Abstract
Objective
As of 3 September 2013, 399 cases of natalizumab-associated progressive multifocal leukoencephalopathy (PML) were confirmed in multiple sclerosis (MS) patients. We evaluated outcomes of natalizumab-treated MS patients who were asymptomatic at PML diagnosis.
Methods
Analyses included data available as of 5 June 2013. Asymptomatic patients diagnosed with PML by magnetic resonance imaging (MRI) findings and JC virus DNA detection in the central nervous system were compared with patients presenting with symptoms at diagnosis. Demographics, MRI, and survival over 12 months were analyzed. Expanded Disability Status Scale (EDSS) and Karnofsky Performance Scale (KPS) scores were recorded pre-PML, at diagnosis, and at 6 and 12 months post-diagnosis.
Results
A total of 372 PML cases were analyzed; 30 patients were asymptomatic and 342 were symptomatic at PML diagnosis. Classifications of PML lesions on MRI in asymptomatic versus symptomatic patients were unilobar in 68% versus 37%, multilobar in 21% versus 24%, and widespread in 11% versus 40%. In both groups with unilobar lesions, frontal lobe lesions predominated. Prior to PML, mean EDSS and KPS scores were similar for asymptomatic and symptomatic patients. At diagnosis, mean EDSS score was significantly lower for asymptomatic patients (4.1; n = 11) than for symptomatic patients (5.4; n = 193; P = 0.038). Six months after PML diagnosis, asymptomatic patients had less functional disability than symptomatic patients. As of 5 June 2013, 96.7% of asymptomatic patients and 75.4% of symptomatic patients were alive.
Interpretation
PML patients asymptomatic at diagnosis had better survival and less functional disability than those who were symptomatic at diagnosis.
Introduction
Natalizumab, a monoclonal antibody directed against α4 integrin, is approved for the treatment of relapsing forms of multiple sclerosis (MS) based on its efficacy in reducing clinical relapses, disability progression, and magnetic resonance imaging (MRI) disease activity measures.1–4 As of 30 September 2013, natalizumab has been used to treat 120,500 patients, corresponding to ≈313,560 patient-years of exposure.5 Natalizumab treatment is associated with an increased incidence of progressive multifocal leukoencephalopathy (PML), a rare, demyelinating opportunistic infection of the central nervous system (CNS) caused by the JC virus (JCV).6–8 Established risk factors for natalizumab-associated PML include the presence of anti-JCV antibodies in the blood, prior immunosuppressive (IS) therapy, and duration of natalizumab treatment greater than 2 years.9 Patients who are anti-JCV antibody negative are at a very low risk of PML (1/10,000). In patients who are anti-JCV antibody positive, PML risk is stratified based on prior IS use and treatment duration.5,9
As per recently published criteria by the American Academy of Neurology, a confirmed diagnosis of PML requires the presence of 3 factors: clinical symptoms, MRI findings suggestive of PML, and the presence of JCV DNA in cerebrospinal fluid (CSF) or brain tissue samples.10 MRI findings suggestive of PML in combination with JCV detection in the CSF but in the absence of symptoms leads to a classification of “probable PML.”10 However, cases of asymptomatic PML can be considered confirmed PML based on an alternative PML classification scheme (Data S1).11
There is increasing evidence that enhanced clinical vigilance including MRI, early PML diagnosis, suspension of natalizumab treatment on suspicion of PML, and treatment of PML complications may optimize outcomes in patients with natalizumab-associated PML.12,13 In an analysis of 35 cases of natalizumab-associated PML, a shorter time from symptom onset to PML diagnosis and localized disease on MRI at diagnosis were associated with improved survival.14
Despite the heterogeneous MRI findings in natalizumab-associated PML patients, MRI has been the most sensitive method for detecting PML before clinical symptoms occur.15,16 The utility of routine MRI for monitoring natalizumab-treated patients has been highlighted in several recent case reports and case series.15,17–25 In some reports, patients were diagnosed with PML in the absence of clinical symptoms when radiologic signs of PML were detected on routine MRI and confirmed by JCV DNA detection in the CSF by polymerase chain reaction.17,18,21,23,24 In others, MRI findings consistent with PML were retrospectively identified on MRI scans obtained several months before clinical symptoms of PML were apparent.19,20,25
The clinical relevance of early PML detection by MRI in asymptomatic patients in terms of its potential association with better survival and/or functional outcomes is unknown. The aim of this study was to analyze all available cases of natalizumab-associated PML as of 5 June 2013, and compare demographic and clinical characteristics, MRI findings, functional status, and survival over 12 months between asymptomatic patients and patients who were symptomatic at the time of PML diagnosis.
Methods
Confirmation of PML
The diagnosis of PML was confirmed by either a positive brain tissue examination showing evidence of viral cytopathic changes on hematoxylin and eosin staining associated with either positive immunohistochemistry for SV40 or in situ hybridization for JCV DNA, or by the presence of JCV DNA in CSF and consistent MRI findings.
If a patient had clinical symptoms, the patient was classified as symptomatic at the time of PML diagnosis. If the patient did not have clinical symptoms, the patient was classified as asymptomatic at the time of PML diagnosis.
Clinical and radiological assessments
Treating physicians were queried at the time of PML case confirmation and every 6 months thereafter for up to 24 months post-PML diagnosis using a standardized PML data collection tool (DCT; Table S1) designed to capture specific information regarding the patient’s PML disease status, vital status (alive or deceased), and additional retrospective data pertinent to the patient’s medical history (e.g., age, gender, MS disease duration, natalizumab exposure, serostatus of anti-JCV antibody, and prior IS use). Data were supplemented by details captured in the natalizumab global safety database. Some treating physicians also provided PML patient information at intervals apart from, and in addition to, the 6-month DCT schedule.
Functional disability status was also assessed by the treating physician using the Expanded Disability Status Scale (EDSS, Table 1)26 and/or the Karnofsky Performance Scale (KPS, Table 2).27 EDSS and KPS scores were assessed pre-PML (on natalizumab therapy), at PML diagnosis, and at 6 and 12 months post-PML diagnosis.
Table 1.
Expanded Disability Status Scale
| EDSS score | Description |
|---|---|
| 0.0 | Normal neurological exam |
| 1.0 | No disability, minimal signs on 1 FS |
| 1.5 | No disability, minimal signs on 2 of 7 FS |
| 2.0 | Minimal disability in 1 of 7 FS |
| 2.5 | Minimal disability in 2 FS |
| 3.0 | Moderate disability in 1 FS; or mild disability in 3–4 FS, though fully ambulatory |
| 3.5 | Fully ambulatory but with moderate disability in 1 FS; mild disability in 1 or 2 FS; moderate disability in 2 FS; or mild disability in 5 FS |
| 4.0 | Fully ambulatory without aid, up and about 12 h a day despite relatively severe disability; able to walk without aid 500 m |
| 4.5 | Fully ambulatory without aid; up and about much of the day; able to work a full day; may otherwise have some limitations of full activity or require minimal assistance; relatively severe disability; able to walk without aid 300 m |
| 5.0 | Ambulatory without aid for about 200 m; disability impairs full daily activities |
| 5.5 | Ambulatory for 100 m; disability precludes full daily activities |
| 6.0 | Intermittent or unilateral constant assistance (cane, crutch, or brace) required to walk 100 m with or without resting |
| 6.5 | Constant bilateral support (cane, crutch, or braces) required to walk 20 m without resting |
| 7.0 | Unable to walk beyond 5 m even with aid, essentially restricted to wheelchair, wheels self, transfers alone; active in wheelchair about 12 h a day |
| 7.5 | Unable to take more than a few steps; restricted to wheelchair; may need aid to transfer; wheels self, but may require motorized chair for full day |
| 8.0 | Essentially restricted to bed, chair, or wheelchair, but may be out of bed much of day; retains self-care functions; generally effective use of arms |
| 8.5 | Essentially restricted to bed much of day; some effective use of arms; retains some self-care functions |
| 9.0 | Helpless bed patient; can communicate and eat |
| 9.5 | Unable to communicate effectively or eat/swallow |
| 10.0 | Death due to MS |
EDSS, Expanded Disability Status Scale; FS, functional scale(s); MS, multiple sclerosis.
Source: Kurtzke.26
Table 2.
Karnofsky Performance Scale
| Progression | Score | Description |
|---|---|---|
| Mild | 100 | Normal; no complaints; no evidence of disease |
| Able to carry on normal activity and to work; no special care needed | 90 | Able to carry on normal activity; minor signs or symptoms of disease |
| 80 | Normal activity with effort; some signs or symptoms of disease | |
| Moderate | 70 | Cares for self; unable to carry on normal activity or do active work |
| Unable to work; able to live at home and care for most personal needs; varying amount of assistance needed | 60 | Requires occasional assistance; able to care for most personal needs |
| 50 | Requires considerable assistance and frequent medical care | |
| Severe | 40 | Disabled; requires special care and assistance |
| Unable to care for self; requires equivalent of institutional or hospital care; disease may be progressing rapidly | 30 | Severely disabled; hospital admission is indicated; death not imminent |
| 20 | Very sick; hospital admission necessary; active supportive treatment necessary | |
| 10 | Moribund; fatal processes progressing rapidly | |
| 0 | Death |
Source: Karnofsky and Burchenal.27
MRI examinations were performed at 1.5 or 3 T using protocols specified at the local site. All examinations included axial fluid attenuated inversion recovery (FLAIR) and/or axial dual echo spin-echo proton-density and T2-weighted images. All available MRIs and MRI reports were reviewed by an individual board-certified radiologist. A subset of the MRI data was also evaluated by an external advisory board15 and a reference center (Image Analysis Center, VU University Medical Center Amsterdam, The Netherlands). The classification of PML lesions on MRI based on review of the MRI reports provided was as follows: unilobar (confined to 1 lobe), multilobar (involving 2 or more contiguous lobes), or widespread (involving 2 or more noncontiguous lobes and/or present in both hemispheres).28
Statistical analyses
Categorical variables were presented as frequencies; continuous variables were reported by mean, median, and range. Functional outcomes, as assessed by available EDSS and KPS scores, were compared in asymptomatic and symptomatic PML patients at each time point using a Mann–Whitney-Wilcoxon test.29,30 Polynomial regression using the locally weighted scatterplot smoothing (LOWESS) algorithm was employed to evaluate functional outcome over time.31 All tests of statistical significance assumed a 2-sided alternative hypothesis and a 0.05 significance level uncorrected for multiple comparisons. All analyses were conducted using SAS/STAT® software, version 9.3, and R, version 2.15.32
A sensitivity analysis of functional outcomes was conducted, matching asymptomatic and symptomatic cases with the same degree of MRI involvement (lesion number and location). All previously described statistical analyses were applied to categorical and continuous variables, respectively, of this subset population.
Results
Patients
As of 5 June 2013, there were 372 confirmed postmarketing cases of natalizumab-associated PML in MS patients worldwide. The majority (70.7%) were female and two-thirds were from outside the United States. The median duration of natalizumab exposure was 39.5 months (range 8–94), and 27.7% of patients had prior IS use.
Thirty patients (8.1%) were classified as asymptomatic and 342 patients (91.9%) as symptomatic at the time of PML diagnosis. Demographic and clinical characteristics were comparable between the two groups (Table 3). More than 80% of asymptomatic PML patients and ≈60% of symptomatic PML patients were from locations outside the United States.
Table 3.
Demographics and clinical characteristics of asymptomatic and symptomatic PML patients
| Asymptomatic PML patients (n = 30) | Symptomatic PML patients1 (n = 342) | All PML patients (n = 372) | |
|---|---|---|---|
| Age at diagnosis, years | |||
| Mean | 42.7 | 45.1 | 44.9 |
| Median (range) | 43.5 (22–61) | 45.0 (14–73) | 45.0 (14–73) |
| (n = 30) | (n = 337) | (n = 367) | |
| Female, n (%) | 21 (70.0) | 242 (70.8) | 263 (70.7) |
| Weight, kg | |||
| Mean | 68.7 | 75.4 | 75.1 |
| Median (range) | 65 (50–98) | 68 (46–163) | 68 (46–163) |
| (n = 12) | (n = 142) | (n = 154) | |
| Duration of MS at diagnosis, years | |||
| Mean | 12.1 | 14.1 | 13.8 |
| Median (range) | 12.0 (4–29) | 12.5 (1–51) | 12.0 (1–51) |
| (n = 19) | (n = 120) | (n = 139) | |
| Natalizumab exposure, months | |||
| Mean | 40.6 | 39.8 | 39.9 |
| Median (range) | 40.5 (24–94) | 40.0 (8–77) | 39.5 (8–94) |
| (n = 30) | (n = 342) | (n = 372) | |
| Prior IS use, yes, % | 23.3 | 28.1 | 27.7 |
| EDSS score on natalizumab pre-PML | |||
| Mean | 3.2 | 3.8 | 3.7 |
| Median (range) | 3.0 (1–6.0) | 4.0 (0–8.5) | 3.5 (0–8.5) |
| (n = 18) | (n = 172) | (n = 190) | |
| KPS score on natalizumab pre-PML | |||
| Mean | 85.0 | 80.2 | 80.6 |
| Median (range) | 90.0 (60–100) | 80.0 (40–100) | 80.0 (40–100) |
| (n = 10) | (n = 112) | (n = 122) | |
| Time to PML diagnosis2, days | |||
| Mean | 37.6 | 44.6 | NA |
| Median (range) | 12 (0–168) | 28 (0–368) | |
| (n = 30) | (n = 330) | ||
| CSF JCV DNA3, copies/mL | |||
| Mean | 277,000 | 185,000 | 192,000 |
| Median (range) | 668 (12–4,970,000) | 510 (1–10,200,000) | 510 (1–10,200,000) |
| (n = 24) | (n = 289) | (n = 313) | |
| Geography, n (%) | |||
| United States | 4 (13.3) | 123 (36.0) | 127 (34.1) |
| Rest of world | 26 (86.7) | 219 (64.0) | 245 (65.9) |
CSF, cerebrospinal fluid; EDSS, Expanded Disability Status Scale; IS, immunosuppressant; JCV, JC virus; KPS, Karnofsky Performance Scale; MS, multiple sclerosis; NA, not applicable; PML, progressive multifocal leukoencephalopathy.
Two Crohn’s disease patients are included.
Time from first suspect MRI (for asymptomatic patients) or PML symptom (for symptomatic patients) to PML diagnosis date, defined as first positive JCV DNA in CSF or positive brain biopsy.
First positive test.
The median time to PML diagnosis, defined as time from first suspect MRI (for asymptomatic patients) or from PML symptoms (for symptomatic patients) to first JCV DNA positive CSF or positive brain biopsy, was 12 days (range 0–168) in asymptomatic patients and 28 days (range 0–368) in symptomatic patients (Table 3). Reported MRI frequencies prior to PML diagnosis in asymptomatic PML patients were every 3 months in one patient, every 4 months in one patient, every 6 months in four patients, and every 12 months in one patient; frequency was not reported in 23 cases. MRI frequencies for most of the symptomatic PML patients were unknown.
Natalizumab was discontinued in all patients upon suspicion of PML and 24 of 30 (80.0%) asymptomatic and 276 of 342 (80.7%) symptomatic PML patients were treated with plasma exchange (PLEX). For the remaining cases, PLEX was not performed or not reported. Immune reconstitution inflammatory syndrome (IRIS) was subsequently reported in 20 asymptomatic PML patients (66.7%) and in 248 symptomatic PML patients (72.5%). PML-IRIS was defined as worsening of clinical symptoms and lesion progression including signs of inflammation and mass effect on MRI, as determined by the reporting physician.
PML symptom patterns
At the time of this analysis, 19 asymptomatic PML patients had at least 6 months of follow-up data available after PML diagnosis. The other 11 cases either had not reached the 6-month follow-up time point (n = 8) or were lost to follow-up (n = 3). Of the 19 asymptomatic patients with at least 6 months of follow-up, 11 (57.9%) remained symptom free over a median of 16.0 months (range 4.8–27.3); the remaining eight asymptomatic PML patients (42.1%) subsequently developed clinical symptoms. One patient who developed symptoms did not have a symptom onset date available. For the other seven patients, the median time from first suspect MRI to the onset of initial symptoms was 20 days (range 1–130). Median follow-up time for the patients who had symptoms at the time of PML diagnosis was 17.5 months (range 7.0–27.0).
The type and frequency of PML symptoms were generally similar in symptomatic patients and in asymptomatic patients who subsequently developed symptoms. Behavioral and/or cognitive and motor symptoms were the most common symptoms overall (seen in 55.6% of asymptomatic and 51.5% of symptomatic PML patients); visual symptoms occurred more frequently in symptomatic patients, consistent with the MRI location of PML lesions (Table 4).
Table 4.
PML symptoms observed in asymptomatic patients who later became symptomatic and in patients symptomatic at PML diagnosis
| PML symptoms1, n (%) | Asymptomatic PML patients (n = 8) | Symptomatic PML patients (n = 342) |
|---|---|---|
| Cognitive/behavioral | 5 (55.6) | 176 (51.5) |
| Motor | 3 (33.3) | 163 (47.7) |
| Speech | 1 (11.1) | 100 (29.2) |
| Visual | 0 (0) | 68 (19.9) |
| Cerebellar | 1 (11.1) | 64 (18.7) |
| Seizure | 1 (11.1) | 27 (7.9) |
| Sensory | 1 (11.1) | 23 (6.7) |
PML, progressive multifocal leukoencephalopathy.
Symptoms reported at a later stage after diagnosis in asymptomatic patients and at diagnosis in symptomatic patients; each patient may have more than one symptom.
MRI findings
Brain MRI results at diagnosis were available for 28 asymptomatic and 298 symptomatic patients. A greater proportion of asymptomatic PML patients compared with symptomatic PML patients had unilobar lesions at diagnosis (Fig. 1). Unilobar frontal lobe lesions were the most common presentation in both asymptomatic and symptomatic patients. Twelve of 17 asymptomatic PML patients (70.6%) and 56 of 107 symptomatic PML patients (52.3%) had unilobar lesions in the frontal lobe. Eleven percent of asymptomatic patients had widespread lesions, compared with 40.0% of symptomatic patients. Figure 2 shows representative MRI scans of progression from asymptomatic to symptomatic PML.
Figure 1.
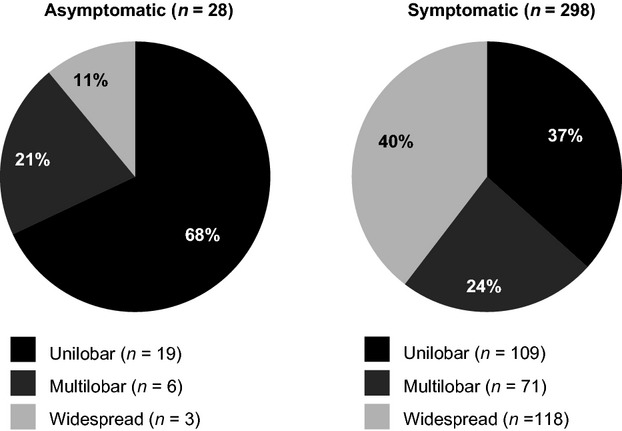
Distribution of PML lesions in asymptomatic and symptomatic PML patients. MRI data for 46 patients, including two asymptomatic patients, were not available. Total percentages may be greater than 100% due to rounding. PML, progressive multifocal leukoencephalopathy; MRI, magnetic resonance imaging.
Figure 2.
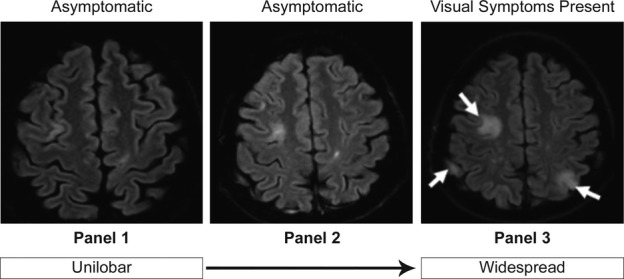
Representative MRI scans of progression from asymptomatic to symptomatic PML. Asymptomatic PML was diagnosed in a 43-year-old woman with no prior IS use who had previously received interferon beta-1a. Twenty-two months after natalizumab initiation, she had no clinical signs of PML, but MRI showed a hyperintense cortical ribbon on both sides of the superior frontal sulcus (panel 1). Four months later the patient was still asymptomatic, but follow-up imaging showed multilobar lesions and natalizumab was discontinued (panel 2). Six months after first visualization of PML on MRI, PML symptoms, primarily visual, had developed and widespread lesions were present on brain MRI scan (panel 3). Anti-JCV antibody was detected in CSF at this time. MRI, magnetic resonance imaging; PML, progressive multifocal leukoencephalopathy; IS, immunosuppressive; JCV, JC virus; CSF, cerebrospinal fluid.
Survival
As of 5 June 2013, 96.7% (29 of 30) of asymptomatic PML patients and 75.4% (258 of 342) of symptomatic PML patients were alive. Mean duration of follow-up was 13.4 months (median [range]: 13.9 [3.4–26.6]; n = 16) in asymptomatic patients and 11.2 months (median [range]: 8.7 [0–34.7]; n = 203) in symptomatic patients. In the nonsurviving asymptomatic PML case, the time to death from diagnosis was 13.2 months; cause of death was suicide likely due to the patient’s preexisting depression. The mean time from PML diagnosis to death in symptomatic PML patients (n = 78) was 4.2 months (median [range]: 2.3 [0.07–35.1]); data were not available for 6 patients.
Functional outcomes
Asymptomatic PML patients had significantly less functional disability at diagnosis and at 6 months post-PML diagnosis compared with symptomatic PML patients (Table 5). Over time, EDSS scores were consistently lower in asymptomatic PML patients than in symptomatic PML patients (Table 5; Fig. 3A). Asymptomatic patients also had less impairment over time as assessed by KPS, with asymptomatic patients having consistently higher KPS scores (Table 5; Fig. 3B). The correlation coefficient for EDSS and KPS scores was 0.712.
Table 5.
Mean EDSS and KPS scores over time in asymptomatic and symptomatic PML patients
| Asymptomatic PML patients | Symptomatic PML patients | P value | |
|---|---|---|---|
| EDSS score | |||
| Pre-PML | 3.2 (n = 21) | 3.7 (n = 179) | 0.336 |
| At diagnosis | 4.1 (n = 11) | 5.4 (n = 193) | 0.038 |
| At 6 months | 4.9 (n = 11) | 6.6 (n = 87) | 0.007 |
| At 12 months | 5.1 (n = 6) | 6.5 (n = 59) | 0.169 |
| KPS score | |||
| Pre-PML | 84.0 (n = 10) | 81.1 (n = 97) | 0.475 |
| At diagnosis | 70.0 (n = 11) | 53.8 (n = 122) | 0.008 |
| At 6 months | 71.5 (n = 10) | 47.1 (n = 108) | <0.001 |
| At 12 months | 56.0 (n = 5) | 46.6 (n = 67) | 0.178 |
P value from Mann–Whitney–Wilcoxon test. EDSS, Expanded Disability Status Scale; KPS, Karnofsky Performance Scale; PML, progressive multifocal leukoencephalopathy.
Bold text indicates statistical significance.
Figure 3.
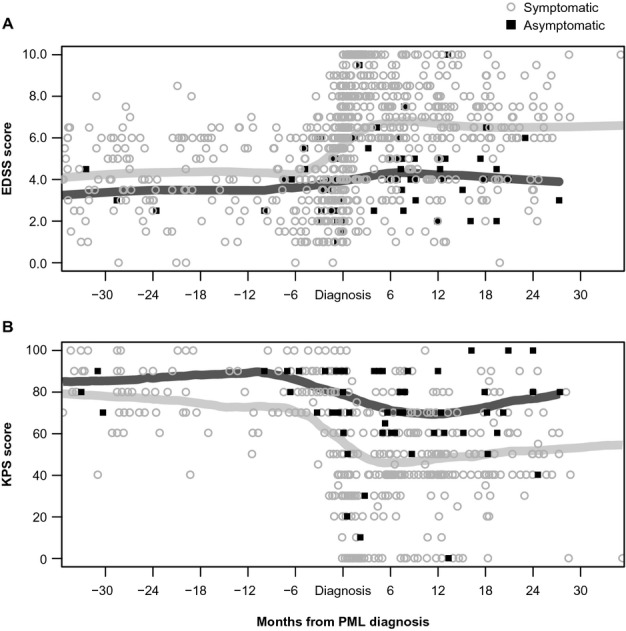
(A) EDSS and (B) KPS scores for asymptomatic and symptomatic PML patients measured over time. Weighted polynomial regression using the LOWESS algorithm. The EDSS and KPS scores for asymptomatic and symptomatic PML patients are shown for time points prior to PML diagnosis, at PML diagnosis, and post-PML diagnosis. Each symbol represents a single patient measurement at a single time point. EDSS and KPS scores were not available for all patients at all time points. Data prior to diagnosis were gathered from medical records. The dark gray lines represent polynomial regression trend-lines (LOWESS curves) for asymptomatic patients; the light gray lines represent polynomial regression trend-lines (LOWESS curves) for symptomatic patients. EDSS, Expanded Disability Status Scale; KPS, Karnofsky Performance Scale; PML, progressive multifocal leukoencephalopathy; LOWESS, locally weighted scatterplot smoothing.
When compared with symptomatic patients who presented with only frontal lobe unilobar involvement (n = 56), asymptomatic patients still had less functional disability (mean EDSS score: 6.5 vs. 4.9) and impairment (mean KPS: 46.0 vs. 71.5) at 6 months.
Discussion
The concept of detecting demyelinating diseases in an asymptomatic stage using a highly sensitive paraclinical tool such as MRI is well established in the field of MS, where the term “radiologically isolated syndromes” has been used to describe subclinical MS.33,34 In the context of opportunistic infections, the current data and a number of recent case reports17,18,21,23,24 suggest that PML may be diagnosed based on brain MRI findings and the presence of JCV DNA in the CSF or on brain biopsy in the absence of clinical symptoms. This observation departs from the traditional 3-part diagnostic algorithm requiring clinical symptoms in combination with MRI findings and JCV DNA detection in the CNS for a diagnosis of definite PML.10,35,36 It also suggests that clinicians with natalizumab-treated MS patients should be aware that clinical symptoms are not required to make the diagnosis of PML; such a requirement could delay timely diagnosis and intervention.
In our study, ≈8% of patients (n = 30) were asymptomatic at the time of PML diagnosis. The average time to PML diagnosis was shorter in asymptomatic patients than in symptomatic patients (12 vs. 28 days). The majority (86.7%) of asymptomatic PML patients were diagnosed outside the United States, which may reflect differences in clinical management of patients during natalizumab treatment. In the United States, best practice recommendations were published in 2009 by an expert panel, which recommended that an MRI scan be conducted at least annually in natalizumab-treated patients, with several panel members reporting that they perform a routine MRI after 6 months of treatment and then annually. All panel members also reported performing an MRI at the time of a relapse or if there were signs or symptoms of PML.37 Meanwhile, European centers may have been performing MRI scans more frequently, as suggested in a PML case report published in 2012 in which the authors described performing brain MRI scans every 6 months for all relapsing MS patients treated with natalizumab.38 The PML case reported by Phan-Ba and colleagues was also included in a case series, in which a group of European authors suggested that “brain MRI scans every 3–4 months could be considered for interval MRI vigilance.”24 Similarly, in a set of recommendations published in 2011 by the International Multiple Sclerosis Expert Forum, it was stated that “more frequent MRI has been suggested (every 3–6 months)” in patients at increased risk for PML.12 Although our data were limited, no clear pattern of frequency of MRI testing was discerned between those patients who were asymptomatic and those who were symptomatic. Clinical characteristics of asymptomatic and symptomatic PML patients were comparable prior to PML diagnosis. Importantly, there were no significant differences in functional disability, as measured by EDSS and KPS prior to PML diagnosis.
In asymptomatic patients, most lesions were unilobar, whereas 63% of PML patients symptomatic at diagnosis had lesions that were multilobar or widespread. However, the majority of unilobar lesions on MRI in both asymptomatic and symptomatic PML patients were in the frontal lobe. Less than 50% of asymptomatic patients subsequently developed PML symptoms, and 11 of 19 patients with follow-up data available remained symptom free over a median 16 months. When symptoms were subsequently observed in asymptomatic patients, they were primarily cognitive/behavioral and motor symptoms and occurred a median of 3 weeks after diagnosis. The general type and pattern of symptoms observed in asymptomatic patients who subsequently became symptomatic generally mirrored that of patients who were symptomatic at PML diagnosis.
Overall, functional disability, measured by both EDSS and KPS, was comparable in both populations up to 30 months prior to PML diagnosis. However, asymptomatic PML patients demonstrated less functional disability at PML diagnosis and at 6 months postdiagnosis compared with symptomatic PML patients. The survival rate was higher in asymptomatic patients than in patients who were symptomatic at diagnosis.
The shorter time to diagnosis of asymptomatic patients compared with symptomatic patients may have allowed more rapid therapeutic intervention (stopping natalizumab and removal of natalizumab via PLEX) to facilitate immune reconstitution. However, whether this led to the improved outcome of the patients remains speculative.
This analysis had several limitations. We had a relatively small number of asymptomatic PML cases, and most of the cases had incomplete data regarding MRI frequency prior to PML diagnosis. It also should be noted that there are still many unknown factors regarding our understanding of PML. The possibility of lead time bias accounting for the observed longer duration of survival in asymptomatic patients cannot be ruled out because of the nonrandomized nature of the analysis. It is also possible that asymptomatic PML patients may represent a cohort of patients who have a slower progression of PML disease than those who were symptomatic at the time of diagnosis and, therefore, have a better prognosis; this could account for the absence of clinical symptoms and better outcomes (length time bias). We have observed in an earlier review of MRIs in natalizumab-treated patients with PML that some patients appear to have rapidly progressive disease (e.g., 2 months), while others appear to have a slower disease progression (e.g., 6 months). This may be due to yet unknown differences in host or viral attributes that may contribute to these observations.39
These data provide evidence of favorable outcomes when PML is diagnosed in the absence of clinical symptoms in natalizumab-treated patients. Clinicians treating patients with natalizumab should be aware that symptoms are not required in order to make a diagnosis of PML. Given that it is currently unclear if more frequent MRI testing contributes to identification of asymptomatic PML and the associated outcomes, it is premature to recommend any specific algorithm that might be used to more effectively identify this cohort of patients. It can be noted, however, our data and the published case reports and case series support the high value of MRI as a tool in detecting PML disease activity at an asymptomatic stage, which appears to be associated with more favorable outcome.
Acknowledgments
Funding for this study was provided by Biogen Idec Inc. Biogen Idec Inc. provided funding for editorial support in the development of this paper; Marie Geissler and Britt Anderson, Ph.D., of Infusion Communications provided writing support based on input from authors, and Jackie Cannon of Infusion Communications copyedited and styled the manuscript per journal requirements. Biogen Idec Inc. reviewed and provided feedback on the paper to the authors. The authors had full editorial control of the paper and provided their final approval of all content.
Authors’ Contributions
T. D. S. and S. G. wrote the first draft of the manuscript. M. W., J. P., J. M., and S. D. did the programming and statistical data analysis. All authors participated in revising subsequent drafts of the manuscript and provided input on the final version of the article.
Conflict of Interest
M.P.W. received speaker honoraria from Biogen Idec, Novartis, Janssen-Cilag, and Bayer HealthCare. He serves as a scientific advisory board member for Biogen Idec and as an editorial board member for European Radiology. All other authors are currently or were at the time of study conduct employees of Biogen Idec.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Sample standardized PML data collection tool (DCT).
Proposed PML classification system using 5 levels of diagnostic certainty and a hierarchy of clinical evidence.
References
- Tysabri (natalizumab) [prescribing information] Cambridge, MA: Biogen Idec, Inc; 2013. [Google Scholar]
- European Medicines Agency. Tysabri (natalizumab) summary of product characteristics. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000603/WC500044686.pdf (accessed 19 June 2014)
- Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- Rudick R, Polman C, Clifford D, et al. Natalizumab: bench to bedside and beyond. JAMA Neurol. 2013;70:172–182. doi: 10.1001/jamaneurol.2013.598. [DOI] [PubMed] [Google Scholar]
- Biogen Idec MedInfo. Available at: https://medinfo.biogenidec.com (accessed 21 January 2014)
- Clifford DB, De Luca A, Simpson DM, et al. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9:438–446. doi: 10.1016/S1474-4422(10)70028-4. [DOI] [PubMed] [Google Scholar]
- Major EO. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med. 2010;61:35–47. doi: 10.1146/annurev.med.080708.082655. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Major EO, Ryschkewitsch C, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med. 2006;354:924–933. doi: 10.1056/NEJMoa054693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80:1430–1438. doi: 10.1212/WNL.0b013e31828c2fa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong-Si T, Weber T, Richert N, et al. Classification of natalizumab cases with progressive multifocal leukoencephalopathy. Neurology. 2012;78:P07.058. [Meeting Abstracts 1]: [Google Scholar]
- Kappos L, Bates D, Edan G, et al. Natalizumab treatment for multiple sclerosis: updated recommendations for patient selection and monitoring. Lancet Neurol. 2011;10:745–758. doi: 10.1016/S1474-4422(11)70149-1. [DOI] [PubMed] [Google Scholar]
- Dahlhaus S, Hoepner R, Chan A, et al. Disease course and outcome of 15 monocentrically treated natalizumab-associated progressive multifocal leukoencephalopathy patients. J Neurol Neurosurg Psychiatry. 2013;84:1068–1074. doi: 10.1136/jnnp-2013-304897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermersch P, Kappos L, Gold R, et al. Clinical outcomes of natalizumab-associated progressive multifocal leukoencephalopathy. Neurology. 2011;76:1697–1704. doi: 10.1212/WNL.0b013e31821a446b. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Pelletier D, Cadavid D, et al. Magnetic resonance imaging pattern in natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2012;72:779–787. doi: 10.1002/ana.23676. [DOI] [PubMed] [Google Scholar]
- Wattjes MP, Richert ND, Killestein J, et al. The chameleon of neuroinflammation: magnetic resonance imaging characteristics of natalizumab-associated progressive multifocal leukoencephalopathy. Mult Scler. 2013;19:1826–1840. doi: 10.1177/1352458513510224. [DOI] [PubMed] [Google Scholar]
- Ayzenberg I, Lukas C, Trampe N, et al. Value of MRI as a surrogate marker for PML in natalizumab long-term therapy. J Neurol. 2012;259:1732–1733. doi: 10.1007/s00415-012-6426-5. [DOI] [PubMed] [Google Scholar]
- Blair NF, Brew BJ, Halpern JP. Natalizumab-associated PML identified in the presymptomatic phase using MRI surveillance. Neurology. 2012;78:507–508. doi: 10.1212/WNL.0b013e318246d6d8. [DOI] [PubMed] [Google Scholar]
- Blinkenberg M, Sellebjerg F, Leffers AM, et al. Clinically silent PML and prolonged immune reconstitution inflammatory syndrome in a patient with multiple sclerosis treated with natalizumab. Mult Scler. 2013;19:1226–1229. doi: 10.1177/1352458513481010. [DOI] [PubMed] [Google Scholar]
- Lindå H, von HA, Major EO, et al. Progressive multifocal leukoencephalopathy after natalizumab monotherapy. N Engl J Med. 2009;361:1081–1087. doi: 10.1056/NEJMoa0810316. [DOI] [PubMed] [Google Scholar]
- Lindå H, von Heijne A. Presymptomatic diagnosis with MRI and adequate treatment ameliorate the outcome after natalizumab-associated progressive multifocal leukoencephalopathy. Front Neurol. 2013;4:11. doi: 10.3389/fneur.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manenti G, Altobelli S, Nezzo M, et al. Early magnetic resonance detection of natalizumab-related progressive multifocal leukoencephalopathy in a patient with multiple sclerosis. Case Rep Radiol. 2013;2013:415873. doi: 10.1155/2013/415873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Govern EM, Hennessy MJ. Asymptomatic progressive multifocal leukoencephalopathy associated with natalizumab. J Neurol. 2013;260:665–667. doi: 10.1007/s00415-012-6759-0. [DOI] [PubMed] [Google Scholar]
- Phan-Ba R, Belachew S, Outteryck O, et al. The earlier, the smaller, the better for natalizumab-associated PML: in MRI vigilance veritas? Neurology. 2012;79:1067–1069. doi: 10.1212/WNL.0b013e31826846b4. [DOI] [PubMed] [Google Scholar]
- Vennegoor A, Wattjes MP, van Munster ET, et al. Indolent course of progressive multifocal leukoencephalopathy during natalizumab treatment in MS. Neurology. 2011;76:574–576. doi: 10.1212/WNL.0b013e31820b7644. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Karnofsky D, Burchenal J. MacLoad CM, ed. Evaluation of chemotherapeutic agents. New York, New York: Columbia University Press; 1949. The clinical evaluation of chemotherapeutic agents in cancer; pp. 191–205. [Google Scholar]
- Richert N, Bloomgren G, Cadavid D, et al. Imaging findings for PML in natalizumab-treated MS patients. Mult Scler. 2012;18(4):27–28. [Google Scholar]
- Chambers JM. Linear models. In: Chambers JM, Hastie TJ, editors. Statistical models in S. Pacific Grove, California: Wadsworth & Brooks/Cole; 1992. pp. 95–138. [Google Scholar]
- Wilkinson GN, Rogers CE. Symbolic description of factorial models for analyis of variance. Appl Stat. 1973;22:392–399. [Google Scholar]
- Cleveland WS. A program for smoothing scatterplots by robust locally weighted regression. Am Stat. 1981;35:54. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- Lebrun C, Bensa C, Debouverie M, et al. Association between clinical conversion to multiple sclerosis in radiologically isolated syndrome and magnetic resonance imaging, cerebrospinal fluid, and visual evoked potential: follow-up of 70 patients. Arch Neurol. 2009;66:841–846. doi: 10.1001/archneurol.2009.119. [DOI] [PubMed] [Google Scholar]
- Okuda DT, Mowry EM, Beheshtian A, et al. Incidental MRI anomalies suggestive of multiple sclerosis: the radiologically isolated syndrome. Neurology. 2009;72:800–805. doi: 10.1212/01.wnl.0000335764.14513.1a. [DOI] [PubMed] [Google Scholar]
- Brew BJ, Davies NW, Cinque P, et al. Progressive multifocal leukoencephalopathy and other forms of JC virus disease. Nat Rev Neurol. 2010;6:667–679. doi: 10.1038/nrneurol.2010.164. [DOI] [PubMed] [Google Scholar]
- Cinque P, Koralnik IJ, Gerevini S, et al. Progressive multifocal leukoencephalopathy in HIV-1 infection. Lancet Infect Dis. 2009;9:625–636. doi: 10.1016/S1473-3099(09)70226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle PK, Foley JF, Fox EJ, et al. Best practice recommendations for the selection and management of patients with multiple sclerosis receiving natalizumab therapy. Mult Scler. 2009;15:S26–S36. [Google Scholar]
- Phan-Ba R, Lommers E, Tshibanda L, et al. MRI preclinical detection and asymptomatic course of a progressive multifocal leucoencephalopathy (PML) under natalizumab therapy. J Neurol Neurosurg Psychiatry. 2012;83:224–226. doi: 10.1136/jnnp-2011-300511. [DOI] [PubMed] [Google Scholar]
- Mancuso R, Saresella M, Hernis A, et al. JC virus detection and JC virus-specific immunity in natalizumab-treated multiple sclerosis patients. J Transl Med. 2012;10:248. doi: 10.1186/1479-5876-10-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sample standardized PML data collection tool (DCT).
Proposed PML classification system using 5 levels of diagnostic certainty and a hierarchy of clinical evidence.


