Abstract
Background
An optimal management of maternal hyperthyroidism is important for positive pregnancy outcome, and to this end, the Endocrine Society published their guidelines in 2007. This survey aimed to investigate to what extent the clinical practice relating to the management of hyperthyroidism during pregnancy in Europe is uniform and consistent with the guidelines. Materials and Methods: We e-mailed an online questionnaire survey based on clinical case scenarios to 605 members of the European Thyroid Association. We analysed 190 responses from 28 European countries.
Results
For a woman with newly diagnosed Graves' disease (GD) and wishing pregnancy, 78% of the responders would initiate antithyroid drugs (ATDs), while 22% would recommend definitive treatment with radioiodine or surgery. In case of a relapsed GD before pregnancy, 80% preferred definitive treatment. For a woman with newly diagnosed GD during pregnancy, 53% would treat with propylthiouracil, 12% with methimazole, and 34% with propylthiouracil initially and switch to methimazole after the first trimester. Responders used several combinations of tests to monitor the dose of ATDs, and the thyroid test results they targeted were inconsistent. For a lactating woman with GD, 68% would give ATDs without stopping lactation.
Conclusions
Variation in the clinical practices surrounding the management of hyperthyroid pregnant women in Europe still exists.
Key Words: European Thyroid Association, Graves' disease, Hyperthyroidism, Management, Pregnancy, Survey, Thyroid
Introduction
The prevalence of hyperthyroidism in pregnancy ranges between 0.1 and 1% [1]. The most common cause of hyperthyroidism in pregnancy is Graves' disease (GD), occurring in about 85% of cases. Gestational transient thyrotoxicosis (GTT), which results from the direct stimulatory effect of human chorionic gonadotrophin on the thyroid gland, is also fairly common and accounts for ∼10% of cases. It most often presents as a subclinical hyperthyroidism in the first half of gestation and this is not associated with an adverse pregnancy outcome [2,3]. GD and GTT must be distinguished from each other because their clinical courses, associated risks for the mother and the fetus, and the management are different. In general the high human chorionic gonadotrophin secretion in the first half of pregnancy is associated with a downward shift of the serum TSH and therefore a need to use trimester-specific reference ranges for serum TSH [4]. Retrospective studies have shown that prolonged and inadequate control of maternal overt thyrotoxicosis in pregnancy is associated with maternal and fetal/neonatal complications [1,5], and these complications can be prevented by early and optimal treatment with antithyroid drugs (ATDs) [1,6]. However, there have been several reports of severe congenital malformations (the ‘methimazole embryopathy’) associated with methimazole (MMI) or carbimazole (CMZ) use in early pregnancy, and recently it has been highlighted that propylthiouracil (PTU) is associated with rare but severe liver failure [7,8]. The Endocrine Society guidelines on the management of thyroid disorders, including hyperthyroidism, during pregnancy were published in 2007 [9]. The aim of this questionnaire survey was to assess how European clinicians manage hyperthyroidism in pregnant women and to what extent their clinical practice is consistent with these guidelines.
Participants and Methods
In December 2010, an electronic questionnaire survey was e-mailed to 605 members of the European Thyroid Association (ETA). This was followed by a reminder e-mail in January 2011. The survey was based on clinical case scenarios, and asked questions about the clinical practices related to the management of hypothyroidism and hyperthyroidism in pregnancy. Two authors (B.V. and K.P.) prepared an initial draft of the survey questionnaire covering key contentious issues on the topic. The draft questionnaire was piloted amongst the co-authors. Following the pilot and feedback from all the co-authors, the questionnaire was revised and finalized for wider distribution. The questionnaire contained 12 multiple-choice questions on management of hyperthyroidism in pregnancy. Responders were asked to select one (11 questions) or multiple (1 question) answers. However, for most questions, they were also able to provide their own answer if it was not included in the questionnaire. A copy of the questionnaire is available on request from the authors. We previously published the results on the management of hypothyroidism during pregnancy [10], and now report the results on the management of hyperthyroidism during pregnancy.
All frequencies were adjusted on a 100% basis excluding the non-responders. Results are predominantly presented as percentages. A statistical analysis was performed by means of Fisher's exact test. All statistical tests were considered statistically significant whenever p < 0.05.
Results
Characteristics of the Responders
We received 210 responses from 32 countries. Fourteen responders were not involved in the management of thyroid diseases in pregnancy. Six responders were from non-European countries (3 USA, 1 China, 1 Australia and 1 not specified), and were not included in the current analysis. Therefore, responses from 190 responders practicing in 28 European countries were analysed. Although the ETA membership database does not distinguish clinician and basic science members, it is estimated that the responders in this survey represent over 50% of practicing clinician members of the association. Most of the responders were endocrinologists (90%); the remaining included nuclear medicine specialists (4%), surgeons (4%), general practitioners (1.5%) and general internists (0.5%).
The majority of responders (71.5%) reported that endocrinologists managed hyperthyroidism in pregnancy in their institutions. In the remaining of the responders' institutions, the condition was managed by endocrinologists and obstetricians jointly in a multidisciplinary clinic (25%), nuclear medicine specialists (2.5%) or obstetricians (1%).
Treatment of GD in Pregnancy
Responders were asked how they would treat a woman with newly diagnosed GD wishing to become pregnant soon. Most responders (78%) reported that they would treat the patient with ATDs, with a slight preference for PTU. For a woman with a third relapse of GD and wishing to become pregnant, most responders preferred a definitive treatment (80%) with surgery or radioiodine (131I) rather than another course of ATDs; p < 0.0001 as compared to responders' preferred treatment for a patient with newly diagnosed GD (table 1).
Table 1.
Responders’ initial treatment of choice for a 26-year-old woman with newly diagnosed GD and a 34-year-old woman with a third relapse of GD, both wishing to become pregnant soon
| Type of treatment | First episode of GD responders, n (%) | Third relapse of GD responders, n (%) | |
|---|---|---|---|
| MMI/CMZ | 62 (36.2) | 15 (8.8) | |
| PTU | 72 (42.1) | 17 (9.9) | |
| Refer for definitive treatment with surgery before pregnancy | 15 (8.8)* | 82 (48) | |
| Refer to definitive treatment with 131I before pregnancy | 22 (12.9)* | 51 (29.8) | |
| Other | – | 6 (3.5)a |
p < 0.0001 compared to third relapse of GD.
131I or surgery depending upon patient choice or timing of planned pregnancy (n = 4), MMI/CMZ followed by surgery (n = 1), previously tolerated ATD (n = 1).
For an 8-week pregnant woman with a newly discovered GD, the majority (53%) of the responders would treat with PTU. However, one third of the responders would treat the woman with PTU in the first trimester, and then switch to MMI/CMZ (table 2).
Table 2.
Responders’ choice of treatment for a 24-year-old woman with newly diagnosed GD and for a 24-year-old woman with GTT, both at 8 weeks’ gestation
| Type of treatment | Newly diagnosed GD responders, n (%) | GTT responders, n (%) |
|---|---|---|
| MMI/CMZ | 21 (12.3) | 11 (6.5) |
| PTU | 90 (52.6) | 31 (18.3) |
| PTU in the first trimester, then change to MMI/CMZ | 58 (33.9) | 28 (16.6) |
| Follow up without treatment | 1 (0.6) | 85 (50.3) |
| Other | 1 (0.6)a | 14 (8.3)b |
β-Blocker (n = 1);
β-blocker (n = 6), miscellaneous (n = 8).
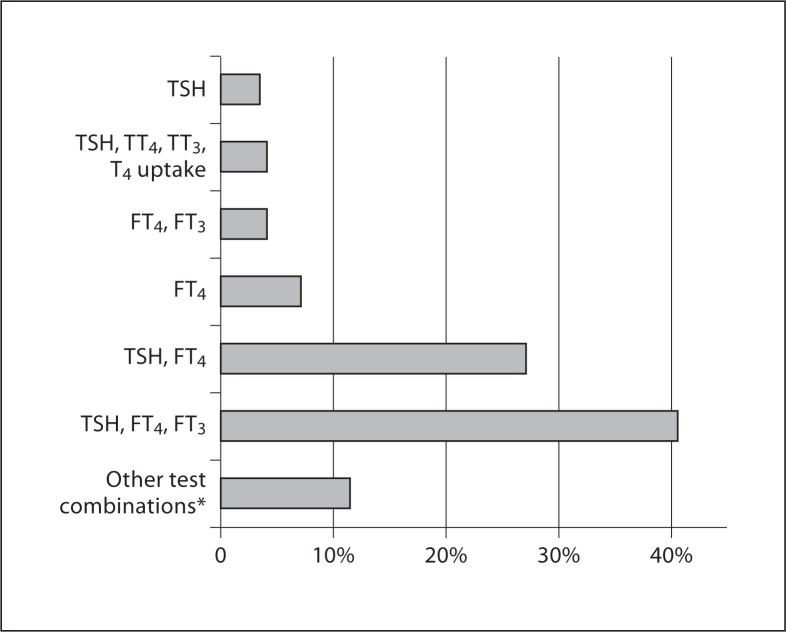
Responders used 18 different combinations of tests to monitor the dose of ATDs during pregnancy. The majority of responders (41%) would measure serum TSH, free T4 (FT4) and free T3 (FT3), whilst 27% would measure serum TSH and FT4 (fig. 1).
Fig. 1.
Different test responders used to monitor the dose of ATDs in pregnancy. FT4 = Free T4; FT3 = free T3; TSH = thyroid-stimulating hormone; TT3 = total T3; TT4 = total T4. * Twelve different test combinations (various combinations of TSH, FT4, FT3, TT4, TT3, T4 uptake and TRAb).
Responders aimed to achieve variable target thyroid function test results, although the majority (64%) would aim for a low serum TSH together with an FT4 or total T4 (TT4) in the upper end of the normal range (table 3).
Table 3.
Target thyroid function tests that responders aim to achieve with ATDs in pregnancy
| Tests | Responders n (%) |
|---|---|
| Low TSH and FT4 (TT4) in the upper end of the normal range | 110 (64.3) |
| Low TSH and FT4 (TT4) in the normal range | 33 (19.3) |
| TSH and FT4 (TT4) in the normal range | 22 (12.8) |
| Low TSH, independent of FT4 (TT4) levels | 3 (1.8) |
| Other | 3 (1.8)a |
Low TSH and high normal FT4 (n = 1), depending upon titre of thyrotropin receptor antibodies (n = 1), miscellaneous (n = 1).
Nearly 90% of the responders would measure the thyrotropin receptor antibodies (TRAb) at least once in a pregnant woman who is being treated with ATDs for GD; however, the responders' recommended timings for the test were variable. In a euthyroid pregnant woman previously treated with 131I or total thyroidectomy for GD, 20% of the responders would never measure TRAb during the pregnancy compared to 11% in women with actively treated GD; p = 0.026 (table 4).
Table 4.
Responders’ recommendations for the measurement of TRAb during pregnancy in a woman with GD on ATDs and in a euthyroid pregnant woman previously treated with 131I or total thyroidectomy for GD
| Timing of measurement | GD on ATDs responders, n (%) | Euthyroid GD after previous 131I or thyroidectomy responders, n (%) |
|---|---|---|
| No (never) | 19 (11.2)* | 35 (20.5) |
| Yes, in each trimester | 29 (17.1) | 17 (9.9) |
| Yes, in the first trimester and third trimester | 25 (14.7) | 21 (12.3) |
| Yes, in the first, and if positive in the third trimester | 71 (41.8) | 68 (39.8) |
| Yes, in the third trimester | 23 (13.5) | 26 (15.2) |
| Other | 3 (1.7)a | 4 (2.3)b |
p = 0.026 compared to euthyroid GD after previous 131I or thyroidectomy.
Second trimester (n = 1), yes, trimester unspecified (n = 1), miscellaneous (n = 1).
Second trimester (n = 1), first trimester and if positive in each trimester (n = 1), depending upon the time lapse between 131I/surgery and pregnancy (n = 1), miscellaneous (n = 1).
In a pregnant woman with GD treated with ATDs, 66% of the responders routinely monitored the fetus with ultrasound scan, whilst 24% carried out fetal ultrasound monitoring if the mother had TRAb and 10% did not carry out fetal ultrasound monitoring.
Treatment of Gestational Hyperthyroidism
For an 8-week pregnant woman with GTT, more than 50% of the responders would follow up without specific treatment; however, 41% would treat the woman with ATDs (table 2).
Treatment of GD during Lactation
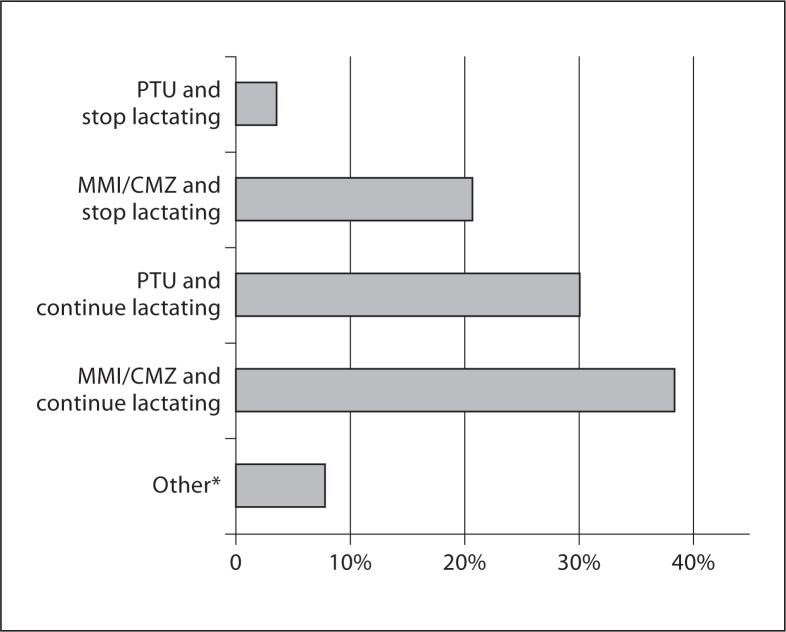
For a woman who is lactating and has a relapsed GD, the majority (68%) of responders would recommend treatment with ATDs without stopping lactation. However, 24% of the responders would treat the woman with ATDs and advise to stop lactation (fig. 2). More responders preferred MMI/CMZ than PTU (59 vs. 36%).
Fig. 2.
Responders' recommendations for a 30-year-old woman who is 3 months postpartum, lactating and has a relapse of GD. * Follow-up without treatment (n = 1), PTU once daily and discarding milk produced in 6 h after the dose (n = 1), PTU and continue lactation as long as the dose is <300 mg daily (n = 3), stop lactation and 131I (n = 1), and miscellaneous (n = 7).
Discussion
This survey has demonstrated that there is wide variation in the clinical practice relating to the management of maternal hyperthyroidism during pregnancy in Europe. Despite the existence of clinical guidelines [9], the survey showed inconsistencies in the treatment of women with GD planning pregnancy, the choice and monitoring of ATDs in pregnant women, treatment of GTT and the choice of ATDs in lactating women.
Although prospective studies on the management of hyperthyroidism in women with hard pregnancy endpoints are scarce it is generally accepted that optimum treatment of overt maternal hyperthyroidism is important for positive pregnancy outcomes [1,9,11]. For a woman with newly diagnosed GD and planning pregnancy, most responders would initiate ATDs, with a slight preference for PTU over MMI/CMZ. Although overt maternal hyperthyroidism itself may increase the risk of congenital malformations in the fetus [12], there is increasing evidence for an association between MMI/CMZ use in early pregnancy and rare ‘embryopathy’ [7,11,13]. Therefore, it is interesting to note that over one third of the responders would prescribe the drug for the woman contemplating pregnancy.
Although the recent ATA guidelines support the use of MMI/CMZ in such a situation and recommend changing MMI/CMZ to PTU once the pregnancy is confirmed [11], it remains unproven if this approach is adequate to prevent MMI/CMZ-associated embryopathy. Therefore, not surprisingly, for a woman with relapsed GD and planning pregnancy, most responders preferred a ‘definitive’ treatment with surgery or 131I before pregnancy. 131I is widely used for the treatment of hyperthyroidism, and the average dose of 10 mCi (370 MBq) used does not lead to an impaired gonadal function. However, it is recommended to avoid conception for 6 months after the administration of 131I [14]. The choice of 131I as treatment thus also depends on the urgency of the pregnancy wish. However, prospective pregnancy outcome data on the optimal treatment in this setting are still lacking.
In view of the association between fetal exposure to MMI/CMZ during early gestation and embryopathy, both the Endocrine Society and the ATA guidelines recommend PTU as the ATD of choice in the first trimester [9,11]. In accordance with these guidelines, only 12% of the responders would prescribe MMI/CMZ for a woman diagnosed with GD in the first trimester. Although PTU has not been convincingly shown to be associated with embryopathy, this drug has been shown to be associated with rare severe liver failure [8]. Therefore, the recent ATA guidelines state that consideration should be given to switch from PTU to MMI after the first trimester [11]; however, this approach is yet to be proven beneficial by well-designed clinical studies. In our survey, only one third of the responders followed this approach, and despite the risk of severe liver failure with PTU, more than one half of the responders preferred to use the drug for treating GD throughout the pregnancy.
Responders of this survey used many different test combinations to monitor the dose of ATDs in pregnancy. This discrepancy between responders could in part be due to availability of different thyroid function tests in the different institutions. It is interesting to note that about 1 in 10 responders used serum FT4 (with or without FT3) without serum TSH for monitoring although some studies, but not all, have questioned the accuracy of the free T4 assays during pregnancy [15,16]. Furthermore, over 60% of the responders included FT3/TT3 for titrating the dose of ATDs; however, some studies suggest that this approach could lead to overtreatment resulting in fetal hypothyroidism [17]. The overall goal of therapy is to control maternal hyperthyroidism as early as possible, with the lowest possible dose of ATDs (avoiding fetal hypothyroidism) to maintain thyroid function at a ‘high euthyroid’ level, reflected by a FT4 at the upper limit of the non-pregnant reference range [9,11]. This may lead to low or undetectable TSH levels; however, suppressed TSH levels do not appear to be associated with adverse pregnancy outcomes [3]. Consistent with the guidelines, the majority (64%) of responders in this survey would aim for low TSH and FT4 at the upper end of the reference range in these patients [9].
The measurement of TRAb in pregnant women with hyperthyroidism is useful in confirming the diagnosis of GD as well as predicting the risk of fetal hyperthyroidism [1,18]. The published guidelines have highlighted the importance of measuring TRAb in pregnant women with hyperthyroidism [9,11]. For pregnant women with GD on ATDs, these guidelines recommend measuring TRAb in the late second trimester or in the third trimester. Most responders (89%) of our survey would measure TRAb during pregnancy in these women; however, in contrast to the guidelines, the majority (74%) would initially measure these during the first trimester. For euthyroid pregnant women with GD previously treated with 131I or thyroidectomy, the European guidelines recommend measuring TRAb in the first trimester and repeating it in the third trimester if the test is positive in the first trimester [19]. In contrast, the Endocrine Society guidelines recommend measuring TRAb at the end of the second trimester [9], and similarly the ATA guidelines at 20–24 weeks' gestation [11]. Most responders (80%) of this survey would measure TRAb in these women, and the majority (65%) would initially measure this in the first trimester, in line with the European guidelines [19]. Even if these women are biochemically euthyroid, they may still be producing TRAb, which may pass through to the fetus resulting in fetal hyperthyroidism. Therefore, it is of concern that over 1 in 5 responders do not measure TRAb in these women, potentially leading to failure in diagnosing fetal or neonatal hyperthyroidism.
It has been recommended that fetal surveillance with repeated ultrasounds should be performed in women who have uncontrolled hyperthyroidism and/or women with persistently elevated TRAb during pregnancy [9,11]. Consistent with these guidelines, about two thirds of the responders in this survey monitored the fetus with ultrasound scan and a further 1 in 4 responders would do an ultrasound in the presence of TRAb; however, 1 in 10 responders would never carry out fetal ultrasound. This heterogeneity in the clinical practice may be related to the variable availability of experienced ultrasonographers in different centres.
GTT is often less severe as compared to GD and is a self-limiting condition [20]. The Endocrine Society guidelines suggest that only few women with severe cases of hyperemesis gravidarum associated with overt biochemical and clinical hyperthyroidism will require treatment [9], and the recent ATA guidelines recommend against using ATDs in GTT [11]. It is therefore remarkable that over 40% of the responders of this survey would treat this condition with ATDs. The setting of this survey does not allow exploring the rationale for this response; it is possible that the responders interpreted the severity of GTT variably and some of them may have intended to treat only for a short period. About 4% of the responders in our survey recommended using β-blockers to control symptoms of hyperthyroidism in GTT. The data in the literature seem to be reassuring when a β-blocker is used for a short period in pregnancy; however, if a long-term treatment with a β-blocker is needed, close fetal growth monitoring is advisable because of a possible association between the drug used in pregnancy with intrauterine growth restriction [21].
Our survey has highlighted the lack of consensus in the use of ATDs in women who are breast-feeding. It is noteworthy that about 25% of the responders would recommend stopping lactation when they use ATDs in a lactating woman. This is in contrast to the recommendations of both the Endocrine Society and the ATA guidelines [9,11], and several studies showing minimal risk to the infants provided that the lactating mothers are treated with moderate doses of ATDs (PTU <300 mg/day or MMI/CMZ 20–30 mg/day) [6,9,11,22]. Furthermore, despite the recently highlighted association between PTU and severe liver injury, about one third of the responders preferred PTU to treat GD in a lactating woman. This practice is not consistent with the recent ATA guidelines, which have recommended MMI as the preferred ATD for a lactating woman [11], although it should be mentioned that this survey was performed before the ATA guidelines were published.
The limitations of our survey are: (a) most of the responders were endocrinologists and none were obstetricians, although one fourth of the responders reported managing hyperthyroidism in pregnancy together with obstetricians in a multidisciplinary setting, (b) only ETA members were included, and (c) heterogeneity in the health and social care in the different European countries. All these factors could have potentially influenced the results of our survey.
In conclusion, this survey has shown that the management of hyperthyroidism in pregnant women is far from being uniform in Europe. This disparity in the clinical practice also reflects the limited evidence in this field and the need for further studies.
Disclosure Statement
The authors have no conflicts of interest to disclose.
Acknowledgements
The authors wish to thank all responders for completing the questionnaire, and the Executive Committee of the European Thyroid Association for giving us permission to carry out this survey amongst its members.
References
- 1.Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31:702–755. doi: 10.1210/er.2009-0041. [DOI] [PubMed] [Google Scholar]
- 2.Lockwood CM, Grenache DG, Gronowski AM. Serum human chorionic gonadotropin concentrations greater than 400,000 IU/l are invariably associated with suppressed serum thyrotropin concentrations. Thyroid. 2009;19:863–868. doi: 10.1089/thy.2009.0079. [DOI] [PubMed] [Google Scholar]
- 3.Casey BM, Dashe JS, Wells CE, McIntire DD, Leveno KJ, Cunningham FG. Subclinical hyperthyroidism and pregnancy outcomes. Obstet Gynecol. 2006;107:337–341. doi: 10.1097/01.AOG.0000197991.64246.9a. [DOI] [PubMed] [Google Scholar]
- 4.Glinoer D, Spencer AC. Serum TSH determinations in pregnancy: how, when and why? Nat Rev Endocrinol. 2010;6:526–529. doi: 10.1038/nrendo.2010.91. [DOI] [PubMed] [Google Scholar]
- 5.Anselmo J, Cao D, Karrison T, Weiss RE, Refetoff S. Fetal loss associated with excess of thyroid hormone exposure. JAMA. 2004;292:691–695. doi: 10.1001/jama.292.6.691. [DOI] [PubMed] [Google Scholar]
- 6.Chan GW, Mandel SJ. Therapy insight: management of Graves' disease during pregnancy. Nat Clin Pract Endocrinol Metab. 2007;3:470–478. doi: 10.1038/ncpendmet0508. [DOI] [PubMed] [Google Scholar]
- 7.Bowman P, Osborne NJ, Sturley R, Vaidya B. Carbimazole embryopathy: implications for the choice of antithyroid drugs in pregnancy. QJM 2011, E-pub ahead of print. [DOI] [PubMed]
- 8.Cooper DS, Rivkees SA. Putting propylthiouracil in perspective. J Clin Endocrinol Metab. 2009;94:1881–1882. doi: 10.1210/jc.2009-0850. [DOI] [PubMed] [Google Scholar]
- 9.Abalovich M, Amino N, Barbour LA, Cobin RH, De Groot LJ, Glinoer D, Mandel SJ, Stagnaro-Green A. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2007;92:S1–S47. doi: 10.1210/jc.2007-0141. [DOI] [PubMed] [Google Scholar]
- 10.Vaidya B, Hubalewska-Dydejczyk A, Laurberg P, Negro R, Vermiglio F, Poppe K. Treatment and screening of hypothyroidism in pregnancy: results of a European survey. Eur J Endocrinol. 2012;166:49–54. doi: 10.1530/EJE-11-0729. [DOI] [PubMed] [Google Scholar]
- 11.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S, Wiersinga W. Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease during Pregnancy and Postpartum. Thyroid. 2011;21:1081–1125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbero P, Valdez R, Rodríguez H, Tiscornia C, Mansilla E, Allons A, Coll S, Liascovich R. Choanal atresia associated with maternal hyperthyroidism treated with methimazole: a case-control study. Am J Med Genet A. 2008;15:2390–2395. doi: 10.1002/ajmg.a.32497. [DOI] [PubMed] [Google Scholar]
- 13.Bournaud C, Orgiazzi J. Antithyroid agents and embryopathies. Ann Endocrinol (Paris) 2003;64:366–369. [PubMed] [Google Scholar]
- 14.Sisson JC, Freitas J, McDougall IR, Dauer LT, Hurley JR, Brierley JD, Edinboro CH, Rosenthal D, Thomas MJ, Wexler JA, Asamoah E, Avram AM, Milas M, Greenlee C. Radiation safety in the treatment of patients with thyroid diseases by radioiodine 131I: practice recommendations of the American Thyroid Association. Thyroid. 2011;21:335–346. doi: 10.1089/thy.2010.0403. [DOI] [PubMed] [Google Scholar]
- 15.Lee RH, Spencer CA, Mestman JH, Miller EA, Petrovic I, Braverman LE, Goodwin TM. Free T4 immunoassays are flawed during pregnancy. Am J Obstet Gynecol. 2009;200:260.e1–e6. doi: 10.1016/j.ajog.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 16.Anckaert E, Poppe K, Van Uytfanghe K, Schiettecatte J, Foulon W, Thienpont LM. FT4 immunoassays may display a pattern during pregnancy similar to the equilibrium dialysis ID-LC/tandem MS candidate reference measurement procedure in spite of susceptibility towards binding protein alterations. Clin Chim Acta. 2010;411:1348–1353. doi: 10.1016/j.cca.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 17.Hamburger JI. Diagnosis and management of Graves' disease in pregnancy. Thyroid. 1992;2:219–224. doi: 10.1089/thy.1992.2.219. [DOI] [PubMed] [Google Scholar]
- 18.Laurberg P, Bournaud C, Karmisholt J, Orgiazzi J. Management of Graves' hyperthyroidism in pregnancy: focus on both maternal and foetal thyroid function, and caution against surgical thyroidectomy in pregnancy. Eur J Endocrinol. 2009;160:1–8. doi: 10.1530/EJE-08-0663. [DOI] [PubMed] [Google Scholar]
- 19.Laurberg P, Nygaard B, Glinoer D, Grussendorf M, Orgiazzi J. Guidelines for TSH-receptor antibody measurements in pregnancy. Eur J Endocrinol. 1998;139:584–586. doi: 10.1530/eje.0.1390584. [DOI] [PubMed] [Google Scholar]
- 20.Glinoer D, De Nayer P, Bourdoux Lemone M, Robyn C, van Steirteghem A, Kinthaert J, Lejeune B. Regulation of maternal thyroid function during pregnancy. J Clin Endocrinol Metab. 1990;71:276–287. doi: 10.1210/jcem-71-2-276. [DOI] [PubMed] [Google Scholar]
- 21.Redmond GP. Propranolol and fetal growth retardation. Semin Perinatol. 1982;6:142–147. [PubMed] [Google Scholar]
- 22.Azizi F, Bahrainian M, Khamseh ME, Khoshniatl M. Intellectual development and thyroid function in children who were breast-fed by thyrotoxic mothers taking methimazole. J Pediatr Endocrinol Metab. 2003;16:1239–1243. doi: 10.1515/jpem.2003.16.9.1239. [DOI] [PubMed] [Google Scholar]