Abstract
Objective
The aim was to examine the effect of dopaminergic medication on stimulus-response learning versus performing decisions based on learning.
Method
To see the effect of dopaminergic therapy on stimulus-response learning and response selection, participants with Parkinson’s disease (PD) were either tested on and/or off their prescribed dose of dopaminergic therapy during different testing days. Forty participants with PD and 34 healthy controls completed the experiment on consecutive days. On Day 1, participants learned to associate abstract images with spoken, “right” or “left” responses via feedback (Session 1). On Day 2, participants recalled these responses (Session 2) and indicated the location (i.e., right or left of center) of previously studied images intermixed with new images (Session 3).
Results
Participants with PD off medication learned stimulus-response associations equally well compared to healthy controls. Learning was impaired by dopaminergic medication. Regardless of medication status, patients recalled the stimulus-response associations from Day 1 as well as controls. In Session 3 off medication, patients demonstrated enhanced facilitation relative to controls and patients on medication, when the stimulus location was congruent with the spoken response that was learned for the stimulus in Session 1.
Interpretation
Learning in PD was comparable to that of healthy controls off medication. Learning was worsened by dopaminergic therapy in PD. We interpret greater facilitation in participants with PD off medication for congruent responses as evidence of greater impulsivity. This motor or reflexive impulsivity was normalized by medication in PD. These findings shed light on the cognitive profile of PD and have implications for dopaminergic treatment.
Introduction
Parkinson’s disease (PD) is a neurodegenerative illness with prominent motor symptoms of tremor, bradykinesia, and rigidity. These motor symptoms result from degeneration of the dopamine-producing cells of the substantia nigra (SN), leading to dopamine deficiency and dysfunction in the dorsal striatum (DS). Cognitive dysfunction has long been recognized as a feature of PD.1 The causes of cognitive impairments in PD are complex and the effect of dopaminergic therapy on cognition is variable.
The cognitive profile in PD has many determinants. Increasingly, it is evident that the striatum itself mediates cognitive functions.2 In PD, some cognitive deficits relate to dopamine depletion in DS and are remediated, at least partially, by dopaminergic therapy. Other cognitive deficits arise as a consequence of dopaminergic therapy.2–5 Increasingly, it is understood that this occurs due to overdose of brain regions that receive dopamine from the ventral tegmental area (VTA) that is relatively spared in PD.2–5 These regions include ventral striatum (VS), prefrontal, and limbic cortices.2 Finally, some abnormalities likely relate to changes in other neurotransmitter systems, cortical degeneration, and Lewy body deposition, and are therefore neither improved nor worsened by dopaminergic therapy.6–8
The current study explored cognition in PD and the effect of dopaminergic medication. We investigated learning and later recalling stimulus-response associations as well as the influence of previously learned associations on response selections. The aim of the study was to understand the effect of dopaminergic medication on learning stimulus-response associations and performing decisions based on that learning.
Dopaminergic therapy has been shown to negatively impact various forms of learning.5,9,10,13 However, studies that examine learning often neglect to separately assess the acquisition of associations between stimuli, responses, and outcomes from response selection processes that rely on the learned associations. For instance, a characteristic stimulus-response learning paradigm proceeds as follows: (1) a stimulus is presented and participants decide amongst the possible responses, (2) feedback pertaining to the accuracy of the response is given, through which the association is learned. An estimation of stimulus-response association learning is obtained by measuring the accuracy of stimulus-specific responses.14,15 Impairment in either learning the associations or using the learned information to decide among a set of responses could yield poor performance in these typical learning scenarios.
Atallah and colleagues16 elegantly address this point. An overwhelming literature exists that implicates DS in mediating learning associations among stimuli, responses and rewards.17,18 However, noting the above confound between learning associations and performance, Atallah and colleagues separated association learning from performing responses based on that learning. In a Y-maze task using odor cues, rats receiving infusions of inhibitory gamma-amino butyric acid (GABA) to DS were unable to consistently choose a rewarded versus unrewarded arm compared to saline-infused rats during the learning period of the experiment. On the surface, this seemed to suggest that inhibitory GABA infusions to DS impaired the rat’s ability to learn the associations between odor cues and rewards. However, the infusions were later stopped and both the experimental and control groups performed the task similarly. This demonstrated that during the learning period, associations were learned equally well for both the experimental and control groups and inhibition of DS by GABA infusions impaired the rat’s ability to use the learned associations to perform consistently rewarded selections. To supplement this finding, a separate experiment performed by the same group found that inhibiting DS by GABA infusions during the test phase impaired selection performance compared to the control (i.e., saline-infused) rats, even though both groups had previously shown identical learning of the odor cue-reward associations during the learning period. Together, these results challenge the notion that DS mediates learning and alternatively suggest a role in performing learned responses.
The literature implicating learning impairment in PD by dopaminergic medication similarly requires reconsideration. Several studies have failed to see impairment in learning due to dopaminergic therapy.19,20 The aim of the present study was to investigate the effect of dopaminergic therapy in PD on learning stimulus-response associations versus enacting the learned responses. In an additional session, we investigated the effect of these variables on how response bias facilitates or interferes with performance.
Materials and Methods
Participants
Forty participants with PD and 34 age- and education-matched healthy controls participated in this experiment. All participants with PD were previously diagnosed by a licensed neurologist, had no coexisting diagnosis of dementia or another neurological or psychiatric disease, and met the core assessment for surgical interventional therapy and the UK Brain Bank criteria for the diagnosis of idiopathic PD.21 All PD and no control participants were treated with dopaminergic therapy. Age- and education-matched controls consisted of friends, spouses, or relatives of participants with PD who were similar in age and education. For the minority of participants with PD who could not recruit a healthy control of their own, participants were recruited from a pool of healthy controls in Sudbury, Ontario, or through advertisements on the University of Western Ontario campus. Healthy controls were required to be within 5 years of age and 5 years of education to the matched PD patient. Participants with PD were recruited through a patient database created in Sudbury, Ontario or the movement disorders database at the London Health Sciences Center. Participants abusing alcohol, prescription or street drugs, or taking cognitive-enhancing medications including donepezil, galantamine, rivastigmine, or memantine were excluded from participating. No participants with PD were diagnosed with an impulse control disorder. Four PD and four control participants performed less than 50% of the associations correctly either in Session 1 or 2, explained below, and a further patient with PD scored less than 20 on the montreal cognitive assessment (MOCA), and therefore his/her data were not included in the analysis.
The motor sub-scale of the Unified Parkinson’s Disease Rating Scale (UPDRS) was scored by a licensed neurologist with sub-specialty training in movement disorders (P. A. M.) to assess the presence and severity of motor symptoms for all patients both on and off dopaminergic medication. Control participants were also screened to rule out undiagnosed neurological illness. Mean group demographics, as well as cognitive and affective screening scores for all patients and controls in each experimental group were recorded (Table 1). UPDRS motor subscale scores on and off dopaminergic therapy, daily doses of dopamine replacement therapy in terms of ι-3,4-dihydroxyphenylalanine (L-dopa) equivalents, and mean duration of PD were also recorded (Table 1). Calculation of daily ι-dopa equivalent dose for each patient was based on the theoretical equivalence to ι-dopa as follows: ι-dopa dose × 1 + ι-dopa controlled release × 0.75 +ι-dopa × 0.33 if on entacapone + amantadine (mg) × 0.5 + bromocriptine (mg) × 10 + cabergoline (mg) × 50 + pergolide (mg) × 100 + pramipexole (mg) × 67 + rasagiline (mg) × 100 + ropinirole (mg) × 16.67 + selegiline (mg) × 10.22
Table 1.
Demographic, clinical information, and screening cognitive and affective measures for participants with PD and controls
| Group | n | Age | Edu | Duration | ι-dopa (mg) | DA (n) | UPDRS ON | UPDRS OFF | ANART | BDI-II | BAI | Apathy | MOCA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | |||||||||||||
| PD | 35 | 64.26 (1.26) | 14.73 (0.47) | 6.36 (0.83) | 574.01 (58.36) | 17 | – | – | 124.71 (1.04) | 10.67 (0.95) | 12.24 (1.39) | 12.07 (0.88) | 25.85 (0.42) |
| ON | 17 | 65.29 (1.81) | 15.35 (0.67) | 5.12 (1.19) | 593.46 (81.51) | 7 | 13.91 (1.25) | – | 123.37 (1.49) | 10.12 (1.36) | 12.77 (1.99) | 10.59 (1.27) | 25.59 (0.60) |
| OFF | 18 | 63.22 (1.76) | 14.11 (0.66) | 7.61 (1.15) | 554.56 (79.21) | 10 | – | 16.25 (1.67) | 126.04 (1.45) | 11.22 (1.32) | 11.72 (1.94) | 13.56 (1.23) | 26.11 (0.58) |
| Control | 30 | 64.66 (1.37) | 14.06 (0.51) | – | – | – | – | – | 123.16 (1.13) | 4.11 (1.03) | 4.00 (1.50) | 10.37 (0.96) | 27.05 (0.45) |
| ON | 16 | 65.31 (1.86) | 14.63 (0.66) | – | – | – | – | – | 123.01 (1.54) | 3.94 (1.40) | 5.50 (2.05) | 10.81 (1.31) | 27.25 (0.61) |
| OFF | 14 | 64.00 (1.99) | 13.50 (0.74) | – | – | – | – | – | 123.31 (1.64) | 4.29 (1.50) | 2.50 (2.19) | 9.93 (1.40) | 26.86 (0.66) |
| Day 2 | |||||||||||||
| PD | 35 | 64.06 (1.23) | 14.71 (0.48) | 6.37 (0.86) | 572.06 (57.05) | 17 | – | – | 124.81 (1.05) | 9.83 (0.88) | 9.46 (1.08) | 11.96 (0.81) | 25.83 (0.42) |
| ON | 16 | 62.13 (1.82) | 14.69 (0.71) | 6.00 (1.26) | 555.80 (84.06) | 9 | 13.19 (0.80) | – | 125.54 (1.55) | 10.88 (1.30) | 9.81 (1.59) | 11.19 (1.20) | 25.56 (0.61) |
| OFF | 19 | 66.00 (1.67) | 14.74 (0.65) | 6.77 (1.16) | 588.32 (77.14) | 8 | – | 18.16 (1.67) | 124.08 (1.42) | 8.79 (1.19) | 9.11 (1.46) | 12.74 (1.10) | 26.11 (0.56) |
| Control | 30 | 64.58 (1.33) | 14.09 (0.52) | – | – | – | – | – | 123.11 (1.13) | 3.21 (0.95) | 2.15 (1.16) | 9.55 (0.88) | 27.08 (0.45) |
| ON | 14 | 62.79 (1.94) | 14.00 (0.76) | – | – | – | – | – | 122.52 (1.66) | 3.86 (1.39) | 2.86 (1.70) | 10.36 (1.28) | 27.29 (0.66) |
| OFF | 16 | 66.38 (1.82) | 14.19 (0.71) | – | – | – | – | – | 123.70 (1.55) | 2.56 (1.30) | 1.44 (1.59) | 8.75 (1.20) | 26.88 (0.61) |
Values are presented as group means (SEM). Screening cognitive and affective measures were completed by participants with PD on medication unless they performed both days off dopaminergic medication. Control participants did not receive dopaminergic therapy during any session of the experiment. Their data are presented to correspond to the ON-OFF order of the patient with Parkinson’s disease to whom they were matched. All control participants presented with normal neurological exams. Day 1 refers to the first day of testing when Session 1 was completed. Day 2 refers to the second day of testing when Sessions 2 and 3 were completed. Edu, years of education; Duration, years since diagnosis of PD; ι-dopa, daily ι-dopa equivalent dose in mg; DA, number of participants with PD taking DA agonists; UPDRS ON, Unified Parkinson’s Disease Rating Scale motor score on medication; UPDRS OFF, Unified Parkinson’s Disease Rating Scale motor score off medication; ANART, National Adult Reading Test IQ Estimation; BDI-II, Beck Depression Inventory II score measured for participants with PD and for matched control participants; BAI, Beck Anxiety Inventory I score measured for participants with PD and for matched control participants; Apathy, Apathy Evaluation Scale score measured for participants with PD and for matched control participants; MOCA, Montreal Cognitive Assessment measured for participants with PD and for matched control participants.
There were no significant demographic differences between PD and control participants (Table 1). Participants with PD scored significantly higher on both Beck Depression Inventory II and Beck Anxiety Inventory compared to controls. Participants with MOCA scores less than 20 were excluded from analysis. No differences were found in terms of depressive or anxiolytic symptoms between participants with PD measured on or off their dopaminergic medication. UPDRS scores were significantly higher in participants with PD measured off relative to on dopaminergic medication, which is expected.
All participants provided informed written consent to the protocol before beginning the experiment, according to the Declaration of Helsinki (2013). All participants with PD were competent and had the capacity to provide informed consent. This study was approved by the Health Sciences Research Ethics Board of the University of Western Ontario and the Ethics Review Board of the Sudbury Regional Hospital.
Experimental design
Participants with PD were randomly divided into four subgroups and all participated in three experimental sessions conducted over two consecutive days, as did their matched healthy controls (Fig. 1). Participants with PD in Group 1 (OFF-ON) performed Session 1, on Day 1, off and Sessions 2 and 3, on Day 2, on dopaminergic medication, whereas patients in Group 2 (ON-OFF) performed Session 1, on Day 1, on medication and Sessions 2 and 3, on Day 2, off medication. Group 3 (OFF-OFF) performed all sessions off dopaminergic therapy, whereas Group 4 (ON-ON) performed all sessions on dopaminergic medication. Despite the four-group design, it was our intention to collapse into ON and OFF groups for Sessions 1 and 2, respectively, to increase power. We found no differences between ON and OFF groups in either Session in terms of age or disease duration. We expected that dopaminergic medication might have an effect on learning in Session 1. Performance in Session 2 depended on how well stimulus-response associations were learned in Session 1. To diminish any carry-over effects from Session 1, we (1) excluded participants who performed less than 50% of the associations correctly in Session 1 or 2 and (2) included a similar number of participants who learned ON as OFF in Session 1, in both the ON and OFF conditions in Session 2.
Figure 1.
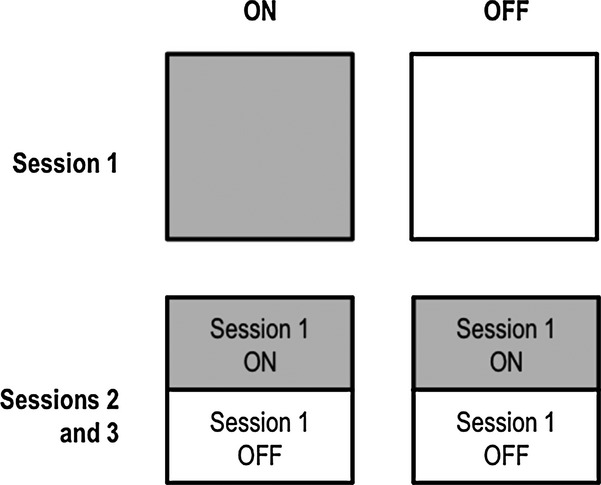
Experimental design. Half of participants completed the learning phase (Session 1) off medication; the other half learned on medication in Session 1. An equal number in each the OFF and ON groups in Sessions 2 and 3 learned the associations off or on medication in Session 1.
Although control participants did not take dopaminergic medication during any session, their data were analyzed to correspond to the medication order of the participants with PD to whom they were matched. Matching was performed prior to data analysis at the time of experimentation. This controlled for possible order, fatigue, and practice effects. Participants with PD took their dopaminergic medication as prescribed by their treating neurologist during ON testing sessions, but abstained from taking all dopaminergic medications including: dopamine precursors such as ι-dopa, aromatic-l-amino-acid decarboxylase inhibitors such as carbidopa, and catechol-O-methyltransferase (COMT) inhibitors such as entacapone (Comtan) for a minimum of 12 to a maximum of 18 h, and dopamine agonists, such as pramipexole (Mirapex), ropinirole (Requip) or pergolide (Permax), as well as amantadine (Symmetrel), rasagiline (Azilect), and selegiline (Eldepryl or Deprenyl) for 16–20 h before beginning OFF testing sessions. All patients confirmed that they complied with these instructions.
All sessions of the experiment were performed using a 14.0″ widescreen laptop (Lenovo T420; Lenovo, Morrisville, North Carolina, USA) running a resolution of 1600 × 900 on the Windows 7 operating system. The screen was placed at a distance of ~50 cm in front of the participant and angled for optimal viewing.
Participants performed a task where they learned to associate six abstract images with one of two spoken responses, either “right” or “left”, via feedback (Session 1). Images consisted of characters taken from the invented Klingon alphabet (Fig. 2). During each trial in Session 1, an image appeared in the center of the computer screen until the participant responded with a verbal response. Images would appear one at a time and in random order. Feedback, either the word “correct” or “incorrect,” was presented after every response. In this way, participants learned to associate each image with the appropriate verbal response through trial and error. Session 1 consisted of 216 image and verbal response trials and at the end of the session participants were given a percentage score, summarizing the number of correct responses provided. Session 1 was completed on the first day of testing, whereas Sessions 2 and 3 were completed on the following day.
Figure 2.
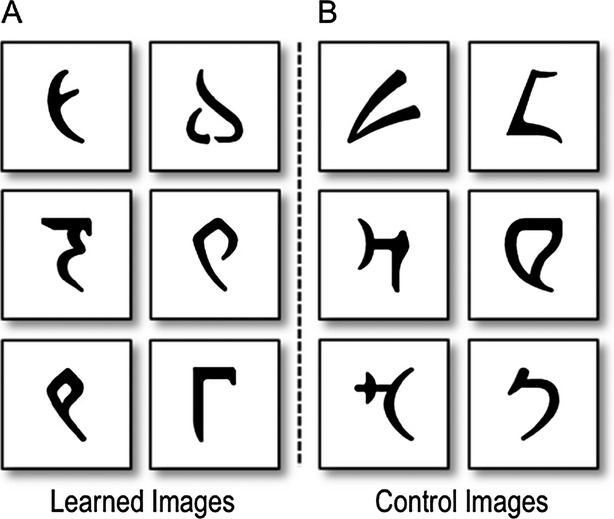
Abstract images presented in the experiment. (A) Learned images refer to the images that were studied and associated with a specific “right” or “left” response via deterministic feedback in Session 1. These learned images were later presented at test in Session 2. In Session 3 these learned images created the conditions for the congruent and incongruent conditions. (B) Control images refer to the images presented only in Session 3 that constituted the control condition.
Session 2 involved recall of the verbal response learned for each of the six images on the previous day. Each image appeared one at a time in random order for a total of 72 trials, or 12 trials per image. No feedback was provided in Session 2 to preclude new, feedback-based learning of the associations.
In Session 3, completed immediately following Session 2, on Day 2 of the experiment, the six images learned in Session 1 were presented with six new Klingon characters one at a time, in random order. These images were presented either on the left or the right side of the screen and the participant responded verbally with the side of the screen on which the image appeared as quickly and accurately as they could. Session 3 consisted of 144 trials. No feedback was provided in this session. Examples of the order of events for trials in each session are presented in Figure 3.
Figure 3.
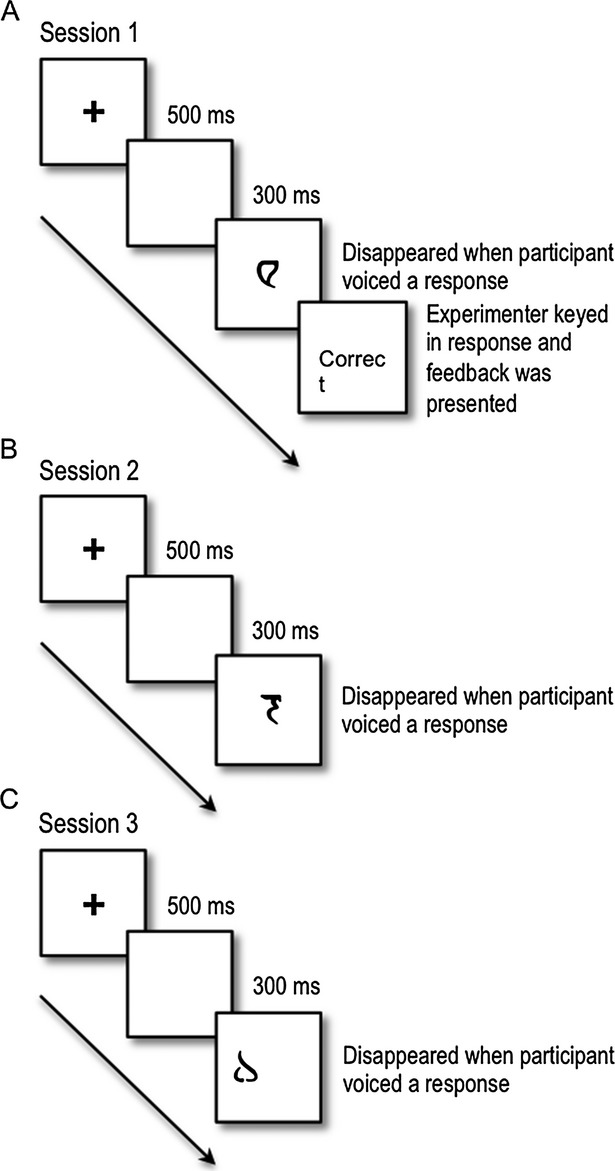
Example of a single trial in Sessions 1, 2, and 3. (A) Participants with PD and age- and education-matched controls learned to associate six abstract images with either a “left” or “right” verbal response in Session 1. The following is an example of a trial: (1) a fixation cross appeared in the center of the computer screen for 700 msec; (2) a blank screen was presented for 300 msec; (3) an image was presented in the center of the computer screen until the participant vocalized a response that was recorded by the microphone; (4) the image disappeared and the experimenter coded the response using a keyboard; (5) feedback, either the word “correct” or “incorrect” was presented for 750 msec before the next trial began. (B) Participants recalled the responses to the learned images in the absence of feedback in Session 2. (C) Images appeared on the left or right side of the screen and participants indicated the location of the images (either left or right of center) with a vocal response. Stimuli included the six learned images presented in Sessions 1 and 2 as well as six new images. Trials in Sessions 2 and 3 were identical to Session 1 except that feedback was omitted in both and the images appeared on the left and right side of the screen in Session 3.
Data analysis
Efficiency of learning stimulus-response associations was measured by calculating the slope of learning in Session 1. Session 1 was divided into 12 discrete blocks of 18 trials. At the end of a block, a score summarizing the number of correct trials was logged but not revealed to the participant. Slope was calculated using the standard slope of the linear regression function in Microsoft Excel (2011), given by the following equation:
where b is the slope, and x and y are the sample means of the number of blocks, and block scores, respectively. Larger slope values signified faster learning of the stimulus-response associations. Session 2 was divided into four discrete blocks of 18 trials and scores summarizing the number of correct trials were logged, as in Session 1. Performance in Session 2 was measured by the average proportion of erroneous responses to the images based on the associations learned in Session 1.
Three conditions – congruent, incongruent, and control – were created in Session 3. In the congruent condition, an image appeared in a location that was consistent with the spoken response that had been learned for that image during Session 1. In the incongruent condition, an image appeared in a location that was opposite to the spoken response that had been learned for that image during Session 1. In the control condition a new image that was not previously associated with “right” or “left” was presented. Session 3 consisted of 48 congruent, 48 incongruent, and 48 control trials that occurred in random order. Response times were measured from the onset of the image until the microphone recorded the participant’s response. The control condition provided a baseline for providing a location response. Facilitation was calculated as mean response times in the congruent condition minus those in the control condition and interference was calculated as mean response times in the incongruent condition minus those in the control condition. Trials with a response time greater than 2.5 standard deviations from the mean were excluded from analysis.
Results
Session 1: Learning phase
The average slope of learning to associate six images from the Klingon alphabet with one of two spoken responses, either “right” or “left”, via feedback was calculated for participants with PD and controls in each of the ON and OFF sessions (Fig. 4A). We performed a 2 × 2 analysis of variance (ANOVA) on the slope. To reiterate, slope was calculated using the percentage scores for the number of correct responses obtained after each of the 12 blocks in Session 1. Group (PD vs. Control) and Medication Session (OFF vs. ON) were between-subject factors. The Group × Medication Session interaction was significant, F1,61 = 3.99, MSE = 0.00, P = 0.050, though the main effect of Group, F1,61 = 1.49, MSE = 0.00, P = 0.226, and of Medication Session, F1,61 < 1, were not.
Figure 4.
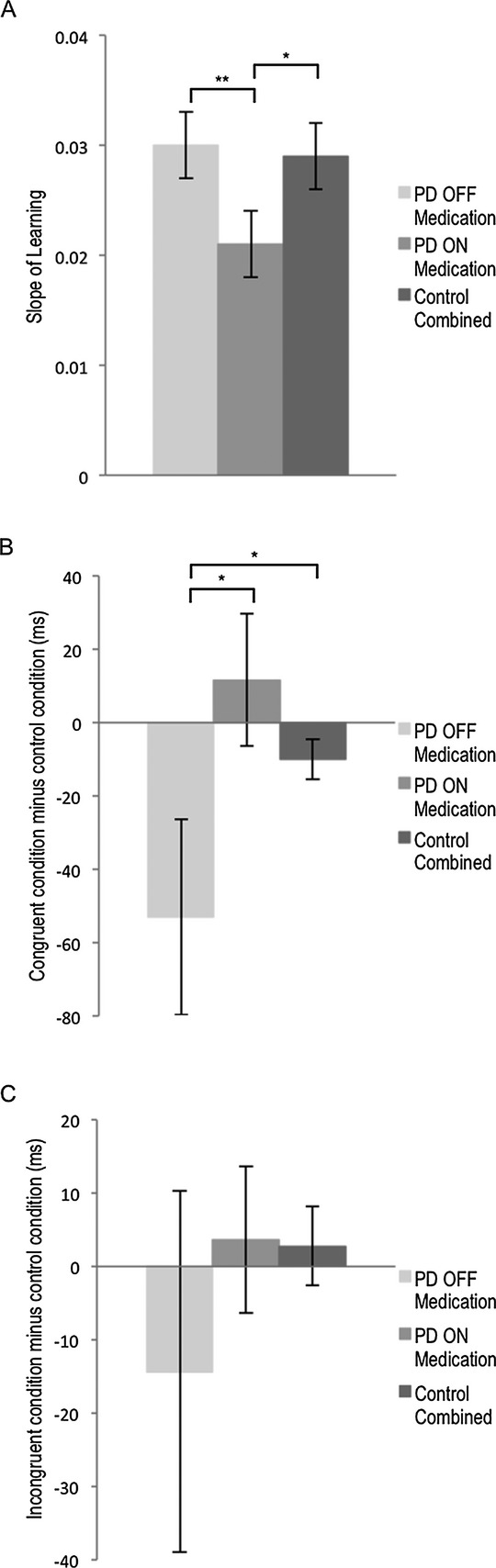
Effect of dopaminergic therapy on association learning, facilitation and interference. (A) Slopes of learning in Session 1 for participants with Parkinson’s disease (PD) and healthy control participants. Average slopes of each medication group are presented. Error bars represent standard error of the mean. Slopes were calculated using the standard slope of the linear regression function in Microsoft Excel (2011). The slope of learning for participants with PD off dopaminergic medication is significantly higher than participants with PD on medication (F1,33 = 4.62, MSE = 0.000, P < 0.050). There was a trend toward a significant difference in terms of learning slopes for PD on medication compared to the collapsed control group, F1,45 = 3.90, MSE = 0.00, P = 0.055, reflecting slower learning for PD patients on dopaminergic medication. (B) Mean facilitation scores for participants with PD and healthy controls. Error bars represent standard error of the mean. Facilitation was calculated as mean response times in the congruent condition minus those in the control condition. The congruent condition involved trials in which the learned, spoken response to an image was the same as the location where it was presented in Session 3. The control condition consisted of new images in the experiment that were not associated with any “right” or “left” responses. Control participants’ performance was equivalent across ON and OFF sessions and therefore we used a combined control group. Participants with PD off medication evidenced greater facilitation than participants with PD on medication (F1,33 = 3.72, MSE = 9766.91, P = 0.062), and controls (F1,47 = 3.74, MSE = 5719.86, P = 0.059) that trended toward significance. (C) Mean interference scores for participants with PD and healthy controls. Interference scores were calculated as mean response times in the incongruent condition minus those in the control condition. The incongruent condition involved trials in which the learned, spoken response to an image was opposite to the location where it was presented in Session 3. There were no significant differences between participants with PD on or off medication or relative to controls. Asterisks indicate level of significance (**P < 0.05; *P < 0.1).
To further explore the significant Group × Medication Session interaction, separate one-way ANOVAs were performed for PD and control participants, with Medication Session (ON vs. OFF) as the between-subject factor. The main effect of Medication Session was significant for participants with PD, F1,33 = 4.62, MSE = 0.000, P < 0.050, reflecting slower learning on relative to off dopaminergic medication, but not for control participants, F1,28 < 1.
We next compared learning slopes for PD patients ON versus OFF medication relative to those of healthy controls. Because control participants’ performance did not differ across the ON versus OFF Medication Sessions, we collapsed into a single control group. As a reminder, control participants did not take dopaminergic medication during any session of this experiment and their pseudo-ON versus pseudo-OFF status was assigned before participation to correspond to the group and order of the PD patient to whom they were matched. Two one-way ANOVAs were performed with Group (PD ON vs. Control; PD OFF vs. Control) as the between-subject factor. There was a trend toward a significant difference in terms of learning slopes for PD ON compared to the collapsed control group, F1,45 = 3.90, MSE = 0.00, P = 0.055, reflecting slower learning for PD patients on dopaminergic medication. There was no significant difference between PD OFF and controls, F < 1.
Session 2: Test phase
We performed a 2 × 2 ANOVA on “right” and “left” spoken response accuracy during Session 2. Group (PD vs. Control) and Medication Session (OFF vs. ON) were between-subject factors. There were no significant main effects (F < 1 for both Group and Medication Session) or interactions, F1,61 = 1.97, MSE = 0.028, P = 0.165. Mean error rates are presented in Table 2. A combined control group was used in Table 2 to serve as a point of comparison between Sessions, given that there were no significant differences between Sessions for controls.
Table 2.
Proportion of errors in Sessions 2 and 3
| Session 3 | ||||
|---|---|---|---|---|
| Session 2 | Congruent condition | Incongruent condition | Control condition | |
| PD | ||||
| OFF | 0.168 (0.036) | 0.022 (0.012) | 0.038 (0.029) | 0.026 (0.015) |
| ON | 0.201 (0.041) | 0.050 (0.009) | 0.058 (0.012) | 0.051 (0.008) |
| Combined control | 0.150 (0.043) | 0.005 (0.003) | 0.010 (0.006) | 0.003 (0.003) |
All values reported are group means (SEM). Proportion of errors in Session 2 was measured by the number of incorrect responses to the images based on the associations learned in Session 1. In Session 3, trials where the participant answered with the incorrect response as to the location of the image (i.e., left or right of center) are considered errors. In the congruent condition, an image appeared in a location that was consistent with the spoken response that had been learned for that image during Session 1. In the incongruent condition, an image appeared in a location that was opposite to the spoken response that had been learned for that image during Session 1. In the control condition, a new image that was not previous associated with “right” or “left” was presented. Control participants’ performance was equivalent across sessions and therefore a combined control group was used. PD = Parkinson’s disease.
Session 3: Facilitation and interference from previous associations
Proportion of errors in the congruent, incongruent, and control condition are presented in Table 2.
We performed a 2 × 2 ANOVA on facilitation scores with Group (PD vs. Control) and Medication Session (ON vs. OFF) as between-subject factors. Facilitation was calculated as mean response times in the congruent condition minus those in the control condition. Again, the congruent condition involved trials in which the learned, spoken response to an image was the same as the location where it was presented in Session 3. The control condition consisted of new images that the participant had not previously associated with a “right” or “left” response. The main effects of Group and Medication Session were not significant, F < 1 and F1,61 = 2.60, MSE = 5700.11, P = 0.112, respectively. The Group × Medication Session interaction trended toward significance, F1,61 = 3.19, MSE = 5700.11, P = 0.073, which was explored further in one-way ANOVAs below.
One-way ANOVAs with Medication Status (ON vs. OFF) as the between-subject factor were performed for PD and Control participants’ data separately. These revealed an ON-OFF difference for participants with PD that trended toward significance, F1,33 = 3.72, MSE = 9766.91, P = 0.062, but no ON-OFF difference for controls, F < 1. Participants with PD showed greater facilitation off relative to on medication (Fig. 4B).
Next, we compared facilitation for participants with PD on versus off medication relative to controls. Control participants’ performance was equivalent across sessions and therefore we used a combined control group. Participants with PD off medication experienced significantly more facilitation from previous associations than did participants with PD on medication or controls, F2,62 = 3.49, MSE = 5610.07, P = 0.037. Comparing PD ON to the combined control group revealed no significant differences in facilitation scores, F1,44 = 2.08, MSE = 2375.17, P > 0.156, whereas the comparison of PD OFF to combined control group trended toward significance, F1,47 = 3.74, MSE = 5719.86, P = 0.059, reflecting enhanced facilitation for PD OFF compared to controls.
We performed analogous analyses on interference scores. Interference scores were calculated as mean response times in the incongruent condition minus those in the control condition. The incongruent condition was composed of trials where the learned spoken response to an image was opposite to the location of the image in Session 3. The main effects of Group and Medication Session, both F1,61 < 1, and the Group × Medication Session interaction, F1,61 < 1, were nonsignificant (Fig. 4C).
Discussion
We showed that stimulus-response association learning in participants with PD, without a coexisting diagnosis of dementia, is comparable to healthy controls off medication. This learning is impaired by dopaminergic medication. Regardless of medication status, recall of previously learned stimulus-response associations and response selection performance for participants with PD were equal to that of age-matched controls. Our four-group design countered any carry-over effects that related to medication status during the learning phase. That is, we ensured that the ON and OFF groups in Sessions 2 and 3 on Day 2 were composed of a similar number of participants with PD who had acquired stimulus-response associations on compared to off dopaminergic therapy in Session 1.
In Session 3, participants named the location of stimuli – “right” or “left” of center – as quickly and accurately as they could. Off medication, participants with PD evidenced greater response facilitation than their counterparts who were tested on medication or controls when the location response in Session 3 was congruent with the response that they had learned for particular stimuli in Session 1. Facilitation from congruent influences on responding has been interpreted as evidence of impulsivity in children23 and adolescents24 relative to adults, as well as in patients with attention deficit hyperactivity disorder,25 schizophrenia,26 and PD.27 Despite this, for all participants, no significant interference occurred in Session 3 when stimuli occurred in the location opposite to the spoken “right” or “left” response paired with them in Session 1. Dissociations between facilitation and interference effects in similar paradigms are commonly noted.11,28,30
Learning and decision performance in PD
We found that stimulus-response association learning is spared in PD but is impaired by dopaminergic therapy. A number of studies have also revealed normal probabilistic, associative, or motor sequence learning in participants with PD at baseline. In these studies, impairments also arose due to dopaminergic medication.5,9–13,31 Others have shown an opposite pattern, however.19,20 Recalling and performing previously learned responses were not affected by PD or dopaminergic medication, suggesting that learning stimulus-response associations and recalling and performing responses based on previous learning are mediated by different brain regions. Cognitive functions worsened by dopaminergic therapy have been widely ascribed to brain regions that are innervated by the VTA.2,32 The VTA is relatively spared compared to the substania nigra SN in PD.2 Dopamine replacement is titrated to the DS-mediated motor symptoms, effectively overdosing VTA-innervated brain regions that are relatively dopamine replete.2,4 These include VS, limbic, and prefrontal cortex. Indeed, using neuroimaging and behavioral methods, VS has been implicated in learning in healthy participants and in participants with PD.11,31,32 In a recent study, we in fact showed that VS activity correlated specifically with stimulus-response association learning at the time of feedback, whereas DS activation was associated with response selection and enactment.33
Impulsivity in PD
Off medication, participants with PD seemed to experience enhanced facilitation for location responses that corresponded to the specific “right” or “left” response that they had associated with an image in Session 1. We interpret this finding as evidence of more impulsive responding for participants with PD off medication. This was relative to participants with PD who were tested on medication and healthy controls. At first glance, this finding seems at odds with the popularly reported problems with impulse control, such as pathological gambling, overeating, or excessive shopping, that arise as side effects of dopaminergic medication, particularly dopamine agonists in PD.34 Understanding that impulsivity is not a unitary concept perhaps accounts for this discrepancy.
One facet of impulsivity involves a predisposition to decisions that favor short-term over long-term rewards or consequences.35,36 This form of impulsivity is captured by experimental paradigms such as the delayed discounting task, which involves choosing between small immediate rewards and larger delayed rewards.37 Participants with PD on dopamine agonists tend to impulsively choose immediate more often than delayed rewards.37 Impulsive choices in these paradigms seem coherent with impulse control disorders. Both are precipitated by dopaminergic medication in PD, and are associated with activity changes in medial frontal, posterior cingulate, as well as VS (i.e., VTA-innervated brain regions).38
Impulsivity also manifests as a failure to inhibit motor responses that are ongoing or prepotent, resulting in impetuous actions (i.e., motor impulsivity) or as premature responding before sensory evidence is adequately sampled (i.e., reflection impulsivity).36 Our experimental paradigm indexes these forms of impulsivity and consistent with our findings, previous studies have shown increased impulsivity in participants with PD at baseline.39 Participants with PD off medication select more prominent stimuli39 or more practised responses40 faster than controls. Further, as we have found here, this enhanced impulsivity is normalized by dopaminergic therapy.39–41 This pattern of findings in participants with PD is consistent with the fact that DS is implicated in mitigating these forms of impulsivity.39–42 In PD, DS is dopamine depleted at baseline, which is remedied by dopaminergic medication.
Consistent with this view, we have previously argued that DS is implicated in decision making, promoting more distributed attention and integration of variable influences on responding.29 DS mediates resisting attentional capture by more salient aspects of a situation or reflexive enactment of more automatic responses to produce more deliberate and considered responses.29 When DS is impaired, as in unmedicated PD, increased motor or reflexive impulsivity is expected. When targets are salient or correct responses are overlearned and automatic, PD patients’ performance is superior to healthy controls. In contrast, poorer performance results when less salient aspects of the context are more relevant to the current goal or less practised responses are demanded.11,41
Summary
At baseline in PD, learning stimulus-response associations is comparable to learning in healthy controls. In contrast, stimulus-response learning is impaired by dopaminergic therapy in PD. Recalling and selecting responses based on previous learning was not affected by PD or dopaminergic medication status. Off medication, however, there was a trend in the data suggesting that participants with PD produced more impulsive responses than controls. This tendency was redressed by dopaminergic therapy. This pattern of results could suggest that learning stimulus-response associations relies on VTA-innervated brain regions. In contrast, the effect of PD and medication on motor and reflexive impulsivity is consistent with the view that these are DS mediated. These brain regions are differentially dopamine depleted and hence dissimilarly affected by dopaminergic therapy in PD.
Here, we add our results to a growing literature that suggests that learning in various forms is impaired by dopaminergic therapy. This effect of dopaminergic medication occurred only when stimulus-response associations were being learned and not when selections were guided by previous learning. A further important insight gained from this study is that some forms of impulsivity occur at baseline in PD. These results highlight the fact that impulsivity is not a unitary concept. Not all facets of impulsivity arise in PD as a consequence of dopaminergic therapy. Indeed, here we present impulsive responding that is normalized by dopaminergic therapy. Our findings have implications for dopaminergic treatment in PD.
Acknowledgments
We thank Nora-Lee Arcand and Tracey Jones for their assistance with recruitment, as well as Allison Partridge and Omer Dost for participant testing. This work was supported by start-up funds and an Opportunity Grant from the Academic Medical Organization of Southwestern Ontario awarded to Penny A. MacDonald.
Author Contributions
Ken N. Seergobin and Penny A. MacDonald designed the experiment; Nole M. Hiebert and Andrew Vo carried out the experiment; Nole M. Hiebert, Ken N. Seergobin, Hooman Ganjavi, and Penny A. MacDonald performed the analysis; Nole M. Hiebert and Penny A. MacDonald wrote the manuscript; All authors edited the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any conflicts of interest.
References
- Brown RG, Marsden CD. How common is dementia in Parkinson’s disease? Lancet. 1984;2:1262–1265. doi: 10.1016/s0140-6736(84)92807-1. [DOI] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Gotham AM, Brown RG, Marsden CD. Levodopa treatment may benefit or impair “frontal functions in Parkinson’s disease. Lancet. 1986;2:970–971. doi: 10.1016/s0140-6736(86)90617-3. [DOI] [PubMed] [Google Scholar]
- Gotham AM, Brown RG, Marsden CD. Frontal’ cognitive function in patients with Parkinson’s disease ‘On’ and ‘Off’ levodopa. Brain. 1988;111:299–321. doi: 10.1093/brain/111.2.299. [DOI] [PubMed] [Google Scholar]
- Swainson R, Rogers RD, Sahakian BJ, et al. Probabilistic learning and reversal deficits in patients with Parkinson’s disease or frontal or temporal lobe lesions: possible adverse effects of dopaminergic medication. Neuropsychologia. 2000;38:596–612. doi: 10.1016/s0028-3932(99)00103-7. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Muller ML, Kotagal V, et al. Heterogeneity of cholinergic denervation in Parkinson’s disease without dementia. J Cereb Blood Flow Metab. 2012;32:1609–1617. doi: 10.1038/jcbfm.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio Y, Hirayama K, Takeda A, et al. Corticolimbic gray matter loss in Parkinson’s disease without dementia. Eur J Neurol. 2010;17:1090–1097. doi: 10.1111/j.1468-1331.2010.02980.x. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Neuropathology of sporadic Parkinson’s disease: evaluation and changes of concepts. Mov Disord. 2012;27:8–30. doi: 10.1002/mds.23795. [DOI] [PubMed] [Google Scholar]
- MacDonald AA, Monchi O, Seergobin KN, et al. Parkinson’s disease duration determines effect of dopaminergic therapy on ventral striatum function. Mov Disord. 2013;28:153–160. doi: 10.1002/mds.25152. [DOI] [PubMed] [Google Scholar]
- Seo M, Beigi M, Jahanshahi M, et al. Effects of dopamine medication on sequence learning with stochastic feedback in Parkinson’s disease. Front Syst Neurosci. 2010;4:1–9. doi: 10.3389/fnsys.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald PA, MacDonald AA, Seergobin KN, et al. The effect of dopamine therapy on ventral and dorsal striatum-mediated cognition in Parkinson’s disease: support from functional MRI. Brain. 2011;134:1447–1463. doi: 10.1093/brain/awr075. [DOI] [PubMed] [Google Scholar]
- MacDonald AA, Seergobin KN, Owen AM, Tamjeedi R, Monchi O, Ganjavi H, MacDonald PA. Differential effects of Parkinson’s disease and dopamine replacement on memory encoding and retrieval. PloS one. 2013;8:e74044. doi: 10.1371/journal.pone.0074044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo A, Hiebert NM. Seergobin KN, Solzc S, Partridge A, Macdonald PA. Dopaminergic therapy affects feedback-based stimulus-response learning in Parkinson’s disease. Front Hum Neurosci. 2014;8:784. doi: 10.3389/fnhum.2014.00784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessup RK, O’Doherty JP. Human dorsal striatal activity during choice discriminates reinforcement learning behavior from the gambler’s fallacy. J Neurosci. 2011;31:6296–6304. doi: 10.1523/JNEUROSCI.6421-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonald RJ, White NM. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav Neurosci. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- Atallah HE, Lopez-Paniagua D, Rudy JW, O’reilly RC. Separate neural substrates for skill learning and performance in the ventral and dorsal striatum. Nat Neurosci. 2007;10:126–131. doi: 10.1038/nn1817. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Ennis JM, Spiering BJ. A neurobiological theory of automaticity in perceptual categorization. Psychol Rev. 2007;114:632–656. doi: 10.1037/0033-295X.114.3.632. [DOI] [PubMed] [Google Scholar]
- Shiner T, Seymour B, Wunderlich K, et al. Dopamine and performance in a reinforcement learning task: evidence from Parkinson’s disease. Brain. 2012;135:1871–1883. doi: 10.1093/brain/aws083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smittenaar P, Chase HW, Aarts E, et al. Decomposing effects of dopaminergic medication in Parkinson’s disease on probabilistic action selection – learning or performance? Eur J Neurosci. 2012;35:1144–1151. doi: 10.1111/j.1460-9568.2012.08043.x. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullner U, Kassubek J, Odin P, et al. Transdermal rotigotine for the perioperative management of Parkinson’s disease. J Neural Transm. 2010;117:855–859. doi: 10.1007/s00702-010-0425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright BC, Wanley A. Adults’ versus children’s performance in the Stroop task: interference and facilitation. Br Psychol Soc. 2003;94:475–485. doi: 10.1348/000712603322503042. [DOI] [PubMed] [Google Scholar]
- Heim S, Benasich AA, Keil A. Distraction by emotion in early adolescence: affective facilitation and interference during the attentional blink. Front Psychol. 2013;4:580. doi: 10.3389/fpsyg.2013.00580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen H, Oades RD. Negative priming within a Stroop task in children and adolescents with attention-deficit hyperactivity disorder, their siblings, and independent controls. J Atten Disord. 2010;13:497–504. doi: 10.1177/1087054708325974. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Valdman O, et al. Enhanced facilitation of spatial attention in schizophrenia. Neuropsychology. 2011;25:76–85. doi: 10.1037/a0020779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ostilio K, Cremers J, Delvaux V, et al. Impaired automatic and unconscious motor processes in Parkinson’s disease. Sci Rep. 2013;3:2095. doi: 10.1038/srep02095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod CM, MacDonald PA. Interdimensional interference in the Stroop effect: uncovering the cognitive and neural anatomy of attention. Trends Cogn Sci. 2000;4:383–391. doi: 10.1016/s1364-6613(00)01530-8. [DOI] [PubMed] [Google Scholar]
- Schrobsdorff H, Ihrke M, Behrendt J, et al. Identity negative priming: a phenomenon of perception, recognition or selection? PLoS One. 2012;7:e32946. doi: 10.1371/journal.pone.0032946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AA, Seergobin KN, Tamjeedi R, Owen AM, Provost J-S, Monchi O, Ganjavi H, MacDonald PA. (2014) Examining dorsal striatum in cognitive effort using Parkinson’s disease and fMRI. Annals of Clinical and Translational Neurology. 2014;1:390–400. doi: 10.1002/acn3.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Geghman KD, et al. L-dopa impairs learning, but spares generalization, in Parkinson’s disease. Neuropsychologia. 2006;44:774–784. doi: 10.1016/j.neuropsychologia.2005.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert NM, Vo A, Hampshire A, et al. Striatum in stimulus-response learning via feedback and in decision making. Neuroimage. 2014;101:448–457. doi: 10.1016/j.neuroimage.2014.07.013. [DOI] [PubMed] [Google Scholar]
- MacDonald PA, Monchi O. Differential effects of dopaminergic therapies on dorsal and ventral striatum in Parkinson’s disease: implications for cognitive function. Parkinsons Dis. 2011;2011:572743. doi: 10.4061/2011/572743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Fernagut P-O, Wickens J, et al. Chronic dopaminergic stimulation in Parkinson’s disease: from dyskinesias to impulse control disorders. Lancet Neurol. 2009;8:1140–1149. doi: 10.1016/S1474-4422(09)70287-X. [DOI] [PubMed] [Google Scholar]
- Hinson JM, Jameson TL, Whitney P. Impulsive decision making and working memory. J Exp Psychol Learn Mem Cogn. 2003;29:298–306. doi: 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Voon V, Reynolds B, Brezing C, et al. Impulsive choice and response in dopamine agonist-related impulse control behaviors. Psychopharmacology. 2010;207:645–659. doi: 10.1007/s00213-009-1697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli F, Ko JH, Miyasaki J, et al. Dopamine-agonists and impulsivity in Parkinson’s disease: impulsive choices vs. impulsive actions. Hum Brain Mapp. 2013;35:2499–2506. doi: 10.1002/hbm.22344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djamshidian A, O’Sullivan SS, Lees A, et al. Stroop test performance in impulsive and non impulsive patients with Parkinson’s disease. Parkinsonism Relat Disord. 2011;17:212–214. doi: 10.1016/j.parkreldis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood AJ, Amador SC, Cain AE, et al. Levodopa slows prosaccades and improves antisaccades: an eye movement study in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:565–570. doi: 10.1136/jnnp.2006.099754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Rogers R, Barker RA, et al. Top-down attentional control in Parkinson’s disease: salient considerations. J Cogn Neurosci. 2010;22:848–859. doi: 10.1162/jocn.2009.21227. [DOI] [PubMed] [Google Scholar]
- Ness V, Beste C. The role of the striatum in goal activation of cascaded actions. Neuropsychologia. 2013;51:2562–2571. doi: 10.1016/j.neuropsychologia.2013.09.032. [DOI] [PubMed] [Google Scholar]


