Abstract
Objective
To identify the prevalence of MRI features of Binswanger’s disease (BD), specifically MRI with diffuse white matter lesions and scattered multiple lacunes (BD-MRI), and to describe neurological features and pathological outcomes of a community-based cohort study.
Methods
Of 697 participants (all 75 years old), 503 completed neurological examinations at baseline and were followed-up every 30 months thereafter with MRIs, the mini-mental state examination (MMSE) and the Unified Parkinson Disease Rating Scale-Motor Section (UPDRSM). Data from participants with BD-MRI were compared with those from participants with predominant white matter lesions (WML-MRI), scattered multiple lacunes (ML-MRI), or normal MRIs.
Results
Fourteen BD-MRI patients (2.8%) were detected at baseline. The mean MMSE scores in the BD-MRI, WML-MRI, ML-MRI, and normal MRIs groups were 26.4, 28.2, 28.4, and 28.5, respectively, and the mean UPDRSM scores were 9.1, 1.3, 3.1, and 1.7, respectively. At the 30-month follow-up, mortality rates in the normal MRIs, WML-MRI and ML-MRI were 4%, 9.1%, and 22.2%, respectively, and follow-up MRIs were available for 80%, 82%, and 61% of the participants, respectively. In the BD-MRI, however, five patients were deceased, and only five follow-up individual MRIs were available (33.3%). Autopsies were performed on six of eight BD-MRI brains, and these brains fulfilled the pathological criteria for BD independent of Alzheimer disease pathology. All these six individuals also showed systemic atherosclerosis and renal arterio-arteriolosclerosis.
Interpretation
The BD-MRI participants had poor prognoses and showed pure BD pathology with advanced systemic vascular disease. BD-MRI appears to be a predictor of vascular neurocognitive impairment.
Introduction
Vascular dementia (VaD) is the second most common cause of dementia after Alzheimer’s disease (AD). VaD is classified into subcortical VaD, cortical VaD, and strategic single infarct dementias.1,2 VaD comprises heterogeneous vascular pathologies that have been classically linked to small vessel and large vessel diseases.3 Cerebrovascular diseases underlying VaD comprise the majority of small vessel diseases, and the rest being large vessel diseases.4 Binswanger’s disease (BD), a major subtype of subcortical VaD, is caused by hypertensive lipo- and fibrohyalinosis of cerebral small vessels, and it leads to widespread diffuse white matter lesions (WML) and scattered multiple lacunes.5–8 The cognitive and clinical features of the BD defined the impairment of attention, volition and executive function as well as the impairment of memory and emotions. Evidence of focal cerebrovascular disease and subcortical cerebral dysfunction (for example, vascular parkinsonism, pseudobulbar palsy or a history of incontinence secondary to a spastic bladder) is also associated.5–7 In a long-term follow-up study of our hypertensive patients with lacunar infarct, concomitant diffuse WML were independent predictors for subsequent development of dementia, while multiple lacunes were independent predictors for vascular events.9 However, it remains unknown whether BD, multiple lacunar state, and diffuse WML are merely a disease process or different categories of small vessel disease, and whether their clinical profiles and brain pathologies are sufficient for vascular neurocognitive impairment.
This study aims to identify the prevalence of MRI features of BD, specifically diffuse WML and scattered multiple lacunes (BD-MRI), to compare the BD-MRI findings with the MRI finding of predominant WML (WML-MRI) and predominant lacunar state with a scattering of more than five lacunes (ML-MRI), and to describe the differences in the clinicopathological features and long-term outcomes between these three subtypes in a community-based birth cohort investigation (The Vienna Trans-Danube Aging Study).10 In this study, we performed the major cognitive and neurological examinations at baseline and at follow-ups every 30 months thereafter. Pathological outcomes were followed for up to 90 months using the pathological diagnostic criteria for BD.
Methods
VITA study
The Vienna Trans-Danube Aging Study (The VITA study) is a prospective cohort study of aging and dementia since 2000 organized by the Ludwig Boltzmann Institute of Aging Research and Danube Hospital. It was approved by the appropriate ethics committee. The participants were all 75-year-old inhabitants of the 21st and 22nd districts of Vienna, an area on the east shore of the Danube River. A total of 1920 individuals (765 males and 1155 females) who were born between May 1925 and June 1926 were identified; the birth data were extracted from official voting registries. At baseline, we investigated 697 inhabitants who agreed to participate, of whom 503 completed extensive neurological examinations, including MRI, the mini-mental state examination (MMSE), trail-making tests A and B, the Unified Parkinson Disease Rating Scale-Motor Section (UPDRSM), the Alzheimer’s Criteria test from the National Institute of Neurological and Communicative Disorders and Stroke, and the Alzheimer’s Disease and Related Disorders Association (ADRDA criteria). The data from participants with BD-MRI were compared with those from individuals with other small vessel disease subtypes (either WML-MRI or ML-MRI) and from individuals with normal MRI. The term “small vessel diseases” encompasses a range of features that are visible on brain imaging, including lacunar infarcts, ischemic WML, microbleeds, and enlarged perivascular spaces.4 MMSE, trail-making tests A and B, and UPDRSM and ADRDA criteria were performed after 30 and 60 months of follow-up and were compared with baseline data. The prognoses in each small vessel disease groups and in the AD and normal MRIs groups were also evaluated after 30, 60, and 90 months of follow-up.
Neuroradiological assessment
The MRI scan was performed using a 1.0-T unit (Siemens Impact Expert; Siemens Medical Systems, Inc., South Iselin, NJ) with a circular polarized skull coil. The following sequences were obtained: transverse proton density and T2-weighted Turbo Spin Echo, and coronary T1-weighted gradient echo sequence. The images were independently assessed by two experienced neurologists (I. A. and Y. S.). The severity of WML on MRI was evaluated from grade 0 to 4 as follows: 0, absent; 1, punctuate; 2, early confluent; 3, confluent; and 4, diffuse.11,12 The severity of the lacunar state was rated from grade 0 to 3 as follows: 0, zero lacuna; 1, one to two lacunes; 2, three to four lacunes; and 3, more than five lacunes.7,8 The MRI criteria for BD-MRI were defined as diffuse WML with a severity score of 3 or 4 with a scattering of multiple lacunes (more than five). The MRI criteria for ML-MRI were defined as a scattering of lacunar infarcts (more than five) and WMLs with a severity score of 0 or 1. The criteria for WML-MRI were defined as predominant WML with a severity score of 3 or 4 and a severity of lacunar state from 0 or 1. AD patients were diagnosed based on interview assessments and NINCDS ADRDA scores of typical of probable AD.13 For these small vessel disease groups and AD, we performed longitudinal neuroradiological examinations at baseline and at follow-ups every 30 months thereafter.
Neuropathological assessment
Six BD-MRI brains were available for autopsy study during the 90 months of follow-up and were evaluated based on diagnostic criteria for BD pathology (Akiguchi & Budka)14, and AD pathology (Braak & Braak, Consortium to establish a registry for Alzheimer’s disease [CERAD] and NIA-Reagan criteria15,16). The following pathological diagnostic procedures and staging criteria were used for BD brains.
Stainings: (A) always done: (1) hematoxylin–eosin and Kluver-Barrera stains, (2) Elastica van Gieson stain, and (3) Bielschowsky stain. (B) Optionally done: (1) HLA-DR or CD68 immunohistochemistry for activated microglia, (2) amyloid precursor protein (APP)-immunohistochemistry for axonal damage, and (3) amyloid staining (Congo Red or Aβ to exclude amyloid vasculopathy).
Diagnostic items: (A) subcortical and periventricular white matter rarefaction, stages, 0 = none, 1 = mild/focal, 2 = moderate/focal, 3 = severe/fronto-parietal, 4 = diffuse. Supporting features (if all are present = raise one stage), 1 = clusters of HLA-DR/CD68-positive microglia, 2 = clusters of APP-positive axons, and 3 = Strategic fiber bundle lesions17 (capsular genu/anterior thalamic peduncle or temporal stem). (B) Multiple lacunes: stages 0 = none, 1 = 1–2 lacunes in total, 2 = 3–4 lacunes, 3 = 5 or more lacunes. Supporting features (if all are present = raise one stage), 1 = clusters of HLA-DR/CD68-positive microglia around the perivascular space, 2 = Clusters of APP-positive axons around perivascular space, and 3 = incomplete lacunes/micro-infarcts.8,18 (C) Small/large vessel diseases (lipo- and fibro-hyalinosis in medullary arteries and atherosclerosis in basal brain arteries), stages, 0 = none, 1 = mild, 2 = moderate, 3 = severe.
Excluded items: (A) chronic major arterial occlusion. (B) Other causes of ischemic diffuse WM diseases (e.g., cerebral amyloid angiopathy, cerebral autosomal dominant arteriopathy with subcortical infarct and leukoencephalopathy, etc.).
Staging criteria: (A) pathological staging criteria for definitive BD required stage 3 for small/large vessel diseases, and either stage 4 for white matter rarefaction with more than stage 2 for multiple lacunes or stage 3 for both white matter rarefaction and multiple lacunes. Probable BD required stages 3 or 4 for white matter rarefaction with multiple lacunes stages 1 or 2 (Fig. 1). (B) The staging criteria used for the lacunar state and ischemic leukoencephalopathy are also shown in Figure 1.
Figure 1.

Staging and pathological diagnostic criteria for BD, lacunar state, and ischemic leukoencephalopathy (Akiguchi & Budka). BD, Binswanger’s disease.
Statistical analysis
The differences in the UPDRSM and MMSE scores and mortality rates between the small vessel disease groups and normal MRIs were analyzed using ANOVA with multiple comparisons. A P < 0.05 was considered to be statistically significant.
Results
Baseline studies and outcome after 30 months of follow-up
Table 1 shows prevalence, baseline major cognitive and neurological studies and mortality rate after 30 months follow-up in participants with BD-MRI and related small-vessel diseases. Fourteen patients with BD-MRI (2.8%), 20 with WML-MRI (4.0%), 23 with ML-MRI (4.6%), and 112 with normal MRIs (22.3%) were detected at baseline. Two observers (I. A. and Y. S.) reviewed the MRI recordings using a set of independent images from all participants of this study blinded to their clinical information. Inter-observer agreements for MRI diagnosis of BD-MRI between two observers from 57 participants with three small vessel disease groups were 91.2%.
Table 1.
Prevalence, baseline studies and mortality rate in BD-MRI and related small-vessel diseases
| Prevalence at baseline | UPDRSMS | MMSE | Gait disturbance | Mortality rate at 30 months | |
|---|---|---|---|---|---|
| Normal MRIs | 112 (22.3%) | 1.87 ± 2.74 | 28.6 ± 1.17 | 5.1% | 4% |
| BD-MRI | 14 (2.8%) | 8.92 ± 0.3* | 26.6 ± 2.40** | 38.5%** | 33.3%* |
| ML-MRI | 23 (4.6%) | 4.36 ± 7.47 | 28.2 ± 1.50 | 13.6% | 22.2% |
| WML-MRI | 20 (4.0%) | 1.25 ± 2.07 | 28.1 ± 1.41 | 10.0% | 9.1% |
Statistically significant compared with normal MRIs and WML-MRI groups (*P < 0.05), and compared with normal MRIs, WML-MRI, and ML-MRI groups (**P < 0.05), respectively.
The mean MMSE in the BD-MRI, WML-MRI, ML-MRI, and normal MRIs groups were 26.6, 28.1, 28.2, and 28.6, respectively, and the mean UPDRSM scores were 8.9, 1.3, 4.4, and 1.9, respectively. The mean trail-making test A and B in the BD-MRI, WML-MRI, ML-MRI, and normal MRIs groups were 62.2 and 219.8 sec, 51.2 and 192.6 sec, 64.9 and 175.1 sec, and 48.3 and 159.4 sec, respectively. MMSE scores in BD-MRI group were significantly lower compared with those in the normal MRIs, WML-MRI, and ML-MRI groups (P < 0.05). The UPDRSM score in the BD-MRI group was significantly higher compared with those in the normal MRIs and WML-MRI groups (P < 0.05). Longer mean performance time in trail-making test A and B were also prominent features of BD-MRI as well as other small vessel disease groups compared to normal MRIs, however, there were no significant differences between these groups. The frequency of gait disturbance in the BD-MRI group was greatest (38.5%) in the small vessel diseases and normal MRIs groups.
After 30 months of follow-up, mortality rates in the WML-MRI, ML-MRI, and normal MRIs groups were 9.1%, 22.2%, and 4%, respectively, and follow-up MRIs were available for 82%, 61%, and 80% of individuals, respectively. In the BD-MRI group, five patients were deceased (33.3%), and only five follow-up MRIs were available, for which all MRI features shows deteriorating WML scores and lacunar states. The mortality rate was significantly higher in the BD-MRI group compared with those in the WML-MRI and normal MRIs groups (P < 0.05).
Assessments after 30 and 60 months and mortality rate at 90 months
Table 2 shows UPDRSM, MMSE, and ADRDA criteria at baseline and after 30 and 60 months of follow-up in BD-MRI participants. UPDRSM gait and total performance scores worsened for all BD-MRI individuals during follow-up. MMSE scores worsened or could not be examined in six of eight BD-MRI participants.
Table 2.
Baseline and 30- or 60-months follow-ups and autopsy results in BD-MRI participants
| Case/sex | Outcome BL/30/60 | UPDRS gait | UPDRS motor | MMSE | ADRDA | Autopsy (1)1 Macroscopic findings | Autopsy (2)2 BD pathology | Autopsy (3) AD pathology |
|---|---|---|---|---|---|---|---|---|
| 1F | BL/N/D | −/− | −/− | 28/− | 0/− | Acute basilar thrombosis, SA, RAS, R-renal infarction | Probable-definitive: W2-3, L3, V3 | None |
| 2M | BL/D | 2 | 27 | 29 | 0 | Pneumonia, AHF, SA, RAS, contracted kidney | Definitive: W3, L3, V3 | Low |
| 3F | BL/N/N | −/−/− | −/−/− | 25/−/0 | 1/− | |||
| 4F | BL/N/N | 0/−/− | 0/−/− | 29/−/− | 0/− | |||
| 5F | BL/D | 1 | 17 | 21 | 1 | No brain | ||
| 6F | BL/N/N | −/−/− | 8/−/− | 27/25/24 | 0/0 | |||
| 7M | BL/D | 0 | 7 | 26 | 0 | No brain | ||
| 8M | BL/D | 2 | 30 | 24 | 0 | Pneumonia, CS, SA, RAS, contracted kidney | Definitive: W4, L3, V3 | Low-intermediate |
| 9M | BL/N/N3 | 0/−/− | 2/−/− | 26/−/− | 0/− | Sepsis, peritonitis, CS, SA, RAS | Definitive: W4, L3, V3 | Intermediate-high4 |
| 10F | BL/N/D | 1/− | 7/− | 29/− | 0/− | AHF, lung edema, CS, SA, RAS | Definitive: W4, L3, V3 | Low-intermediate |
| 11F | BL/F/F | 0/0/1 | 2/5/8 | 27/27/28 | 0/0 | |||
| 12F | BL/F/F | 0/0/0 | 0/2/3 | 29/30/30 | 0/0 | |||
| 13F | BL/F/N | 0/1/− | 4/23/− | 25/27/− | 0/1 | |||
| 14M | BL/D | 0 | 3 | 29 | 0 | AHF, lung edema, SA, RAS, cystic kidney | Definitive: W3, L3, V3 | None |
BL, baseline; F, followed up/tested; N, not tested (home visit, telephone interview, or refusal), D, died.
SA, systemic atherosclerosis; RAS, renal arterio-arteriolosclerosis, AHF, acute heart failure, CS, coronary sclerosis.
W3, L3, V3, white matter rarefaction: stage 3, lacunar state: stage 3, and small/large vessel diseases: stage 3.
Died between the 60- and 90-months follow-ups.
Associated AGD Stage III and mild Lewy-body pathology.
Eight of 14 BD-MRI patients (57.1%), nine of 18 AD patients (50%) and six of 23 ML-MRI patients (26.1%) were deceased by the 90-months follow-up. Only two deaths occurred in the WML-MRI (10%), and 11 of 75 (14.7%) patients died in the control groups. The mortality rate in the BD-MRI group was significantly higher compared with those of the normal MRIs, WML-MRI, and ML-MRI groups (P < 0.05). Thus both the 30-month and 90-month prognoses for the BD-MRI group were extremely poor. The 90-month prognoses for other small vessel disease categories (i.e., WML-MRI, and ML-MRI), were not as poor, and those of the AD group were also poor, second to the BD-MR group.
Autopsy findings
Autopsies were performed on six of eight BD-MRI brains. All these six individuals showed systemic atherosclerosis, renal arterio-arteriolosclerosis and renovascular or cardiovascular lesions. All six brains fulfilled the diagnostic criteria for BD: three of six brains, pure BD pathology; two brains, BD pathology with low-intermediate likelihood AD pathology; one brain, both latter pathologies with argyrophilic grain disease. The autopsy summaries for the six BD-MRI (case numbers 1, 2, 8, 9, 10, and 14) and MRIs at baseline and pathological findings of the three BD-MRI brains with pure BD pathology (case numbers 1, 2, and 14) are shown in Table 2, Figures 2 and 3.
Figure 2.
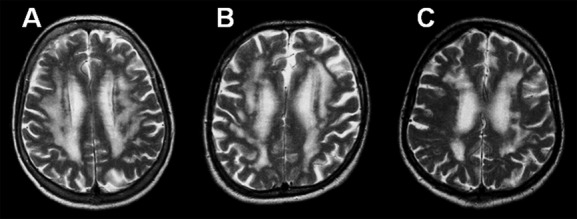
MRIs in three BD-MRI brains. MRIs at baseline in Case 1 (A), Case 2 (B), and Case 14 (C). BD, Binswanger’s disease.
Figure 3.

Autopsy findings in three BD-MRI brains with pure BD pathology. Case 1: probable-definitive BD pathology, (A) subcortical lacuna and the surrounding and extending WML (bar, 50 μm) and (D) incomplete lacuna/micro-infarct in deep white matter (bar, 50 μm) in Kluver-Barrera stain; AD pathology none. Case 2: definitive BD pathology, (B); subcortical lacuna and the surrounding and neighboring WML, (E) perivascular APP-positive microglial clusters and microinfarct (bar, 50 μm). AD pathology (Braak & Braak, stage ll; CERAD, moderate NP; NIA-Reagan, low). Case 14: definitive BD pathology, (C) lacuna with surrounding WML in the deep white matter, (F) cluster of HLA-DR-positive activated microglia around the perivascular spaces (bar, 25 μm). AD pathology (Braak & Braak, stage l; CERAD, slight NP; NIA-Reagan, no AD). BD, Binswanger’s disease; WML, white matter lesions.
Discussion
BD is characterized pathologically by a combination of diffuse WML and lacunar infarcts in the basal ganglia and white matter. The vascular mechanisms underlying BD and subcortical VaD are likely chronic cerebral ischemia caused by both hypertensive lipohyalinotic small artery disease/arteriolar-capillary fibrohyalinosis6,8,19 and intracranial arterial dolichoectasia/dilatative arteriopathy,20 which may ultimately cause multiple lacunes in the basal ganglia and the white matter and diffuse WML by altering glia and axons.8,21 In BD brains, we previously demonstrated that cerebrovascular WML were associated with compromised axonal transport and blood–brain barrier function, regressive changes in astroglia, frequent infiltration of T lymphocytes, and activated microglia.8,22 However, the prognoses and pathological outcomes of MRI features of BD such as diffuse WML with multiple lacunes have not yet been revealed.
Subcortical VaD and BD have long been considered rare; however, because of the relatively high prevalence of VaD and its treatable causes, such as nocturnal hypertension and chronic kidney disease, increasing attention has been paid to subcortical VaD/BD not only in Europe and Japan, but also in North America.1,6,8,23–27 In our cohort study of 75-year-old individuals, the prevalence of BD-MRI was 2.8%, which was two-thirds the rate of probable AD based on ADRDA criteria (4.0%). Thus, our study, in agreement with data from Japan,23,24 confirms a quite high prevalence of BD features on MRI in a Western community. However, there is considerable disagreement on the epidemiology and prevalence of VaD and subcortical VaD/BD. In clinical studies, the prevalence of VaD ranges from 4.5% to 39%, and in Western memory clinic- and population-based series, the mean are between 8% and 15.8%, with standardized incidence rates of between 0.1 and 2.68, which increase with age.28,29 VaD composes heterogeneous vascular pathologies that have been classically linked to small vessel and large vessel diseases.3,4 It has recently been proposed that patients with subcortical VaD/BD, the majority of which arises from small vessel disease, represent a highly prevalent 57.4% of VaD in autopsy series from demented elderly individuals in Austria and 51.3% of VaD in Japan,23,24 and comprise a relatively homogeneous group.8,24
In addition, the prognosis for BD-MRI in our study was extremely poor, based on the high, one-third mortality rate by 30 months, and the low, one-third follow-up MRI study rate, for which all MRI features deteriorated at the 30-months follow up. More than half of BD-MRI participants were deceased by the 90-months follow-up thereafter. Frisoni et al. also noted that ~30% of patients in a memory clinic with mild cognitive impairment of the vascular type died during the follow-up period at an average of 33 months. However, less information was presented to enable identification of the clinical course and pathological outcome.30 Melkas et al. showed that in an ischemic stroke cohort with ultra-long (12-year) follow-ups, acute index stroke attributable to cerebral small vessel disease was associated with worsened long-term survival and a higher risk of cardiac death than other stroke subtypes due to large vessel diseases.2 Limitations, however, were imposed by the authors’ use of a risk-factor based stroke classification and a selection bias of only using hospitalized patients.3 It is clear that small vessel diseases tend to slip through conventional stroke classifications, particularly in early stages of illness, because these diseases occur incidentally and without overt manifestations. Thus, a longitudinal epidemiological cohort study is required to attempts to clarify the true clinical profile and outcome of cerebral small vessel diseases. In subcortical VaD/BD patients, concomitant chronic kidney disease, systemic vascular disease, cardiac insufficiency, and a hypercoagulation state may further accelerate their poor prognosis.8,26,31,32
The neuropsychological assessment used during this study are very limited, however, BD-MRI individuals showed significantly lower MMSE as well as higher UPDRSM scores compared with those suffering from other small vessel diseases and controls. BD-MRI also showed longer performance time in trail-making tests, which correspond to deteriorated executive function in cognitive domains. In contrast to recently refined pathological criteria used for the diagnosis of AD and other degenerative dementias, no validated pathological criteria for the VaD brain have been established thus far.28 Because of the high variability in pathological findings and the multi-factorial pathogeneses,33,34 it is difficult to establish generally accepted morphologic schemes for quantifying vascular brain injury in VaD.28 Thus, we focused on pathological staging and criteria for BD brains that are assumed to show relatively homogenous small vessel disease pathology. In proposing a new pathological staging for BD-related cerebral small vessel disease, we refer to the following publications: (1) the grading scheme for small vessel disease35; (2) the scoring system for small vessel-associated disease36; and (3) the cerebrovascular disease pathology-scoring system.37 Briefly, scoring systems in (1) and (2) consider whether pathology of the perivascular space exists. Gliosis, perivascular pallor, hyaline thickening, nerve fiber loss, and multiple lacunes must also be evaluated. The scoring system in (3) quantifies hippocampal sclerosis rather than pathology of the perivascular space.
The functional anatomy and pathophysiological basis of impaired cognitive function of the following items in BD brains must also be considered in pathological diagnostic criteria. Impairment of attention, volition and executive function are of paramount importance. The underlying associated structures are the ascending reticular formation including anterior thalamic peduncle/capsular genu,17 nonspecific and specific thalamic nuclei and the frontal subcortical circuits.38,39 These structures are primarily involved in diffuse frontal/parietal WM rarefactions because of hypertensive small vessel disease/chronic cerebral hypoperfusion and small infarcts in the thalamus and the related fiber bundle lesions.17,39 Impairment of memory and emotions may also be reflected in evaluations of BD pathology. The underlying associated structures include the limbic system (hippocampal complex, amygdala, and temporal stem), the anterior and dorsomedial thalamic nuclei and other structures in the Papez and the basolateral limbic circuits.39 These structures are primarily involved in lacunes, branch atheromatous diseases, hippocampal sclerosis, and fiber bundle lesions related to these structures. Macro-pathological involvements in these structures and micro-cellular pathologies because of hypertensive small vessel diseases and chronic hypoperfusion (including microinfarcts, perivascular space pathologies, axonal damage, and immune-inflammatory responses) are essential for estimating pathological diagnosis scores.8,17,18,26
Our recent in vivo neuroimaging studies revealed several possible measures for discrimination between AD and BD brains such as different topographic patterns of brain atrophy in voxel-based morphometry,40 absolute quantification of N-acetylaspartate in proton magnetic resonance spectroscopy,41 and different profile of hippocampal metabolites measured by proton magnetic resonance spectroscopy.42 In this prospective cohort study, we confirm the existence of “pure” BD pathology, and all six BD-MRI brains available for autopsy fulfilled our pathological diagnostic criteria for BD (three of six brains, pure BD pathology; two brains, BD pathology with low-intermediate likelihood of AD pathology; one brain, both BD and AD pathologies with argyrofilic grain disease). Moreover, all these six individuals showed systemic atherosclerosis, renal arterio-arteriolosclerosis and renovascular or cardiovascular lesions. Jellinger et al. observed 12.3% “pure”VaD (because of cerebrovascular disease without other concomitant pathologies; neuritic Braak stages 1.2–1.6) in 1700 retrospective hospital-based autopsy cases of demented elderly individuals in Austria.25 In the Honolulu Asia Aging Study, Launer et al. also stressed that the burden of vascular lesions and AD-type lesions are independent, and are consistent with an additive effect of the two lesion types on cognitive impairment.43 However, MRI used to acquire baseline and follow-up data in this study was a 1.0 T, which is not the gold-standard for imaging research and is associated with a very low signal-to-noise image. Further study will be necessary to clarify the significance of MRI-based markers of small vessel diseases in prognosis and pathological outcome of VaD using higher field strength (3 T or 7 T).
In summary, we compared the clinical profiles of BD-MRI cases with diffuse WML and multiple lacunes, participants with predominant WML and those with predominant lacunar state. These three MRI subtypes of cerebral small vessel diseases showed different clinical features at baseline and in the prognoses at long-term follow-up. Prognosis of BD-MRI is highly poor and showed corresponding BD pathology independent of AD pathology, and advanced systemic vascular disease at autopsy.
Acknowledgments
We acknowledge Yoshimi Fukuda for his contributions to the manuscript.
Author Contributions
Dr Ichiro Akiguchi – Study concept and design, acquisition of data and analysis and interpretation. Dr Yoshitomo Shirakashi – Acquisition of data. Dr Herbert Budka – Study supervision. Dr Adelheid Woehrer – Acquisition of data. Dr Toshiyuki Watanabe – Analysis and interpretation. Dr Akihiko Shiino – Analysis and interpretation. Dr Yasumasa Yamamoto – Analysis and interpretation. Dr Yasuhiro Kawamoto – Analysis and interpretation. Dr Susanne Jungwirth – Acquisition of data. Dr Wolfgang Krampla – Acquisition of data. Dr Peter Fischer – Study supervision.
Conflict of Interest
None declared.
References
- Erkinjuntti T. Diagnosis and management of vascular cognitive impairment and dementia. J Neural Transm Suppl. 2002;63:91–109. doi: 10.1007/978-3-7091-6137-1_6. [DOI] [PubMed] [Google Scholar]
- Melkas S, Putaala J, Oksala NK, et al. Small-vessel disease relates to poor poststroke survival in a 12-year follow-up. Neurology. 2011;76:734–739. doi: 10.1212/WNL.0b013e31820db666. [DOI] [PubMed] [Google Scholar]
- Potter GM, Román G. Cerebral small-vessel disease: what lies beyond the early years? Neurology. 2011;76:684–685. doi: 10.1212/WNL.0b013e31820eb127. [DOI] [PubMed] [Google Scholar]
- Staekenborg SS, van Straaten EC, van der Flier WM, et al. Small vessel versus large vessel vascular dementia: risk factors and MRI findings. J Neurol. 2008;255:1644–1651. doi: 10.1007/s00415-008-0944-1. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Gilley DW, Fox JH. Clinical diagnosis of Binswanger’s disease. J Neurol Neurosurg Psychiatry. 1990;53:961–965. doi: 10.1136/jnnp.53.11.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan LR. Binswanger’s disease – revisited. Neurology. 1995;45:626–633. doi: 10.1212/wnl.45.4.626. [DOI] [PubMed] [Google Scholar]
- Román GC, Erkinjuntti T, Wallin A, et al. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- Akiguchi I, Tomimoto H, Suenaga T, et al. Alterations in glia and axons in the brains of Binswanger’s disease patients. Stroke. 1997;28:1423–1429. doi: 10.1161/01.str.28.7.1423. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Akiguchi I, Oiwa K, et al. Twenty-four-hour blood pressure and MRI as predictive factors for different outcomes in patients with lacunar infarct. Stroke. 2002;33:297–305. [PubMed] [Google Scholar]
- Fischer P, Jungwirth S, Krampla W, et al. Vienna Transdanube Aging “VITA”: study design, recruitmentstrategies and level of participation. J Neural Transm Suppl. 2002;62:105–116. doi: 10.1007/978-3-7091-6139-5_11. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Niederkorn K, Schmidt R, et al. White matter signal abnormalities in normal individuals: correlation with carotid ultrasonography, cerebral blood flow measurements, and cerebrovascular risk factors. Stroke. 1988;19:1285–1288. doi: 10.1161/01.str.19.10.1285. [DOI] [PubMed] [Google Scholar]
- Shinohara Y, Tohgi H, Hirai S, et al. Effect of the Ca Antagonist nilvadipine on stroke occurrence or recurrence and extension of asymptomatic cerebral infarction in hypertensive patients with or without history of stroke (PICA Study) Cerebrovasc Dis. 2007;24:202–209. doi: 10.1159/000104478. [DOI] [PubMed] [Google Scholar]
- Tierney MC, Fisher RH, Lewis AJ, et al. The NINCDS-ADRDA Work Group criteria for the clinical diagnosis of probable Alzheimer’s disease: a clinicopathologic study of 57 cases. Neurology. 1988;38:359–364. doi: 10.1212/wnl.38.3.359. [DOI] [PubMed] [Google Scholar]
- Akiguchi I, Budka H, Shirakashi Y, et al. Do specific MRI features of Binswanger’s disease also reveal cognitive/motor impairments and the corresponding neuropathology? The Vienna Trans-Danube Aging (VITA) study. Brain Pathology. 2010;20(Suppl. 1):23. [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997;18(4 suppl):S1–S2. [PubMed] [Google Scholar]
- Akiguchi I, Tomimoto H, Wakita H, et al. Topographical and cytopathological lesion analysis of the white matter in Binswanger’s disease brains. Acta Neuropathol. 2004;107:563–570. doi: 10.1007/s00401-004-0850-2. [DOI] [PubMed] [Google Scholar]
- Westover MB, Bianchi MT, Yang C, et al. Estimating cerebral microinfarct burden from autopsy samples. Neurology. 2013;80:1365–1369. doi: 10.1212/WNL.0b013e31828c2f52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JX, Tomimoto H, Akiguchi I, et al. Vascular cell components of the medullary arteries in Binswanger’s disease brains: a morphometric and immunoelectron microscopic study. Stroke. 2000;31:1838–1842. doi: 10.1161/01.str.31.8.1838. [DOI] [PubMed] [Google Scholar]
- Pico F, Jacob MP, Labreuche J, et al. Matrix metalloproteinase-3 and intracranial arterial dolichoectasia. Ann Neurol. 2010;67:508–515. doi: 10.1002/ana.21922. [DOI] [PubMed] [Google Scholar]
- Tomimoto H, Ihara M, Wakita H, et al. Chronic cerebral hypoperfusion induces white matter lesions and loss of oligodendroglia with DNA fragmentation in the rat. Acta Neuropathol. 2003;106:527–534. doi: 10.1007/s00401-003-0749-3. [DOI] [PubMed] [Google Scholar]
- Akiguchi I, Tomimoto H, Suenaga T, et al. Blood–brain barrier dysfunction in Binswanger’s disease; an immunohistochemical study. Acta Neuropathol. 1998;95:78–84. doi: 10.1007/s004010050768. [DOI] [PubMed] [Google Scholar]
- Yoshitake T, Kiyohara Y, Kato I, et al. Incidence and risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population: the Hisayama Study. Neurology. 1995;45:1161–1168. doi: 10.1212/wnl.45.6.1161. [DOI] [PubMed] [Google Scholar]
- Yanagihara T. Vascular dementia in Japan. Ann NY Acad Sci. 2002;977:24–28. doi: 10.1111/j.1749-6632.2002.tb04795.x. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Prevalence and pathology of vascular dementia in the oldest-old. J Alzheimers Dis. 2010;21:1283–1293. doi: 10.3233/jad-2010-100603. [DOI] [PubMed] [Google Scholar]
- Akiguchi I, Yamamoto Y. Vascular mechanisms of cognitive impairment: roles of hypertension and subsequent small vessel disease under sympathetic influences. Hypertens Res. 2010;33:29–31. doi: 10.1038/hr.2009.189. [DOI] [PubMed] [Google Scholar]
- Tomonaga M, Yamanouchi H, Tohgi H, Kameyama M. Clinicopathologic study of progressive subcortical vascular encephalopathy (Binswanger type) in the elderly. J Am Geriatr Soc. 1982;30:524–529. doi: 10.1111/j.1532-5415.1982.tb01691.x. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Pathology and pathogenesis of vascular cognitive impairment-a critical update. Front Aging Neurosci. 2013;5:1–19. doi: 10.3389/fnagi.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imfeld P, Brauchli Pernus YB, Jick SS, Meier CR. Epidemiology, co-morbidities, and medication use of patients with Alzheimer’s disease or vascular dementia in the UK. J Alzheimers Dis. 2013;35:565–573. doi: 10.3233/JAD-121819. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Galluzzi S, Bresciani L, et al. Mild cognitive impairment with subcortical vascular features: clinical characteristics and outcome. J Neurol. 2002;249:1423–1432. doi: 10.1007/s00415-002-0861-7. [DOI] [PubMed] [Google Scholar]
- Akiguchi I, Tomimoto H, Kinoshita M, et al. Effects of antithrombin on Binswanger’s disease with antiphospholipid antibody syndrome. Neurology. 1999;52:398–401. doi: 10.1212/wnl.52.2.398. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Ohara T, Nagakane Y, et al. Chronic kidney disease, 24-h blood pressure and small vessel diseases are independently associated with cognitive impairment in lacunar infarct patients. Hypertens Res. 2011;34:1276–1282. doi: 10.1038/hr.2011.118. [DOI] [PubMed] [Google Scholar]
- Deramecourt V, Slade JY, Oakley AE, et al. Staging and natural history of cerebrovascular pathology in dementia. Neurology. 2012;78:1043–1050. doi: 10.1212/WNL.0b013e31824e8e7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaria RN. Cerebrovascular disease and mechanisms of cognitive impairment: evidence from clinicopathological studies in humans. Stroke. 2012;43:2526–2534. doi: 10.1161/STROKEAHA.112.655803. [DOI] [PubMed] [Google Scholar]
- Esiri MM, Wilcock GK, Morris JH. Neuropathological assessment of the lesions of significance in vascular dementia. J Neurol Neurosurg Psychiatry. 1997;63:749–753. doi: 10.1136/jnnp.63.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans JC, Daniel SE, Hughes AJ, et al. Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Mov Disord. 2004;19:630–640. doi: 10.1002/mds.20083. [DOI] [PubMed] [Google Scholar]
- Chui HC, Zarow C, Mack WJ, et al. Cognitive impact of subcortical vascular and Alzheimer’s disease pathology. Ann Neurol. 2006;60:677–687. doi: 10.1002/ana.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Akiguchi I, Ino T, Nabatame H, et al. Acute-onset amnestic syndrome with localized infarct on the dominant side–comparison between anteromedial thalamic lesion and posterior cerebral artery territory lesion. Jpn J Med. 1987;26:15–20. doi: 10.2169/internalmedicine1962.26.15. [DOI] [PubMed] [Google Scholar]
- Shiino A, Akiguchi I, Watanabe T, et al. Morphometric characterization of Binswanger’s disease: comparison with Alzheimer’s disease. Eur J Radiol. 2012;81:2375–2379. doi: 10.1016/j.ejrad.2011.05.031. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Shiino A, Akiguchi I. Absolute quantification in proton magnetic resonance spectroscopy is superior to relative ratio to discriminate Alzheimer’s disease from Binswanger’s disease. Dement Geriatr Cogn Disord. 2008;26:89–100. doi: 10.1159/000144044. [DOI] [PubMed] [Google Scholar]
- Shiino A, Watanabe T, Shirakashi Y, et al. The profile of hippocampal metabolites differs between Alzheimer’s disease and subcortical ischemic vascular dementia, as measured by proton magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 2012;32:805–815. doi: 10.1038/jcbfm.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launer LJ, Petrovitch H, Ross GW, et al. Brain pathology: vascular origins? Results from the HAAS autopsy study. Neurobiol Aging. 2008;29:1587–1590. doi: 10.1016/j.neurobiolaging.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


