Abstract
Objective
Olfactory impairment is a potential marker for impending phenoconversion to Parkinson disease (PD) that may precede the development of disease by several years. Because of low specificity, it may be of greater predictive value in those with genetic mutations and its potential as a marker for developing LRRK2 PD should be evaluated.
Methods
We examined olfactory identification in 126 LRRK2 G2019S mutation carriers with PD, 125 mutation carriers not manifesting PD, 126 noncarriers with idiopathic PD, 106 noncarrier family members without PD, and 35 unrelated controls. We compared olfactory performance and performed mixture modeling to identify possible subgroups of olfactory performance in LRRK2 PD and nonmanifesting carriers.
Results
Adjusting for sex, age, cognitive score, site, and smoking history, LRRK2 PD had better olfactory scores compared to idiopathic PD (mean olfaction difference: −3.7, P < 0.001), and both LRRK2 PD and idiopathic PD had worse olfaction than controls (−12.8, −9.1, both P < 0.001). LRRK2 PD were less likely to be hyposmic than idiopathic PD (54.8% vs. 80.2%, P < 0.001). Nonmanifesting carriers and noncarrier family members did not differ. Mixture model analysis identified three classes in the LRRK2 PD and nonmanifesting carriers, suggesting that there are subgroups with poor olfactory identification in both LRRK2 PD and nonmanifesting carriers.
Interpretation
Therefore, olfactory identification deficit is less likely to be an obligate feature in LRRK2 PD than idiopathic PD, and while a relevant marker in some, a subset of carriers who eventually phenoconvert may proceed directly to PD without prior impaired olfaction.
Introduction
Smell impairment is present in over 85% of Parkinson disease (PD)1 and may discriminate idiopathic PD from atypical parkinsonism and essential tremor.2 Impaired olfaction has been postulated to be related to alpha-synuclein deposition in the olfactory bulb and primary olfactory cortex, although additional structures also mediate olfactory disturbance,2 and olfactory loss can be observed in other neurodegenerative disorders, such as tauopathies.3 Even among PD cases, olfaction is not uniformly affected: it is preserved in some genetic forms of PD without prominent synuclein deposition, such as that due to biallelic parkin mutations.4,5
Olfactory loss has been described in LRRK2 mutation PD. However, reports are conflicting.6 While most studies demonstrate olfaction impairment, some find similar7,8 or less disturbance compared to idiopathic PD.9–15 Furthermore, it is not clear whether it is heterogeneous – composed of groups with better and worse olfaction – or whether loss of olfaction affects most LRRK2 mutation PD but is milder overall compared to idiopathic PD. It has been difficult to dissect this relationship as studies with systematic olfactory testing are limited to samples of <50 subjects with LRRK2 mutations.
As impaired olfaction may precede the onset of PD by several years16 and olfactory loss is observed in family members of PD subjects,17 it has been proposed as a biomarker for incipient disease. LRRK2 mutation carriers without motor signs of PD but with olfactory loss may constitute a group at especially high risk for phenoconversion.10 Apparently contradicting this supposition is the lack of findings of olfactory impairment in nonmanifesting carriers in other reports.12,14,15 However, because of the reduced penetrance of LRRK2 mutations, if olfactory impairment precedes phenoconversion to PD by a short period, olfactory loss would be expected to evolve in only that subset of LRRK2 mutation carriers (~20–30%) who develop PD in their lifetimes.18–20 Furthermore, if it is a state marker associated with impending disease, hyposmia might only occur in the subgroup who are within a decade or less of onset of PD.
In order to better delineate the frequency and severity of olfactory loss in LRRK2 PD, and to determine whether olfaction is a potential preclinical marker for LRRK2 PD, we systematically evaluated the olfactory phenotype of PD, at-risk and control groups. We assessed LRRK2 G2019S mutation PD (LRRK2 PD), unaffected family members (both nonmanifesting carriers [NMC] and noncarrier family members [NC-F]), LRRK2 G2019S and GBA1 mutation-negative PD (IPD), and controls without PD (control) from the Ashkenazi Jewish LRRK2 Consortium sites of Tel-Aviv Medical Center, Tel-Aviv, Israel (Tel-Aviv), and Columbia University Medical Center (Columbia) and Mount Sinai Beth Israel Medical Center (Beth Israel), both in New York, NY, U.S.A. In addition to overall differences, we assessed for latent classes of subgroups within the LRRK2 PD and NMC.
Materials and Methods
Subjects
Participants in primary analyses included those enrolled in the Ashkenazi Jewish LRRK2 Consortium who completed olfactory assessments. LRRK2 G2019S mutation status was determined.21 DNA was also screened for the common GBA1 mutations as previously described,5,23,24 and GBA1 heterozygous and homozygous mutation carriers were excluded. The study procedures were approved by the respective internal review boards at Beth Israel, Columbia, and Tel-Aviv Medical Center, and all subjects gave informed consent.
A total of 252 subjects with PD (126 LRRK2 carriers and 126 nonmutation carriers), 125 NMC, 106 unaffected noncarrier family members (including 227 first-degree relatives and 4 second-degree relatives of probands), and 35 noncarrier unrelated controls were studied. Diagnosis of PD was as previously reported.21 Briefly, a diagnostic checklist was completed, and only those examined subjects rated as having met stringent diagnostic criteria for PD were included in the LRRK2 PD and nonmutation PD groups. Participants were recruited if they reported at least two Ashkenazi Jewish grandparents and were diagnosed with PD by a movement disorder specialist based on United Kingdom Parkinson’s Disease Brain Bank criteria (except individuals who had a family history of PD were not excluded).22 Blood-related family members were separated into groups of NMC with G2019S mutations and noncarrier family members without G2019S mutations. In both New York sites, unaffected Ashkenazi Jewish spouses and friend controls were also evaluated. Seventeen of the LRRK2 PD, five of the NMC, and two of the controls from Beth Israel were previously reported.10
Olfaction
Olfactory identification was measured using the full University of Pennsylvania Smell Identification Test (UPSIT), which includes 40 encapsulated odors.25 Tests were self-administered. Subjects were instructed to choose a response from the four choices listed. Most UPSITs were completed at the study visit, although a small portion was performed remotely and returned by mail after the on-site visit. Any potential subject with a known respiratory tract infection or active allergies at the time of testing was excluded.
Additional assessments
While the primary goal of this report was to assess the olfaction, additional measures were collected as part of the Ashkenazi Jewish LRRK2 Consortium Study, and motor and cognitive assessments, as well as smoking history, were used as covariates in the primary analysis. Motor Function was assessed with the Unified PD Rating Scale (UPDRS).21,26 Cognition was assessed using the Montreal Cognitive Assessment (MoCA).27 Cigarette smoking history was assessed using the PD Risk Factor Questionnaire (RFQ-U CRFs, Version 1.0 Epidemiology Working Group of the Collaborative Centers for PD Environmental Research). Ever regular smoking was defined as smoking one cigarette/day for 6 months or longer.
Analysis
Raw UPSIT scores were calculated as the number of correct identifications, ranging from 0 to 40, with 40 representing perfect olfaction. For the six subjects completing <40 responses, the weighted score was tabulated from the total items completed. Analyses were performed first on the raw UPSIT scores as the primary outcome. As UPSIT is known to be worse with increasing age and in male gender, percentiles based on age and gender have been established in over three thousand subjects (1819 males, 2109 females).25 UPSIT scores were also categorized using normative data for age and gender as previously reported with a dichotomous cut at the 15th percentile for age and gender corresponding to labeling as hyposmic.25
T-tests or Mann–Whitney tests for continuous variables and chi-square or Fisher’s exact tests for categorical variables were performed for univariate comparisons among groups for demographic features, olfaction, and smoking. For the primary outcome of UPSIT olfactory performance, linear mixed-effects models were applied to compare continuous UPSIT scores among the different groups of nonmutation PD, LRRK2 PD, NMC, noncarrier family members and controls, adjusting for age, gender, site (Tel-Aviv vs. both New York sites), ever regularly smoked, and total MoCA score. Linear mixed-effects models accounted for the possible correlations of measurements among subjects from the same family through random effects.28 Robust empirical variance estimates were used due to the skewness in the data. Models were repeated excluding current smokers. Presence of hyposmia was assessed using generalized estimating equations (GEEs) adjusting for age and gender, site, family, and total MoCA score, with a logistic link for dichotomized hyposmia.
To determine whether there are clusters of subgroups within the LRRK2 PD and nonmanifesting carrier groups, Gaussian mixture models were performed for UPSIT olfactory score using M-plus (statmodel.com).43 Vuong-Lo-Mendell-Rubin likelihood ratio tests were used to test hypotheses on the number of classes.29 Each subject was assigned to the latent class with the largest probability of belonging to the class. Post-hoc comparisons of features between the identified class memberships were performed using parametric or nonparametric tests accordingly.
Results
Demographic, olfactory scores, and smoking differed between groups, and univariate comparisons are reported in Table 1. The most notable differences in the univariate comparisons were that nonmutation Parkinson cases (64.3 ± 11.4 years), LRRK2 mutation-related PD (67.4 ± 9.3 years), and unrelated controls (69.4 ± 10.6 years) were overall older than NMC (52.6 ± 15.1) and noncarrier family members (50.7 ± 16.4 years) (all comparisons P < 0.001). LRRK2 PD were more likely to have longer duration of disease than nonmutation cases (8.4 ± 6.9 vs. 4.8 ± 5.4, P < 0.001), and nonmutation PD (38.1%) and manifesting carriers (44.4%) were less likely than controls (74.3%) to be female (P < 0.001, P = 0.002). These are consistent with features noted in the cross-sectional sample of LRRK2 PD previously reported.21 Nonmutation PD participants were less likely to have regularly smoked than controls (34.8% vs. 54.3%, P = 0.04) and LRRK2 PD (51.2%, P = 0.01), but there was no significant difference between LRRK2 PD and controls. As ever regular smoker, or current smoker, did not correlate with olfactory performance, only one proxy for smoking, ever regular smoker, was included in the primary analysis.
Table 1.
Demographics, olfactory raw scores, and % hyposmic
| IPD | LRRK2 PD | Control | NMC | NC-F | |
|---|---|---|---|---|---|
| Overall (n) | 126 | 126 | 35 | 125 | 106 |
| US site | 48 | 69 | 35 | 47 | 32 |
| Israel | 78 | 57 | 78 | 74 |
| Demographic | IPD vs. LRRK2 PD (P value) | NMC vs. NC-F | |||||
|---|---|---|---|---|---|---|---|
| Age (y), mean ± SD | 64.3 ± 11.4 | 67.4 ± 9.3 | 69.4 ± 10.6 | 0.02 | 52.6 ± 15.1 | 50.7 ± 16.4 | 0.22 |
| Gender (% women) | 38.1 | 44.4 | 74.3 | 0.31 | 55.2 | 50.0 | 0.43 |
| Disease duration (years) | 4.8 ± 5.4 | 8.4 ± 6.9 | <0.001 | ||||
| UPDRS-III | 19.2 ± 11.3 | 22.0 ± 12.4 | 1.9 ± 3.3 | 0.06 | 3.0 ± 5.0 | 2.2 ± 3.2 | 0.37 |
| MoCA | 25.2 ± 3.8 | 25.4 ± 3.0 | 26.3 ± 2.9 | 0.97 | 26.5 ± 2.6 | 26.4 ± 2.7 | 0.56 |
| Smoking ever (%) | 34.8 | 51.2 | 54.3 | 0.01 | 38.5 | 36.4 | 0.74 |
| Current smoker (%) | 3.2 | 7.1 | 0.0 | 0.15 | 9.6 | 11.3 | 0.67 |
| UPSIT | 18.6 ± 7.1 | 22.8 ± 8.7 | 33.1 ± 5.7 | <0.001 | 32.7 ± 5.3 | 31.8 ± 5.3 | 0.08 |
| Hyposmic (%)1 | 80.2 | 54.8 | 11.4 | <0.001 | 28.8 | 36.8 | 0.21 |
IPD, Idiopathic PD, no LRRK2 G2019S or GBA mutation; LRRK2 PD, Manifesting Carrier; NMC, nonmanifesting carrier; NC-F, noncarrier family member; US, United States site; FDR, first-degree relative; UPDRS, Universal Parkinson’s Disease Rating Scale; MoCA, Montreal Cognitive Assessment; UPSIT, University of Pennsylvania Smell Identification Test.
Hyposmic defined as 15th percentile or less for age and gender.
Overall olfaction scores were worse in the nonmutation PD (18.6 ± 7.1) versus LRRK2 PD (22.8 ± 8.7, P < 0.001), the LRRK2 PD versus NMC (32.7 ± 5.3, P < 0.001), nonmutation PD versus controls (33.1 ± 5.7, P < 0.001), and LRRK2 PD versus controls (P < 0.001). NMC were not worse than noncarrier family members (31.8 ± 5.3, P = 0.08), and did not differ from controls (P = 0.31) although noncarrier family members performed worse than controls (P = 0.04). In parallel, percent hyposmic was greater in the nonmutation group (80.2%) compared with LRRK2 PD (54.8%) (P < 0.001), LRRK2 PD versus controls (11.4%) (P < 0.001), and in LRRK2 PD versus NMC (28.8%) (P < 0.001), but not greater in NMC versus noncarrier family members (36.8%) (P = 0.21), and greater in the noncarrier family members compared with controls (P = 0.005). Distributions of raw UPSIT scores can be seen (Fig. 1), and because UPSIT is a forced choice response of four options with 25% chance of getting an answer correct, as expected, there was a floor effect at a score of 10 (25% of 40) for all groups.
Figure 1.
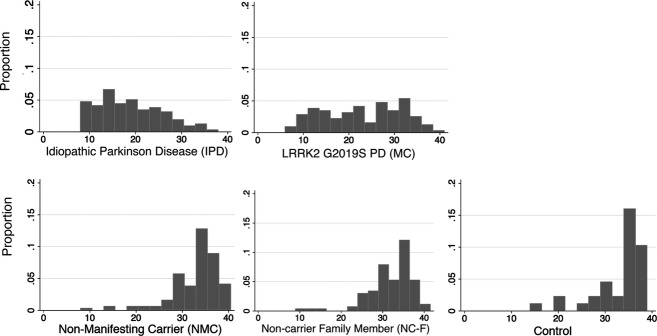
LRRK2 PD demonstrate less of a unomodal distribution, suggesting subgroups among the group, which are apparent in the latent class analysis.
Olfactory identification scores were lower (worse) in Israel than in the combined New York sites for all groups except nonmutation Parkinson cases, which were slightly lower in NY (nonmutation PD: New York mean UPSIT 17.7 ± 7.0 vs. Israel 19.2 ± 7.1; manifesting carriers: 24.1 ± 8.6 vs. 21.2 ± 8.6; NMC 34.2 ± 6.4 vs. 31.7 ± 4.4; and noncarrier family members 32.5 ± 6.2 vs. 31.5 ± 4.8). The direction of the effects was maintained across all groups; however, the two-point average difference in UPSIT score between nonmutation PD and manifesting carriers was not significant in the Israeli group (19.2 ± 7.1 vs. 21.2 ± 8.6, P = 0.16), when evaluated in isolation.
In an adjusted model, older age (P < 0.001), male gender (P < 0.001), Israel as site (P < 0.01), and lower MoCA score (P = 0.04) were associated with worse performance on UPSIT, but ever regular smoking was not (Table 2). Ever regular smoking was also not significant in a model assessing an interaction with site or with status category (not shown); manifesting carriers scored higher on UPSIT (better olfactory identification) than nonmutation PD (P < 0.001), and lower on UPSIT (worse identification) than NMC (P < 0.001). There was no difference between NMC and noncarrier family members (P = 0.12). However, noncarrier family members scored significantly lower on UPSIT than controls (P = 0.04). The effects persisted when current smokers were excluded from the analysis.
Table 2.
Comparison of continuous UPSIT score: mixed-effect model
| Predictors and group comparisons1 | Β | SE | P | 95% Confidence interval |
|---|---|---|---|---|
| Sex | 2.94 | 0.55 | <0.001 | (1.87, 4.01) |
| Age at UPSIT | −0.16 | 0.02 | <0.001 | (−0.20, −0.12) |
| MoCA | 0.18 | 0.09 | 0.06 | (0.01, 0.36) |
| US site | 2.00 | 0.64 | 0.002 | (0.75, 3.24) |
| Ever smoke | 0.30 | 0.55 | 0.59 | (−0.79, 1.39) |
| IPD vs. control | −12.75 | 1.12 | <0.001 | (−14.95, −10.54) |
| MC vs. control | −9.08 | 1.14 | <0.001 | (−11.31, −6.84) |
| NMC vs. control | −1.22 | 1.08 | 0.26 | (−3.34, 0.91) |
| NC-F vs. control | −2.19 | 1.08 | 0.04 | (−4.31, −0.07) |
| Further group comparisons2 | ||||
| IPD vs. MC | −3.67 | 1.01 | <0.001 | (−5.66, −1.68) |
| MC vs. NMC | −7.86 | 0.98 | <0.001 | (−9.78, −5.93) |
| MC vs. NC-F | −6.88 | 0.99 | <0.001 | (−8.82, −4.95) |
| NMC vs. NC-F | 0.97 | 0.62 | 0.12 | (−0.24, 2.18) |
| IPD vs. NC-F | −10.55 | 0.86 | <0.001 | (−12.23, −8.98) |
SE, standard error; US, United States site; UPSIT, University of Pennsylvania Smell Identification Test; MoCA, Montreal Cognitive Assessment; IPD, idiopathic PD; MC, manifesting carrier; NMC, nonmanifesting carrier; NC-F, noncarrier family member.
Group comparisons and the covariates were evaluated in the same model (using control group as the reference)
Additional group comparisons were deduced from the main model.
Using a GEE with logit link for binary outcomes to estimate the odds of being hyposmic (≤15th percentile) with respect to subject status, accounting for correlation of data within each family, and adjusting for gender, age, MoCA score, ever smoker status, and site of examination, the associations with hyposmia and PD and gene status were maintained. The odds of being hyposmic for nonmutation PD were 2.57 times that of manifesting carriers (95% CI: 1.4–4.8; P = 0.003); and for manifesting carriers 9.8 times that of NMC (95% CI: 4.9–19.6; P < 0.0001). The odds of being hyposmic were not significantly different among NMC, noncarrier family members, and controls. Results from the mixed-effects models as well as the GEE were maintained when sites were limited to New York alone.
The Gaussian mixture analysis among manifesting carriers suggested three latent classes (Table 3): Group 1 (MC1, mean UPSIT ± SD, 12.7 ± 3.2), Group 2 (MC2, 20.7 ± 1.2), and Group 3 (MC3, 30.1 ± 4.1). Among the NMC, three latent classes were also identified: Group 1 (NMC1, 18.4 ± 6.0), Group 2 (NMC2, 30.0 ± 2.5), and Group 3 (NMC3, 35.6 ± 2.0). Because of resulting small sample sizes from post hoc assignment, analyses were performed evaluating characteristics of the two worst classes combined and compared to the best. The two manifesting carrier groups with worse olfaction (MC1 and MC2) differed from the best performing manifesting carrier group (MC3) in that the proportion of participants with recent onset (disease <3 years) was greater in the best olfaction group (30.6% vs. 15.9%, P = 0.05), and motor performance was also better (UPDRS-III, 18.6 ± 9.7 vs. 25.3 ± 13.7, P = 0.01). Among NMC, comparing two classes of worse (n = 44) to the better olfaction group (n = 81), the two groups with worse olfaction were more likely to be older (57.2 vs. 50.1 years) (P = 0.01), less likely to be from New York (15.9% vs. 49.4%) (P < 0.001), more likely to have a lower MoCA score (25.7 vs. 27.0) (P < 0.01), and more likely to have a worse UPDRS-III (3.9 ± 4.4 vs. 2.4 ± 5.3) (P = 0.001).
Table 3.
Univariate summary of clusters from mixture analysis
| Group (n) | MC1 (42) | MC2 (21) | MC3 (63) | Lowest 2 vs. highest | NMC1 (7) | NMC2 (37) | NMC3 (81) | Lowest 2 vs. highest |
|---|---|---|---|---|---|---|---|---|
| UPSIT | 12.6 ± 3.0 | 20.6 ± 1.0 | 30.3 ± 3.9 | <0.001 | 16.4 ± 4.9 | 29.3 ± 2.0 | 35.6 ± 1.9 | <0.001 |
| Hyposmia (%) | 97.6 | 71.4 | 20.6 | <0.001 | 71.4 | 59.5 | 11.1 | <0.001 |
| Women (%) | 40.5 | 38.1 | 49.2 | 0.28 | 42.9 | 43.3 | 61.7 | 0.05 |
| Age | 67.7 ± 9.1 | 68.3 ± 8.7 | 67.0 ± 9.7 | 0.78 | 68.9 ± 20.0 | 55.0 ± 13.3 | 50.1 ± 14.5 | 0.01 |
| Duration PD (years) | 9.9 ± 8.0 | 8.9 ± 6.4 | 7.2 ± 6.0 | 0.07 | ||||
| Duration ≥3 years (%) | 83.3 | 85.7 | 69.4 | 0.05 | ||||
| Age onset | 56.8 ± 10.6 | 57.6 ± 10.8 | 58.2 ± 10.3 | 0.40 | ||||
| US (%) | 45.2 | 52.4 | 61.9 | 0.11 | 42.9 | 10.8 | 49.4 | <0.001 |
| Ever smoke (%) | 56.1 | 52.4 | 47.5 | 0.42 | 42.9 | 31.4 | 41.3 | 0.39 |
| MoCA | 25.6 ± 3.1 | 24.8 ± 3.1 | 25.4 ± 2.8 | 0.80 | 24.3 ± 2.9 | 25.9 ± 2.6 | 27.0 ± 2.5 | <0.01 |
| UPDRS-III | 23.5 ± 12.4 | 28.7 ± 15.5 | 18.6 ± 9.7 | 0.01 | 6.4 ± 4.8 | 3.4 ± 4.3 | 2.4 ± 5.3 | 0.001 |
All values are mean ± SD. MC, manifesting carrier; NMC, nonmanifesting carrier; 3, lowest scoring group; 1, highest scoring group; UPSIT, University of Pennsylvania Smell Identification Test; MoCA, Montreal Cognitive Assessment; UPDRS, Unified Parkinson’s Disease Rating Scale.
Discussion
We report the largest multisite systematic evaluation of olfactory identification in LRRK2 mutation carriers and, consistent with other studies, show that overall olfactory identification scores are better in LRRK2 PD than in PD without LRRK2 G2019S mutations, but worse in PD overall than controls.9–15 A unique feature of this study is that because of the large sample size we are able to evaluate the distribution of olfactory scores. The multimodal appearance together with the simple mixture model supports three distinct groups, including a cluster of approximately one third of manifesting carrier cases that have relatively preserved olfaction. Of interest, the two worst latent classes were more likely to have higher UPDRS-III and a trend toward longer disease duration, suggesting that in LRRK2 PD, the severity of olfactory loss is correlated with measures of disease burden.
The pathophysiology of olfactory identification loss in both nonmutation PD and related to LRRK2 G2019S mutations is uncertain. Olfaction disturbance in PD may be related to synuclein deposition, either in the olfactory bulb3 or olfactory cortical areas.30 There is significant olfactory loss in GBA1 mutation PD, where synuclein deposition is more extensive and more likely to extend to the cortex,31,32 and preservation of olfactory dysfunction in biallelic parkin related PD, where synuclein deposition is classically not a prominent feature.33 However, clinical olfactory loss may not correspond to Lewy body deposition, and/or might be attributed to additional factors.30 Changes in the olfactory bulb and tract have also been demonstrated in other neurodegenerative diseases, such as progressive supranuclear palsy and Alzheimer disease, where synuclein deposition is not the prominent feature,1 and the latter is attributed to neurofibrillary tangles in entorhinal cortex. While olfaction is preserved in a subset of nonmutation PD, it is a smaller group than in manifesting carriers, and it remains unclear what the pathophysiologic correlate in the better performing subset is. It could be either a lighter load of synuclein deposition, or it may be factors other than synuclein that are pathologically different. It has been argued that an association of olfactory loss and I-metaiodobenzylguanidine cardiac uptake segregating together in a study of LRRK2 mutation carriers supports presence of Lewy body pathology in this group.13 If synuclein deposition detectable on autopsy was solely responsible for mediating the olfactory deficit, then most nonmutation PD should demonstrate hyposmia, as the majority of autopsy cases of nonmutation PD report both nigral degeneration and brainstem Lewy bodies.34 Furthermore, in hyposmic PD patients with staining of the olfactory bulb for synuclein, there was both reduced olfaction and olfactory bulb synuclein deposition.7 Additional autopsy studies and synuclein-specific imaging in vivo are needed to further clarify.
Of tremendous interest in PD is a potential clinical pathologic correlate between onset of olfactory dysfunction, which may precede diagnosis of PD by only 4 years,16 and the Braak hypothesis of staged progression of disease whereby olfactory synuclein deposition precedes nigral involvement.37 Because most nonmutation PD demonstrates impaired olfaction, it is presumed to be a promising marker of developing the condition.36,37 However, as the better performing group of LRRK2 PD has presumably proceeded to clinically evident PD without prior olfactory disturbance, this suggests that the negative predictive value of olfaction for mutation carriers to develop PD may be limited.
Nonmanifesting mutation carriers do not overall have impaired olfaction, suggesting that olfactory loss is not a trait of harboring a LRRK2 mutation. However, given that there is a latent class of NMC that performs worse on olfactory testing, there may be some predictive value to olfactory loss in the subset of carriers who develop it. Individuals in this group are more likely to have subtle motoric features (as demonstrated by worse UPDRS scores), tend be older, and have lower cognitive scores. While each of these factors may be independently associated with worse olfaction, it is tantalizing to consider that taken together these markers might have some utility in predicting more imminent phenoconversion in the small subgroup of unaffected carriers with abnormal olfactory scores. Thus, while olfactory identification does not discriminate the group overall, it may have some potential to serve as a marker for impending parkinsonism, and in theory could improve the discriminative characteristics if included as part of a biomarker battery, possibly with subtle motor features and cognitive assessment. Longitudinal study with other markers is therefore necessary to allow dissection of the temporal relationship of olfactory disturbance in LRRK2 G2019S mutation carriers.
A factor that may be considered in a combined battery is cognitive scores, although the relationship between cognition, LRRK2 mutations, and olfaction is complex. LRRK2 mutation-related PD demonstrates better attention and executive function than idiopathic PD,21 and NMC may have different compensatory executive function.38 In the overall model, worse MoCA score correlated with worse performance on UPSIT, although in comparing nonmutation PD to LRRK2-related cases, the olfactory difference persisted even when adjusting for MoCA, suggesting that the olfactory difference is not solely attributable to differences measured with MoCA.
Analysis of continuous UPSIT scores showed that noncarrier family members have worse olfaction than controls. This finding could be partially explained by the demographic differences in the groups. Even though adjusted for in the models, NC-F were more likely to be male than the controls, and controls were not available from the Tel Aviv site. While controls were older, this should contribute to worse UPSIT, and should bias away from a finding in the raw UPSIT scores. Another possible explanation is that family members, even noncarriers are more susceptible to PD than controls. Other markers of presumed susceptibility, increased UPDRS motor scores, and substantia nigra hyperechogenicity have been demonstrated in LRRK2 noncarrier family members without PD.12,14 Further supporting this point is that phenocopies (PD cases in relatives who do not harbor the family mutation) are increased in LRRK2 families.44 Mechanistically, as LRRK2 has incomplete penetrance, there are presumably additional genetic and/or environmental modifiers that are increased in LRRK2 probands who have manifested disease. By virtue of shared familial environment as well as genetics, these “susceptibility” factors may thus be overrepresented in family members regardless of whether they harbor a mutated gene, and these factors may by themselves increase risk of parkinsonism. They could be genetic, associated with another gene in trans that also increases risk of parkinsonism, or could be epigenetic factors affecting expression of other genes that increase penetrance. Thus, in complex disorders such as LRRK2 where there may be additional genetic and environmental modifiers, future studies should consider analyses including a nonfamily control arm.
A potential drawback is that two of the instruments, UPSIT and MoCA, may demonstrate cultural differences, with slightly worse MoCA scores using the Hebrew translation,45 as well as the potential for worse olfaction because the smells were developed for US participants. While UPSIT has been used in research from Israel,46 large sets of normative data have not been collected. UPSIT assessment has been found to be reliable and is easily administered with little subject burden. Olfactory performance did vary by country, with worse olfaction in most groups from Israel, suggesting that UPSIT does not perform uniformly across cultures. Even though we adjusted for site, it is possible that hyposmia cuts are limited by the fact that norms may differ among countries, and that this could have influenced results. However, when primary mixed-effects models were repeated limited to US sites only, the results were maintained, suggesting that the data are robust. Furthermore, the overall relationships were maintained across sites.
In summary, olfaction in LRRK2 G2019S PD is less impaired than in nonmutation disease with a large subgroup not demonstrating hyposmia; furthermore, as a group, NMC do not significantly differ from controls or noncarrier family members. While this supports that olfaction alone will not have great sensitivity as a marker, it is possible that it may have merit if it shows longitudinal change or if it is combined with other markers.39,40 Therefore, longitudinal studies are required to dissect the temporal relation between manifesting disease and developing olfactory deficits in LRRK2 PD. Among NMC, only a small fraction of cases are likely to phenoconvert in a several year period that might constitute the study duration for an interventional trial. If preventing phenoconversion is the outcome, then it is possible that enriching the cohort for individuals with olfactory loss might enhance identification of some individuals at risk to imminent phenoconversion. Furthermore, combining with additional marker abnormalities, such as imaging abnormalities in NMC, motor dysfunction as assessed with gait variability,41 abnormalities in spiral drawing or executive dysfunction could aid in improving the discriminative ability to predict individuals at highest risk for phenoconversion in a shorter window close to the development of PD.39,42
Acknowledgments
The AJ LRRK2 Consortium includes the following individuals: Alcalay R. N., Mirelman A., Saunders-Pullman R., Tang M. X., Mejia Santana H., Raymond D., Roos E., Orbe-Reilly M., Gurevich T., Bar Shira A., Gana Weisz M., Yasinovsky K., Zalis M., Thaler A., Deik A., Barrett M. J., Cabassa J., Groves M., Hunt A. L., Lubarr N., San Luciano M., Miravite J., Palmese C., Sachdev R., Sarva H., Severt L., Shanker V., Swan M. C., Soto-Valencia J., Johannes B., Doan N., Pomerantz A., Ortega R., Fahn S., Cote L., Waters C., Mazzoni P., Ford B., Louis E., Levy O., Rosado L., Ruiz D., Dorovski T., Pauciulo M., Nichols W., Orr-Urtreger A., Ozelius L., Clark L., Giladi N., Bressman S., Marder K. S. The authors are grateful to the study participants who graciously donated their time and energy for this study and to Mariel Pullman for her work in preparation of the manuscript.
Author Roles
Rachel Saunders-Pullman drafted the manuscript, performed data collection and analysis, and was involved in overall study design. Anat Mirelman, Roy Alcalay, Karen Marder, Nir Giladi and Susan Bressman, and Avi Orr-Utreger, Lorraine Clark, and Laurie Ozelius were responsible for overall study design, data collection, and revision of the manuscript. Deborah Raymond and Helen Mejia-Santana were responsible for data collection and revision of the manuscript. Cuiling Wang, Marta San Luciano, and Robert Ortega were responsible for analysis and revision of the manuscript.
Conflict of Interest
Dr. San Luciano reports grants from Michael J Fox Foundation, during the conduct of the study. Dr. Saunders-Pullman reports grants from Michael J Fox Foundation, grants from NIH NS073836 during the conduct of the study. Dr. Marder reports grants from MJ Fox, grants from NIH NS036630, grants from Parkinson’s Disease Foundation, during the conduct of the study; grants from NIH, grants from Huntington’s Disease Society of America, grants from CHDI, personal fees from Pfizer (invited lecture), personal fees from Springer -section editor, outside the submitted work. Dr. Bressman reports grants from NIH, grants from Michael J. Fox Foundation, grants from Bigglesworth Foundation, during the conduct of the study. Ms. Raymond reports grants from NIH, grants from Michael J. Fox Foundation, grants from Bigglesworth Foundation, during the conduct of the study. Nir Giladi – Prof. Giladi serves as Associate Editor of the Journal of Neural Transmissions, and as member of the Editorial Board for the Current Treatment Options in Neurology Journal and the Journal of Parkinson’s Disease. He serves as consultant to Teva-Lundbeck, IntecPharma, Neuroderm, Armon Neuromedical Ltd. And Pharma Two B. Received payment for lectures at Teva-Lundbeck, Novartis and UCB and receives research support from the Michael J Fox Foundation, the National Parkinson Foundation, the European Union 7th Framework Programme and the Israel Science. Foundation.
References
- Stern MB, Doty RL, Dotti M, et al. Olfactory function in Parkinson’s disease subtypes. Neurology. 1994;44:266–268. doi: 10.1212/wnl.44.2.266. [DOI] [PubMed] [Google Scholar]
- Doty RL. Olfactory dysfunction in Parkinson’s disease. Nat Rev Neurosci. 2012;8:329–339. doi: 10.1038/nrneurol.2012.80. [DOI] [PubMed] [Google Scholar]
- Duda JE. Olfactory system pathology as a model of Lewy body neurodegenerative disease. J Neurol Sci. 2010;289:49–54. doi: 10.1016/j.jns.2009.08.042. [DOI] [PubMed] [Google Scholar]
- Khan NL, Katzenschlager R, Watt H, et al. Olfaction differentiates parkin disease from early-onset parkinsonism and PD. Neurology. 2004;62:1224–1226. doi: 10.1212/01.wnl.0000118281.66802.81. [DOI] [PubMed] [Google Scholar]
- Alcalay RN, Caccappolo E, Mejia-Santana H, et al. Cognitive performance of GBA mutation carriers with early-onset PD: The CORE-PD study. Neurology. 2012;78:1434–1440. doi: 10.1212/WNL.0b013e318253d54b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NL, Jain S, Lynch JM, et al. Mutations in the gene LRRK2 encoding dardarin (PARK8) cause familial Parkinson’s disease: clinical, pathological, olfactory and functional imaging and genetic data. Brain. 2005;128:2786–2796. doi: 10.1093/brain/awh667. [DOI] [PubMed] [Google Scholar]
- Silveira-Moriyama L, Guedes LC, Kingsbury A, et al. Hyposmia in G2019S LRRK2-related parkinsonism: clinical and pathologic data. Neurology. 2008;71:1021–1026. doi: 10.1212/01.wnl.0000326575.20829.45. [DOI] [PubMed] [Google Scholar]
- Trinh J, Amouri R, Duda JE, et al. A comparative study of Parkinson’s disease and leucine-rich repeat kinase 2 p. G2019S parkinsonism. Neurobiol Aging. 2014;35:1125–1131. doi: 10.1016/j.neurobiolaging.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Healy DG, Falchi M, O’Sullivan SS, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case–control study. Lancet Neurol. 2008;7:583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders-Pullman R, Stanley K, Wang C, et al. Olfactory dysfunction in LRRK2 G2019S mutation carriers. Neurology. 2011;77:319–324. doi: 10.1212/WNL.0b013e318227041c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira-Moriyama L, Munhoz RP, de J Carvalho M, et al. Olfactory heterogeneity in LRRK2 related Parkinsonism. Mov Disord. 2010;25:2879–2883. doi: 10.1002/mds.23325. [DOI] [PubMed] [Google Scholar]
- Marras C, Schuele B, Munhoz RP, et al. Phenotype in parkinsonian and nonparkinsonian LRRK2 G2019S mutation carriers. Neurology. 2011;77:325–333. doi: 10.1212/WNL.0b013e318227042d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallderiola F, Gaig C, Muxi A, et al. 123I-MIBG cardiac uptake and smell identification in parkinsonian patients with LRRK2 mutations. J Neurol. 2011;258:1126–1132. doi: 10.1007/s00415-010-5896-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra M, Sánchez-Juan P, Martínez-Rodríguez MI, et al. Olfaction and imaging biomarkers in LRRK2 G2019S-associated PD. Neurology. 2013;80:621–626. doi: 10.1212/WNL.0b013e31828250d6. [DOI] [PubMed] [Google Scholar]
- Johansen KK, Warø BJ, Aasly JO. Olfactory dysfunction in sporadic Parkinson’s disease and LRRK2 carriers. Acta Neurol Scand. 2014;129:300–306. doi: 10.1111/ane.12172. DOI: 10.1111/ane.12172. [DOI] [PubMed] [Google Scholar]
- Ross GW, Petrovitch H, Abbott RD, et al. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann Neurol. 2008;63:167–173. doi: 10.1002/ana.21291. [DOI] [PubMed] [Google Scholar]
- Ponsen MM, Stoffers D, Wolters EC, et al. Olfactory testing combined with dopamine transporter imaging as a method to detect prodromal Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2010;81:396–399. doi: 10.1136/jnnp.2009.183715. [DOI] [PubMed] [Google Scholar]
- Clark LN, Wang Y, Karlins E, et al. Frequency of LRRK2 mutations in early- and late-onset PD. Neurology. 2006;67:1786–1791. doi: 10.1212/01.wnl.0000244345.49809.36. [DOI] [PubMed] [Google Scholar]
- Ozelius LJ, Senthil G, Saunders-Pullman R, et al. LRRK2 G2019S as a cause of Parkinson’s disease in Ashkenazi Jews. N Engl J Med. 2006;354:424–425. doi: 10.1056/NEJMc055509. [DOI] [PubMed] [Google Scholar]
- Ferreira JJ, Guedes LC, Rosa MM, et al. High prevalence of LRRK2 mutations in familial and sporadic Parkinson’s disease in Portugal. Mov Disord. 2007;22:1194–1201. doi: 10.1002/mds.21525. [DOI] [PubMed] [Google Scholar]
- Alcalay RN, Mirelman A, Saunders-Pullman R, et al. PD phenotype in Ashkenazi Jews with and without LRRK2 G2019S mutations. Mov Disord. 2013;28:1966–1971. doi: 10.1002/mds.25647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinic-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan-Or Z, Bar-Shira A, Gurevich T, et al. Homozygosity for the MTX1 c.184T>A (p. S63T) alteration modifies the age of onset in GBA-associated Parkinson’s disease. J Neurogenet. 2011;12:325–332. doi: 10.1007/s10048-011-0293-6. [DOI] [PubMed] [Google Scholar]
- Barrett MJ, Hagenah J, Dhawan V, et al. Transcranial sonography and functional imaging in glucocerebrosidase mutation PD. Parkinsonism Relat Disord. 2013;19:186–191. doi: 10.1016/j.parkreldis.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL. The Smell Identification Test™ administration manual. 3. Haddon Heights, NJ: Sensonics, Inc; 1995. [Google Scholar]
- Fahn S, Marsden CD, Calne DB, et al. Recent developments in Parkinson’s disease. Florham Park, NJ: Mcmillan Healthcare Information; 1987. pp. 153–163. [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment (MoCA): a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Vuong QH. Likelihood ratio tests for model selection and non-nested hypotheses. Econometrica. 1898;57:307–333. [Google Scholar]
- Silveira-Moriyama L, Holton JL, Kingsbury A, et al. Regional differences in the severity of Lewy body pathology across the olfactory cortex. Neurosci Lett. 2009;453:77–80. doi: 10.1016/j.neulet.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Brockmann K, Srulijes K, Hauser AK, et al. GBA-associated PD presents with non-motor characteristics. Neurology. 2011;77:276–280. doi: 10.1212/WNL.0b013e318225ab77. [DOI] [PubMed] [Google Scholar]
- Saunders-Pullman R, Hagenah J, Dhawan V, et al. Gaucher disease ascertained through a Parkinson’s center: imaging and clinical characterization. Mov Dis. 2010;25:1364–1372. doi: 10.1002/mds.23046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlden H, Singleton A. The genetics and neuropathology of Parkinson’s disease. Acta Neuropathol. 2012;124:325–338. doi: 10.1007/s00401-012-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulopoulos M, Cortes E, Vonsattel JP, et al. Clinical and pathological characteristics of LRRK2 G2019S patients with PD. J Mol Neurosci. 2012;47:139–143. doi: 10.1007/s12031-011-9696-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del TK, Rub U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Siderowf A, Jennings D, Connolly J, et al. Risk factors for Parkinson’s disease and impaired olfaction in relatives of patients with Parkinson’s disease. Mov Disord. 2007;22:2249–2255. doi: 10.1002/mds.21707. [DOI] [PubMed] [Google Scholar]
- Siderowf A, Jennings D, Eberly S, et al. Impaired olfaction and other prodromal features in the Parkinson At-Risk Syndrome Study. Mov Disord. 2012;27:406–412. doi: 10.1002/mds.24892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler A, Mirelman A, Helmich RC, et al. Neural correlates of executive functions in healthy G2019S LRRK2 mutation carriers. Cortex. 2013;49:2501–2511. doi: 10.1016/j.cortex.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Berg D, Godau J, Seppi K, et al. The PRIPS study: screening battery for subjects at risk for Parkinson’s disease. Eur J Neurol. 2013;20:102–108. doi: 10.1111/j.1468-1331.2012.03798.x. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Aarsland D, Barone P, et al. Identifying prodromal Parkinson’s disease: pre-motor disorders in Parkinson’s disease. Mov Disord. 2012;27:617–626. doi: 10.1002/mds.24996. [DOI] [PubMed] [Google Scholar]
- Mirelman A, Gurevich T, Giladi N, et al. Gait alterations in healthy carriers of the LRRK2 G2019S mutations. Ann Neurol. 2011;69:193–197. doi: 10.1002/ana.22165. [DOI] [PubMed] [Google Scholar]
- Ortega R, Deik A, Battista J, et al. Progression of motor dysfunction in elderly LRRK2 carriers, including prior to phenoconversion to PD [abstract] Neurology. 2013;80 IN2-2-005. [Google Scholar]
- Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- Klein C, Chuang R, Marras C, Lang AE. The curious case of phenocopies in families with genetic Parkinson’s disease. Mov Disord. 2011;26:1793–1802. doi: 10.1002/mds.23853. [DOI] [PubMed] [Google Scholar]
- Quinn TP, Alcalay RN, Saunders-Pullman R, et al. 2013. The Montreal Cognitive Assessment across English and Hebrew. World Parkinson’s Congress, Montreal.
- Djaldetti R, Nageris BI, Lorberboym M, et al. [123-I]-FP-CIT SPECT and olfaction test in patients with combined postural and rest tremor. J Neural Transm. 2008;115:469–472. doi: 10.1007/s00702-007-0851-0. [DOI] [PubMed] [Google Scholar]


