
- 6.Fleishman JA, Yehia BR, Moore RD, Gebo KA. The economic burden of late entry into medical care for patients with HIV infection. Med Care. 2010 Dec;48(12):1071–1079. doi: 10.1097/MLR.0b013e3181f81c4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Missed opportunities for earlier diagnosis of HIV infection--South Carolina, 1997–2005. MMWR Morb Mortal Wkly Rep. 2006 Dec 1;55(47):1269–1272. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Rapid HIV testing in emergency departments--three U.S. sites, January 2005-March 2006. MMWR Morb Mortal Wkly Rep. 2007 Jun 22;56(24):597–601. [PubMed] [Google Scholar]
- 10.Centers for Disease Control Prevention. Results of the Expanded HIV Testing Initiative --- 25 Jurisdictions, United States, 2007--2010. MMWR Morb Mortal Wkly Rep. 2011 Jun 24;60(24):805–810. [PubMed] [Google Scholar]
- 11.Haukoos JS, Lyons MS. Idealized models or incremental program evaluation: translating emergency department HIV testing into practice. Acad Emerg Med. 2009 Nov;16(11):1044–1048. doi: 10.1111/j.1553-2712.2009.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothman RE, Lyons MS, Haukoos JS. Uncovering HIV infection in the emergency department: a broader perspective. Acad Emerg Med. 2007 Jul;14(7):653–657. doi: 10.1197/j.aem.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Rothman RE, Lyons MS. HIV screening in emergency departments. Introduction. Ann Emerg Med. 2011 Jul;58(1) Suppl 1 doi: 10.1016/j.annemergmed.2011.03.049. S1. [DOI] [PubMed] [Google Scholar]
- 14.Torres GW, Heffelfinger JD, Pollack HA, Barrera SG, Rothman RE. HIV Screening Programs in US Emergency Departments: A Cross-Site Comparison of Structure, Process, and Outcomes. Ann Emerg Med. 2011 Jul;58(Suppl 1):S104–S113. doi: 10.1016/j.annemergmed.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 15.Kecojevic A, Lindsell CJ, Lyons MS, et al. Public Health and Clinical Impact of Increasing Emergency Department-Based HIV Testing: Perspectives From the 2007 Conference of the National Emergency Department HIV Testing Consortium. Ann Emerg Med. 2011 Jul;58(Suppl 1):S151–S159. doi: 10.1016/j.annemergmed.2011.03.040. e151. [DOI] [PubMed] [Google Scholar]
- 16.Holtgrave DR. Assessing the Population-Level Prevention Effect of Emergency Department-Based HIV Testing in the United States: A Research Framework and Commentary. Ann Emerg Med. 2011 Jul;58(Suppl 1):S164–167. doi: 10.1016/j.annemergmed.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 17.Haukoos JS, Hopkins E, Conroy AA, et al. Routine Opt-Out Rapid HIV Screening and Detection of HIV Infection in Emergency Department Patients. Jama. 2010 Jul 21;304(3):284–292. doi: 10.1001/jama.2010.953. [DOI] [PubMed] [Google Scholar]
- 18.Lyons MS, Lindsell CJ, Haukoos JS, et al. Nomenclature and definitions for emergency department human immunodeficiency virus (HIV) testing: report from the 2007 conference of the National Emergency Department HIV Testing Consortium. Acad Emerg Med. 2009 Feb;16(2):168–177. doi: 10.1111/j.1553-2712.2008.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyons MS, Lindsell CJ, Hawkins DA, Raab DL, Trott AT, Fichtenbaum CJ. Contributions to early HIV diagnosis among patients linked to care vary by testing venue. BMC Public Health. 2008;8:220. doi: 10.1186/1471-2458-8-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyss SB, Branson BM, Kroc KA, Couture EF, Newman DR, Weinstein RA. Detecting Unsuspected HIV Infection With a Rapid Whole-Blood HIV Test in an Urban Emergency Department. J Acquir Immune Defic Syndr. 2007 Apr 1;44(4):435–442. doi: 10.1097/QAI.0b013e31802f83d0. [DOI] [PubMed] [Google Scholar]
- 21.Kellermann AL. Crisis in the emergency department. N Engl J Med. 2006 Sep 28;355(13):1300–1303. doi: 10.1056/NEJMp068194. [DOI] [PubMed] [Google Scholar]
- 22.Kellermann AL. Defining the future of emergency care. Annals of emergency medicine. 2006 Aug;48(2):135–137. doi: 10.1016/j.annemergmed.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Institue of Medicine. Hospital-Based Emergency Care: At the Breaking Point. Washington, DC: Institute of Medicine; Jun 13, 2006. 2006. [Google Scholar]
- 24.Lyons MS, Lindsell CJ, Fichtenbaum CJ, Camargo CA., Jr. Interpreting and implementing the 2006 CDC recommendations for HIV testing in health-care settings. Public Health Rep. 2007 Sep-Oct;122(5):579–583. doi: 10.1177/003335490712200504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irvin Flagel, Fox The Emergency Department is Not the Ideal Place for Routine HIV Testing. Ann Emerg Med. 2007;49(5):722–722. doi: 10.1016/j.annemergmed.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Kelen GD. Public Health Initiatives in the Emergency Department: Not So Good for the Public Health? Academic Emergency Medicine. 2008;15(2):194–197. doi: 10.1111/j.1553-2712.2008.00068.x. [DOI] [PubMed] [Google Scholar]
- 27.Rhodes KV, Gordon JA, Lowe RA. Preventive care in the emergency department, Part I: Clinical preventive services--are they relevant to emergency medicine? Society for Academic Emergency Medicine Public Health and Education Task Force Preventive Services Work Group. Acad Emerg Med. 2000 Sep;7(9):1036–1041. doi: 10.1111/j.1553-2712.2000.tb02097.x. [DOI] [PubMed] [Google Scholar]
- 28.Clancy CM, Eisenberg JM. Emergency medicine in population-based systems of care. Ann Emerg Med. 1997 Dec;30(6):800–803. doi: 10.1016/s0196-0644(97)70052-0. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez RM, Kreider WJ, Baraff LJ. Need and desire for preventive care measures in emergency department patients. Ann Emerg Med. 1995 Nov;26(5):615–620. doi: 10.1016/s0196-0644(95)70014-5. [DOI] [PubMed] [Google Scholar]
- 30.Pollock DA, Lowery DW, O'Brien PM. Emergency medicine and public health: new steps in old directions. Ann Emerg Med. 2001 Dec;38(6):675–683. doi: 10.1067/mem.2001.119457. [DOI] [PubMed] [Google Scholar]
- 31.Rothman RE, Bloomfield PJ, Kelen GD. Emergency Department HIV Testing: Sounds Good, but…?: Response. Acad Emerg Med. 2003 Dec 1;10(12):1416–1417. doi: 10.1111/j.1553-2712.2003.tb00023.x. 2003. [DOI] [PubMed] [Google Scholar]
- 32.Peckler B. Emergency Department HIV Testing: Sounds Good, but…? Acad Emerg Med. 2003 Dec 1;10(12) doi: 10.1111/j.1553-2712.2003.tb00023.x. 2003 1415-a-1416. [DOI] [PubMed] [Google Scholar]
- 33.Brown J, Shesser R, Simon G. Establishing an ED HIV Screening Program: Lessons from the Front Lines. Academic Emergency Medicine. 2007;14(7):658–661. doi: 10.1197/j.aem.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 34.Mumma BE, Suffoletto BP. Less Encouraging Lessons From the Front Lines: Barriers to Implementation of an Emergency Department-Based HIV Screening Program. Ann Emerg Med. 2011 Jul;58(Suppl 1):S44–S48. doi: 10.1016/j.annemergmed.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Haukoos JS. The Impact of Nontargeted HIV Screening in Emergency Departments and the Ongoing Need for Targeted Strategies: Comment on "Modest Public Health Impact of Nontargeted Human Immunodeficiency Virus Screening in 29 Emergency Departments". Arch Intern Med. 2011 Oct 24; doi: 10.1001/archinternmed.2011.538. [DOI] [PubMed] [Google Scholar]
- 36.Cunningham CO, Doran B, DeLuca J, Dyksterhouse R, Asgary R, Sacajiu G. Routine opt-out HIV testing in an urban community health center. AIDS Patient Care STDS. 2009 Aug;23(8):619–623. doi: 10.1089/apc.2009.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minniear TD, Gilmore B, Arnold SR, Flynn PM, Knapp KM, Gaur AH. Implementation of and barriers to routine HIV screening for adolescents. Pediatrics. 2009 Oct;124(4):1076–1084. doi: 10.1542/peds.2009-0237. [DOI] [PubMed] [Google Scholar]
- 38.Brown J, Shesser R, Simon G, et al. Routine HIV screening in the emergency department using the new US Centers for Disease Control and Prevention Guidelines: results from a high-prevalence area. J Acquir Immune Defic Syndr. 2007 Dec 1;46(4):395–401. doi: 10.1097/qai.0b013e3181582d82. [DOI] [PubMed] [Google Scholar]
- 39.d’Almeida KW, Kierzek G, de Truchis P, et al. Modest Public Health Impact of Nontargeted Human Immunodeficiency Virus Screening in 29 Emergency Departments. Arch Intern Med. 2011 Oct 24; doi: 10.1001/archinternmed.2011.535. [DOI] [PubMed] [Google Scholar]
- 40.Holtgrave DR. Costs and Consequences of the US Centers for Disease Control and Prevention's Recommendations for Opt-Out HIV Testing. PLoS Med. 2007 Jun 01;4(6):e194. doi: 10.1371/journal.pmed.0040194. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moran GJ, Talan DA. Processes and Models for HIV Screening in the Emergency Department: Can and Should We Do This? Ann Emerg Med. 2011;58(1):S172–S173. doi: 10.1016/j.annemergmed.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 42.Christopoulos KA, Kaplan B, Dowdy D, et al. Testing and linkage to care outcomes for a clinician-initiated rapid HIV testing program in an urban emergency department. AIDS patient care and STDs. 2011 Jul;25(7):439–444. doi: 10.1089/apc.2011.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kennedy LA, Gordin FM, Kan VL. Assessing targeted screening and low rates of HIV testing. Am J Public Health. 2010 Sep;100(9):1765–1768. doi: 10.2105/AJPH.2009.182790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duffus WA, Weis K, Kettinger L, Stephens T, Albrecht H, Gibson JJ. Risk-based HIV testing in South Carolina health care settings failed to identify the majority of infected individuals. AIDS Patient Care STDS. 2009 May;23(5):339–345. doi: 10.1089/apc.2008.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jenkins TC, Gardner EM, Thrun MW, Cohn DL, Burman WJ. Risk-Based Human Immunodeficiency Virus (HIV) Testing Fails to Detect the Majority of HIV-Infected Persons in Medical Care Settings. Sex Transm Dis. 2006 Mar 16; doi: 10.1097/01.olq.0000194617.91454.3f. [DOI] [PubMed] [Google Scholar]
- 46.Greenwald JL, Hall J, Skolnik PR. Approaching the CDC’s guidelines on the HIV testing of inpatients: physician-referral versus nonreferral-based testing. AIDS Patient Care STDS. 2006 May;20(5):311–317. doi: 10.1089/apc.2006.20.311. [DOI] [PubMed] [Google Scholar]
- 47.Walensky RP, Losina E, Steger-Craven KA, Freedberg KA. Identifying undiagnosed human immunodeficiency virus: the yield of routine, voluntary inpatient testing. Arch Intern Med. 2002 Apr 22;162(8):887–892. doi: 10.1001/archinte.162.8.887. [DOI] [PubMed] [Google Scholar]
- 48.Bartlett JG, Branson BM, Fenton K, Hauschild BC, Miller V, Mayer KH. Opt-out testing for human immunodeficiency virus in the United States: progress and challenges. Jama. 2008 Aug 27;300(8):945–951. doi: 10.1001/jama.300.8.945. [DOI] [PubMed] [Google Scholar]
- 49.Freeman AE, Sattin RW, Miller KM, Dias JK, Wilde JA. Acceptance of rapid HIV screening in a southeastern emergency department. Acad Emerg Med. 2009 Nov;16(11):1156–1164. doi: 10.1111/j.1553-2712.2009.00508.x. [DOI] [PubMed] [Google Scholar]
- 50.Walensky RP, Reichmann WM, Arbelaez C, et al. Counselor- Versus Provider-Based HIV Screening in the Emergency Department: Results From the Universal Screening for HIV Infection in the Emergency Room (USHER) Randomized Controlled Trial. Ann Emerg Med. 2011 Jul;58(Suppl 1):S126–S132. doi: 10.1016/j.annemergmed.2011.03.023. e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lubelchek RJ, Kroc KA, Levine DL, Beavis KG, Roberts RR. Routine, rapid HIV testing of medicine service admissions in the emergency department. Ann Emerg Med. 2011 Jul;58(1) Suppl 1:S65–S70. doi: 10.1016/j.annemergmed.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 52.Centers for Disease Control Prevention. Routine jail-based HIV testing - Rhode Island, 2000–2007. MMWR Morb Mortal Wkly Rep. 2010 Jun 25;59(24):742–745. [PubMed] [Google Scholar]
- 53.Calderon Y, Leider J, Hailpern S, et al. High-volume rapid HIV testing in an urban emergency department. AIDS Patient Care STDS. 2009 Sep;23(9):749–755. doi: 10.1089/apc.2008.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White DA, Scribner AN, Schulden JD, Branson BM, Heffelfinger JD. Results of a rapid HIV screening and diagnostic testing program in an urban emergency department. Ann Emerg Med. 2009 Jul;54(1):56–64. doi: 10.1016/j.annemergmed.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 55.Silva A, Glick NR, Lyss SB, et al. Implementing an HIV and sexually transmitted disease screening program in an emergency department. Ann Emerg Med. 2007 May;49(5):564–572. doi: 10.1016/j.annemergmed.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 56.Wilbur L, Huffman G, Lofton S, Finnell JT. The Use of a Computer Reminder System in an Emergency Department Universal HIV Screening Program. Ann Emerg Med. 2011 Jul;58(Suppl 1):S71–S73. doi: 10.1016/j.annemergmed.2011.03.028. e71. [DOI] [PubMed] [Google Scholar]
- 57.Temkin E, Marsiglia VC, Hague C, Erbelding E. Screening for acute human immunodeficiency virus infection in Baltimore public testing sites. Sex Transm Dis. 2011 May;38(5):374–377. doi: 10.1097/OLQ.0b013e31820279bd. [DOI] [PubMed] [Google Scholar]
- 58.Sattin RW, Wilde JA, Freeman AE, Miller KM, Dias JK. Rapid HIV Testing in a Southeastern Emergency Department Serving a Semiurban-Semirural Adolescent and Adult Population. Ann Emerg Med. 2011;58(1):S60–S64. doi: 10.1016/j.annemergmed.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 59.Hoxhaj S, Davila JA, Modi P, et al. Using Nonrapid HIV Technology for Routine, Opt-out HIV Screening in a High-Volume Urban Emergency Department. Ann Emerg Med. 2011;58(1):S79–S84. doi: 10.1016/j.annemergmed.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 60.Lyons MS, Lindsell CJ, Hawkins DA, et al. Contributions to early HIV diagnosis among patients linked to care vary by testing venue. BMC Public Health. 2008 Jun;8(220) doi: 10.1186/1471-2458-8-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lyons MS, Lindsell CJ, Ledyard HK, et al. Emergency Department HIV Testing and Counseling: An Ongoing Experience in a Low-Prevalence Area. Ann Emerg Med. 2005 Jul;46(1):22–28. doi: 10.1016/j.annemergmed.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 62.Lyons MS, Lindsell CJ, Ledyard HK, et al. Health Department Collaboration with Emergency Departments as a Model for Public Health Programs Among At-risk Populations. Public Health Reports. 2005 May-Jun;120:259–264. doi: 10.1177/003335490512000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohio Department of Health Bureau of HIV/AIDS. [Accessed June 16, 2008];HIV/AIDS Statistics Reporting by County in Ohio through December 31, 2005. 2005 http://www.odh.ohio.gov/healthStats/disease/hivann/hcty1.aspx.
- 64.Ko YC, Lan SJ, Chiang TA, Yen YY, Hsieh CC. Successful use of pooled sera to estimate HIV antibody seroprevalence and eliminate all positive cases. Asia Pac J Public Health. 1992;6(3):146–149. doi: 10.1177/101053959200600305. [DOI] [PubMed] [Google Scholar]
- 65.Monzon OT, Paladin FJ, Dimaandal E, Balis AM, Samson C, Mitchell S. Relevance of antibody content and test format in HIV testing of pooled sera. AIDS. 1992 Jan;6(1):43–48. doi: 10.1097/00002030-199201000-00005. [DOI] [PubMed] [Google Scholar]
- 66.Mehta SR, Nguyen VT, Osorio G, Little S, Smith DM. Evaluation of pooled rapid HIV antibody screening of patients admitted to a San Diego Hospital. J Virol Methods. 2011 Jun;174(1–2):94–98. doi: 10.1016/j.jviromet.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fertel BS, Hart KW, Lindsell CJ, Ryan RJ, Lyons MS. Toward understanding the difference between using patients or encounters in the accounting of emergency department utilization. Ann Emerg Med. 2012 Jun;60(6):693–698. doi: 10.1016/j.annemergmed.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lyons MS, Lindsell CJ, Ruffner AH, Trott AT, Fichtenbaum CJ. Relationship of self-reported prior testing history to undiagnosed HIV positivity and HIV risk. Curr HIV Res. 2009 Nov;7(6):580–588. doi: 10.2174/157016209789973646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lyons MSLC, Raab DL, Ruffner AH, Trott AT, Fichtenbaum CJ. Comparison of emergency department HIV testing data with visit or patient as the unit of analysis. J Med Screen. 2009;16(1):29–32. doi: 10.1258/jms.2009.008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pisculli ML, Reichmann WM, Losina E, et al. Factors associated with refusal of rapid HIV testing in an emergency department. AIDS Behav. 2011 May;15(4):734–742. doi: 10.1007/s10461-010-9837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Merchant RC, Seage GR, Mayer KH, Clark MA, DeGruttola VG, Becker BM. Emergency department patient acceptance of opt-in, universal, rapid HIV screening. Public Health Rep. 2008 Nov-Dec;123(Suppl 3):27–40. doi: 10.1177/00333549081230S305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ubhayakar ND, Lindsell CJ, Raab DL, et al. Risk, reasons for refusal, and impact of counseling on consent among ED patients declining HIV screening. Am J Emerg Med. 2011 May;29(4):367–372. doi: 10.1016/j.ajem.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Merchant RC, Clark MA, Langan TJt, Mayer KH, Seage GR, 3rd, Degruttola VG. Can Computer-Based Feedback Improve Emergency Department Patient Uptake of Rapid HIV Screening? Ann Emerg Med. 2011 Jul;58(Suppl 1):S114–S119. doi: 10.1016/j.annemergmed.2011.03.035. e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Plitt SS, Singh AE, Lee BE, Preiksaitis JK. HIV seroprevalence among women opting out of prenatal HIV screening in Alberta, Canada: 2002–2004. Clin Infect Dis. 2007 Dec 15;45(12):1640–1643. doi: 10.1086/523730. [DOI] [PubMed] [Google Scholar]
- 75.Czarnogorski M, Brown J, Lee V, et al. The Prevalence of Undiagnosed HIV Infection in Those Who Decline HIV Screening in an Urban Emergency Department. AIDS Res Treat. 2011:879065. doi: 10.1155/2011/879065. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haukoos JS, Hopkins E, Bender B, Sasson C, Al-Tayyib AA, Thrun MW. Comparison of Enhanced Targeted Rapid HIV Screening Using the Denver HIV Risk Score to Nontargeted Rapid HIV Screening in the Emergency Department. Ann Emerg Med. 2013 Jan 3; doi: 10.1016/j.annemergmed.2012.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haukoos JS, Lyons MS, Lindsell CJ, et al. Derivation and validation of the Denver Human Immunodeficiency Virus (HIV) risk score for targeted HIV screening. Am J Epidemiol. 2012 Apr 15;175(8):838–846. doi: 10.1093/aje/kwr389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lyons MS, Lindsell CJ, Wayne DB, et al. Comparison of Missed Opportunities for Earlier HIV Diagnosis in 3 Geographically Proximate Emergency Departments. Ann Emerg Med. 2011 Jul;58(Suppl 1):S17–S22. doi: 10.1016/j.annemergmed.2011.03.018. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lindsell CJ, Hart KW, Lyons MS. A Simple Method for Estimating the Prevalence of Undiagnosed HIV Infection in an Emergency Department. Ann Emerg Med. 2011 Jul;58(Suppl 1):S23–S27. doi: 10.1016/j.annemergmed.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haukoos JS, White DA, Lyons MS, et al. Operational Methods of HIV Testing in Emergency Departments: A Systematic Review. Ann Emerg Med. 2011 Jul;58(Suppl 1):S96–S103. doi: 10.1016/j.annemergmed.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.