Abstract
Background
To eliminate the medical risks and logistical challenges of transporting infants from the neonatal intensive care unit (NICU) to the radiology department for magnetic resonance imaging, a small-footprint 1.5-T MRI scanner has been developed for neonatal imaging within the NICU. MRI is known to be noisy, and exposure to excessive acoustic noise has the potential to elicit physiological distress and impact development in the term and preterm infant.
Objective
To measure and compare the acoustic noise properties of the NICU MRI system against those of a conventional 1.5-T MRI system.
Materials and methods
We performed sound pressure level measurements in the NICU MRI scanner and in a conventional adult-size whole-body 1.5-T MRI system. Sound pressure level measurements were made for six standard clinical MR imaging protocols.
Results
The average sound pressure level value, reported in unweighted (dB) and A-weighted (dBA) decibels for all six imaging pulse sequences, was 73.8 dB and 88 dBA for the NICU scanner, and 87 dB and 98.4 dBA for the conventional MRI scanner. The sound pressure level values measured on the NICU scanner for each of the six MR imaging pulse sequences were consistently and significantly (P=0.03) lower, with an average difference of 14.2 dB (range 10–21 dB) and 11 dBA (range 5–18 dBA). The sound pressure level frequency response of the two MR systems showed a similar harmonic structure above 200 Hz for all imaging sequences. The amplitude, however, was appreciably lower for the NICU scanner, by as much as 30 dB, for frequencies below 200 Hz. Conclusion The NICU MRI system is quieter than conventional MRI scanners, improving safety for the neonate and facilitating siting of the unit within the NICU.
Keywords: Magnetic resonance imaging, Neonatal intensive care unit, Acoustic noise, Safety, Neonates
Introduction
Acoustic noise
MRI exams are known to be loud. Sound pressure level (SPL) is typically measured in decibels (dB) or in A-weighted decibels (dBA) that incorporate the frequency response (i.e. transfer function) of the human auditory system. Human sensitivity to sound starts at 0 dB and discomfort or pain occurs when the sound pressure level exceeds 120–140 dB [1–3]. Normal conversation levels range from 50–60 dBA [4]. Sound pressure levels of 81–117 dB are common in clinical 1.5-T MRI exams [5] but can be as high as 131 dB for high-speed acquisitions such as echo planar imaging (EPI) [6]. Because noise exposure can cause hearing loss, acoustic noise is an MRI safety parameter that is regulated by the U.S. Food and Drug Administration. For standard clinical MRI exams, the FDA requires that peak unweighted sound pressure levels do not exceed 140 dB and that A-weighted root mean square SPLs do not exceed 99 dBA with hearing protection in place. Hence, hearing protection is required for MR scanning and is typically accomplished with earplugs, which provide roughly 29- to 32-dB attenuation in noise exposure. In addition, noise-attenuating headphones are available for adults and children older than 3 months, and depending on size, materials and fit, can provide approximately 18–33 dB protection. However, the size and snug fit of pediatric headphones are not appropriate for the small and malleable skulls of newborns. Hence, earmuffs are often used to provide sound attenuation in the neonatal population and can provide up to 7–12 dB protection [3].
Acoustic noise is a critical issue in neonatal MRI because it can elicit autonomic instability in both term and preterm neonates [7]. Excessive sound pressure level is also believed to have a detrimental effect, both direct and indirect, on the growth and neurological development of term and preterm infants [8–10]. Several adverse noise-induced health effects have been identified in the preterm population. These include hearing impairment, sleep disturbance, somatic effects and impaired auditory perception and emotional development [3]. Sleep and normal sleep cycles, which emerge at approximately 28–30 weeks’ gestation, are critical for early sensory development and the creation of the permanent neuronal circuits that support the primary sensory systems, the creation of long-term memories, and learning [10, 11]. From 28 weeks of gestational age to 6 months, infants require large amounts of REM (rapid eye movement) sleep, which is necessary for the development of the tonotopic organization of the auditory nerves and auditory cortex and the tuning of the hair cells of the cochlea, all crucial for frequency discrimination [10]. Hence activity that elevates ambient sound level within the NICU has the potential to disrupt sleep and adversely affect patients. In addition, background sound levels greater than 60 dB interfere with the infant’s ability to discriminate voice, language, music and other meaningful environmental sounds from background noise levels [10]. These considerations have profound implications for those considering siting an MRI unit within the NICU environment.
Although there are no regulatory guidelines defining sound pressure level limits for neonates [5], ad hoc limits are frequently employed in the NICU environment. For example, it is recommended that neonatal noise exposure during inter-institutional transport be held below 60 dB [12]. For the NICU environment, the American Academy of Pediatrics Committee on Environmental Health (1997) recommends a daily noise level of 45 dB. Alternatively, Graven and Browne [9] recommend an Leq (hourly time-averaged equivalent continuous A-weighted sound level) of 50 dBA, an L10 (sound pressure level exceeded for no more than 10% of an hour) of 55 dBA, and an Lmax (the single highest sound pressure level over an hour monitoring period) of 70 dBA. Similarly, the Committee to Establish Recommended Standard for Newborn ICU Design (September 18, 2012) developed guidelines for acoustic noise exposure in the NICU that advocate that the combination of continuous background sound and operational sound in the infant rooms not exceed an Leq of 45 dBA and an hourly L10 of 50 dBA [13]. In addition, to prevent physiological stress Lmax should not exceed 65 dBA [13]. In light of the lack of firm guidelines limiting short-term noise exposure over a single finite time interval (e.g., such as during an MRI procedure), we have chosen a conservative target acoustic noise threshold of 65 dBA (with hearing protection) during neonatal MRI. This threshold is difficult to achieve with conventional adult-size MRI systems.
Similarly, there are no established standards regarding the types of hearing protection used to achieve target sound pressure level exposures for newborns. Passive hearing protection is typically a part of every neonatal noise reduction strategy and has been developed and used primarily to promote sound sleep. Foam earplugs and soft-shell earmuffs are the most commonly used passive noise attenuators for neonatal MRI exams. Because infant-size earplugs are not available, adult earplugs are often used. In practice, earplugs must be cut down to size for the neonate, and even then proper insertion can be difficult. The reduced size and poor fit compromise the noise attenuation. To improve the hearing protection attenuation level, a combination of soft-shell earmuffs (MiniMuffs™, Natus Medical Inc., San Carlos, CA) and earplugs can be used. MiniMuffs™ are made of soft foam secured around the ear with hydrogel and are easily attached and removed [3]. According to the manufacturer, the earmuffs can reduce noise exposure by at least 7 dB (noise reduction rating=7 dB); however some users have reported that the same earmuffs were effective in reducing the noise levels by 7–12 dB [3].
The attenuation provided by the combined use of earplugs and earmuffs is better than either device alone, but it is typically less than the combined ratings of the two methods [14, 15]. This is due in part to mechanical coupling of the plug and the muff to the body tissues and the direct conduction of sound through the skull to the inner ear. For most adults, bone conduction pathways limit the maximum amount of attenuation obtainable with passive hearing protection to 35–50 dB [15]. The degree of bone conduction differs between newborns and adults because of the size and thinness of the infant skull [16]. However the maximum limit of hearing protection using passive means has not been determined for the neonatal population. Thus the only guaranteed way to reduce noise exposure for an infant undergoing MRI is to perform scanning more quietly.
A new neonatal MRI system
A small footprint high-field MRI scanner for neonatal imaging that can be easily installed within the NICU was developed [17, 18]. The scanner is an adaptation of an OPTIMA™ MR430s (GE Healthcare, Waukesha, WI). The MRI system has a maximum patient bore diameter of 21.8 cm (without radiofrequency coil), and roughly twice the gradient performance of conventional adult whole-body systems.
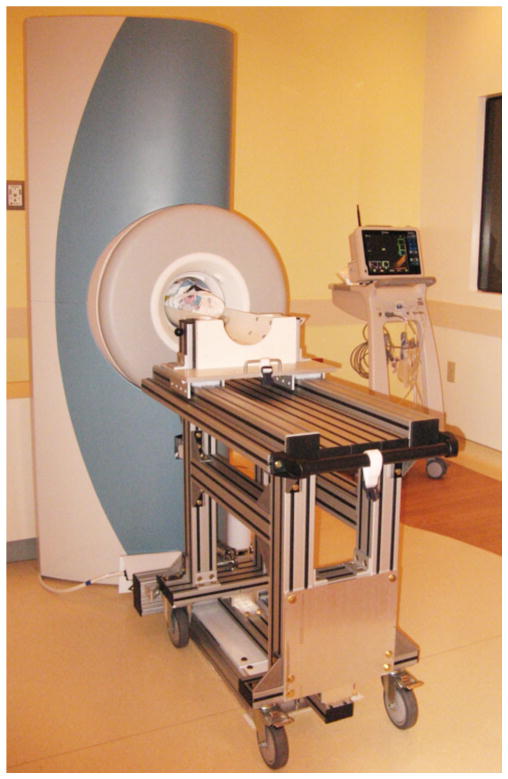
For neonatal imaging, the OPTIMA™ magnet was raised and leveled. The chair used for extremity imaging was replaced with a customized patient table (Fig. 1). In addition, the control electronics and radiofrequency system from a state-of-the-art 1.5-Tesla GE scanner were integrated with the basic OPTIMA™ MRI system. The end result is a small-footprint 1.5-T scanner with imaging capabilities equivalent to those of a high-end adult-size 1.5-T MRI system. The enhanced NICU MRI platform supports the full spectrum of advanced imaging techniques (e.g., MR spectroscopy, diffusion tensor imaging, functional MRI, arterial spin labeling and phase contrast angiography) as well as electrocardiogram and respiratory gating/triggering. The control and scan room of the scanner are off the main corridor of the NICU, immediately adjacent to and across from the patient rooms.
Fig. 1.
Neonatal MRI system installed in the NICU at our institution. NICU neonatal intensive care unit
The purpose of the present study was to compare the noise characteristics of the NICU MRI system with that of a conventional adult-size 1.5-T MR scanner. As a consequence of the smaller spatial dimensions of the gradient coil, for identical operating conditions (e.g., sequence and acquisition parameters), we anticipated that the NICU system would be appreciably quieter than an adult-size 1.5-T MR unit.
Materials and methods
Acoustic noise evaluation of the NICU and adult-size MR systems
We performed sound pressure level measurements on the new NICU MRI scanner and on a conventional adult-size whole-body 1.5-T HDxt GE MRI system (GE Healthcare, Waukesha, WI). A Brüel & Kjær model 2250 sound level meter (Brüel & Kjær Sound & Vibration Measurement A/S, Denmark) was used to perform the sound pressure level measurements for six standard MR scans (spin echo, conventional gradient recalled echo, echo planar imaging, fast radiofrequency spoiled gradient echo, balanced steady state free precession, and diffusion-weighted) using acquisition parameters consistent with clinical neonatal protocols (Table 1). Unmodified product pulse sequences were used for the acoustic noise evaluation of both MRI scanners. For both MR systems, the sensor was placed at the isocenter of the empty bore. The MR sequences, acquisition parameters, noise measurement equipment and methodology were identical for the two systems. Because the same model imaging electronics were used for each MR system, gradient amplitudes, waveforms and pulse sequence timing were identical for each system. The average sound pressure level in units of dBA was recorded for each of the MR acquisition and MR system combinations evaluated. We also documented the sound pressure level in units of dB as a function of frequency over the range of 50–20,000 Hz for each MR acquisition and MR system tested and calculated the associated average dB sound level. The sound pressure levels measured at the isocenter of the empty magnet bore in this study were dictated by the specific hardware configuration, sequence type and acquisition parameters being evaluated (Table 1). As such, the sound pressure level (and hence, the measured value) for a given sequence/MR system, was expected to be constant across repeated measurements within the tolerance of the measuring device. Consequently, only one set of measurements was obtained for each sequence/MR system evaluated. The average sound pressure levels measured in dB and dBA for each system as a function of sequence were submitted to a two-tailed nonparameteic Wilcoxon signed rank test to determine whether any differences in the sound pressure levels measured for each of the MRI systems were statistically significant.
Table 1.
MR protocols used for the acoustic noise evaluation of the NICU and adult-size MR systems
| Sequence | TR (ms) | TE (ms) | FA (degrees) | FOV (mm) | Matrix | Slice thickness (mm) | # slices | Receiver bandwidth (±kHz) |
|---|---|---|---|---|---|---|---|---|
| SE – Ax | 400 | 10 | 90/180 | 160 | 256×256 | 3 | 20 | 15.63 |
| GRE – Ax | 384 | 13 | 60 | 160 | 256×256 | 3 | 9 | 15.63 |
| bSSFP – Ax | 10.5 | 1.6 | 70 | 180 | 256×256 | 3 | 1 | 125 |
| EPI – Ax | 2,000 | 35 | 90 | 160 | 256×256 | 3 | 30 | 125 |
| SPGR – Ax | 384 | 1.5 | 60 | 180 | 256×256 | 3 | 9 | 125 |
| DWI – Ax (b=1,000) | 6,000 | 67.6 | 90/180 | 160 | 256×256 | 3 | 32 | 125 |
Ax axial plane, DWI diffusion-weighted imaging, EPI echo planar imaging, FA flip angle, bSSFP balanced steady state free precession, FOV field of view, GRE gradient recalled echo, SE spin echo, SPGR spoiled gradient echo, TE echo time, TR repetition time
Acoustic noise evaluation of clinical NICU MRI protocols
Upon completion of the acoustic noise evaluation of the NICU MRI system, we conducted a pilot study to evaluate the safety and image quality in human neonates. We evaluated 15 medically and thermally stable pre-term infants (post-menstrual age at scan 30 weeks+2 days to 46 weeks+3 days; weight at scan 1.60–3.36 kg). In accordance with the institutional review board, written informed consent was obtained from at least one of the parents of each of the infants prior to imaging. The MRI exams were performed without sedation using a feed-and-sleep, a.k.a. feed-and-swaddle approach [19, 20]. With the exception of one infant, who was nil per os for medical reasons, the infants were fed approximately 1 h before the exam. The infants were then swaddled with blankets, hearing protection was applied, including earplugs (E·A·R Classic™, 3M, St. Paul, MN) and earmuffs (MiniMuffs™, Natus Medical Inc., San Carlos, CA), and the MR room lights were lowered. Electrocardiography, pulse oximetry, respiration and skin temperature were monitored continuously throughout the exam using MRI-compatible monitoring equipment (Invivo, Orlando, FL). MRI images of the brain, chest and abdomen were obtained with protocols identical to those used on the conventional large-bore MRI scanners. The average A-weighted sound pressure level for each of the clinical MR protocols was obtained for the NICU scanner in an empty bore using the same sound-level meter and sensor placement used to perform the initial acoustic noise evaluation of the system (Table 2). As was done for the MR system sound pressure level comparison, one SPL measurement was made for each of the clinical sequence protocols evaluated.
Table 2.
Clinical MR protocols used for the acoustic noise evaluation of the NICU MR system
| Sequence | TR (ms) | TE (ms) | FA (degrees) | FOV (mm) | Matrix | Slice thickness (mm) | # slices | Receiver bandwidth (±kHz) | Other |
|---|---|---|---|---|---|---|---|---|---|
| Brain | |||||||||
| T1 FLAIR – Sag | 2,200 | 27 | 180/90/180 | 160 | 256×320 | 3 | 18 | 31.25 | TI=750 ms |
| PD/T2 FSE – Ax | 4,000 | 10/121 | 90/180 | 160 | 256×256 | 3 | 27×2 | 20.83 | ETL=12 |
| T2* MPGR – Ax | 600 | 30 | 20 | 160 | 256×256 | 4 | 1 | 15.6 | |
| T1 IR prepared 3-D SPGR – Sag | 6.5 | 3.1 | 14 | 160 | 256×256 | 1.2 | 192 | 15.6 | TI=650 ms |
| DTI – Ax | 10,000 | 99 | 90/180 | 180 | 128×128 | 3 | 32 | 250 | 15 directions, b=800 s/mm2; ETL=128 |
| BOLD EPI – Ax | 2,000 | 35 | 90 | 180 | 64×64 | 3 | 32 | 250 | ETL=64 |
| Abdomen/chest | |||||||||
| 3-D T1 LAVA – Sag | 5.5 | 2.32 | 12 | 180 | 160×288 | 3.4 | 68 | 62.5 | |
| bSSFP – Ax and Cor | 4.2 | 1.3 | 70 | 180 | 224×256 | 4 | 28 | 62.5 | |
| SSFSE – Cor and Ax | 1,160 | 103 | 90/180 | 180 | 192×256 | 4 | 21 | 62.5 | ETL=96; 0.5 NEX |
| bSSFP cine cardiac – Obl | 3.6 | 1.3 | 45 | 250 | 192×192 | 5 | 1 | 62.6 | 20 phases |
Ax axial plane, BOLD blood oxygenation level dependent, bSSFP balanced steady state free precession, Cor coronal plane, DTI diffusion tensor imaging, EPI echo planar imaging, ETL echo train length, FA flip angle, FLAIR fluid-attenuated fast spin echo, FOV field of view, FS fat saturated, FSE fast spin echo, IR inversion recovery, LAVA liver acquisition volume acceleration, MPGR multiplanar gradient recalled acquisition in the steady state, NEX number of excitations, Obl oblique plane, PDW proton-density-weighted, Sag sagittal plane, SPGR spoiled gradient echo, SSFSE single-shot fast spin echo, T1 T1-weighted, T2 T2-weighted, T2* T2*-weighted, TE echo time, TI inversion time, TR repetition time
Results
Acoustic noise evaluation of the NICU and adult-size MR systems
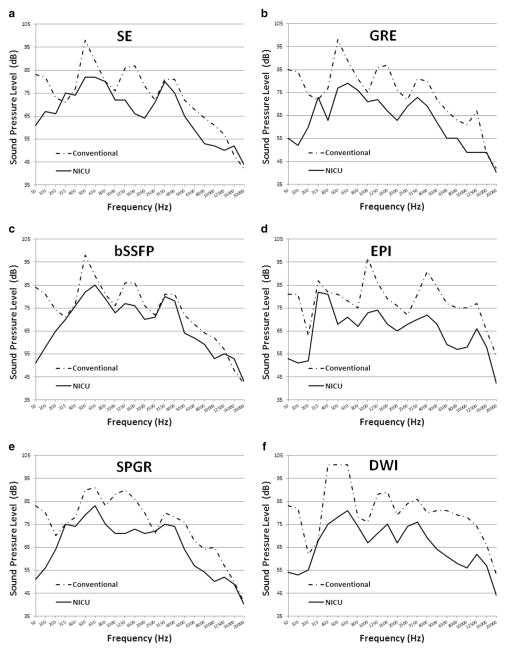
The sound pressure level values measured for the NICU MRI during each of the six MR acquisitions were consistently lower than those of the conventional adult-size MR scanner (Table 3). The differences in sound pressure levels measured for the two systems reported in both unweighted dB and A-weighted dB were statistically significant (dB: P=0.03; dBA: P=0.03, two-tailed nonparametric Wilcoxon signed rank test). On average, the small NICU scanner was approximately 14.2 dB (range 10–21 dB) and 11 dBA (range 5–18 dBA) quieter. For the NICU MRI system, the highest sound pressure level value was measured for the balanced steady-state free precession (bSSFP) sequence (76 dB and 91 dBA). Alternatively, for the adult-size scanner, the highest sound pressure level value was measured to be 93 dB and 103 dBA for the diffusion-weighted imaging (DWI) sequence. The harmonic measurements performed for each of the MR sequence/system combinations evaluated are presented in Fig. 2. The measured sound pressure level as a function of frequency showed a similar harmonic structure above 200 Hz between the two MR systems for all sequences. However the amplitude was consistently lower for the NICU MRI system. Notably, the adult-size MR system was substantially louder (as much as 30 dB) at frequencies below 200 Hz.
Table 3.
The average sound pressure level in units of (a) unweighted decibels (dB) and (b) A-weighted decibels (dBA) recorded for each of the MR acquisition/MR system combinations evaluated
| System/sequencea | SE | GRE | bSSFP | EPI | SPGR | DWI | Average |
|---|---|---|---|---|---|---|---|
| (a) Average sound pressure level (dB) | |||||||
| NICU MRI | 75 | 71 | 76 | 73 | 74 | 72 | 73.8 |
| Conventional MRI | 86 | 86 | 86 | 86 | 84 | 93 | 88 |
| (b) Average sound pressure level (dBA) | |||||||
| NICU MRI | 86 | 83 | 91 | 86 | 86 | 85 | 86.9 |
| Conventional MRI | 97 | 96 | 96 | 98 | 95 | 103 | 98.4 |
bSSFP balanced steady state free precession, DWI diffusion-weighted imaging, EPI echo planar imaging, GRE gradient recalled echo, SE spin echo, SPGR spoiled gradient echo
Acquisition parameters consistent with clinical protocols (Table 1)
Fig. 2.
Harmonic behavior of the NICU and adult-size conventional MRI scanners measured for each of the standard MRI sequences tested: (a) spin echo (SE), (b) gradient recalled echo (GRE), (c) balanced steady state free precession (bSSFP), (d) echo planar imaging (EPI), (e) spoiled gradient echo (SPGR) and (f) diffusion-weighted imaging (DWI). NICU neonatal intensive care unit
NICU MR clinical protocol evaluation
The average A-weighted dB sound pressure level values of each of the clinical imaging protocols measured at the isocenter of the empty bore of the NICU MR system are reported in Table 4. Ideally, passive hearing protection used for infants should be selected to provide the maximum amount of noise attenuation (i.e. up to 29 dBA for the products currently available). Assuming that hearing protection provides a noise reduction factor of approximately 29 dBA, the noise levels experienced by the infants during a clinical MRI exam in the NICU MRI system fall near or below the target maximum noise level of 65 dBA. These findings are consistent with the observation that all 15 infants evaluated in the pilot study were able to complete the MR exam without sedation and with minimal motion artifact. Image quality in all cases was equivalent to if not better than that obtained on an adult-size 1.5-T MR scanner [18]. Nevertheless, some babies did exhibit a startle reflex upon the initiation of scans, indicating that these infants were able to hear scanner noise.
Table 4.
The average sound pressure level in units of A-weighted decibels (dBA) recorded for each of the clinical NICU MR protocols without hearing protection
| Sequencea | NICU MRI system (without hearing protection) |
|---|---|
| Brain | dBA |
| T1 FLAIR – Sag | 92 |
| PD/T2 FSE – Ax | 88 |
| T2* MPGR – Ax | 85 |
| T1 IR prepared 3-D SPGR – Sag | 86 |
| DTI – Ax | 86 |
| BOLD EPI – Ax | 87 |
| Abdomen/chest | dBA |
| T1 LAVA – Sag | 94 |
| bSSFP – Ax and Cor | 94 |
| Single-shot FSE – Cor and Ax | 86 |
| Cardiac bSSFP cine – Obl | 92 |
Ax axial plain, BOLD blood-oxygen-level-dependent, bSSFP balanced steady state free precession, Cor coronal plane, DTI diffusion tensor imaging, EPI echo planar imaging, FLAIR fluid-attenuated inversion recovery, FSE fast spin echo, IR inversion recovery, LAVA liver acquisition with volume acceleration, MPGR multiplanar gradient recalled acquisition in the steady state, Obl oblique plane, PD proton-density weighted, Sag sagittal plane, T1 T1-weighted, T2 T2-weighted, T2* T2*-weighted
Acquisition parameters consistent with clinical protocols
Discussion
In this study the acoustic noise properties of the new NICU MRI scanner were investigated as part of the initial safety evaluation of the system. A similar assessment was performed for a conventional whole-body adult-size MRI system using identical imaging protocols. In addition, because of the heightened concern for the exposure of preterm and term infants to excessive noise levels, we evaluated the A-weighted sound pressure level for each of the imaging protocols for brain, chest and abdominal neonatal MR exams in the NICU conducted on the NICU MR system. The results of these evaluations were interpreted within the context of the targeted acoustic noise level of 65 dBA with hearing protection (ear-plugs and soft-shell earmuffs) for the NICU MRI protocols.
Sensory stimulation such as acoustic noise can elicit autonomic instability in term and preterm neonates. The noise levels of standard 1.5-T MR exams range from 81 dB to 117 dB on average, but can be as high as 131 dB for echo planar imaging techniques [5, 6]. Even with hearing protection these levels are well in excess of the recommended guidelines for maximum sound pressure levels within the NICU environment [13]. Largely as a consequence of the smaller spatial dimensions, the NICU scanner is on average 11 dBA quieter than an adult-size 1.5-T scanner for identical imaging sequences and acquisition parameters. In addition, the transmission of MR-related noise through bone conduction is minimized in the NICU MRI system by placement of a large amount of soft padding around the infant’s head; the patient table can also be cantilevered within the magnet bore to avoid direct physical contact with the noise-producing gradient coils. Hence, although not strictly additive, the noise attenuation provided by the combined use of earplugs and soft-shell earmuffs is estimated to reduce the sound pressure level by approximately 29 dBA.
We found that the NICU MRI system created less acoustic noise than conventional adult-size MRI scanners. The NICU scanner was on average 14.2 dB and 11 dBA quieter than the conventional MRI system. The lower noise level is due in large part to the fact that the NICU gradient coil is considerably smaller than its adult-size counterpart. The lower acoustic noise level of the NICU system has implications not only for improved safety for the NICU patients but it also further facilitates siting of the unit in the NICU, which has its own set of acoustic noise guidelines for constant background as well as transient noise levels [13]. Of particular relevance is the recommendation that in order to provide for uninterrupted sleep and sleep cycles (critical for early development of the sensory systems [11] and the development of the tonotopic columns in the auditory cortex and tuning of the hair cells of the cochlea [10]) and to protect physiological stability in premature and critically ill infants, the combination of continuous background sound and operational sound in the infant rooms should not exceed an Leq of 45 dBA [13]. This low ambient sound level also promotes the NICU patients’ ability to discriminate voice, language, music and other meaningful environmental sounds from background noise. The harmonic behavior of the two MR systems evaluated was similar above 200 Hz; however the NICU scanner was quieter across all frequencies evaluated. The fact that the rank ordering of the quietest to loudest sequence measured for the NICU and adult-size MRI systems differed (Table 3) reflects the different acoustic response functions (Fig. 2) of the two scanners as well as the acoustic properties of the scan rooms themselves [21–23].
Although the acoustic noise evaluation was performed for the adult-size GE 1.5-T MR 60-cm bore system, the results for comparable operating conditions (bore size, sequence type and acquisition parameters) should be similar for comparable adult-size systems (60-cm bore size, 1.5 T) manufactured by the other vendors because the sound pressure levels for identical sequence types and acquisition parameters are largely determined by the dimensions of the gradient coils. In general the acoustic noise levels of MRI systems increase with field strength [23], but the details in scanner construction make it difficult to extrapolate the current 1.5-T results to systems with higher field strengths such as 3 T.
The average difference in sound pressure level between the adult-size and NICU scanners was observed to be 14.2 dB and 11 dBA. Because A-weighting is greatest for frequencies less than 1 kHz, the discrepancies between dB and dBA measurements are dominated by lower-frequency components [24]. Assuming that an infant’s hearing sensitivity is comparable to that of an adult for the low frequencies [8], A-weighted dB measurements are more physiologically relevant than un-weighted measurements. Furthermore, NICU guidelines for noise exposure are given in dBA and thus dBA measurements provide a more realistic measure of acoustic performance than unweighted sound pressure levels.
In utero the fetus is protected from frequencies above 2 kHz [25]. Hence, after birth the preterm infant is exposed to frequencies atypical for his or her level of neurological development. Consequently, it is prudent that exposure to background noise in frequencies above 2 kHz be restricted as much as possible. In addition, because the 500-Hz to 2-kHz frequency range is critical for discrimination of speech [26, 27], excessive exposure to high sound levels in this frequency band should be minimized. Furthermore, frequencies of 500 Hz and below (particularly <200 Hz), although often inaudible, are extremely effective at producing vibrotactile stimulation [12, 28]. It has been reported that prolonged exposure to vibrotactile stimulation can cause physiological stress in adults [12, 28] and therefore should also be mitigated when possible for the neonatal population. Notably, the NICU MRI scanner was found to be as much as 30 dB quieter for frequencies of 200 Hz and below (Fig. 2).
The images [18] and associated sound pressure level measurements demonstrate that the NICU MRI is capable of performing high-quality diagnostic MRI exams at acoustic noise levels near or below the target value of 65 dBA when appropriate hearing protection is applied. Although not measured explicitly, the comparable sound pressure level values for the same set of imaging protocols executed on the adult-size whole-body MRI unit are on average 11 dBA higher and would expose a neonate to sound levels well above the target noise level of 65 dBA even with the use of hearing protection. The lower noise levels reduce the probability of physiological stress, thereby reducing the medical risk of the MRI exam to the neonate. In addition, the lower noise levels of the NICU MR system are less disruptive to sleep. This is not only important developmentally for all of the NICU patients, but it also increases the probability that the MRI exam can be completed without sedation. The latter is particularly beneficial in light of the growing evidence that sedation in the neonatal population poses significant risk, including neuronal apoptosis, apnea and bradycardia [29–34]. Although the NICU MR scanner is substantially quieter than the conventional MR systems used for neonatal imaging today, measures to further reduce the acoustic noise for the NICU system are being explored, including the development of alternative hearing protection strategies and quieter pulse sequences. The NICU MRI system is currently being used for routine diagnostic exams as well as for clinical research.
Conclusion
The results of the acoustic noise evaluation together with the clinical image data collected to date have demonstrated that with appropriate hearing protection, state-of-the-art high-quality MRI exams can be performed on the NICU MRI unit without exposing infants to acoustic noise above a sound pressure level of 65 dBA. The NICU MRI unit was found to be on average 14.2 dB and 11 dBA quieter than a conventional scanner and approximately 30 dB quieter in the vibrotactile band below 200 Hz. This lower acoustic noise not only reduces the risk of noise-induced autonomic instability, it also increases the probability that the MRI exam can be performed without sedation. Last, the lower acoustic noise levels of the NICU MRI system further facilitate siting of the unit in the NICU, which is governed by its own set of recommended acoustic noise guidelines [13].
Footnotes
Conflicts of interest None
Contributor Information
Jean A. Tkach, Email: jean.tkach@cchmc.org, Imaging Research Center, Department of Radiology, Cincinnati Children’s Hospital Medical Center, 240 Albert Sabin Way, MLC 5033, Cincinnati, OH 45229, USA
Yu Li, Imaging Research Center, Department of Radiology, Cincinnati Children’s Hospital Medical Center, 240 Albert Sabin Way, MLC 5033, Cincinnati, OH 45229, USA.
Ronald G. Pratt, Imaging Research Center, Department of Radiology, Cincinnati Children’s Hospital Medical Center, 240 Albert Sabin Way, MLC 5033, Cincinnati, OH 45229, USA
Kelly A. Baroch, Division of Audiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA
Wolfgang Loew, Imaging Research Center, Department of Radiology, Cincinnati Children’s Hospital Medical Center, 240 Albert Sabin Way, MLC 5033, Cincinnati, OH 45229, USA.
Barret R. Daniels, Imaging Research Center, Department of Radiology, Cincinnati Children’s Hospital Medical Center, 240 Albert Sabin Way, MLC 5033, Cincinnati, OH 45229, USA
Randy O. Giaquinto, Imaging Research Center, Department of Radiology, Cincinnati Children’s Hospital Medical Center, 240 Albert Sabin Way, MLC 5033, Cincinnati, OH 45229, USA
Stephanie L. Merhar, Division of Neonatology and Pulmonary Biology, Perinatal Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA
Beth M. Kline-Fath, Department of Radiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA
Charles L. Dumoulin, Imaging Research Center, Department of Radiology, Cincinnati Children’s Hospital Medical Center, 240 Albert Sabin Way, MLC 5033, Cincinnati, OH 45229, USA
References
- 1.Franks JR, Stephenson MR, Merry CJ. Preventing occupational hearing loss—a practical guide. U.S. Department of Health and Human Services; 1996. [Accessed 2 Jan 2014]. http://www.cdc.gov/niosh/docs/96-110/pdfs/96-110.pdf. [Google Scholar]
- 2.Morris BH, Philbin MK, Bose C. Physiological effects of sound on the newborn. J Perinatol. 2000;20:S55–S60. doi: 10.1038/sj.jp.7200451. [DOI] [PubMed] [Google Scholar]
- 3.Watts C, Trim E, Metherall J, et al. Neonatal transport—the comfort zone. Neonatal Transp. 2008;4:4. [Google Scholar]
- 4.Occupational Safety and Health Administration. [Accessed 2 Jan 2014];OSHA fact sheet: laboratory safety noise. 2011 https://www.osha.gov/Publications/laboratory/OSHAfactsheet-laboratory-safety-noise.pdf.
- 5.Nordell A, Lundh M, Horsch S, et al. The acoustic hood: a patient-independent device improving acoustic noise protection during neonatal magnetic resonance imaging. Acta Paediatr. 2009;98:1278–1283. doi: 10.1111/j.1651-2227.2009.01339.x. [DOI] [PubMed] [Google Scholar]
- 6.Amaro E, Jr, Williams SC, Shergill SS, et al. Acoustic noise and functional magnetic resonance imaging: current strategies and future prospects. J Magn Reson Imaging. 2002;16:497–510. doi: 10.1002/jmri.10186. [DOI] [PubMed] [Google Scholar]
- 7.Philbin MK, Taber KH, Hayman LA. Preliminary report: changes in vital signs of term newborns during MR. AJNR Am J Neuroradiol. 1996;17:1033–1036. [PMC free article] [PubMed] [Google Scholar]
- 8.Lasky RE, Williams AL. The development of the auditory system from conception to term. Neo Reviews. 2005;6:141–152. [Google Scholar]
- 9.Graven SN, Browne JV. Auditory development in the fetus and infant. Newborn Infant Nurs Rev. 2008;8:187–193. [Google Scholar]
- 10.Graven SN, Browne JV. Sensory development in the fetus, neonate and infant. Newborn Infant Nurs Rev. 2008;8:169–172. [Google Scholar]
- 11.Graven SN, Browne JV. Sleep and brain development, neonate and infant. Newborn Infant Nurs Rev. 2008;8:173–179. [Google Scholar]
- 12.Macnab A, Chen Y, Gagnon F, et al. Vibration and noise in pediatric emergency transport vehicles: a potential cause of morbidity? Aviat Space Environ Med. 1995;66:212–219. [PubMed] [Google Scholar]
- 13.White RD. Report of the eighth census conference on newborn ICU design. [Accessed 16 Jan 2014];Standard 27—acoustic environment. 2012 http://www3.nd.edu/~nicudes/stan%2027.html.
- 14.Berger EH. Attenuation of earplugs worn in combination with earmuffs. Occup Health Saf. 1984 May;:72–73. [PubMed] [Google Scholar]
- 15.Witt B. Dual protection. Bacou-Dalloz Hearing Safety Group. [Accessed 2 Jan 2014];Sound Source. 2007 http://www.howardleight.com/images/pdf/0000/0270/Sound_Source_11a_Dual_Protection.pdf.
- 16.Small SA, Stapells DR. Multiple auditory steady-state response thresholds to bone-conduction stimuli in young infants with normal hearing. Ear Hear. 2006;27:219–228. doi: 10.1097/01.aud.0000215974.74293.b9. [DOI] [PubMed] [Google Scholar]
- 17.Tkach JA, Hillman NH, Jobe AH, et al. An MRI system for imaging neonates in the NICU: initial feasibility study. Pediatr Radiol. 2012;42:1347–1356. doi: 10.1007/s00247-012-2444-9. [DOI] [PubMed] [Google Scholar]
- 18.Tkach JA, Merhar SL, Kline-Fath BM, et al. MR imaging in the neonatal intensive care unit: initial experience using a small footprint 1.5 T system. AJR Am J Roentgenol. 2014;202:W95–W105. doi: 10.2214/AJR.13.10613. [DOI] [PubMed] [Google Scholar]
- 19.Windram J, Grosse-Wortmann L, Shariat M. Cardiovascular MRI without sedation or general anesthesia using a feed-and-sleep technique in neonates and infants. Pediatr Radiol. 2012;42:183–187. doi: 10.1007/s00247-011-2219-8. [DOI] [PubMed] [Google Scholar]
- 20.Mathur AM, Neil JJ, McKinstry RC, et al. Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol. 2008;38:260–264. doi: 10.1007/s00247-007-0705-9. [DOI] [PubMed] [Google Scholar]
- 21.Hurwitz R, Lane SR, Bell RA, et al. Acoustic analysis of gradient-coil noise in MR imaging. Radiology. 1989;173:545–548. doi: 10.1148/radiology.173.2.2798888. [DOI] [PubMed] [Google Scholar]
- 22.McJury MJ. Acoustic noise levels generated during high field MR imaging. Clin Radiol. 1995;50:331–334. doi: 10.1016/s0009-9260(05)83427-0. [DOI] [PubMed] [Google Scholar]
- 23.Price DL, De Wilde JP, Papadaki AM, et al. Investigation of acoustic noise on 15 MRI scanners from 0.2 T to 3 T. J Magn Reson Imaging. 2001;13:288–293. doi: 10.1002/1522-2586(200102)13:2<288::aid-jmri1041>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 24.Occupational Safety and Health Administration, U.S. Department of Labor. Noise and hearing conservation. [Accessed 10 Dec 2013];Appendix I: A-4. A-weighted network. 2013 https://www.osha.gov/dts/osta/otm/noise/health_effects/soundpressure_aweighted.html.
- 25.Gerhardt KJ, Abrams RM. Fetal exposures to sound and vibroacoustic stimulation. J Perinatol. 2000;20:S21–S30. doi: 10.1038/sj.jp.7200446. [DOI] [PubMed] [Google Scholar]
- 26.Boothroyd A. Pro-ed studies in communication disorders. PRO-ED; Austin, TX: 1986. Speech acoustics and speech perception. [Google Scholar]
- 27.Boothroyd A. Room acoustics and speech perception. Semin Hear. 2004;25:155–166. [Google Scholar]
- 28.Berglund B, Hassmen P, Job RF. Sources and effects of low-frequency noise. J Acoust Soc Am. 1996;99:2985–3002. doi: 10.1121/1.414863. [DOI] [PubMed] [Google Scholar]
- 29.Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beauve B, Dearlove O. Sedation of children under 4 weeks of age for MRI examination. Paediatr Anaesth. 2008;18:892–893. doi: 10.1111/j.1460-9592.2008.02580.x. [DOI] [PubMed] [Google Scholar]
- 31.Allegaert K, Naulaers G. Procedural sedation of neonates with chloral hydrate: a sedation procedure does not end at the end of the acquisition of the images. Paediatr Anaesth. 2008;18:1270–1271. doi: 10.1111/j.1460-9592.2008.02792.x. [DOI] [PubMed] [Google Scholar]
- 32.Koch BL. Avoiding sedation in pediatric radiology. Pediatr Radiol. 2008;38:S225–S226. doi: 10.1007/s00247-008-0807-z. [DOI] [PubMed] [Google Scholar]
- 33.Cote CJ. Safety after chloral hydrate sedation of former pre-term and term infants for magnetic resonance imaging: are the data clear? Anesth Analg. 2010;110:671–673. doi: 10.1213/ANE.0b013e3181cd4428. [DOI] [PubMed] [Google Scholar]
- 34.Etzel-Hardman D, Kapsin K, Jones S, et al. Sedation reduction in a pediatric radiology department. J Healthc Qual. 2009;31:34–39. doi: 10.1111/j.1945-1474.2009.00035.x. [DOI] [PubMed] [Google Scholar]