Abstract
Background
Pregnancy-induced utero-placental growth, angiogenic remodeling, and enhanced vasodilation are all partly regulated by estradiol-17β-mediated activation of endothelial nitric oxide synthase (eNOS) and nitric oxide (NO) production. However, very little is known about the effects of alcohol on these maternal utero-placental vascular adaptations during pregnancy and its potential role in the pathogenesis of Fetal Alcohol Spectrum Disorders (FASD). In this study, we hypothesized that in vitro chronic binge-like alcohol will decrease uterine arterial endothelial eNOS expression and alter its multi-site phosphorylation activity state via disruption of AKT signaling. To study the direct effects of alcohol on uterine vascular adaptations, we further investigated the effects of alcohol on estradiol-17β-induced uterine angiogenesis in vitro.
Methods
Uterine artery endothelial cells were isolated from pregnant ewes (gestational day 120-130; term = 147), Fluorescence Activated Cell sorted, validated, and maintained in culture to passage 4. To mimic maternal binge drinking patterns, cells were cultured in the absence or presence of a lower (LD) or higher dose (HD) of alcohol in a compensating sealed humidified chamber system equilibrated with aqueous alcohol for 3 h on 3 consecutive days. Immunoblotting was performed to assess expression of NO system-associated proteins and eNOS multi-site phosphorylation. Following this treatment paradigm, control and binge alcohol treated cells were passaged, grown for two days, and then treated with increasing concentrations of estradiol-17β (0.1, 1, 10, 100 nM) in the absence or presence of LD or HD alcohol to evaluate estradiol-17β-induced angiogenesis index using BrdU Proliferation Assay.
Results
LD and HD binge-like alcohol decreased uterine arterial eNOS expression (P=0.009). eNOS multi-site phosphorylation activation state was altered: P635eNOS was decreased (P=0.017), P1177 eNOS was not altered, and P495 eNOS exhibited an inverse U shaped dose-dependent relationship with alcohol. LD and HD alcohol decreased the major eNOS-associated protein cav-1 (P<0.001). However, the commonly implicated AKT pathway did not correlate with eNOS post-translational modifications. Assessment of uterine vascular adaptation via angiogenesis demonstrated that alcohol abrogated the dose-dependent proliferative effects of estradiol-17β and thus blunted angiogenesis.
Conclusions
Thus, the maternal uterine vasculature during pregnancy may be vulnerable to chronic binge-like alcohol. Altered eNOS multi-site phosphorylation also suggests that alcohol produces specific effects at the level of post-translational modifications critical for pregnancy-induced uterine vascular adaptations. Finally, the alcohol and estradiol-17β data suggest a negative impact of alcohol on estrogen actions on the uterine vasculature.
Keywords: Estrogen, Angiogenesis, FASD, eNOS, Phosphorylation, Caveolin
INTRODUCTION
Maternal alcohol consumption during pregnancy may lead to pre- and postnatal growth restriction that persists through adolescence (Day et al., 1989; Day et al., 2002). The overall whole body structural deficits are compounded by a wide range of neuroanatomical (Archibald et al., 2001), behavioral (Mattson and Riley, 1998), and memory deficits (Mattson et al., 1996) and is termed as Fetal Alcohol Spectrum Disorders (FASD). These observations in children are supported by specific phenotypes in several animal model systems especially relating to the deleterious effects of alcohol on the developing brain (Abdollah and Brien, 1995; Bonthius et al., 1992; Cudd, 2005; Weinberg et al., 2008). Candidate mechanisms include disrupted fetal neuronal cellular energetics, dysregulated developmental timing, impaired cell-cell interactions, and altered gene/protein expression (Goodlett et al., 2005). However, very little is known about disruption of maternal-fetal interface as a potential source of FASD and in particular about the effects of alcohol on maternal uterine vascular adaptations during pregnancy.
By the third trimester of gestation, uterine vascular resistance decreases by 70 fold (Rosenfeld, 1977), accompanied by extensive angiogenesis (Reynolds and Redmer, 1995; Zygmunt et al., 2003) and extracellular matrix remodeling (Osol and Mandala, 2009) resulting in a 53 fold increase in uterine blood flow compared to the nonpregnant state (Magness, 1998; Rosenfeld, 1977). These adaptations are directly controlled by concurrent elevations in estradiol-17β-induced nitric oxide (NO) production (Losordo and Isner, 2001; Magness, 1998; Magness et al., 2005; Matsubara et al., 2008; Rosenfeld et al., 1996; Rubanyi et al., 2002). We have recently demonstrated that estradiol-17β produces bi-phasic dose-dependent proliferation of uterine artery endothelial cells derived from pregnant but not nonpregnant ewes (Jobe et al., 2010). Estrogen acts on the membrane estradiol-17β receptors located on caveolar lipid rafts to induce complex regulation of endothelial NO synthase (eNOS) leading to NO production (Chen et al., 2004; Losordo and Isner, 2001). eNOS activation involves a cascade of events that involve PI3-Kinase AKT activation, eNOS posttranslational modification via multi-site phosphorylation, and eNOS translocation to non-caveolar sub-cellular compartment (Michell et al., 2002; Shaul, 2002). However, the effects of alcohol on the uterine vascular endothelial NO system during pregnancy are not well studied.
In this study, we hypothesized that chronic binge-like alcohol will decrease uterine arterial endothelial eNOS expression and alter its multi-site phosphorylation activation state via the AKT signaling pathway. To study the direct effects of alcohol on uterine vascular adaptations, we further investigated the interactive effects of alcohol and estradiol-17β on uterine angiogenesis in vitro. We specifically utilized ovine uterine artery endothelial cells obtained during the third trimester-equivalent of human pregnancy, a period during which alcohol-associated maladaptations have been previously reported in both ovine maternal and fetal vascular compartments (Cook et al., 2001; Falconer, 1990; Parnell et al., 2007).
MATERIALS AND METHODS
Alcohol Binging
The Animal Care and Use Committee of the University of Wisconsin-Madison approved procedures for obtaining uterine arteries from pregnant ewes (Day 120-130; term = 147) for endothelial cells isolation using collagenase digestion procedures (Bird et al., 2000). For each of the studies described, three replicates from four different pregnant ewes were utilized. Additionally, for endothelial proliferation studies, assays were performed in quadruplicates from each individual preparation. In brief, cells were purified using fluorescence activated cell sorting (FACS), devoid of vascular smooth muscle cell contamination and maintained in culture to passage 4. To mimic maternal binge drinking patterns, uterine artery endothelial cells were cultured to 70% confluence in the absence (0 mg/dl; Control, Ctrl) or presence of two doses of alcohol. To achieve a magnitude that is similar to the peak blood alcohol concentrations (BACs) obtained in previously published Fetal Alcohol Spectrum Disorders (FASD) studies performed using the sheep model system (Cudd et al., 2001; Ramadoss et al., 2008; West et al., 2001), we utilized a lower dose (LD, 300 mg/dl) group. In addition, to mimic clinically relevant abusive patterns of drinking in women of child-bearing age and those who are admitted to emergency wards (Church and Gerkin, 1988; Hammond et al., 1973; Urso et al., 1981; Wells and Barnhill, 1996), we utilized a higher dose (HD, 600 mg/dl) group. Cells were exposed to alcohol in sealed, humidified chambers equilibrated with aqueous alcohol for 3 h on 3 consecutive days (Eysseric et al., 1997; Ramadoss et al., 2010; Ramadoss et al., 2007a; Ramadoss et al., 2007b), a pattern common among drinking women of child bearing age (Caetano et al., 2006; Gladstone et al., 1996; Maier and West, 2001). Alcohol concentrations were validated using an enzymatic assay kit (Quantichrom® ethanol assay kit, BioAssay Systems, Hayward, CA; data not shown). As part of preliminary studies, cell viability was validated. At the end of the experiment, the endothelial cells were scraped and collected in a lysis buffer containing Na4P2O7 (4 mM), HEPES (50 mM), NaCl (100 mM), EDTA (10 mM), NaF (10 mM), Na3VO4 (2 mM), pH (10.5), with freshly added PMSF (2 mM), Triton X100 (1% V/V), aprotinin (5 μg/ml), leupeptin (5 μg/ml), and microcystin (4 μl in 10 ml). The lysate was sonicated and centrifuged at 13,200 RPM for five minutes at 4 degree Celsius. The supernatant was assayed for protein concentration using a BCA assay kit (BCA® Protein Assay, Thermo Scientific, Rockford, IL).
Immunoblotting
Proteins (15 μg) along side of Rainbow molecular weight markers (Bio-Rad Laboratories, Inc.) were resolved on 4-20% gradient denaturing 18-well polyacrylamide gels with 0.1% SDS at 100 V for 1.5 h at room temperature before transfer onto Immobilon-P membranes at 100 V for 45 min. Non-specific binding was blocked with 5% fat-free milk in TBST (50 mm Tris-HCl, pH 7.5, 0.15 m NaCl, 0.05% Tween-20) for 120 minutes and incubated with primary antibodies in TBST + 1% BSA for 120 minutes or overnight at 4 degrees Celsius. The primary antibodies were monoclonal eNOS (1:750; BD Transduction Labs; #610312), polyclonal actin (1:3000; #4970), total caveolin-1 (1:10,000; #3251S), total AKT (1:1000; #9272), P1177eNOS (1:1000; #9570S), and P473 AKT (1:1000; #9271S) from Cell Signaling Inc., and polyclononal P635eNOS (1:1000; #07562), and P495eNOS (1:1000; #07384) from Millipore Inc. After washing, the membrane was incubated with secondary antibodies (goat anti-rabbit/HRP conjugate or Sheep anti-mouse/HRP conjugate) at 1:3000 dilution and detected with the Pierce ECL or ECL plus detection kits (Thermo Scientific, Waltham, MA). Protein expression was quantified by scanning densitometry (Bio-Rad 670 scanning densitometer) and normalized to β actin.
Assessment of estradiol-17β-induced uterine vascular adaptation
Following the end of binge paradigm, cells were passaged, plated, and grown in phenol red free media (DVal EBM with 20% FBS, 100 mg/ml penicillin, and 100 mg/ml streptomycin) on a 96 well plate for 24 hours as previously described (Jobe et al., 2010). Subsequently, cells were serum starved for 24 hours and treated with 0, 0.1 nM, 1 nM, 10 nM, or 100 nM of estradiol-17β for 24 hours in the absence or presence of LD or HD alcohol. BrdU label was added at the beginning of the steroid treatment and this in vitro index of proliferation was utilized as an estimate of angiogenesis, a specific uterine vascular adaptation (Jobe et al., 2010; Wulff et al., 2002). Plates were read using Synergy HT Multi-Mode Microplate Reader. Proliferation results were expressed as fold increases over untreated control after subtracting the value of the blank (wells incubated without BrdU loading). Validation of cell number increase and cytoxicity after treatment with estradiol-17β was previously performed using ViaLight Plus High Sensitivity Cell Proliferation and Cytotoxicity Kit (Lonza Inc., Rockland, ME) according to manufacturer’s instructions (Jobe et al., 2010). In addition, cell viability at different doses of estradiol-17β was validated as follows: after 24 hour starvation and subsequent treatment with estradiol-17β in white opaque 96 well plates (24-hours), cells were lysed with Lysis Reagent (10 mins) to extract ATP from cells. Then the appropriate amount of ATP Monitoring Reagent Plus was added (2 mins) in each well to generate luminescent signal. Plates were read using Synergy HT Multi-Mode Microplate Reader to determine luminescence and results expressed in Relative Light Units as fold increases over untreated control after subtracting the value of the blank against an ATP standard curve (Jobe et al., 2010).
Statistical Analyses
Data are expressed as mean ± SEM. Protein expression data were expressed as a ratio to actin. A two or a one way ANOVA was performed as appropriate. Further multiple pairwise comparisons were performed when appropriate using Fisher’s protected least significant difference (PLSD). The α level was established a priori at P < 0.05 for all analyses.
RESULTS
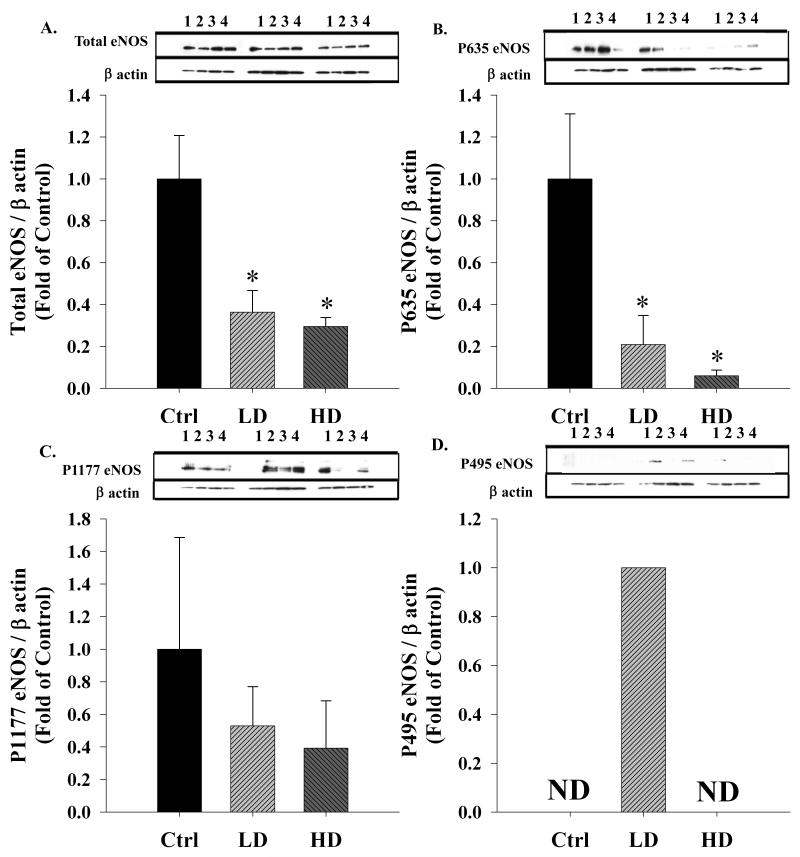
Chronic binge-like alcohol significantly decreased (one way ANOVA, P = 0.009) the expression of total eNOS, the rate liming enzyme for NO production in the uterine artery endothelium (Figure 1A). Compared to the Control (Ctrl) group, total eNOS expression was significantly decreased in both the Lower Dose (LD; P = 0.009) and Higher Dose (HD; P = 0.005) groups. Subsequently, eNOS multi-site phosphorylation state was examined by specifically assessing the expression of excitatory Ser 635, excitatory Ser 1177, and inhibitory Thr 495 residues (Kukreja and Xi, 2007; Mount et al., 2007). P635eNOS, an excitatory phosphorylation site, and the best estimate of the activity of the enzyme in the uterine endothelium was significantly decreased by alcohol (one way ANOVA, P = 0.017); compared to the Ctrl group, P635eNOS was significantly decreased in the LD (P = 0.019) and was barely detectable in the HD (P = 0.008) group (Figure 1B). The decrease in P635eNOS was in excess of total eNOS demonstrating that eNOS was barely phosphorylated at the Ser 635 residue. Surprisingly, the excitatory P1177eNOS was not different among treatment groups (Figure 1C). The classic inhibitory P495eNOS exhibited an inverse U shaped relationship and was barely detectable in the Ctrl and HD groups whereas it was detectable in the LD group (Figure 1D).
Figure 1. Effect of chronic binge-like alcohol on eNOS expression and multi-site phosphorylation.
(A) Compared to the control (Ctrl) group, total eNOS expression was significantly (*, P < 0.05) decreased in both the lower dose (LD) and higher dose (HD) groups. (B) The excitatory P635eNOS was significantly decreased in the LD group and was barely detectable in the HD group. (C) The excitatory P1177eNOS was not different among treatment groups. (D) The classic inhibitory P495eNOS exhibited an inverse U shaped relationship and was barely detectable (ND) in the Ctrl and HD groups whereas it was detectable in the LD group. Optical Density (OD) data are expressed as a ratio to β actin OD.
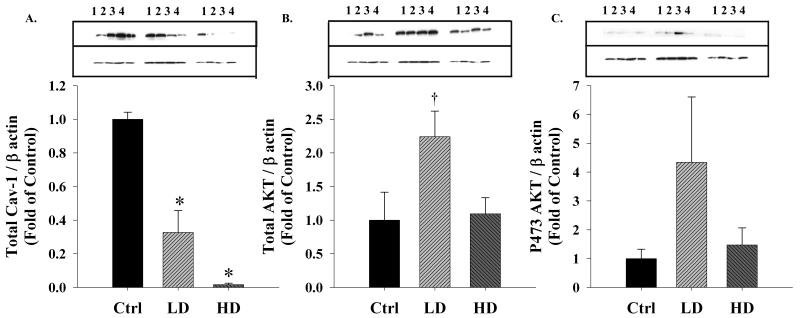
The caveolar scaffolding protein caveolin-1 (total cav-1) that is bound to eNOS in its inactive state (Shaul, 2002) was significantly different (one way ANOVA, P < 0.001) among treatment groups (Figure 2A). Compared to the Ctrl group, total cav-1 expression was significantly decreased in the LD (P = 0.002) and was barely detectable in the HD (P < 0.001) group. Total AKT, a protein widely implicated in modulating the phosphorylation and activity states of eNOS exhibited an inverse U shaped relationship and trended to be increased in response to LD alcohol (one way ANOVA, P = 0.063; Figure 2B). pAKT was not significantly different among treatment groups (Figure 2C). Therefore, under the present conditions, there was no direct correlation between total AKT and eNOS expression (R2 = 0.122) or their phosphorylation.
Figure 2. Effect of chronic binge-like alcohol on eNOS associated proteins.
(A) Compared to the control (Ctrl) group, the caveolar scaffolding protein cav-1 was significantly (*, P < 0.05) decreased in both the lower dose (LD) and higher dose (HD) groups. (B) Total AKT, a protein widely implicated in the eNOS phosphorylation exhibited an inverse U shaped relationship and trended (†) to be elevated in response to LD alcohol (P = 0.063). (C) However, activated pAKT was not different among treatment groups. Optical Density (OD) data are expressed as a ratio to β actin OD.
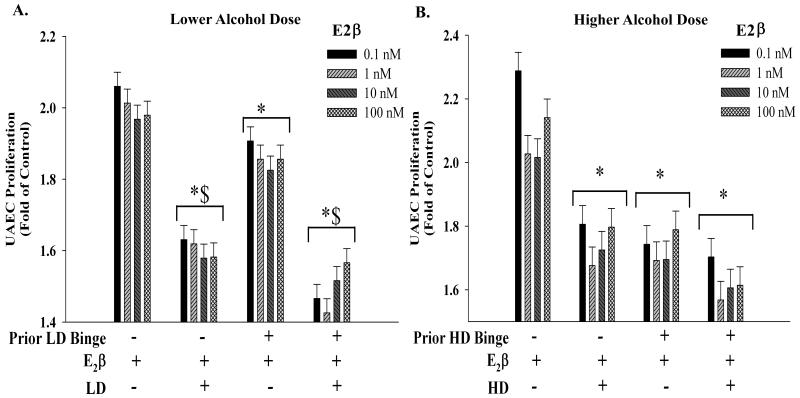
To study the direct effects of alcohol on uterine vascular adaptations, we then investigated the interactive effects of alcohol and estradiol-17β on uterine angiogenesis in vitro. Figure 3A depicts estradiol-17β-induced proliferative response in the LD group compared to the Ctrl. Bi-phasic dose-dependent proliferative effects of estradiol-17β that we recently reported (Jobe et al., 2010) were abolished by alcohol. The presence of LD alcohol significantly decreased estradiol-17β-induced proliferation; however, this was independent of whether the cells were previously exposed to binge-like LD alcohol. Specifically, the estradiol-17β-induced proliferation was significantly decreased in the binge-like LD alcohol group even if alcohol was not present with estradiol-17β in the system. However, this decrease was significantly lower compared to when alcohol was present with estradiol-17β. Figure 3B depicts estradiol-17β-induced proliferative response in the HD group compared to the Ctrl. Similar to the LD group, the presence of HD alcohol with estradiol-17β significantly decreased proliferation independent of whether the cells were previously exposed to binge-like HD alcohol. However, unlike the LD group, there was no difference in the binge-like HD alcohol groups even if alcohol was not present with estradiol-17β in the system compared to when alcohol was present.
Figure 3. Interactive effects of estradiol-17β and alcohol on uterine arterial endothelial proliferative function.
(A) Estradiol 17β-induced uterine artery endothelial cell (UAEC) proliferative response in the lower dose (LD) alcohol group compared to the controls (Ctrl). Dose-dependent proliferative effects of estradiol-17β that we previously reported (Jobe et al., 2010) were abolished by alcohol. The presence of LD alcohol with estradiol-17β significantly (*) decreased proliferation independent of whether the cells were previously exposed to binge-like LD alcohol. Specifically, the estradiol-17β-induced proliferation was significantly decreased in the binge-like LD alcohol group even if alcohol was not present with estradiol-17β in the system. However, this decrease ($) was significantly lower compared to when alcohol was present with estradiol-17β. (B) Estradiol-17β-induced proliferative response in the higher dose (HD) group compared to the Ctrl. Similar to the LD group, the presence of HD alcohol with estradiol-17β significantly decreased (*) proliferation independent of whether the cells were previously exposed to binge-like HD alcohol. However, unlike the LD group, there was no difference in the binge-like HD alcohol groups even if alcohol was not present with estradiol-17β in the system compared to when alcohol was present.
DISCUSSION
This is the first study to systematically investigate the effects of alcohol on the uterine NO system and associated vascular adaptations at the level of endothelium. The current study demonstrates that the maternal uterine vasculature is vulnerable to chronic binge-like alcohol and that these effects are in part mediated by alterations in the NO system. Alcohol decreased uterine arterial eNOS expression and negatively impacted its multi-site phosphorylation state and associated proteins cav-1 and AKT, indicating reduced eNOS enzyme activity. However, the commonly implicated AKT pathway did not directly correlate with eNOS post-translational modifications. Furthermore, assessment of uterine vascular adaptation via angiogenesis using this in vitro model demonstrated strong negative effects of alcohol on estradiol-17β-induced proliferation alcohol abolished dose-dependent proliferative effects of estradiol-17β and blunted angiogenesis.
Uterine vascular adaptations during pregnancy including vasodilation, angiogenesis, and extracellular matrix remodeling are in part controlled by NO (Osol and Mandala, 2009; Zygmunt et al., 2003). eNOS protein expression is dramatically increased in pregnancy compared to the nonpregnant state in the uterine artery endothelium whereas such dramatic pregnancy-associated adaptations are not observed in systemic arteries (Magness et al., 1997; Magness et al., 2001). The increase in uterine endothelial eNOS expression is accompanied by a simultaneous elevation in eNOS activity in vitro (Magness et al., 1997; Yi et al., 2005) and the circulating levels of NOx and cGMP ex vivo (Magness et al., 2001; Rosenfeld et al., 1996; Vonnahme et al., 2005). It has been previously reported that the deficiency in NO production renders the developing fetal neuronal cells more vulnerable to the toxic effects of alcohol and that the nitric oxide-cGMP-PKG pathway has a protective effect against alcohol-induced injury (Bonthius et al., 2008; Bonthius et al., 2003; Bonthius et al., 2009). However, no previous study has reported the effects of alcohol on the NO system in the maternal uterine compartment. Herein, we report for the first time that in vitro chronic binge-like alcohol blunts total eNOS protein expression. These data directly point to possible uterine vascular maladaptations during the third trimester-equivalent of human gestation when uterine blood flow is the highest and when the fetal brain growth velocity peaks.
The decrease in total eNOS expression was accompanied by a dramatic simultaneous alteration in the multi-site phosphorylation state markers of eNOS activation. In this study, we demonstrate that alcohol blunts phosphorylation of Ser 635, the best estimate of the activity of eNOS in the uterine vasculature. Ser 635, a well-known excitatory site, is located in the flavin mononucleotide binding domain of the enzyme (Michell et al., 2002; Mount et al., 2007) and is important for sustained NO production without requiring rises in intracellular calcium (Boo et al., 2003). The nearly complete depletion of Ser 635 phosphorylation in the HD group suggests the deleterious effects of alcohol on eNOS activity. In contrast, alcohol did not have an effect on Ser 1177 phosphorylation. Ser1177 is a widely examined AKT-dependent site located in the reductase domain in the vicinity of the C-terminal end of eNOS, preventing the electron transfer between the two monomers (Mount et al., 2007). Although previous reports have also been skeptical of the possible role of Ser 1177 in uterine vasculature as opposed to the systemic vasculature (Cale and Bird, 2006), it could also mean that alcohol may only affect specific phosphorylation sites. We also observed that inhibitory Thr 495 phosphorylation exhibited an inverse U shaped relationship and was barely detectable in the Ctrl and HD groups whereas it was detectable in the LD group. Thr 495 is located in the calcium/calmodulin binding domain of eNOS and its phosphorylation inhibits eNOS-calmodulin interaction and the activity of the enzyme (Cale and Bird, 2006; Mount et al., 2007). In summary, alcohol adversely affects eNOS multi-site phosphorylation suggesting lowered enzyme activity state and uterine NO production.
In resting conditions, eNOS is primarily located in specialized protein-rich plasmalemmal invaginations called caveolae, where its activity is inhibited by direct binding to the scaffolding protein cav-1 (Michell et al., 2002; Sessa, 2004; Shaul, 2002). The scaffolding domain (amino acids 61–101) and to a lesser extent the C-terminal tail (amino acids 135–178) of cav-1 directly interact with eNOS (Garcia-Cardena et al., 1997). In this study, we observed that total cav-1 was significantly decreased in the LD alcohol group and was barely detected in the HD group. Our data support earlier studies where alcohol has been demonstrated to disrupt the caveolae by caveolar cholesterol/lipid depletion and disruption of caveolar assembly of proteins (Mao et al., 2009; Ramadoss et al., 2010; Ronis et al., 2007). Collectively, these data suggest that chronic binge-like alcohol has deleterious effects on the caveolar lipid rafts. Thus, we hypothesize that alcohol will negatively impact the temporal and spatial partitioning of eNOS between caveolar and non-caveolar sub-cellular compartments. We observed that total AKT expression trended to be biphasic, with higher levels in the LD group compared to the Control and the HD group. However, the phosphorylation of Ser 473 residue of AKT was not different among groups. The protein kinase AKT is the most cited kinase protein in the context of eNOS phosphorylation and is an important downstream target of PI3 kinase (Dimmeler et al., 1999; Michell et al., 1999; Michell et al., 2002; Mount et al., 2007; Tanimoto et al., 2002; Tsang et al., 2004). In the systemic endothelium, inhibition of the AKT pathway or mutation of the AKT site on eNOS protein (at Ser 1177) attenuates the serine phosphorylation and prevents the activation of eNOS (Dimmeler et al., 1999). In contrast, it has been reported previously that AKT may not play a significant role in uterine vascular eNOS activity (Bird et al., 2003). Therefore, the next step will be to investigate if alcohol mediates altered eNOS multi-site phosphorylation via alternate signaling cascades like the ERK and the P38 MAPK pathway.
Finally, we further investigated uterine vascular adaptation by assessing estradiol-17β-mediated endothelial proliferation. We specifically studied mitogenic responses to estradiol-17β, a hormone that is significantly elevated in pregnancy (Carnegie and Robertson, 1978; Magness, 1998) and acts via specifically upregulating uterine eNOS expression and activity (Vagnoni et al., 1998). Further, we recently demonstrated that estradiol-17β increased uterine artery endothelial cell proliferation in a bi-phasic dose-dependent pattern (Jobe et al., 2010). In the present study, we observed that alcohol abolished the mitogenic effects of estradiol-17β. However, in both HD and LD groups, the presence of alcohol with estradiol-17β significantly decreased proliferation independent of whether the cells were previously exposed to binge-like alcohol. Unlike the effects of HD alcohol, despite prior binge-like LD alcohol exposure, estradiol-17β induced proliferation was less affected when LD alcohol was not present together with estradiol-17β in the system. This observation is easily attributable to dose-dependent effects of alcohol or the partial recovery of proliferative response in the LD compared to the HD group. Collectively these data support the notion that chronic binge-like alcohol inhibits uterine angiogeneic indices. In support of the fact that all models of Intrauterine Growth Restriction (IUGR) developed to date are associated with some degree of reduction in uterine blood flow (Reynolds et al., 2006), our data suggests that FASD-associated IUGR must also be accompanied by a severely compromised uterine vascular function. Whether this is mediated by abrogation of the estradiol-17β mediated uterine angiogenesis or reductions in vasodilator NO production remains to be determined. Other studies do show an interaction between estradiol-17β and alcohol as well as a sexually dimorphic differential response. Associations between alcohol and estradiol-17β have also been noted with reference to the cytochrome P450 CYP19 aromatase activity; perinatal alcohol exposure increases aromatase activity in select brain regions in male but not female rats (McGivern et al., 1988). In humans, elevated estradiol-17β is associated with increases in acetaldehyde levels in women but not men (Eriksson et al., 1996). In summary, we demonstrate here that there is a negative effect of alcohol on estradiol-17β-induced NO associated vascular endothelial proliferation in the uterine endothelium.
We conclude that chronic binge alcohol modulates specific uterine vascular adaptations during pregnancy including the NO system. Effects of alcohol range from altered uterine endothelial expression of total eNOS to disrupted eNOS multi-site phosphorylation and altered expression of major NO-associated proteins including cav-1 and AKT. Furthermore, alcohol negatively modulates estrogen-induced uterine angiogenesis. As part of this publication series, we will investigate the mechanistic perspectives underlying alcohol-induced alterations in uterine vascular adaptations in the pregnant state in an attempt to understand the role played by the maternal uterine vasculature in the pathogenesis of FASD.
Acknowledgements
We wish to thank Gladys Lopez, Benjamin Hofeld, Tess Becker, Ryan Robin, Daniel Faust, Tim Morschauser, Mayra B. Pastore, Mary Y. Sun, Cindy Goss and Jason Austin for their assistance in this study.
Supported by NIH grants AA19446 (JR); HL49210, HD38843, HL89144 (RRM).
REFERENCES
- Abdollah S, Brien JF. Effect of chronic maternal ethanol administration on glutamate and N-methyl-D-aspartate binding sites in the hippocampus of the near-term fetal guinea pig. Alcohol. 1995;12(4):377–82. doi: 10.1016/0741-8329(95)00021-i. [DOI] [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43(3):148–54. [PubMed] [Google Scholar]
- Bird IM, Sullivan JA, Di T, Cale JM, Zhang L, Zheng J, Magness RR. Pregnancy-dependent changes in cell signaling underlie changes in differential control of vasodilator production in uterine artery endothelial cells. Endocrinology. 2000;141(3):1107–17. doi: 10.1210/endo.141.3.7367. [DOI] [PubMed] [Google Scholar]
- Bird IM, Zhang L, Magness RR. Possible mechanisms underlying pregnancy-induced changes in uterine artery endothelial function. Am J Physiol Regul Integr Comp Physiol. 2003;284(2):R245–58. doi: 10.1152/ajpregu.00108.2002. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, Bonthius NE, Li S, Karacay B. The protective effect of neuronal nitric oxide synthase (nNOS) against alcohol toxicity depends upon the NO-cGMP-PKG pathway and NF-kappaB. Neurotoxicology. 2008;29(6):1080–91. doi: 10.1016/j.neuro.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, Bonthius NE, Napper RM, West JR. Early postnatal alcohol exposure acutely and permanently reduces the number of granule cells and mitral cells in the rat olfactory bulb: a stereological study. J Comp Neurol. 1992;324(4):557–66. doi: 10.1002/cne.903240408. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, Karacay B, Dai D, Pantazis NJ. FGF-2, NGF and IGF-1, but not BDNF, utilize a nitric oxide pathway to signal neurotrophic and neuroprotective effects against alcohol toxicity in cerebellar granule cell cultures. Brain Res Dev Brain Res. 2003;140(1):15–28. doi: 10.1016/s0165-3806(02)00549-7. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, Luong T, Bonthius NE, Hostager BS, Karacay B. Nitric oxide utilizes NF-kappaB to signal its neuroprotective effect against alcohol toxicity. Neuropharmacology. 2009;56(3):716–31. doi: 10.1016/j.neuropharm.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Boo YC, Sorescu GP, Bauer PM, Fulton D, Kemp BE, Harrison DG, Sessa WC, Jo H. Endothelial NO synthase phosphorylated at SER635 produces NO without requiring intracellular calcium increase. Free Radic Biol Med. 2003;35(7):729–41. doi: 10.1016/s0891-5849(03)00397-6. [DOI] [PubMed] [Google Scholar]
- Caetano R, Ramisetty-Mikler S, Floyd LR, McGrath C. The epidemiology of drinking among women of child-bearing age. Alcohol Clin Exp Res. 2006;30(6):1023–30. doi: 10.1111/j.1530-0277.2006.00116.x. [DOI] [PubMed] [Google Scholar]
- Cale JM, Bird IM. Dissociation of endothelial nitric oxide synthase phosphorylation and activity in uterine artery endothelial cells. Am J Physiol Heart Circ Physiol. 2006;290(4):H1433–45. doi: 10.1152/ajpheart.00942.2005. [DOI] [PubMed] [Google Scholar]
- Carnegie JA, Robertson HA. Conjugated and unconjugated estrogens in fetal and maternal fluids of the pregnant ewe: a possible role for estrone sulfate during early pregnancy. Biol Reprod. 1978;19(1):202–11. doi: 10.1095/biolreprod19.1.202. [DOI] [PubMed] [Google Scholar]
- Chen DB, Bird IM, Zheng J, Magness RR. Membrane estrogen receptor-dependent extracellular signal-regulated kinase pathway mediates acute activation of endothelial nitric oxide synthase by estrogen in uterine artery endothelial cells. Endocrinology. 2004;145(1):113–25. doi: 10.1210/en.2003-0547. [DOI] [PubMed] [Google Scholar]
- Church MW, Gerkin KP. Hearing disorders in children with fetal alcohol syndrome: findings from case reports. Pediatrics. 1988;82(2):147–54. [PubMed] [Google Scholar]
- Cook JL, Zhang Y, Davidge ST. Vascular function in alcohol-treated pregnant and nonpregnant mice. Am J Physiol Regul Integr Comp Physiol. 2001;281(5):R1449–55. doi: 10.1152/ajpregu.2001.281.5.R1449. [DOI] [PubMed] [Google Scholar]
- Cudd TA. Animal model systems for the study of alcohol teratology. Exp Biol Med (Maywood) 2005;230(6):389–93. doi: 10.1177/15353702-0323006-06. [DOI] [PubMed] [Google Scholar]
- Cudd TA, Chen WJ, Parnell SE, West JR. Third trimester binge ethanol exposure results in fetal hypercapnea and acidemia but not hypoxemia in pregnant sheep. Alcohol Clin Exp Res. 2001;25(2):269–76. [PubMed] [Google Scholar]
- Day NL, Jasperse D, Richardson G, Robles N, Sambamoorthi U, Taylor P, Scher M, Stoffer D, Cornelius M. Prenatal exposure to alcohol: effect on infant growth and morphologic characteristics. Pediatrics. 1989;84(3):536–41. [PubMed] [Google Scholar]
- Day NL, Leech SL, Richardson GA, Cornelius MD, Robles N, Larkby C. Prenatal alcohol exposure predicts continued deficits in offspring size at 14 years of age. Alcohol Clin Exp Res. 2002;26(10):1584–91. doi: 10.1097/01.ALC.0000034036.75248.D9. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399(6736):601–5. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Eriksson CJ, Fukunaga T, Sarkola T, Lindholm H, Ahola L. Estrogen-related acetaldehyde elevation in women during alcohol intoxication. Alcohol Clin Exp Res. 1996;20(7):1192–5. doi: 10.1111/j.1530-0277.1996.tb01110.x. [DOI] [PubMed] [Google Scholar]
- Eysseric H, Gonthier B, Soubeyran A, Bessard G, Saxod R, Barret L. There is not simple method to maintain a constant ethanol concentration in long-term cell culture: keys to a solution applied to the survey of astrocytic ethanol absorption. Alcohol. 1997;14(2):111–5. doi: 10.1016/s0741-8329(96)00112-7. [DOI] [PubMed] [Google Scholar]
- Falconer J. The effect of maternal ethanol infusion on placental blood flow and fetal glucose metabolism in sheep. Alcohol Alcohol. 1990;25(4):413–6. [PubMed] [Google Scholar]
- Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem. 1997;272(41):25437–40. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- Gladstone J, Nulman I, Koren G. Reproductive risks of binge drinking during pregnancy. Reprod Toxicol. 1996;10(1):3–13. doi: 10.1016/0890-6238(95)02024-1. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Horn KH, Zhou FC. Alcohol teratogenesis: mechanisms of damage and strategies for intervention. Exp Biol Med (Maywood) 2005;230(6):394–406. doi: 10.1177/15353702-0323006-07. [DOI] [PubMed] [Google Scholar]
- Hammond KB, Rumack BH, Rodgerson DO. Blood ethanol. A report of unusually high levels in a living patient. Jama. 1973;226(1):63–4. doi: 10.1001/jama.226.1.63. [DOI] [PubMed] [Google Scholar]
- Jobe SO, Ramadoss J, Koch JM, Jiang Y, Zheng J, Magness RR. Estradiol-17beta and its cytochrome P450- and catechol-O-methyltransferase-derived metabolites stimulate proliferation in uterine artery endothelial cells: role of estrogen receptor-alpha versus estrogen receptor-beta. Hypertension. 2010;55(4):1005–11. doi: 10.1161/HYPERTENSIONAHA.109.146399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukreja RC, Xi L. eNOS phosphorylation: a pivotal molecular switch in vasodilation and cardioprotection? J Mol Cell Cardiol. 2007;42(2):280–2. doi: 10.1016/j.yjmcc.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losordo DW, Isner JM. Estrogen and angiogenesis: A review. Arterioscler Thromb Vasc Biol. 2001;21(1):6–12. doi: 10.1161/01.atv.21.1.6. [DOI] [PubMed] [Google Scholar]
- Magness RR. The Endocrinology of Pregnancy Bazer. Humana Press; 1998. Maternal cardiovascular and other physiologic responses to the endocrinology of pregnancy; pp. 507–539. [Google Scholar]
- Magness RR, Phernetton TM, Gibson TC, Chen DB. Uterine blood flow responses to ICI 182 780 in ovariectomized oestradiol-17beta-treated, intact follicular and pregnant sheep. J Physiol. 2005;565(Pt 1):71–83. doi: 10.1113/jphysiol.2005.086439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magness RR, Shaw CE, Phernetton TM, Zheng J, Bird IM. Endothelial vasodilator production by uterine and systemic arteries. II. Pregnancy effects on NO synthase expression. Am J Physiol. 1997;272(4 Pt 2):H1730–40. doi: 10.1152/ajpheart.1997.272.4.H1730. [DOI] [PubMed] [Google Scholar]
- Magness RR, Sullivan JA, Li Y, Phernetton TM, Bird IM. Endothelial vasodilator production by uterine and systemic arteries. VI. Ovarian and pregnancy effects on eNOS and NO(x) Am J Physiol Heart Circ Physiol. 2001;280(4):H1692–8. doi: 10.1152/ajpheart.2001.280.4.H1692. [DOI] [PubMed] [Google Scholar]
- Maier SE, West JR. Drinking patterns and alcohol-related birth defects. Alcohol Res Health. 2001;25(3):168–74. [PMC free article] [PubMed] [Google Scholar]
- Mao H, Diehl AM, Li YX. Sonic hedgehog ligand partners with caveolin-1 for intracellular transport. Lab Invest. 2009;89(3):290–300. doi: 10.1038/labinvest.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara K, Matsubara Y, King AG, Z J, Abe E, Ito M, Magness RR. In: Regulation of endothelial cell proliferation by estrogen in reproductive organs, in Cell growth in processes: new research. Kimura D, editor. Nova Science Publishers, Inc.; Hauppauge: 2008. pp. 159–182. [Google Scholar]
- Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22(2):279–94. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Delis DC, Stern C, Jones KL. Verbal learning and memory in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20(5):810–6. doi: 10.1111/j.1530-0277.1996.tb05256.x. [DOI] [PubMed] [Google Scholar]
- McGivern RF, Roselli CE, Handa RJ. Perinatal aromatase activity in male and female rats: effect of prenatal alcohol exposure. Alcohol Clin Exp Res. 1988;12(6):769–72. doi: 10.1111/j.1530-0277.1988.tb01342.x. [DOI] [PubMed] [Google Scholar]
- Michell BJ, Griffiths JE, Mitchelhill KI, Rodriguez-Crespo I, Tiganis T, Bozinovski S, de Montellano PR, Kemp BE, Pearson RB. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr Biol. 1999;9(15):845–8. doi: 10.1016/s0960-9822(99)80371-6. [DOI] [PubMed] [Google Scholar]
- Michell BJ, Harris MB, Chen ZP, Ju H, Venema VJ, Blackstone MA, Huang W, Venema RC, Kemp BE. Identification of regulatory sites of phosphorylation of the bovine endothelial nitric-oxide synthase at serine 617 and serine 635. J Biol Chem. 2002;277(44):42344–51. doi: 10.1074/jbc.M205144200. [DOI] [PubMed] [Google Scholar]
- Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol. 2007;42(2):271–9. doi: 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 2009;24:58–71. doi: 10.1152/physiol.00033.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell SE, Ramadoss J, Delp MD, Ramsey MW, Chen WJ, West JR, Cudd TA. Chronic ethanol increases fetal cerebral blood flow specific to the ethanol-sensitive cerebellum under normoxaemic, hypercapnic and acidaemic conditions: ovine model. Exp Physiol. 2007;92(5):933–43. doi: 10.1113/expphysiol.2007.038091. [DOI] [PubMed] [Google Scholar]
- Ramadoss J, Liao WX, Chen DB, Magness RR. High-throughput caveolar proteomic signature profile for maternal binge alcohol consumption. Alcohol. 2010 doi: 10.1016/j.alcohol.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Lunde ER, Chen WJ, West JR, Cudd TA. Temporal vulnerability of fetal cerebellar Purkinje cells to chronic binge alcohol exposure: ovine model. Alcohol Clin Exp Res. 2007a;31(10):1738–45. doi: 10.1111/j.1530-0277.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- Ramadoss J, Lunde ER, Ouyang N, Chen WJ, Cudd TA. Acid-sensitive channel inhibition prevents fetal alcohol spectrum disorders cerebellar Purkinje cell loss. Am J Physiol Regul Integr Comp Physiol. 2008;295(2):R596–603. doi: 10.1152/ajpregu.90321.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Lunde ER, Pina KB, Chen WJ, Cudd TA. All three trimester binge alcohol exposure causes fetal cerebellar purkinje cell loss in the presence of maternal hypercapnea, acidemia, and normoxemia: ovine model. Alcohol Clin Exp Res. 2007b;31(7):1252–8. doi: 10.1111/j.1530-0277.2007.00422.x. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Caton JS, Redmer DA, Grazul-Bilska AT, Vonnahme KA, Borowicz PP, Luther JS, Wallace JM, Wu G, Spencer TE. Evidence for altered placental blood flow and vascularity in compromised pregnancies. J Physiol. 2006;572(Pt 1):51–8. doi: 10.1113/jphysiol.2005.104430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Utero-placental vascular development and placental function. J Anim Sci. 1995;73(6):1839–51. doi: 10.2527/1995.7361839x. [DOI] [PubMed] [Google Scholar]
- Ronis MJ, Wands JR, Badger TM, de la Monte SM, Lang CH, Calissendorff J. Alcohol-induced disruption of endocrine signaling. Alcohol Clin Exp Res. 2007;31(8):1269–85. doi: 10.1111/j.1530-0277.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CR. Distribution of cardiac output in ovine pregnancy. Am J Physiol. 1977;232(3):H231–5. doi: 10.1152/ajpheart.1977.232.3.H231. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CR, Cox BE, Roy T, Magness RR. Nitric oxide contributes to estrogen-induced vasodilation of the ovine uterine circulation. J Clin Invest. 1996;98(9):2158–66. doi: 10.1172/JCI119022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi GM, Johns A, Kauser K. Effect of estrogen on endothelial function and angiogenesis. Vascul Pharmacol. 2002;38(2):89–98. doi: 10.1016/s0306-3623(02)00131-3. [DOI] [PubMed] [Google Scholar]
- Sessa WC. eNOS at a glance. J Cell Sci. 2004;117(Pt 12):2427–9. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- Shaul PW. Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol. 2002;64:749–74. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- Tanimoto T, Jin ZG, Berk BC. Transactivation of vascular endothelial growth factor (VEGF) receptor Flk-1/KDR is involved in sphingosine 1-phosphate-stimulated phosphorylation of Akt and endothelial nitric-oxide synthase (eNOS) J Biol Chem. 2002;277(45):42997–3001. doi: 10.1074/jbc.M204764200. [DOI] [PubMed] [Google Scholar]
- Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res. 2004;95(3):230–2. doi: 10.1161/01.RES.0000138303.76488.fe. [DOI] [PubMed] [Google Scholar]
- Urso T, Gavaler JS, Van Thiel DH. Blood ethanol levels in sober alcohol users seen in an emergency room. Life Sci. 1981;28(9):1053–6. doi: 10.1016/0024-3205(81)90752-9. [DOI] [PubMed] [Google Scholar]
- Vagnoni KE, Shaw CE, Phernetton TM, Meglin BM, Bird IM, Magness RR. Endothelial vasodilator production by uterine and systemic arteries. III. Ovarian and estrogen effects on NO synthase. Am J Physiol. 1998;275(5 Pt 2):H1845–56. doi: 10.1152/ajpheart.1998.275.5.H1845. [DOI] [PubMed] [Google Scholar]
- Vonnahme KA, Wilson ME, Li Y, Rupnow HL, Phernetton TM, Ford SP, Magness RR. Circulating levels of nitric oxide and vascular endothelial growth factor throughout ovine pregnancy. J Physiol. 2005;565(Pt 1):101–9. doi: 10.1113/jphysiol.2004.082321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20(4):470–88. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells DJ, Jr., Barnhill MT., Jr. Unusually high ethanol levels in two emergency medicine patients. J Anal Toxicol. 1996;20(4):272. doi: 10.1093/jat/20.4.272. [DOI] [PubMed] [Google Scholar]
- West JR, Parnell SE, Chen WJ, Cudd TA. Alcohol-mediated Purkinje cell loss in the absence of hypoxemia during the third trimester in an ovine model system. Alcohol Clin Exp Res. 2001;25(7):1051–7. [PubMed] [Google Scholar]
- Wulff C, Wilson H, Wiegand SJ, Rudge JS, Fraser HM. Prevention of thecal angiogenesis, antral follicular growth, and ovulation in the primate by treatment with vascular endothelial growth factor Trap R1R2. Endocrinology. 2002;143(7):2797–807. doi: 10.1210/endo.143.7.8886. [DOI] [PubMed] [Google Scholar]
- Yi FX, Magness RR, Bird IM. Simultaneous imaging of [Ca2+]i and intracellular NO production in freshly isolated uterine artery endothelial cells: effects of ovarian cycle and pregnancy. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R140–8. doi: 10.1152/ajpregu.00302.2004. [DOI] [PubMed] [Google Scholar]
- Zygmunt M, Herr F, Munstedt K, Lang U, Liang OD. Angiogenesis and vasculogenesis in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2003;110(Suppl 1):S10–8. doi: 10.1016/s0301-2115(03)00168-4. [DOI] [PubMed] [Google Scholar]