Abstract
Background
Mirror therapy has been demonstrated to reduce phantom limb pain (PLP) experienced by unilateral limb amputees. Research suggests that the visual feedback of observing a limb moving in the mirror is critical for therapeutic efficacy.
Objective
Since mirror therapy is not an option for bilateral lower limb amputees, the purpose of this study was to determine if direct observation of another person’s limbs could be used to relieve PLP.
Methods
We randomly assigned 20 bilateral lower limb amputees with PLP to visual observation (n = 11) or mental visualization (n = 9) treatment. Treatment consisted of seven discrete movements which were mimicked by the amputee’s phantom limbs moving while visually observing the experimenter’s limbs moving, or closing the eyes while visualizing and attempting the movements with their phantom limbs, respectively. Participants performed movements for 20 min daily for 1 month. Response to therapy was measured using a 100-mm visual analog scale (VAS) and the McGill Short-Form Pain Questionnaire (SF-MPQ).
Results
Direct visual observation significantly reduced PLP in both legs (P < 0.05). Amputees assigned to the mental visualization condition did not show a significant reduction in PLP.
Interpretation
Direct visual observation therapy is an inexpensive and effective treatment for PLP that is accessible to bilateral lower limb amputees.
Introduction
Most individuals who have survived major limb loss experience the resurrection of their amputated limb as a phantom.1 In addition to feeling as if their amputated limb is still present, 80-90% of amputees experience phantom limb pain (PLP). While imaging studies and case series reports have shed light on neuroanatomical and perceptual correlates of phantom limbs, the etiology of phantom phenomena remains unknown.2,3 One of the leading theories contends that phantom phenomena are the result of maladaptive neuroplasticity that is driven by neuronal deafferentation or that such changes are caused by the experience of PLP itself.4–9 Alternatively, the absence of null sensory feedback to cancel inappropriate stimulation of pain by pain memories, a mismatch in visual and proprioceptive feedback from the amputated limb could cause PLP.10–12
While it is the most common therapeutic approach, pharmaceutical treatment of PLP is supported mainly by circumstantial reports and studies have not demonstrated efficacy placebo-controlled, randomized studies.13,14 A promising alternative for unilateral amputees is mirror therapy, which allows patients to “see” their phantom limb as a reflection of the intact limb. Ramachandran and Rogers-Ramachandran pioneered this therapy in a well-documented case series of 10 amputees.15 Most subjects described that they could “feel” their phantom moving when viewing the reflection of their intact hand.15 PLP was relieved through different modalities: five subjects who reported chronic cramping prior to therapy discovered newfound kinesthetic abilities, and one experienced the disappearance of the phantom limb itself.15
The efficacy of mirror therapy was confirmed in a randomized controlled trial of 22 unilateral lower extremity amputees in which 93% reported reduced PLP after 1 month of therapy, while sham (i.e., covered) mirror and mental visualization therapies did not provide relief. This suggests that visual feedback is the essential component of mirror therapy necessary to reducing PLP.16 A major disadvantage of mirror therapy is that it is a treatment only accessible to unilateral amputees, since bilateral amputees do not have an intact limb to view. This leaves a gap in noninvasive, low-cost therapeutic options available to bilateral amputees with PLP. Approximately 30% of the 1645 US military combat amputees have bilateral lower extremity amputations. Although the prevalence of bilateral amputation is lower in the general population, this number will likely rise with increasing rates of diabetes and vascular disease.17–19
In a case series of four patients with unilateral upper-limb amputations, Ramachandran reported that watching an assistant wiggle the fingers of the hand that corresponded to the amputated extremity evoked the same movement in their phantom limb.20 Similarly, two subjects in the Chan et al. study reported phantom limb movements when viewing an instructional demonstration of movements by an investigator (unpublished data from study).16 If similar kinesthetic sensations to those induced by mirror therapy could be stimulated by observing another person’s limbs moving, this suggests that pain could be relieved by a more targeted treatment regimen. We investigated the feasibility of a novel therapy, the direct observation of another person’s limbs moving, to relieve PLP experienced by bilateral lower extremity amputees.
Methods
Subjects
Twenty male bilateral, lower limb amputees were enrolled at Walter Reed Army Medical Center between January 2008 and June 2012. The total number of participants needed was calculated based on our previous research demonstrating the efficacy of mirror therapy. Power calculations demonstrated that five subjects per group would have 81% power to detect a similar difference (i.e., mirror, −25 mm on a 100-mm visual analog scale [VAS] versus mental visualization, +12 mm) in pain score changes. However, to ensure that if the effect of visual observation on pain levels was less robust than visual feedback from viewing the reflected image of a limb moving using mirror therapy, we sought to enroll a minimum of seven subjects per group. Volunteers did not have any significant Axis I or II diagnoses and had at least three PLP episodes each week of a minimum pain level of three of 10 in one or both of their lower limbs and were a maximum of 2 years since amputation. Effort was assessed using the test of memory malingering (TOMM),21 with all study participants scoring within the normal range (>42/50). Using a predetermined randomization table, subjects were assigned to one of two groups for 20 min daily treatment for 1 month – direct visual observation of another person’s lower limbs and feet moving coupled with the subject moving his phantom limbs and feet mimicking the same movements (n = 11) or mental visualization of moving the subject’s phantom limbs and feet (n = 9). Participants were asked to continue their normal rehabilitative practices and medications. This normal process did not include any concurrent, nonmedication therapies for PLP. This study was approved by the Walter Reed Army Medical Center Institutional Review Board and registered on clinicaltrials.gov [NCT00639431].
Visual observation/mental visualization therapy
Participants in both groups received an initial training session where they watched the investigator perform a series of foot, ankle, and leg movements. A total of seven discrete movements were performed each session, lasting 2–3 min each: abduction and adduction of the great toe, flexion and extension of the foot, inversion and eversion of the foot, flexion and extension of the toes, foot rotation around the ankle, and knee flexion and extension (for above knee amputees). Subjects in the visual observation condition observed the investigator’s lower limb movements while simultaneously attempting to replicate the movements with their phantom limbs. Subjects in the mental visualization group were asked to close their eyes and attempt to move their phantom limbs while visualizing each of the movements as prompted by the investigator. All subjects in both conditions were allowed to move their residual limbs during each movement.
Outcome measures
Prior to daily treatment, subjects were asked to indicate their phantom pain severity using a 100-mm VAS, with 0 as “no pain” and 100 being “worst pain,” and the Short-Form McGill Pain Questionnaire (SF-MPQ),22 and report the total number and duration of PLP episodes in each leg/foot over the past 24 h. Changes in usage of analgesic medications were also recorded during the therapeutic period. Due to rehabilitation schedule, some subjects were unable to complete all planned treatment sessions. Consequently, an intent-to-treat (ITT) last observation carry forward analytical method was used.
Statistical analysis
Results were analyzed using SPSS 21.0 for Windows (IBM, Armonk, NY). To determine whether a significant decrease in PLP had been achieved with either therapy, paired samples t-tests were performed comparing outcome measures (VAS and SF-MPQ, for each side) assessed at baseline and final treatment session within each treatment group.
Results
The mean age of the 20 volunteers was 26.7 ± 5.6 years (range: 19–41), and the mean length of time experiencing PLP was 5.7 ± 5.6 months (range: 1.4–19.2). Subjects in the visual observation group experienced pain for 7.2 ± 5.7 months, while subjects in the mental visualization group experienced pain for 4.2 ± 5.3 months (P = 0.26). Participants demonstrated significant differences in pain severity at baseline for right and left limbs based on their treatment assignment, with participants assigned to the visual observation group reporting more severe PLP prior to treatment than those assigned to mental visualization. This discrepancy prevented comparison of the two treatment effects, so each treatment was assessed separately.
The visual observation group showed a significant decrease in severity of PLP for both the left (P = 0.001) and right (P = 0.002) lower limbs as measured using the VAS. Subjects assigned to perform movements under mental visualization did not show a significant decrease in their reported PLP for either left (P = 0.107) or right (P = 0.420) limbs (Fig. 1). A similar pattern emerged on the SF-MPQ. The visual observation group showed a decrease in PLP of both left (P = 0.026) and right (P = 0.009) limbs. The mental visualization group did not experience a significant PLP reduction for either left (P = 0.320) or right (P = 0.231) limbs (Fig. 2). Examination of individual differences further distinguishes the two groups. Eight of 11 participants (73%) assigned to direct visual observation had a clinically meaningful decrease in PLP (defined as a minimum 20-mm decrease on the VAS), with four having reductions in pain of 50%. In contrast, none of the nine participants assigned to mental visualization showed a clinically meaningful decrease in PLP. Both treatment groups demonstrated a reduction in the total duration of reported time in pain (number of pain episodes X duration of each episode) and analgesia usage, which were not significant for either treatment or limb.
Figure 1.
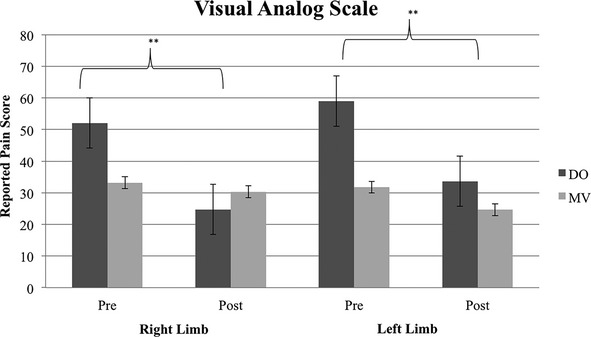
Visual analog scale (VAS) scores reported by subjects assigned to either the direct observation (DO) or mental visualization (MV) treatment groups pre- and posttherapeutic intervention. VAS scores range from 0 to 100 with greater numbers indicating higher reported pain levels. Data are presented as the mean (SD) for each group at two time points - pre- and post-treatment after 20 sessions. ***P < 0.01.
Figure 2.

Cumulative Short-Form McGill Pain Questionnaire scores reported by subjects assigned to either the direct visual observation (DO) or mental visualization (MV) treatment groups pre- and posttherapeutic intervention. The McGill short form is scored out of a total of 45, with greater scores indicating more severe phantom limb pain symptoms. Data are presented as the mean (SD) for each group at two time points - pre- and post-treatment after 20 sessions. *P < 0.05, **P < 0.01.
As a comparison to these results we examined the natural time course of PLP resolution in a separate group of 31 bilateral lower limb military amputees enrolled in a different study. We found that one amputee (3%) had no history of PLP, two (6%) had pain lasting <1 month (average severity by VAS = 36/100), 11 (35%) had pain lasting between 1 and 6 months (average severity by VAS = 37/100), and 17 (55%) had been experiencing pain for over 6 months (average severity by VAS = 37/100).
Discussion
This is the first randomized, controlled study to use direct observation as a modality of visual feedback to relieve PLP. We found that PLP was significantly reduced in the visual observation group, and eight of 11 subjects reported a reduction of pain of at least 20 mm on the VAS. These findings have promising implications for the treatment of PLP in bilateral amputees since visual observation therapy allows such individuals to access the benefits of visuomotor feedback therapy with an easily administered, cost-efficient approach.
The pain-relieving effect of the visual observation intervention supports the importance of visualizing an intact limb when treating pain in the phantom limb. The dominance of vision over the proprioceptive or touch senses could explain why vision is sufficient to override painful signals (or the lack of null signals that could be responsible for pain).12 Like mirror therapy, this intervention could have led to reduction in pain in several ways based on the unclear pathophysiology of PLP itself.
After amputation, the motor cortex remains intact; therefore, afferent motor signals corresponding to the missing limb continue to propagate from the brain. However, since the limb is no longer present, there is a mismatch between the motor commands to and proprioceptive or somatosensory inputs from the limb.23 Seeing a limb that is no longer present may provide feedback that corresponds to the efferent motor commands to override the error signal triggered by the incomplete somatosensory–motor feedback loop.23 This theory could explain the immediate relief felt by four subjects in the visual observation group, who reported a decline of pain by 50% after a single treatment session (data not shown).
Like visual therapy, motor imagery practice has also been proposed to resolve maladaptive neuroplastic changes. Lotze et al. reported greater overall activation in the primary contralateral sensorimotor cortices in a sample of 14 amputees during phantom hand movement compared with healthy controls during motor imagery task performance.6 This increase in neuronal activity could reflect the loss of inhibitory feedback from the limb that was amputated.24 Similarly, MacIver and colleagues found a direct correlation between PLP and hand area sensorimotor activation during phantom hand movement in 13 upper extremity amputees.25 After an intervention of six sessions of meditation and motor imagery, the later study showed that both pain and cortical activation associated with reorganization were reduced, which the authors attributed to improved neuronal efficiency and precision.25 This suggests exercises that improve motor control may decrease inappropriate cortical activation and also pain.
However, it is possible that motor imagery elicits a response similar to visually mediated relief since, when closing their eyes, subjects likely pictured their amputated extremity moving. A vivid image of the limb may have served as sufficient visual feedback. Conversely, the execution of phantom movements may underlie PLP resolution in both motor imagery and visuomotor therapy, since it is implicit in both. Our findings did not show efficacy of mental visualization on PLP reduction. Despite similarities between the mechanisms of action of visuomotor techniques and motor imagery, the results of the current study and those from the Chan et al., study, in which some subjects in the mental visualization group reported an increase in PLP, do not support the idea that mental visualization alone has a strong effect on relieving PLP.
Vision could also reverse pathological “unmasking” of the mirror neuron system (MNS) due to the absence of sensory feedback from the amputated limb. The MNS was first described by Rizolatti and is located in the frontal and parietal cortex.20,26 These neurons are a subset of motor neurons normally recruited to perform a specific action that also fire when observing another individual perform the same motion.20 Pain mirror neurons are constitutively suppressed in intact limbs by somatosensory feedback that confirms no painful stimuli are present. However, in an individual with amputation, the mirror system is disinhibited by the removal of “null” feedback, allowing these neurons to reach threshold and fire.20 Whether this failed inhibition of the mirror system is responsible for PLP is challenged in a study by Fitzgibbon et al. exploring mirror-touch synesthesia in amputees. A group of 12 self-reported mirror-touch synesthetes reported that the location of the stimulus and the perceived pain did not always correspond to the phantom.27
Questions regarding whether MNS dysregulation may underlie PLP will require further research, but perhaps the MNS can be employed through observation of nonpainful events to relieve pain. Ramachandran and Altschuler reported that in one upper extremity amputee, a somatosensory response was triggered when watching someone else’s arm being touched. Watching this person’s arm being massaged actually relieved the patient’s PLP.12 Similarly, Weeks et al. found that watching someone else massaging their intact limb that corresponded to the patient’s phantom limb relieved PLP only when the patient was experiencing PLP.28 This suggests that hyperactivation of the MNS could be modulated by the experience of pain itself. It may be that when the observation of another person’s limb is unhindered by conflicting somatosensory feedback (i.e., that the patient’s corresponding limb is not, in fact, moving, or that no stimulus is present), as is the case in amputees, MNS activation can relieve painful sensations. Since it resides near the motor system, MNS activation may explain the resolution of immobility, often accompanied by pain, in the phantom limb.
While heterogeneity in volunteers’ clinical characteristics and clinical management make prediction of the natural resolution of PLP difficult, there is evidence that persistence of PLP is predicted in absence of interventions that seek to address the neurological effects of amputation. One study reported greater reduction in PLP after completion of a combined motor imagery and mirror therapy program than management with physical therapy and medication.29 From data collected for a separate study, we found 55% of 31 bilateral lower limb amputees experienced PLP for more than 6 months and at similar severity to those who experienced PLP lasting between 1 and 6 months, which suggests a greater persistence of PLP in patients who did not receive therapy addressing the neurological correlates of PLP. These data along with the study results showing that eight of 11 (73%) amputees had a pain reduction of at least 20 mm on the VAS, lends further credence to the concept that visual observation treatment is the reason for PLP reduction rather than a spontaneous remission of PLP.
A major limitation of this study was that baseline PLP severity differed between the two treatment groups, which resulted from baseline pain levels not being available prior to randomization. Subjects in the visual observation group reported higher severity of pain in both left and right lower limbs than subjects in the mental visualization group. Additionally, we were unable to assess long-term treatment efficacy of treatment. Finally, due to the small sample size, confounding factors including comorbid conditions could not be included in the analyses.
Nonetheless, we believe that our results represent an extension of visuomotor feedback therapy, where providing visual feedback to agree with afferent motor signals was likely critical to relieving PLP. Importantly, this therapy is both effective and accessible to bilateral amputees. Virtual reality systems also aim to provide visual feedback and could be used by bilateral amputees; however, this technology is expensive and often requires setup and administration by trained technicians.12 Given the rise in complex battlefield injuries in the military and chronic conditions requiring amputation in the general population, such as diabetes and vascular disease, this new and easily administered therapy may offer a low-cost alternative to pharmacological treatment or could be used to complement existing physical rehabilitation techniques. Several of our volunteers reported the ability to train more rigorously immediately following a visual observation therapy session (i.e., walk further distances, perform extra activities, and run faster). Direct observation therapy also offers practical feasibility from the perspective of patient adherence, since individuals may find visual observation to be more natural and require less cognitive effort than virtual reality systems that require the patient to embody the limbs of a computerized avatar.
Acknowledgments
This material is based upon work supported by the Department of Defense to the Military Amputee Research Program at Walter Reed Army Medical Center, Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. and the Center for Rehabilitation Science Research, Department of Physical Medicine & Rehabilitation, Uniformed Services University of Health Sciences.
Author Contributions
M. T. assisted in data analysis, manuscript writing, and editing. I. M., S. G., A. A., L. H., K. H., S. W., V. M. and J. Y. assisted in study design and data collection. P. P. and J. T. contributed to study conception and design, data analysis, and manuscript editing.
Conflict of Interest
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Navy, the Department of the Army, or the Department of Defense.
References
- Manchikanti L, Singh V. Managing phantom pain. Pain Physician. 2004;7:365–376. [PubMed] [Google Scholar]
- Flor H, Elbert T, Knecht S, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375:482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- Roux FE, Lotterie JA, Cassol E, et al. Cortical areas involved in virtual movement of phantom limbs: comparison with normal subjects. Neurosurgery. 2003;53:1342–1353. doi: 10.1227/01.neu.0000093424.71086.8f. [DOI] [PubMed] [Google Scholar]
- Giraux P, Sirigu A. Illusory movements of the paralyzed limb restore motor cortex activity. Neuroimage. 2003;20:S107–S111. doi: 10.1016/j.neuroimage.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Grüsser SM, Winter C, Schaefer M, et al. Perceptual phenomena after unilateral arm amputation: a pre-post-surgical comparison. Neurosci Lett. 2001;302:13–16. doi: 10.1016/s0304-3940(01)01606-8. [DOI] [PubMed] [Google Scholar]
- Lotze M, Montoya P, Erb M, et al. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: an fMRI study. J Cogn Neurosci. 1999;11:491–501. doi: 10.1162/089892999563553. [DOI] [PubMed] [Google Scholar]
- Flor H, Nikolajsen L, Jensen TS. Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci. 2006;7:873–881. doi: 10.1038/nrn1991. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Stewart M, Rogers-Ramachandran D. Perceptual correlates of massive cortical reorganization. NeuroReport. 1992;3:583–586. doi: 10.1097/00001756-199207000-00009. [DOI] [PubMed] [Google Scholar]
- Makin TR, Scholz J, Filippini N, et al. Phantom pain is associated with preserved structure and function in the former hand area. Nat Commun. 2013;4:1570. doi: 10.1038/ncomms2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson-Barnes VC, McAuliffe C, Swanberg KM, Tsao JW. Phantom limb pain – a phenomenon of proprioceptive memory? Med Hypotheses. 2009;73:555–558. doi: 10.1016/j.mehy.2009.05.038. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Hirstein W. The perception of phantom limbs. The DO Hebb lecture. Brain. 1998;121:1603–1630. doi: 10.1093/brain/121.9.1603. [DOI] [PubMed] [Google Scholar]
- Ramachandran V, Altschuler EL. The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain. 2009;132:1693–1710. doi: 10.1093/brain/awp135. [DOI] [PubMed] [Google Scholar]
- McCormick Z, Chang-Chien G, Marshall B, et al. Phantom limb pain: a systematic neuroanatomical-based review of pharmacologic treatment. Pain Med. 2013;15:292–305. doi: 10.1111/pme.12283. [DOI] [PubMed] [Google Scholar]
- Rothgangel AS, Braun SM, Beurskens AJ, et al. The clinical aspects of mirror therapy in rehabilitation: a systematic review of the literature. Int J Rehabil Res. 2011;34:1–13. doi: 10.1097/MRR.0b013e3283441e98. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc R Soc Lond B Biol Sci. 1996;263:377–386. doi: 10.1098/rspb.1996.0058. [DOI] [PubMed] [Google Scholar]
- Chan BL, Witt R, Charrow AP, et al. Mirror therapy for phantom limb pain. N Engl J Med. 2007;357:2206–2207. doi: 10.1056/NEJMc071927. [DOI] [PubMed] [Google Scholar]
- Penn-Barwell JG. Outcomes in lower limb amputation following trauma: a systematic review and meta-analysis. Injury. 2011;42:1474–1479. doi: 10.1016/j.injury.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, et al. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422–429. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Su PF, Gard SA, Lipschutz RD, Kuiken TA. Gait characteristics of persons with bilateral transtibial amputations. J Rehabil Res Dev. 2007;44:491–501. doi: 10.1682/jrrd.2006.10.0135. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Brang D. Sensations evoked in patients with amputation from watching an individual whose corresponding intact limb is being touched. Arch Neurol. 2009;66:1281. doi: 10.1001/archneurol.2009.206. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN. The Test of Memory Malingering (TOMM): normative data from cognitively intact and cognitively impaired individuals. Psychol Assess. 1997;9:260. [Google Scholar]
- Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- Reilly KT, Sirigu A. The motor cortex and its role in phantom limb phenomena. Neuroscientist. 2008;14:195–202. doi: 10.1177/1073858407309466. [DOI] [PubMed] [Google Scholar]
- Marconi B, Koch G, Pecchioli C, et al. Breakdown of inhibitory effects induced by foot motor imagery on hand motor area in lower-limb amputees. Clin Neurophysiol. 2007;118:2468–2478. doi: 10.1016/j.clinph.2007.08.021. [DOI] [PubMed] [Google Scholar]
- MacIver K, Lloyd D, Kelly S, et al. Phantom limb pain, cortical reorganization and the therapeutic effect of mental imagery. Brain. 2008;131:2181–2191. doi: 10.1093/brain/awn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Fitzgibbon BM, Enticott PG, Rich AN, et al. High incidence of ‘synaesthesia for pain’in amputees. Neuropsychologia. 2010;48:3675–3678. doi: 10.1016/j.neuropsychologia.2010.07.029. [DOI] [PubMed] [Google Scholar]
- Weeks SR, Tsao JW. Incorporation of another person’s limb into body image relieves phantom limb pain: a case study. Neurocase. 2010;16:461–465. doi: 10.1080/13554791003730592. [DOI] [PubMed] [Google Scholar]
- Moseley GL. Graded motor imagery for pathologic pain A randomized controlled trial. Neurology. 2006;67:2129–2134. doi: 10.1212/01.wnl.0000249112.56935.32. [DOI] [PubMed] [Google Scholar]


