Abstract
Objective
Explosive blast mild traumatic brain injury (mTBI) is associated with a variety of symptoms including memory impairment and posttraumatic stress disorder (PTSD). Explosive shock waves can cause hippocampal injury in a large animal model. We recently reported a method for detecting brain injury in soldiers with explosive blast mTBI using magnetic resonance spectroscopic imaging (MRSI). This method is applied in the study of veterans exposed to blast.
Methods
The hippocampus of 25 veterans with explosive blast mTBI, 20 controls, and 12 subjects with PTSD but without exposure to explosive blast were studied using MRSI at 7 Tesla. Psychiatric and cognitive assessments were administered to characterize the neuropsychiatric deficits and compare with findings from MRSI.
Results
Significant reductions in the ratio of N-acetyl aspartate to choline (NAA/Ch) and N-acetyl aspartate to creatine (NAA/Cr) (P < 0.05) were found in the anterior portions of the hippocampus with explosive blast mTBI in comparison to control subjects and were more pronounced in the right hippocampus, which was 15% smaller in volume (P < 0.05). Decreased NAA/Ch and NAA/Cr were not influenced by comorbidities – PTSD, depression, or anxiety. Subjects with PTSD without blast had lesser injury, which tended to be in the posterior hippocampus. Explosive blast mTBI subjects had a reduction in visual memory compared to PTSD without blast.
Interpretation
The region of the hippocampus injured differentiates explosive blast mTBI from PTSD. MRSI is quite sensitive in detecting and localizing regions of neuronal injury from explosive blast associated with memory impairment.
Introduction
Mild traumatic brain injury (mTBI) is a significant manifestation in warfighters exposed to explosive blasts.1 Explosive blasts generate high-intensity shock waves sometimes exposing nearby personnel to pressure loading, which can affect the brain often without externally visible injury to the head. Up to about 20% of all returning veterans have a history of traumatic brain injury.2 Some estimates report that 20–50% of this group suffers some form of ongoing dysfunction 1 year post injury.3–5 Although mTBI from explosive blast may present with postconcussive symptoms similar to head impact TBI, the physics and pathophysiology of explosive blast mTBI is likely to be different.1 Among the symptoms of explosive blast mTBI is a very high incidence of memory complaint6,7 and often comorbid with posttraumatic stress disorder (PTSD). The relationship between the two remains controversial.8
Despite previous studies in warfighters with explosive blast mTBI using several imaging techniques, little is known about its pathology. No clear lesions are seen with conventional magnetic resonance imaging (MRI). Abnormalities in white matter tracts are reported with diffusion tensor imaging (DTI) in about 30% of veterans with explosive blast mTBI compared to veterans exposed to blast but without mTBI,9 but these changes cannot unequivocally be attributed to blast as the subjects had also experienced concomitant head impact. A study with high angular diffusion imaging (HARDI) reports widely distributed white matter differences between controls and veterans with explosive blast mTBI comorbid with PTSD and suggest that these differences correlate with the duration of loss of consciousness, but not PTSD.10 However, these white matter imaging findings are not always replicated and there is a lack of correlation with clinical symptoms.11 A positron emission tomographic (PET) study12 on veterans with explosive blast mTBI without a history of head injury or loss of consciousness ≥30 min finds reductions in fluordeoxyglucose (FDG) uptake in the cerebellum, vermis, pons, and medial temporal lobe compared to a significantly older group of controls. Since 10 of their 12 subjects are comorbid for PTSD, the study is unable to differentiate the effect of shock wave pressures alone.
Animal studies confirm that pure explosive blast shock wave pressures can cause brain injury.13 We recently reported a method for detecting brain injury in soldiers with explosive blast mTBI using magnetic resonance spectroscopic imaging (MRSI) at 7 T.14 In this study, we report further on MRSI studies of the hippocampus of warfighters with self-reported memory impairments, and compare these with findings on a group of veterans with PTSD but without exposure to blast. The studies demonstrate clear evidence of hippocampal injury in subjects with memory impairment and explosive blast and point to regional differences in hippocampal injury from subjects with PTSD without blast exposure. The findings help differentiate blast mTBI from PTSD and may potentially impact current understanding of underlying neural mechanisms.
Materials and Methods
Selection of subjects
Informed consent was obtained from subjects participating in this study. Participants were recruited during secondary TBI screen at the tertiary Veterans Administration (VA) hospital. Subjects with explosive blast mTBI were enrolled in the study. They were classified as having mTBI based on the criteria proposed by the “Mild Traumatic Brain Injury Committee, ACRM, Head injury Interdisciplinary Special Interest Group, Definition of mild traumatic brain injury.”15 According to their definition, a patient with mTBI is a person who has had a traumatically induced physiological disruption of brain function, as manifested by at least one of several criteria and severity criteria consistent with the definition. All subjects enrolled were consistent with this definition. In general, Glasgow Coma Scales (GCS) were unavailable due to battlefield conditions. All subjects reported only brief changes in consciousness, walked away from the blast explosion, and none had speech problems. Four subjects were retired Special Forces personnel from outside the VA hospital who volunteered for study. Subjects with a history of neurosurgery, active substance dependence, active psychotic illness, or contraindication to MRI imaging were excluded. Subjects included 24 men and one woman, who had an average age of 34 years (SD ± 9) and 14 years of education (SD ± 2.2). Three subjects reported only one explosive blast exposure and the remainder reported multiple blast exposures. Twelve subjects (eight men, four women) with PTSD with an average age of 43 (SD ± 9) and no-blast exposure were attendees at the VA PTSD clinic. They were diagnosed with PTSD based on Diagnostic and statistical manual of mental disorder-IV (DSM IV) criteria. The exact duration of PTSD could not be accurately ascertained, as most of the subjects were uncertain when symptoms first appeared. Twenty subjects (12 men, eight women), with an average age of 32 (SD ± 12 years) without a history of mTBI or other neurologic or psychiatric disorder served as controls. The control group was recruited from Yale community (laboratory and administrative staff members, students), as well as Craig’s list volunteers. Their educational level was bimodal as in the experimental groups. An analysis variance (ANOVA) of the ages of the subjects in the three groups showed no significant difference even though the mean age of the PTSD-only group is slightly higher than the other groups. All subjects were screened and evaluated by a Board Certified neurologist and psychiatrist for enrollment in the study. The studies were approved under Yale protocol HIC#0708002993 and VA protocol IRB HH0001.
Clinical characterization and symptom measures
Clinical variables such as handedness, age, sex, marital status, current and past medical and medication history, history of head injury before and after deployment, detailed history of explosive blast exposure(s) as well as other impact head injuries, and specific treatment of TBI were obtained. A standardized TBI screening tool was used to assess the degree of neuropsychiatric complaints of each veteran. Psychiatric Evaluation: Military version of the PTSD Check List (PCL-M), Beck Depression Inventory (BDI) and the Hamilton Rating Scale for Depression (HRSD), Beck Anxiety Inventory (BAI), and a Structured Clinical Interview for Diagnostic Statistical Manual-IV (SCID). The patient demographics of explosive blast mTBI subjects along with their psychiatric assessments are listed in Table 1. The medications the subjects were on at the time of the study are also listed in the table. There was no correlation between the mediations the subjects were on and NAA ratios or with their comorbid conditions.
Table 1.
Patient demographics and psychiatric assessments
| Subject # | Gender | Age | Trauma | PTSD | Depress | Anxiety | Effort | Meds |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 46 | BB | Yes | Yes | No | No | Clonazepam |
| Citalopram | ||||||||
| 2 | M | 52 | B | No | No | No | No | Not known |
| 3 | M | 52 | B | Yes | Yes | No | Yes | Not known |
| 4 | M | 21 | BB | Yes | Yes | No | No | Duloxetin |
| Buproprion | ||||||||
| 5 | M | 34 | BB | Yes | Yes | No | NA | Prazosin HCL |
| Aripiprazole | ||||||||
| Duloxetine | ||||||||
| 6 | M | 44 | BB | No | Yes | No | Yes | Mirtazipine |
| Alprazolam | ||||||||
| Sertraline | ||||||||
| Trazadone | ||||||||
| 7 | M | 24 | BB | No | Yes | Yes | No | Sertraline |
| Trazadone | ||||||||
| Aripiprazole | ||||||||
| 8 | M | 37 | B | Yes | Yes | Yes | Yes | Clonazepam |
| Mirtazipine | ||||||||
| 9 | M | 38 | BB | No | No | No | Yes | None |
| 10 | M | 26 | BB | No | No | No | Yes | None |
| 11 | M | 22 | BB | Yes | No | No | NA | Trazadone |
| 12 | M | 45 | B | No | Yes | Yes | Yes | Clonazepam |
| 13 | M | 26 | B | Yes | No | Yes | Yes | Citalopram |
| Trazadone | ||||||||
| 14 | M | 40 | BB | Yes | No | No | Yes | Sertraline |
| Quetiapine fumarate | ||||||||
| 15 | M | 30 | BB | Yes | Yes | Yes | No | Divalproex |
| Buspirone HCL | ||||||||
| Fluoxetine HCL | ||||||||
| Risperidone | ||||||||
| Temazepam | ||||||||
| 16 | M | 46 | BB | Yes | Yes | Yes | Yes | Citalopram |
| Venlafaxine | ||||||||
| 17 | M | 26 | BB | No | No | Yes | NA | None |
| 18 | M | 33 | B | No | No | No | No | Trazadone |
| 19 | M | 29 | BB | Yes | Yes | Yes | No | Citalopram |
| Trazadone | ||||||||
| Prazosin HCL | ||||||||
| 20 | M | 28 | BB | Yes | Yes | No | No | Divalproex |
| 21 | M | 28 | BB | Yes | Yes | Yes | No | Trazadone |
| Prazosin HCL | ||||||||
| 22 | M | 32 | BB | Yes | Yes | No | No | Mirtazipine |
| 23 | M | 44 | B | No | No | No | No | Not known |
| 24 | F | 28 | BB | Yes | Yes | Yes | Yes | Citalopram |
| Lorazepam | ||||||||
| 25 | M | 30 | B | No | No | No | Yes | None |
Gender: M, male; F, female; Age – years; Trauma: B, explosive blast only; BB, explosive blast and blunt trauma at time of exposure; PTSD, posttraumatic stress disorder; Depress, depression; Anxiety; Effort, Yes passed effort testing during cognitive evaluations; NA, subject did not complete examination; Meds, medications on at the time of study.
Cognitive assessments included evaluations of: General Intelligence: Wechsler Adult Intelligence Scale-Fourth Edition (Selected Subtests) (WAIS-IV), Wechsler Test of Adult Reading; Verbal Skills: Boston Naming Test, Semantic Fluency and Controlled Oral Word Association (FAS); Visiospatial: Judgment of Line Orientation; Memory: California Verbal Learning Test-Second Edition (CVLT), Rey-Osterrieth Complex Figure Test; Executive Function/Processing Speed: Trail Making Test, Wisconsin Card Sorting, Grooved Pegboard, Digit Span (WAIS-IV), Symbol Search (WAIS-IV), Digit Symbol (WAIS-IV); and Effort Testing: Test of Memory Malingering and Green’s Word Memory Test. Performance estimates were corrected where appropriate for demographic variables including age, race, gender, and education.
Magnetic resonance spectroscopic imaging
Details of the MRSI techniques adopted for this study are published in Hetherington et al.14 In brief, all MR data at 7 T were acquired using an eight-coil transceiver array.16 This work used the phase sensitive reconstruction described in our initial papers. In brief, an unsuppressed rapid water SI (TR = 300 msec) is acquired using the same plane selection, plane thickness, field of view, and spatial resolution as the metabolite SI and a SI is reconstructed for each coil in each subject. The phase of the water resonance for each pixel from each coil is calculated. This pixel by pixel, coil by coil phase correction is then applied to the SI data acquired for each coil of the metabolite acquisition. The phase corrected metabolite SI data from each coil are then recombined using the sensitivity matrix, measured from the water SI. This scheme is independent of the brain region studied, since it utilizes a matched water reference acquisition with each subject’s study.
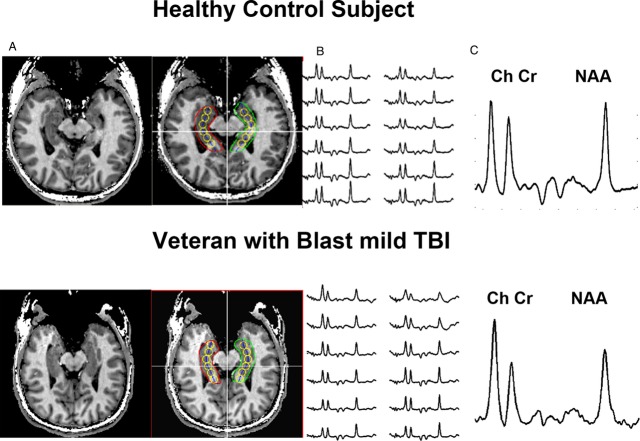
MRSI data were acquired with a single slice acquisition sequence17 using a 10-mm-thick slice angulated along the temporal pole. Metabolic images were acquired with 24 × 24 phase encoding steps over a field of view 192 × 192 mm providing ~1 cc resolution after postacquisition spatial filtering. A repetition time of 1.5 sec was used yielding an acquisition time of 14.4 min. An echo time of 40 msec was used to minimize spectral overlap of NAA, Cr, and Ch with amino acids and macromolecule resonances.18 To improve the accuracy of their measurement by correcting for naturally occurring variations in metabolite content along the hippocampal formation, image-guided single voxel reconstructions19 were used to provide reproducible sampling across subjects for the hippocampus (see Table 2). Briefly, the hippocampal formations were manually outlined (red and green Region of Interest-ROIs), and a midline (yellow) was automatically calculated. The aqueduct was then manually identified (intersection of white lines) and six voxels (yellow circles), three anterior, and two posterior to the aqueduct are reconstructed at 9-mm intervals along the midline (Fig. 1).
Table 2.
Group MRSI data from control subjects, veterans with explosive blast mTBI and PTSD with no blast
| Left | Right | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | |
| NAA/Cr | ||||||||||||
| Blast TBI (N = 25) | ||||||||||||
| Avg | 1.50 | 1.31 | 1.29 | 1.27 | 1.15 | 1.18 | 1.46 | 1.30 | 1.29 | 1.20 | 1.06 | 1.05 |
| SD | 0.17 | 0.11 | 0.16 | 0.20 | 0.15 | 0.19 | 0.16 | 0.08 | 0.10 | 0.12 | 0.13 | 0.18 |
| P value | NS | NS | NS | NS | NS | NS | NS | NS | 3.97E-02 | 3.52E-03 | 2.74E-02 | 5.59E-04 |
| PTSD (N = 12) | ||||||||||||
| Avg | 1.48 | 1.25 | 1.25 | 1.21 | 1.16 | 1.20 | 1.47 | 1.28 | 1.31 | 1.26 | 1.10 | 1.13 |
| SD | 0.26 | 0.13 | 0.13 | 0.15 | 0.15 | 0.30 | 0.18 | 0.08 | 0.14 | 0.17 | 0.17 | 0.18 |
| P value | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | 3.97E-02 |
| Control (N = 20) | ||||||||||||
| Avg | 1.47 | 1.32 | 1.33 | 1.32 | 1.21 | 1.21 | 1.46 | 1.34 | 1.37 | 1.31 | 1.17 | 1.29 |
| SD | 0.15 | 0.10 | 0.12 | 0.17 | 0.17 | 0.17 | 0.16 | 0.10 | 0.12 | 0.11 | 0.16 | 0.22 |
| NAA/Ch | ||||||||||||
| Blast TBI (N = 25) | ||||||||||||
| Avg | 1.69 | 1.36 | 1.24 | 1.12 | 0.95 | 0.86 | 1.68 | 1.37 | 1.26 | 1.11 | 0.92 | 0.81 |
| SD | 0.23 | 0.14 | 0.15 | 0.16 | 0.15 | 0.18 | 0.20 | 0.14 | 0.16 | 0.17 | 0.14 | 0.19 |
| P value | NS | NS | NS | NS | 1.04E-02 | 1.25E-02 | NS | 2.62E-02 | 1.03E-02 | 2.05E-02 | 3.72E-03 | 1.78E-05 |
| PTSD (N = 12) | ||||||||||||
| Avg | 1.60 | 1.27 | 1.21 | 1.13 | 1.07 | 0.95 | 1.63 | 1.35 | 1.24 | 1.10 | 1.00 | 0.91 |
| SD | 0.26 | 0.13 | 0.16 | 0.16 | 0.21 | 0.26 | 0.17 | 0.14 | 0.18 | 0.19 | 0.17 | 0.20 |
| P value | NS | 3.92E-03 | 3.84E-02 | NS | NS | NS | 3.80E-02 | 3.19E-02 | 3.85E-02 | NS | NS | 3.00E-02 |
| Control (N = 20) | ||||||||||||
| Avg | 1.71 | 1.43 | 1.33 | 1.22 | 1.08 | 0.99 | 1.77 | 1.46 | 1.38 | 1.22 | 1.05 | 1.07 |
| SD | 0.18 | 0.14 | 0.12 | 0.20 | 0.16 | 0.16 | 0.15 | 0.11 | 0.12 | 0.13 | 0.13 | 0.16 |
The numbers in bold black have a P < 0.05 two-tailed t-test, false discovery rate (FDR) P > 0.05. Those in italics P < 0.05 two-tailed t-test, FDR P < 0.05. The P values shown are for comparisons of controls versus Blast TBI or PTSD. The FDR test takes into account the comparison of a large number of measures and is more effective than an ANOVA analysis. There are no significant differences between the PTSD-only group and the blast TBI group measures compared against each other. MRSI, magnetic resonance spectroscopic imaging; mTBI, mild traumatic brain injury; PTSD, posttraumatic stress disorder.
Figure 1.
Representative MRSI data acquired from a control subject and a veteran with explosive blast mTBI. Scout image (A) displaying the loci of the reconstructed voxels (yellow circles). Loci numbered 1 to 6 from posterior to anterior. Displayed in (B) are the spectra. Displayed in (C) are spectra from the most anterior pixel from the right hippocampal formation. The labeled resonances are NAA N-acetyl aspartate, Cr – Creatine, and Ch – choline. MRSI, magnetic resonance spectroscopic imaging; mTBI, mild traumatic brain injury.
Hippocampal volumes (HcV) were determined by manual delineation of the hippocampi on 1.5-mm isotropic T1-weighted images.20 As there was only one female in the explosive blast mTBI group, to eliminate possible bias associated with gender differences in HcV, the data from only the male controls (n = 12) were compared with those of the male veterans. The HcV asymmetry index – HcVAI = (right HcV − left HcV)/(right HcV + left HcV) was calculated to correct for potential volume differences between individuals.
Statistical analysis
The MRSI data were grouped according to location (1–6), side (right or left), and patient versus control subject. An ANOVA was first done when multiple groups were compared, and where significance was found, a two-tailed Student’s t-test was carried out to evaluate for potential statistical differences between equivalent loci. The data were then divided into two families of data, N-acetyl aspartate to creatine (NAA/Cr) and N-acetyl aspartate to choline (NAA/Ch) ratios. To correct for potential type I errors and retain statistical power to avoid type II errors for each family of measures, a Benjamini–Hochberg test21 was used with the threshold for false discovery set to be 5%. Neuropsychological data for subject groups were compared with the Student’s t-test for independent samples.
Results
MRSI and volumetric data in explosive blast mTBI subjects
A single voxel reconstruction process, which uses anatomical landmarks to accurately position the center of the reconstructed spectra thereby minimizing anatomic variations, was used to account for the natural variation in the NAA/Ch ratios seen in control subjects. The data acquired from a control subject and veteran with explosive blast mTBI who did not have PTSD are shown in Figure 1. The displayed spectra highlight two important features. First, there was substantial variation in the NAA/Ch ratio in the control subject with significant declines in the NAA/Ch ratio seen along the hippocampal formation, ranging from a value of ~1.7 in the posterior hippocampus (pHPC) to ~1.0 in anterior regions of the hippocampal formation. Second, the data from the veterans showed a dramatic decline in the NAA/Ch ratio from the anterior pixels, with the NAA/Ch ratio reaching a value of 0.6, more than 2 SDs from the control mean. Table 2 displays pooled data from 20 controls and 25 patients with explosive blast mTBI. The loci were numbered sequentially from the most posterior locus #1, to the most anterior locus #6. After applying the false discovery test, NAA/Ch was significantly decreased in (loci 5 and 6) from the left hippocampus, and in loci 2–6 on the right. A significant decline in NAA/Cr was also seen from loci #4 and #6 of the right hippocampus.
As a group, the right hippocampus was smaller (15%) in the explosive blast mTBI group compared to controls (3.39 ± 0.36 vs. 4.01 ± 0.36 cc, P < 0.001), consistent with a more severely affected hippocampus. No volume difference was seen on the left when compared with patients and controls (3.73 ± 0.35 vs. 3.83 ± 0.35 cc). Notably, the apparent selective reduction in right HcV resulted in a statistically significant (P < 0.001) reversal of the HcVAI from +0.02 (SD ± 0.02) in controls to −0.05 (SD ± 0.05) in the mTBI veterans.
Of the group of 25 veterans with blast exposure, eight reported no secondary head impact. Five of these eight had no comorbidity with PTSD. Those belonging to the latter group, as well as all eight with no secondary head impact, showed as much brain injury as those with both blast and impact (Table 3). Furthermore, while most of our subjects had exposure to multiple explosions, three reported only one exposure. Though a small sample, the mean NAA/Ch in these three subjects for the right anterior hippocampal loci (#3 – 1.24, #4 – 1.05, #5 – 0.88, #6 – 0.76) were comparable to the mean of the combined explosive blast mTBI and lower than those of the control group (see Table 2). The mean NAA/Ch values for left loci #5 (1.03) and #6 (1.10) were somewhat higher than the group mean and closer to the values in controls. A single blast exposure thus while causing significant changes in the right anterior hippocampus (aHPC) appears to have a lesser effect on the left.
Table 3.
Comparison of metabolic changes throughout the hippocampus in explosive blast mTBI with and without comorbidities and controls
| 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HC locus | NAA/Cr (Left) | NAA/Cr (Right) | |||||||||||
| Explosive blast mTBI (n = 5), noncomorbid | Avg | 1.58 | 1.28 | 1.24 | 1.31 | 1.08 | 1.05 | 1.51 | 1.29 | 1.26 | 1.15 | 1.07 | 0.98 |
| SD | 0.28 | 0.17 | 0.24 | 0.24 | 0.13 | 0.16 | 0.21 | 0.06 | 0.05 | 0.07 | 0.16 | 0.26 | |
| Explosive blast mTBI (n = 20), comorbid | Avg | 1.49 | 1.32 | 1.3 | 1.26 | 1.16 | 1.21 | 1.45 | 1.3 | 1.3 | 1.21 | 1.06 | 1.07 |
| SD | 0.14 | 0.09 | 0.13 | 0.19 | 0.15 | 0.19 | 0.15 | 0.09 | 0.11 | 0.13 | 0.13 | 0.15 | |
| Explosive blast mTBI (n = 25), combined | Avg | 1.5 | 1.31 | 1.29 | 1.27 | 1.15 | 1.18 | 1.46 | 1.3 | 1.29 | 1.2 | 1.06 | 1.05 |
| SD | 0.17 | 0.11 | 0.16 | 0.2 | 0.15 | 0.19 | 0.16 | 0.08 | 0.1 | 0.12 | 0.13 | 0.18 | |
| Controls | Avg | 1.47 | 1.32 | 1.33 | 1.32 | 1.21 | 1.21 | 1.46 | 1.34 | 1.37 | 1.31 | 1.17 | 1.29 |
| SD | 0.15 | 0.1 | 0.12 | 0.17 | 0.17 | 0.17 | 0.16 | 0.1 | 0.12 | 0.11 | 0.16 | 0.22 | |
| NAA/Ch (Left) | NAA/Ch (Right) | ||||||||||||
| Explosive blast mTBI (n = 5), noncomorbid | Avg | 1.75 | 1.32 | 1.17 | 1.12 | 0.91 | 0.71 | 1.6 | 1.29 | 1.2 | 1.02 | 0.9 | 0.68 |
| SD | 0.34 | 0.18 | 0.21 | 0.24 | 0.21 | 0.15 | 0.22 | 0.09 | 0.13 | 0.13 | 0.2 | 0.27 | |
| Explosive blast mTBI (n = 20), comorbid | Avg | 1.67 | 1.36 | 1.26 | 1.12 | 0.96 | 0.89 | 1.7 | 1.39 | 1.28 | 1.13 | 0.92 | 0.84 |
| SD | 0.2 | 0.13 | 0.13 | 0.14 | 0.14 | 0.17 | 0.19 | 0.15 | 0.17 | 0.17 | 0.13 | 0.16 | |
| Explosive blast mTBI (n = 25), combined | Avg | 1.69 | 1.36 | 1.24 | 1.12 | 0.95 | 0.86 | 1.68 | 1.37 | 1.26 | 1.11 | 0.92 | 0.81 |
| SD | 0.23 | 0.14 | 0.15 | 0.16 | 0.15 | 0.18 | 0.2 | 0.14 | 0.16 | 0.17 | 0.14 | 0.19 | |
| Controls | Avg | 1.71 | 1.43 | 1.33 | 1.22 | 1.08 | 0.99 | 1.77 | 1.46 | 1.38 | 1.22 | 1.05 | 1.07 |
| SD | 0.18 | 0.14 | 0.12 | 0.2 | 0.16 | 0.16 | 0.15 | 0.11 | 0.12 | 0.13 | 0.13 | 0.16 | |
The “explosive blast” group in this table includes those subjects only exposed to explosive blast and without secondary trauma or PTSD. They are compared to those with explosive blast and one or more comorbidities of PTSD, depression, anxiety, and secondary trauma, the “explosive blast comorbid group” and the values of all subjects with explosive blast, the “combined group”. The highlighted regions (gray) are those showing significant change in the combined group compared to controls. Values in noncomorbid group are generally lower than in the group with comorbidities. NAA/Ch, N-acetyl aspartate to choline; NAA/Cr, N-acetyl aspartate to creatine; mTBI, mild traumatic brain injury; PTSD, posttraumatic stress disorder.
MRSI data in subjects with PTSD but no explosive blast mTBI
Table 2 shows the pooled MRSI data for 12 subjects with PTSD but no exposure to explosive blast and the 20 controls. On average, NAA/Ch values were intermediate to explosive blast mTBI and controls. The NAA/Ch ratios were lower than in the controls as a group reaching significance for posterior hippocampal loci #2 and 3 on the left and loci #1, 2, 3, and 6 in the right hippocampus when a two-tailed t-test was applied. Applying a more stringent false discovery rate test only locus #2 was significant. However, due to the smaller number of PTSD samples, the power of the false discovery test was much reduced. In general, injury tended to be in the pHPC in comparison to subjects with explosive blast mTBI where injury was focused anteriorly.
Psychiatric evaluations and MRSI data in explosive blast mTBI
Of the 25 explosive blast mTBI veterans, 16 met DSM-IV criteria for PTSD, 15 veterans had a history of depressive disorder, and 10 had an anxiety disorder. The average BDI and PCL–M scores were both high: 24.9 (SD ± 9.6) and 55.0 (SD ± 14.3), respectively. To evaluate the role of the various comorbidities (PTSD, depression, and anxiety) with explosive blast mTBI, we compared the MRSI data for five explosive blast mTBI subjects who had no comorbidities with the values for those with comorbidities and the group as a whole (Table 3). The mean values for these five subjects across the hippocampal loci were even lower than for the 20 with comorbidities and the combined group, suggesting that comorbidities were not a factor in the reduced metabolite levels. We also grouped the data according to each of their psychiatric evaluations. To minimize the number of total comparisons, we restricted our analysis to those regions, which showed the greatest changes in MRSI, the two most anterior loci (#5, #6) of the right and left hippocampi. NAA/Ch ratios were somewhat smaller, on average, in the groups without a comorbid diagnosis of PTSD, depression, or anxiety, in comparison to those with the diagnoses, but none of these comparisons yielded statistically significant differences (Table 4). These observations suggested that the reductions seen in NAA/Ch in the blast exposure mTBI group were probably not due to a comorbid psychiatric disorder.
Table 4.
Comorbidity versus anterior hippocampal ratios in explosive blast mTBI group
| NAA/Cr | NAA/Ch | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | L5 | L6 | R5 | R6 | L5 | L6 | R5 | R6 | |
| PTSD | |||||||||
| Yes | 16 | 1.16 | 1.20 | 1.06 | 1.08 | 0.95 | 0.88 | 0.92 | 0.84 |
| No | 9 | 1.11 | 1.16 | 1.09 | 1.02 | 0.95 | 0.85 | 0.93 | 0.76 |
| Depression | |||||||||
| Yes | 15 | 1.15 | 1.22 | 1.07 | 1.08 | 0.97 | 0.89 | 0.94 | 0.87 |
| No | 10 | 1.13 | 1.14 | 1.07 | 1.02 | 0.93 | 0.84 | 0.90 | 0.74 |
| Anxiety | |||||||||
| Yes | 10 | 1.08 | 1.12 | 1.04 | 1.08 | 0.97 | 0.90 | 0.94 | 0.87 |
| No | 15 | 1.18 | 1.23 | 1.09 | 1.04 | 0.94 | 0.84 | 0.91 | 0.77 |
| Effort | |||||||||
| Yes | 11 | 1.11 | 1.17 | 1.08 | 1.04 | 0.95 | 0.87 | 0.95 | 0.82 |
| No | 11 | 1.17 | 1.15 | 1.09 | 1.10 | 0.96 | 0.80 | 0.92 | 0.83 |
There are no statistically significant differences in MRSI measures with or without any of the particular comorbidities. mTBI, mild traumatic brain injury; NAA/Cr, N-acetyl aspartate to choline; PTSD, posttraumatic stress disorder; MRSI, magnetic resonance spectroscopic imaging.
Cognitive evaluations, memory, and MRSI data in explosive blast mTBI
Evaluation of broad cognitive functioning indicated a population with premorbid intellectual skills that were estimated to be average or better. Intellectual skills were consistent with premorbid estimates with scores consistently in the average range across the sample. Memory skills and executive skills were relatively reduced, on average, across the sample by ~0.5–1.0 standard deviation compared to normative expectations. However, 11 of the subjects failed one or more performance validity measures designed to evaluate effort level. This could potentially suggest that the cognitive decrements noted may be the result of factors other than a reduction in their true ability. When the subjects were subdivided into those passing or failing the effort tests, there was no significant difference between the groups in their MRSI measures (Table 4). That is, even subjects who failed the effort test had clear hippocampal injury on MRSI assessment. Thus, MRSI data provide an objective measure of injury free from motivational issues, which can affect neuropsychological testing.
Memory function in PTSD (no blast) and explosive blast mTBI
The 12 subjects with PTSD without blast exposure also underwent neuropsychiatric and neurocognitive evaluation. Nine of these subjects had a positive diagnosis of depression and four had a diagnosis of anxiety. The latter four also had depression. Eight subjects passed tests of effort level. All subjects with explosive blast mTBI had a self-reported history of reduction in memory function. Given the observed difference in the hippocampal region injured in the explosive blast mTBI and PTSD without blast exposure group based on MRSI measures and the greater likelihood of impact on memory functioning following injury to these structures, we compared the tests for verbal memory (California Verbal Learning Test) and visual memory (Rey-Osterrieth Complex Figure Test) across the two groups. Only subjects that passed the effort test were included in the comparison. The subjects were comparable in general intelligence measures (as estimated by WAIS4 vocabulary, Table 5). Comparison across the groups on measures of memory suggested greater reductions in visual memory performance across an extended time delay within the blast mTBI group when compared to those with PTSD (P = 0.026, Table 5) with immediate recall of the same material showing a tendency in the same direction (P = 0.068, Table 5). The verbal memory test scores in the CVLT did not, in general, show statistically significant differences with the exception of the recognition component of the test, in which the explosive blast mTBI group tended to show reduced verbal recognition function (P = 0.053, Table 5) when compared to the PTSD group. Thus, subjects with explosive blast mTBI and anterior hippocampal injury show deficits in visual memory with much lesser effects on verbal memory.
Table 5.
Performance measures on verbal and visual memory tests
| Test | Group | N | Mean ± SD | P (2-tailed) |
|---|---|---|---|---|
| *CVLT – TotalWrdsSS | PTSD only | 8 | 49.75 ± 7.440 | NS |
| Blast TBI | 14 | 44.93 ± 14.478 | ||
| **CVLT – SDFreeSS | PTSD only | 8 | 0.063 ± 1.016 | NS |
| Blast TBI | 14 | −0.357 ± 1.447 | ||
| ***CVLT – LDFreeSS | PTSD only | 8 | 0.000 ± 0.655 | NS |
| Blast TBI | 14 | 0.536 ± 1.538 | ||
| **CVL – RecognitionSS | PTSD only | 8 | −0.188 ± 0.843 | 0.053 |
| Blast TBI | 14 | −1.393 ± 1.521 | ||
| *ReyImmediateSS | PTSD only | 8 | 58.75 ± 9.301 | 0.068 |
| Blast TBI | 14 | 47.29 ± 18.503 | ||
| *ReyDelaySS | PTSD only | 8 | 59.88 ± 9.746 | 0.026 |
| Blast TBI | 14 | 46.00 ± 17.369 | ||
| ***WAIS4.Vocabulary | PTSD only | 8 | 12.25 ± 2.915 | NS |
| Blast TBI | 14 | 11.57 ± 3.155 |
The performance measures of only the subjects of the two groups that passed the effort test are compared in this table. The P values were determined with t-test for independent samples. CVLT, California Verbal Learning Test (2nd Edition); Rey, Rey-Osterrieth Complex Figure Test; WAIS4, Wechsler Adult Intelligence Scale (4th Edition); LD, long delay; SD, short delay; PTSD, posttraumatic stress disorder; TBI, traumatic brain injury.
T-Score (mean = 50; standard deviation = 10)
Z-score (mean = 0; standard deviation = 1)
Scaled Score (mean = 10; standard deviation = 3).
Discussion
Hippocampal injury in explosive blast mTBI and PTSD
These studies on explosive blast-exposed warfighters with mTBI identify the hippocampus as a brain region particularly vulnerable to the effects of explosive blast shock wave pressures. This vulnerability of the hippocampus has also been confirmed in large animal studies.13 In spite of the subjectivity of the reported brain injury history, as well as unremarkable clinical MRI findings, subjects who reported explosive blast mTBI and memory complaints demonstrated significant decreases in NAA/Ch in both hippocampi, with the right hippocampus being generally more severely affected. In contrast to the bilateral changes seen in NAA/Ch, albeit numerically more severe on the right, a statistically significant reduction in NAA/Cr was seen only in the right hippocampus, and was accompanied by a statistically significant reduction in right HcV and a reversal of the sign of the HcV asymmetry index. Thus, as a whole, the anterior portion of the right hippocampus seems to be more profoundly affected in this group and suggests neuronal impairment and loss. It is known that NAA synthesis is localized to neuronal mitochondria22 and is correlated with bioenergetics measures in model systems and the human brain.23 The finding of reduced NAA/Cr is consistent with the findings of oxidative injury in rat models of blast.24 The findings of right worse than left medial temporal lobe metabolic dysfunction in explosive blast mTBI are striking and raise important questions on the cause of this asymmetry and may be inherent to the functional asymmetry of the human brain. Data on the sidedness of the explosive blast itself are difficult to obtain but might also shed information on the asymmetric brain response. Reasons for the asymmetry need further investigation.
Our data also address three other issues of considerable interest in respect of explosive blast mTBI. (1) Is the injury seen in explosive blast mTBI due to an associated comorbid condition for example, PTSD? In the explosive blast mTBI group, there were no statistical differences in the NAA/Ch and NAA/Cr ratios from the anterior loci of both hippocampi between those veterans with and without a diagnosis of PTSD. Similarly, symptoms of depression and anxiety did not significantly affect metabolite ratios. Regardless of the nature of the causal relationship between explosive blast mTBI and PTSD in this group, the hippocampus is similarly injured, thus pointing to a primary role of blast pressure rather than a comorbid mechanism in the causation of the injury. (2) Does explosive blast shock wave pressure alone (without head impact) cause pathological changes in the brain? Our finding in a subgroup of veterans with explosive blast mTBI but no secondary head impact showed as much hippocampal injury as those with both blast and impact; thus, again pointing to explosive blast shock wave pressure as a principal cause of brain injury. (3) Is exposure to a single blast sufficient to cause injury? Although albeit a small subgroup of our study, the three veterans who reported only one blast exposure, had mean NAA/Ch measures in the right anterior hippocampal loci that were comparable to the mean of the combined explosive blast mTBI group and lower than those of the control group. The mean NAA/Ch values for left loci #5 (1.03) and #6 (1.10) were somewhat higher than the group mean and closer to the values in controls. A single blast exposure thus while causing significant changes in the right aHPC has a lesser effect on the left. Large animal studies provide evidence that whereas a single exposure produces mild injury, two and three exposures amplify the degree of injury (unpublished findings).
In contrast to the pattern of hippocampal injury in the explosive blast mTBI group, the veterans with a diagnosis of PTSD without a history of exposure to blast indicated injury more commonly, in the posterior hippocampal loci (#1–3). Our findings of injury to the aHPC with reduced right HcV in those sustaining explosive mTBI are inconsistent with observations in most studies of PTSD. A meta-analysis of MRI estimates of HcV revealed smaller volumes of both left and right hippocampi in chronic PTSD.25 A further analysis reveals a significantly smaller pHPC in PTSD with no difference in volumes of aHPC or subiculum.26 This finding is consistent with the MRSI findings in our subjects in the PTSD-only group, where NAA/Ch ratios were reduced more in the pHPC.
The foregoing observations point to differences in hippocampal vulnerability in explosive blast mTBI and PTSD without blast. In the former, the aHPC being more vulnerable, while in the latter the pHPC. The human hippocampus is shown to possess long-axis specialization of anatomical connections with differences between an aHPC and pHPC (reviewed in 27). The aHPC and pHPC also have a variety of functional specializations.27 The aHPC has strong reciprocal connections with the amygdala.28 It is thus possible that the selective vulnerability of hippocampal regions in these two conditions may be related to their anatomical organization and connectivity patterns.
Memory in explosive blast mTBI and PTSD
The hippocampus has been demonstrated to be associated with declarative memory.29 The amygdala while connected to the hippocampus is primarily involved with emotion and is not essential for declarative memory, but is thought to be important for emotional aspects of memory.30 Beyond the neurological injury, the veterans with explosive blast mTBI also complained of memory difficulties in their everyday lives. However, neurocognitive testing suggested only mild reductions in memory efficiency. Complicating this finding were scores in one half of subjects below conservative cutoffs on performance validity measures suggesting suboptimal effort and thus questioning the validity of their scores for cognitive ability. However, in this group that failed effort, NAA/Ch ratios in the most anterior loci in the right hippocampus were still reduced by almost 2 SDs in comparison to control subjects. The HcV were also reduced. Thus, in these individuals, absent a valid cognitive evaluation, the MRSI data provide an objective indication of significant injury. Comparison with Veterans who were diagnosed with PTSD revealed worse performance on specific measures of visual memory by those experiencing blast even once the effects of poor effort are removed. This finding would be consistent with current conceptualization of the right hippocampus processing visual memory preferentially.31
It is useful to consider if the antero-posterior differences in the hippocampus are associated with the processing of visual and verbal memory. Functional imaging studies have demonstrated novelty-dependent activation of the aHPC,32 with the function of the aHPC postulated to register mismatch between expectation and experience.33 The aHPC responds to generic novelty, whereas the pHPC responds to familiarity to stimuli that have behavioral relevance.34 The aHPC bilaterally is shown through event-related fMRI studies to be crucial for successful associative encoding and the degree of coordination between hippocampal and neocortical activity predicting the likelihood of subsequent memory.35 How these postulated functions of the aHPC might contribute to visual memory encoding needs further investigation.
We did not carry out memory testing in our control group to be able to directly compare with the PTSD-only group, as the decision to include functional cognitive evaluation occurred after the completion of their participation in earlier imaging studies. They were part of a database of controls acquired for other prior studies. However, it is possible that verbal memory is associated with the pHPC and may be reduced with mild pHPC injury as in the PTSD group without blast exposure in comparison to controls. Multiple preclinical and clinical studies have demonstrated verbal memory deficits in PTSD following traumatic stress, visual memory being spared.36,37 A post hoc analysis had found a significantly smaller pHPC in PTSD patients (an observation consistent with our MRSI findings) with no volume difference in the aHPC.26 Furthermore, encoding and retrieval of verbal stimuli are shown to activate the middle and pHPC more strongly than the aHPC.38 It thus appears that the transmission of explosive blast shock pressure waves into the brain injures the hippocampus regionally and has greater effects on visual than verbal memory, a pattern of injury that results in functional memory differences from that in PTSD.
Acknowledgments
The DARPA PREVENT Research Program, National Institutes of Health: NIBIB R01-EB-011639; NIBIB R01-EB-009871, NINDS R01–NS-081772 supported this work. The views, opinions, and/or findings contained in this study are those of the authors and should not be interpreted as representing the official views or policies, either expressed or implied, of the Defense Advanced Research Projects Agency or the Department of Defense. The authors declare no competing financial interests. H. Hetherington and J. Pan are currently affiliated with the Departments of Radiology and Neurology respectively, University of Pittsburgh Medical School, Pittsburgh, PA 15213.
Conflict of Interest
None declared.
References
- Ling G, Bandak FA, Armonda R, et al. Explosive blast neurotrauma. J Neurotrauma. 2009;26:815–825. doi: 10.1089/neu.2007.0484. [DOI] [PubMed] [Google Scholar]
- RAND. Invisible wounds of war: psychological and cognitive injuries, their consequences and services to assist recovery. Santa Monica: RAND Corporation; 2008. [Google Scholar]
- Rosenfeld JV, Ford NL. Bomb blast, mild traumatic brain injury and psychiatric morbidity: a review. Injury. 2010;41:437–443. doi: 10.1016/j.injury.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Sayer NA. Traumatic brain injury and its neuropsychiatric sequelae in war veterans. Annu Rev Med. 2012;63:405–419. doi: 10.1146/annurev-med-061610-154046. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Verfaellie M, Sullivan KD. Mild traumatic brain injury and posttraumatic stress disorder in returning veterans: perspectives from cognitive neuroscience. Clin Psychol Rev. 2009;29:674–684. doi: 10.1016/j.cpr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Flynn GF. Memory impairment after mild traumatic brain injury. Continuum. 2010;16:79–109. doi: 10.1212/01.CON.0000391454.15052.e4. [DOI] [PubMed] [Google Scholar]
- Kontos AP, Kotwal RS, Elbin RJ, et al. Residual effects of combat-related mild traumatic brain injury. J Neurotrauma. 2013;30:680–686. doi: 10.1089/neu.2012.2506. [DOI] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, et al. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- MacDonald CL, Johnson AM, Cooper D, et al. Detection of blast-related traumatic brain injury in U.S. military personnel. N Engl J Med. 2011;364:2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Haswell CC, Selgrade ES, et al. Effects of chronic mild traumatic brain injury on white matter integrity in Iraq and Afganistan war veterans. Hum Brain Mapp. 2013;34:2986–2999. doi: 10.1002/hbm.22117. doi: 10.1001/hbm.22117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HS, Wilde E, Troyanskaya M, et al. Diffusion tensor imaging of mild to moderate blast-related traumatic brain inury andits sequelae. J Neurotrauma. 2010;27:683–694. doi: 10.1089/neu.2009.1073. [DOI] [PubMed] [Google Scholar]
- Peskind ER, Petrie EC, Cross DJ, et al. Cerebrocerebellar hypometabolism associated with repetitive blast exposure mild traumatic brain injury in 12 Iraq war Veterans with persistent post-concussive symptoms. Neuroimage. 2010;27:683–694. doi: 10.1016/j.neuroimage.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lanerolle NC, Bandak FA, Kang D, et al. Characteristics of an explosive blast-induced brain injury in an experimental model. J Neuropathol Exp Neurol. 2011;70:1046–1057. doi: 10.1097/NEN.0b013e318235bef2. [DOI] [PubMed] [Google Scholar]
- Hetherington HP, Hamid H, Kulas J, et al. MRSI of the medial temporal lobe at 7T in explosive blast mild traumatic brain injury. Magn Reson Med. 2014;71:1358–1367. doi: 10.1002/mrm.24814. doi: 10.1002/mrm.24814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay T, Havvington DE, Adams R, et al. Definition of truamatic brain injury. J. Head Trauma Rehabil. 1993;8:86–87. [Google Scholar]
- Avdievich NI, Pan JW, Baehring JM, et al. Short echo spectroscopic imaging of the human brain at 7T using transceiver arrays. Magn Reson Med. 2009;62:17–25. doi: 10.1002/mrm.21970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington HP, Avdievich NI, Kuznetsov AM, Pan JW. RF shimming for spectroscopic localization in the human brain at 7T. Magn Reson Med. 2010;63:9–19. doi: 10.1002/mrm.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar KL, Ogino T. Characterization of macromolecule resonances in the 1H NMR spectrum of rat brain. Magn Reson Med. 1993;30:38–44. doi: 10.1002/mrm.1910300107. [DOI] [PubMed] [Google Scholar]
- Hetherington HP, Kuzniecky RI, Vives K, et al. A subcortical network of dysfunction in TLE measured by magnetic resonance spectroscopy. Neurology. 2007;69:2256–2265. doi: 10.1212/01.wnl.0000286945.21270.6d. [DOI] [PubMed] [Google Scholar]
- Cavus I, Pan JW, Hetherington HP, et al. Decreased hippocampal volume on MRI is associated with increased extracellular glutamate in epilepsy patients. Epilepsia. 2008;49:1358–1366. doi: 10.1111/j.1528-1167.2008.01603.x. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B. 1995;57:289–300. [Google Scholar]
- Goldstein F. The enzymatic synthesis of N-acetyl-aspartic acid by subcellular preparation of rat brain. J Biol Chem. 1969;244:4. [PubMed] [Google Scholar]
- Pan JW, Takahashi K. Interdependence of N-acetyl aspartate and high-energy phosphates in healthy human brain. Ann Neurol. 2005;57:92–97. doi: 10.1002/ana.20317. [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Dixon CE, Shellington DK, et al. Screeing of biochemical and molecular mechanisms of secondary injury and repair in the brain after experimental blast-induced brain injury in rats. J Neurotrauma. 2013;30:920–937. doi: 10.1089/neu.2013.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama N, Vaccarino V, Kutner M, et al. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Bonne O, Vythilingam M, Inagaki M, et al. Reduced posterior hippocampal volume in posttraumatic stress disorder. J Clin Psychiatry. 2008;69:1087–1091. doi: 10.4088/jcp.v69n0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17:230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G, Akirav I. Amygdala-hippocampus dynamic interaction in relation to memory. Mol Neurobiol. 2000;22:11–20. doi: 10.1385/MN:22:1-3:011. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT. The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci. 2011;34:259–288. doi: 10.1146/annurev-neuro-061010-113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Alvarez-Royo P, Clower RP. Independence of memory functions and emotional behavior: separate contributions of the hippocampal formation and the amygdala. Hippocampus. 1991;1:207–220. doi: 10.1002/hipo.450010208. [DOI] [PubMed] [Google Scholar]
- Lee PL, Clason CL. Classification of seizure disorders and syndromes, and neuropsychological impairments in adults withepilepsy. In: Morgan JE, Ricker JH, editors. Textbook of clinical psychology. New York: Taylor & Francis; 2008. pp. 437–465. [Google Scholar]
- Strange BA, Duggins A, Penny W, et al. Information theory, novelty and hippocampal responses: unpredicted or unpredictable? Neural Netw. 2005;18:225–230. doi: 10.1016/j.neunet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ. Adaptive anterior hippocampal responses. Hippocampus. 2001;11:690–698. doi: 10.1002/hipo.1084. [DOI] [PubMed] [Google Scholar]
- Strange BA, Fletcher PC, Henson RNA, et al. Segregating the functions of human hippocampus. Proc Natl Acad Sci USA. 1999;96:4034–4039. doi: 10.1073/pnas.96.7.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Chua E, Cocchiarella A, et al. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20:1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci. 2006;8:445–461. doi: 10.31887/DCNS.2006.8.4/jbremner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga BM, Bremner JD. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J Affect Disord. 2002;70:1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Boyett-Anderson JM, et al. Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study. Hippocampus. 2003;13:164–174. doi: 10.1002/hipo.10064. [DOI] [PubMed] [Google Scholar]