Abstract
Molecular mechanisms regulating lymphangiogenesis may be exploited as potential treatments for disorders of lymphatic circulation.
The lymphatic system is generally underappreciated until something goes wrong. It controls fluid balance, dietary lipid absorption, and immune surveillance (1). When disrupted in adults, the consequences can be lethal, arising from infl ammation, infection, and fi brosis. Impaired lymphatic vessel development causes fluid and protein accumulation in tissues, resulting in lymphedema, a disfiguring and disabling swelling of the extremities and other parts of the body. Moreover, lymph-edema and chronic inflammation are aggravating factors in cardiovascular disease. Therapeutic agents that can promote lymphatic vessel formation (lymphangiogenesis) should prevent lymphedema and benefi t individuals with cardiovascular pathologies. Both blood-vascular and lymphatic circulatory systems are closely intermingled and share many molecular signaling mechanisms for their development (2). At a recent meeting (3), advances in our understanding of these mechanisms indicate that selectively targeting lymphatic vessels without affecting blood vessels remains a challenge.
The major growth factors that regulate angiogenesis (new blood vessel formation from existing vessels) and lymphangiogenesis include vascular endothelial growth factor A and C (VEGF-A and -C). A deficiency in VEGF-A is lethal in early embryonic mice due to nearly complete failure of blood vessel development. Mice lacking VEGF-C die later in embryogenesis from the absence of lymphatic vessel sprouting. Thus, there are critical, nonoverlapping functions for VEGF-A and -C in the development of both circulatory systems. However, VEGF-C also affects angiogenesis (4), and although it is currently the most promising avenue to treat lymphedema (5), it may also induce blood vessel growth and unwanted side effects in patients. Developing selective therapies to promote lymphangiogenesis requires better knowledge about the context-dependent signaling of VEGF-C in blood and lymphatic vessels.
During embryonic life, lymphatics develop from veins after the onset of circulation. This requires a subset of blood-vascular endothelial cells (BECs) in the jugular vein to acquire lymphatic identity. These lymphatic endothelial cells (LECs) must then migrate out of the cardinal vein and form the jugular lymphatic sacs, which through expansion and sprouting give rise to the entire lymphatic vasculature.
The cellular signaling events determining lymphatic fate specification involve sequential activation of two transcription factors, SOX18 and PROX1, in the cardinal vein. In the absence of Sox18 or Prox1 function in the mouse, venous endothelial cells fail to express LEC-specific “identity” markers, leading to failure of lymphatic programming (6, 7). Prox1 is required throughout life to maintain LEC identity; deletion of the Prox1 gene at any stage of mouse development reverses LEC to BEC identity, and leads to the fusion of LECs with blood vessels (8). How Sox18 expression is limited to a subset of cells in the cardinal vein is unknown. Sox18 and Prox1 are expressed in vein endothelial cells for a defined developmental period, but how this determines the total number of LECs generated is also unknown.
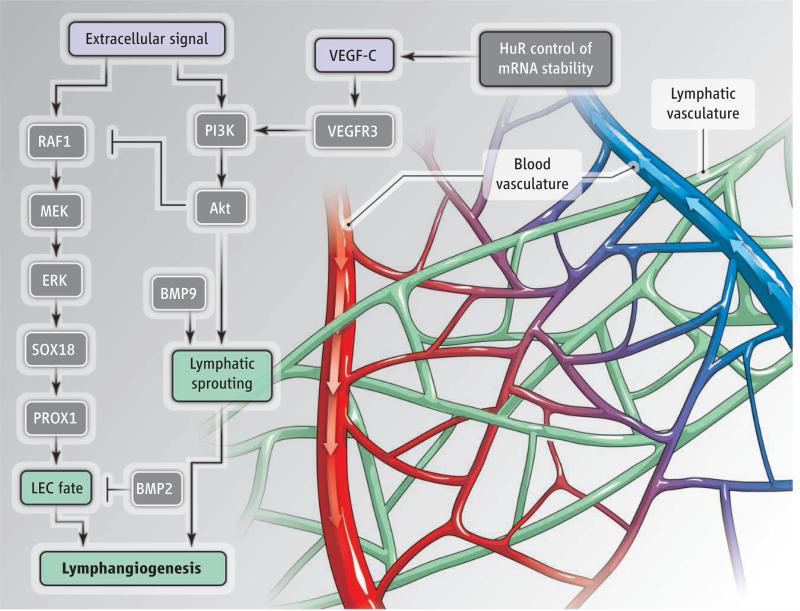
Two key findings provide new insight into how LEC specification is controlled. Signaling by bone morphogenetic protein 2 (BMP2) is required for venous sprouting angiogenesis, without affecting arteries (9). This increase in venous cells may occur at the expense of LEC formation; BMP2 blocks Prox1 expression, which suppresses LEC specification (3). Factors turning off BMP2 signaling may hence represent the elusive inducers of LEC differentiation. Another fi nding is that increased signaling by extracellular signal–regulated kinase (ERK) enhances Sox18 expression and LEC differentiation, leading to sustained LEC specification and outmigration from the cardinal vein. The result is pathologically smaller veins, enlarged lymphatic vessels, and lymphangiectasia (dilation of lymph vessels) (10). Together, these findings suggest that the BEC decision to become lymphatic or remain venous is determined by a balance between different signaling inputs, and reveal at least two variables that control LEC specification–BMP2 and ERK signaling. Thus, the simultaneous suppression of BMP2 and activation of ERK should markedly increase this specification (see the figure).
Lymphatic vessels.
The blood-vascular system and lymphatic system are closely intertwined in tissues. The growth factor VEGF-C promotes both angiogenesis and lymphangiogenesis. The signaling pathways that promote lymphangiogenesis are shown. MEK, mitogen-activated protein kinase kinase; RAF1, rapidly accelerated fibrosarcoma 1.
The process of LEC sprouting is also mysterious. VEGF-C and its cognate receptor VEGFR3 constitute the only signaling system thus far implicated in this process (1, 2). In the absence of functional VEGFR3, LECs become properly specified but cannot sprout from the cardinal vein. However, VEGFR3 also affects angio-genesis (4). It is expressed on both BECs and LECs during early mouse embryonic development, and its expression decreases in BECs after LEC specification while remaining high in LECs. How VEGFR3 signaling acts in several distinct contexts during both blood-vascular and lymphatic development is unknown. In BECs, VEGFR3 regulates sprouting angiogenesis through crosstalk with the signaling pathway controlled by Notch protein, but the role of Notch in LECs is not fully understood (3, 11).
VEGF-C binding induces VEGFR3 dimerization and subsequent receptor auto-phosphorylation of tyrosine residues within its cytoplasmic domain. VEGFR3 activates two signaling pathways—phosphatidylinositol 3-kinase (PI3K)–Akt and ERK—through different phosphorylated tyrosines, which suggests that each output may lead to distinct cellular responses at the different developmental steps (3, 12). However, little is known about the in vivo requirement for either signaling branch. Mice lacking pik3r1, which encodes the regulatory subunit of PI3K, display defects in lymphatic remodeling and maturation, whereas lymphatic vessels show an increased expression of BEC markers such as endoglin (13). It may be that down-regulation of ERK activity (by the enzyme Akt) could serve as a critical control point in LEC sprouting and also contribute to LEC differentiation and the maintenance of lymphatic identity (3).
It is likely that VEGR3 signaling outputs are further modulated by other signaling molecules expressed at the same time. The receptors for BMPs and transforming growth factor–β (TGFβ) emerge as interesting candidates in this context. Inducible, endothelial-specific deletion of TGFβ receptors (TGFβR2 and Alk5) suppresses LEC sprouting at the expense of LEC proliferation by altering the expression of VEGFR3 and another VEGF-C receptor called neuropilin-2 (3). BMP9, which binds with high affi nity to a receptor complex composed of BMPR2 and Alk1, also affects lymphangiogenesis (14, 15). Alk1 inhibition using a “ligand trap” blocked sprouting angiogenesis and decreased lymphangiogenesis (15, 16), which suggests that BMP9 may be a potential target for promoting lymphangiogenesis without stimulating growth of blood vessels. Further studies are required to determine how BMPs and TGFβ affect lymphangiogenesis.
Other important recent discoveries in the field include the RNA binding protein called human antigen R (HuR) in maintaining the stability of mRNA encoding VEGF-C and -A. Depending on the cell type in which HuR is deleted, this results in impairment of lymphangiogenesis or angiogenesis (3). LEC mechanosensing is also emerging as a critical regulator of lymphangiogenesis and lymphatic valve formation, although many details are yet to be filled in (17, 18). And exciting results suggest that cutaneous lymph capillaries are important for systemic blood pressure control by locally modulating skin electrolyte composition (19).
Unlike the blood system, much about the lymphatic system remains elusive. As our understanding of its development and pathology grows, we should begin to finally develop treatments for lymphedema and other disorders of lymphatic circulation— progressive, lifelong conditions that affect millions of people, for whom curative treatments are currently not available.
Acknowledgments
We thank J. Bender, T. Hla, Y. Mukouyama, S.-W. Jin, and W. Sessa for permission to cite findings that were presented at the conference (3).
References and Notes
- 1.Alitalo K. Nat. Med. 2011;17:1371. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 2.Koltowska K, Betterman KL, Harvey NL, Hogan BM. Development. 2013;140:1857. doi: 10.1242/dev.089565. [DOI] [PubMed] [Google Scholar]
- 3.Yale-North American Vascular Biology Organization (NAVBO) Lymphatic Circulation in Health and Disease meeting. New Haven, CT. 2013 May 3 to 4; [Google Scholar]
- 4.Tammela T, et al. Nat. Cell Biol. 2011;13:1202. doi: 10.1038/ncb2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tammela T, et al. Nat. Med. 2007;13:1458. doi: 10.1038/nm1689. [DOI] [PubMed] [Google Scholar]
- 6.Wigle JT, Oliver G. Cell. 1999;98:769. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 7.François M, et al. Nature. 2008;456:643. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- 8.Johnson NC, et al. Genes Dev. 2008;22:3282. doi: 10.1101/gad.1727208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiley DM, et al. Nat. Cell Biol. 2011;13:686. doi: 10.1038/ncb2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng Y, et al. J. Clin. Invest. 2013;123:1202. doi: 10.1172/JCI63034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murtomaki A, et al. Development. 2013;140:2365. doi: 10.1242/dev.083865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Covassin LD, Villefranc JA, Kacergis MC, Weinstein BM, Lawson ND. Proc. Natl. Acad. Sci. U.S.A. 2006;103:6554. doi: 10.1073/pnas.0506886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mouta-Bellum C, et al. Dev. Dyn. 2009;238:2670. doi: 10.1002/dvdy.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levet S, et al. Blood. 2013 10.1182/blood-2012-12-472142. [Google Scholar]
- 15.Niessen K, et al. Blood. 2010;115:1654. doi: 10.1182/blood-2009-07-235655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larrivée B, et al. Dev. Cell. 2012;22:489. doi: 10.1016/j.devcel.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C-Y, et al. J. Clin. Invest. 2012;122:2006. doi: 10.1172/JCI57513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabine A, et al. Dev. Cell. 2012;22:430. doi: 10.1016/j.devcel.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 19.Wiig H, et al. J. Clin. Invest. 2013;123:2803. doi: 10.1172/JCI60113. [DOI] [PMC free article] [PubMed] [Google Scholar]