Abstract
Early diabetic neuropathy is characterized by loss of unmyelinated axons, resulting in pain, numbness, and progressive decline in intraepidermal nerve fiber density. Patients with type 2 diabetes, without neuropathy, were assigned to quarterly lifestyle counseling (N = 40) or structured, supervised weekly exercise (N = 60) for 1 year. Distal leg IENFD significantly increased in the exercise cohort and remained unchanged in the counseling cohort (1.5 ± 3.6 vs. −0.1 ± 3.2 fibers/mm, P = 0.03). These results suggest preclinical injury to unmyelinated axons is potentially reversible, and that IENFD may be a responsive biomarker useful in future neuropathy prevention clinical trials.
Introduction
Diabetic neuropathy is characterized by early injury to small unmyelinated axons resulting in pain, sensory loss, and distal sudomotor dysfunction. Intraepidermal nerve fiber density (IENFD) is a sensitive, quantitative, and reproducible measure of early injury to small unmyelinated fibers for which age-specific normative values have been established.1,2 Accumulating epidemiological and clinical data suggest obesity, dyslipidemia, and metabolic syndrome are important risk factors for neuropathy in type 1 and type 2 diabetes.3,4 Animal models of isolated obesity, dyslipidemia, and Metabolic Syndrome demonstrate similar selective injury to distal small fibers.5–8 Exercise improves multiple facets of metabolic syndrome and has shown promise as a therapeutic strategy to slow progression of neuropathy in patients with diabetes and prediabetes.9,10 The objective of this study was to determine if exercise and lifestyle counseling could prevent the natural history of IENFD decline in presymptomatic diabetic patients and to further validate IENFD as a neuropathy biomarker for future use in neuropathy prevention or early intervention trials.
Participants and Methods
Participants with type 2 diabetes were recruited by direct mail solicitation, and from University of Utah Diabetes and Primary Care clinics. The University of Utah Institutional Review Board approved the study, and all participants provided written informed consent. A Data Safety and Monitoring Officer was appointed and reviewed all potentially significant adverse events within 1 week of occurrence. Participants were 30–70 years of age at study enrollment. Body mass index (BMI), blood pressure, fasting glucose, hemoglobin A1c and lipids were performed at screening to confirm the participant’s metabolic status. Use of medications intended to control lipids or blood pressure were accepted as evidence of dyslipidemia and hypertension.
Participants with symptoms of distal lower extremity sensory loss, numbness or neuropathic pain consistent with peripheral neuropathy were excluded. All participants completed a validated neuropathy questionnaire, the Michigan Neuropathy Screening Instrument (MNSI)11 and underwent a focused standardized physical examination performed by an Investigator using the Utah Early Neuropathy Scale (UENS)12 to exclude asymptomatic neuropathy. Those with a UENS score >4 were excluded. Participants taking coumadin were excluded because of bleeding risk from the biopsies. Pregnant women were excluded because the dramatic metabolic changes associated with pregnancy would interfere with interpretation of testing. Each participant’s primary care provider was asked to approve participation in the proposed exercise program, and each participant was asked to undergo standardized exercise tolerance testing conforming to American College of Sports Medicine guidelines.13 Those with significant cardiovascular disease or whose primary care provider did not provide approval were excluded.
Participants who met all enrollment criteria completed a baseline evaluation designed to measure nerve function and fitness. After local anesthesia with subdermal 1% lidocaine, a 3 mm punch biopsy was obtained from the left ankle 10 cm above the lateral malleolus and from the left proximal lateral thigh 20 cm below the pelvic rim. Using previously described methods,1 skin biopsy tissue was sectioned into 50 micron thick sections, stained with PGP 9.5, and IENFD determined by counting the number of derma1–epidermal crossing fibers and dividing by epidermal length. The technician was blinded to the biopsy’s site and the participant’s status. Nerve conduction studies (NCS) were performed after bringing the tested limbs (usually left, as above) to between 32 and 34°C using a Viasys Viking NCS machine (Natus, Middleton, WI). Sural and radial sensory responses, tibial and peroneal motor responses, proximal conduction velocities and F-responses were obtained by the Investigators or an American Board of Electrodiagnostic Medicine certified technician. Six Minute Walk test was performed over a standardized course.14 Quadriceps isometric peak force (in Newtons) was measured using a KinCom dynamometer (KinCom 500H; Isokinetic International, Chattanooga, TN) and lower extremity extension power (in Watts) was measured using the Nottingham Power Rig (Nottingham, UK).15
Participants were assigned to either quarterly counseling sessions emphasizing principles of diet and moderate home exercise, or to a mentored program of exercise and dietary counseling based on that used in the Diabetes Prevention Program.16 Each participant completed a baseline 3-day food diary, reviewed this with a dietician, and received individualized counseling on dietary habits. For those assigned to the mentored program, exercise was supervised for 30–90 min weekly, supplemented by assigned home exercise, and emphasized a balanced mix of aerobic and resistance training tailored to the participant’s baseline fitness and any orthopedic, cardiac or pulmonary limitations. At baseline, each exercise participant met individually with the exercise physiologist to review their medical, cardiac, orthopedic and exercise, and to discuss the participant’s perception of current exercise tolerance. An age-predicted maximum heart rate was calculated using 220-age for cardiovascular exercise. Participants were taught to perform home exercise at a moderate exertion level, 11–14 on the validated Borg “Rate of Perceived Exertion” (RPE) scale, and to record exercise in a diary as a counseling aid.17 While performing weekly mentored exercise, participants followed a schedule for progression of cardiovascular exercise, ramping from 65% of calculated maximum heart rate for 30 min in week one to 50 min of exercise at 85% of calculated maximum heart rate by study week 7. If participants were not able to follow this recommended intensity progression, they were asked to maintain RPE of 12–16 during each mentored session. Similarly, for resistance exercise, baseline strength testing was performed to determine maximum weight lifted for one repetition (1RM) on leg press and biceps curls. Participants began resistance training at 60% of 1RM, and worked to increase strength and endurance sufficiently to perform three sets of 12 repetitions at this weight before progressing to two sets of 12 repetitions at the next weight setting.
During the initial 12 weeks, participants in the active exercise cohort had biweekly access to a nutritionist at the mentored exercise venue who provided additional dietary counseling. After 12 weeks of mentored exercise active cohort participants joined monthly group meetings designed to provide additional dietary education and promote home exercise. Both groups received quarterly study progress visits.
Blood pressure, pulse, weight, BMI, metabolic measures, neuropathy scales, NCS, and distal leg and proximal thigh biopsy for IENFD were repeated at the completion of 12 months of follow up. The Investigator performing exam scales and NCS was blinded to the participant’s study assignment.
Results
174 diabetic participants provided consent and were screened to identify 100 without baseline symptoms or exam signs of peripheral neuropathy who met all study entry criteria and were deemed safe to exercise. The most frequent reasons for screen failure are enumerated in Figure 1, and included symptoms of, or exam evidence of neuropathy following review with the Investigator (N = 28), lack of required permission to exercise from their primary physician, failure to complete standardized cardiac exercise treadmill testing, failure to return for or complete baseline assessment, and unacceptable cardiac or orthopedic exercise risk based on results of exercise tolerance testing or on baseline evaluation by the exercise physiologist. At baseline, participants were randomly assigned to exercise (N = 60) or lifestyle counseling (N = 40) in a 3/2 ratio, anticipating greater dropout from the exercise cohort. Analysis was intent-to-treat, and all participants were invited to return quarterly and at 12 months regardless of their participation in assigned aspects of the intervention. 88 participants could be evaluated after 12 months, representing roughly 12% attrition from each group. Student’s t tests compared baseline demographics, metabolic, fitness and nerve measures between groups, and pairwise change in these measures after 12 months (Table 1). Both groups experienced a modest (but not statistically significant) improvement in Six Minute Walk distance, which was greater in those in the supervised exercise group. The only significant improvement in metabolic function was increased HDL (P < 0.05) in the exercise cohort (Table 1).
Figure 1.
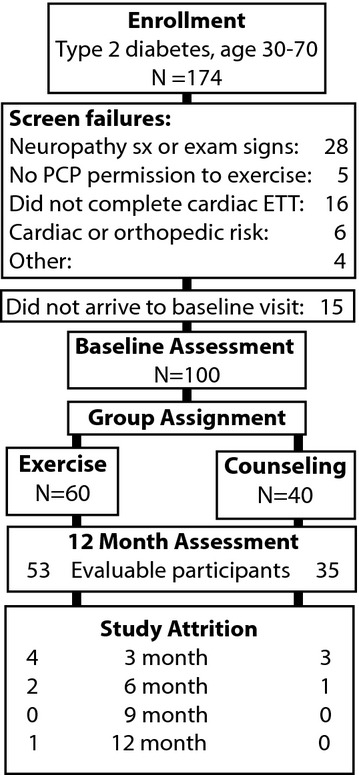
Study progress, with numbers of participants screened, evaluated at baseline, assigned to study groups, and completing 12 month assessment.
Table 1.
Selected comparisons between study groups
| Counseling N = 35 | Exercise N = 53 | Comparison P value | |
|---|---|---|---|
| Baseline characteristics of participants completing 12 months of study | |||
| Age (years) | 58.4 ± 6.7 | 56.4 ± 6.9 | 0.16 |
| Fraction male | 18/35 (0.51) | 31/53 (0.58) | 0.66* |
| Diabetes duration (months) | 81 ± 64 | 87 ± 54 | 0.62 |
| Metabolic, fitness and strength | |||
| Body mass index | 32.5 ± 5.7 | 33.9 ± 6.8 | 0.30 |
| Hemoglobin A1c | 6.8 ± 1.0 | 6.8 ± 1.2 | 0.81 |
| Fasting glucose | 114 ± 32 | 120 ± 34 | 0.48 |
| Fraction taking any glucose lowering agent | 30/35 (0.85) | 46/53 (0.87) | 0.99* |
| Fraction taking insulin | 6/35 (0.17) | 8/53 (0.15) | 0.76* |
| HDL (mg/dL) | 46 ± 12 | 43 ± 10 | 0.21 |
| Quadriceps peak force (N) | 386 ± 131 | 410 ± 135 | 0.45 |
| Six minute walk distance (m) | 497 ± 61 | 492 ± 61 | 0.65 |
| Nerve function | |||
| UENS score | 0.7 ± 1.0 | 0.8 ± 1.1 | 0.79 |
| Peroneal conduc velocity (m/sec) | 44.9 ± 4.0 | 44.7 ± 4.4 | 0.87 |
| Tibial F-response latency (msec) | 52.7 ± 6.9 | 53.1 ± 5.7 | 0.74 |
| IENFD ankle (fibers/mm) | 6.9 ± 4.4 | 6.4 ± 4.4 | 0.59 |
| IENFD prox thigh (fibers/mm) | 9.4 ± 4.6 | 10.4 ± 6.1 | 0.38 |
| Change in measures at 12 months | |||
| Metabolic, fitness and strength | |||
| Hemoglobin A1c | 0.11 ± 1.0 | 0.20 ± 1.3 | 0.81 |
| Body mass index | 0.0 ± 1.4 | −0.2 ± 2.0 | 0.76 |
| HDL (mg/dL) | −2.2 ± 5.5 | 0.2 ± 6.0 | 0.05 |
| Quadriceps peak force (N) | 4.3 ± 71.2 | 19.3 ± 53.8 | 0.36 |
| Six minute walk distance (m) | 16 ± 47 | 24 ± 44 | 0.45 |
| Nerve function | |||
| UENS score | 1.1 ± 1.8 | 0.4 ± 1.7 | 0.08 |
| Number (fraction) UENS >4 | 6/35 (0.17) | 3/53 (0.06) | 0.15* |
| Peroneal conduct velocity (m/sec) | 2.4 ± 8.3 | 0.8 ± 7.7 | 0.36 |
| Tibial F-response latency (msec) | 0.7 ± 4.0 | −0.2 ± 2.9 | 0.28 |
At baseline, data columns represent mean values for each measure ±1 standard deviation. Twelve-month data columns represent mean change from baseline by pairwise comparison, ±1 standard deviation. For both data sets p values are derived from between-group t-test comparisons assuming unequal variances, and significant differences are bolded. Sex ratio, and 12 months fraction of participants with UENS score >4 were compared by two-sided Fisher’s exact test (*). Fraction with UENS >4 was 0 for both groups at baseline by study entry criteria definition. IENFD, intraepidermal nerve fiber density; UENS, Utah Early Neuropathy Scale.
Exercisers increased IENFD at both ankle and proximal thigh, while controls showed a small decline in IENFD at both sites (Fig. 2). This difference between groups was statistically significant at the ankle, P < 0.03. IENFD at the proximal thigh was significantly greater at 12 months for exercisers than controls (12.1 ± 4.8 vs. 9.6 ± 4.7, P = 0.02). While other neuropathy measures were not significantly different between groups at 12 months there was a trend toward slower progression of UENS and other neuropathy measures in the exercise group. Six of 35 controls (17%) but only three of 53 exercisers (5.7%) progressed to an UENS score more than four over 1 year (P < 0.08 by Fishers one-tailed exact test). For the small number progressing to UENS more than four in either group there was a nonsignificant trend toward lower thigh IENFD and worsening of nerve conduction study values (Fig. 2).
Figure 2.
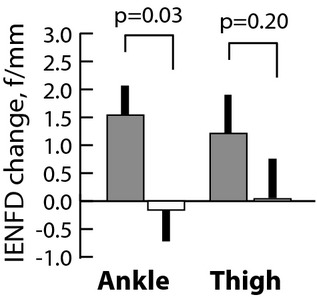
Supervised exercise over 12 months improves intraepidermal nerve fiber density (IENFD) in non-neuropathic patients with diabetes. Bars represent change in IENFD 0–12 months ± SEM at ankle or proximal thigh for participants assigned to supervised exercise (solid), or counseling (unfilled) groups. Participants receiving standard-of-care counseling showed stasis or slow decline in fiber density.
Discussion
In this prospective, single-blinded cohort study of lifestyle intervention for the prevention of diabetic neuropathy, a program of sustained, mentored exercise resulted in a significant increase in IENFD among diabetic patients without peripheral neuropathy, whereas counseling alone resulted in stability. While there were too few participants to assess impact of intervention on risk of incident neuropathy, those in the counseling cohort experienced a threefold higher rate of progression to abnormal neurological examination compared to the exercise group (17% vs. 5.7%). Together, these results support the concept that distal nerve fiber injury may occur in patients with diabetes prior to neuropathy symptom onset, and that cutaneous axons can regenerate in response to metabolic improvement.
Improvement in IENFD was associated with a trend toward improvement in metabolic syndrome features, and change in HDL was significant between groups. Previous, unblinded studies have found that exercise alone, or in combination with dietary counseling, results in improved cutaneous innervation and reduced neuropathy symptoms in patients with neuropathy associated with either prediabetes or early diabetes.9,10 The lack of significant metabolic improvement suggests that exercise may mediate enhanced peripheral nerve regenerative capacity via mechanisms other than weight loss and improved glycemic control. One study of 17 participants with diabetic neuropathy found significant improvement in neuropathy symptoms and epidermal nerve branching following mixed aerobic exercise and strength training, despite no change in BMI.9 While there was an improvement in HgbA1c from 7.8% to 7.3%, participants in the current study had better baseline glycemic control (mean A1c of 6.8%).
These results further validate IENFD as a potentially powerful surrogate measure for use in future clinical trials. Therapeutic development for diabetic neuropathy is significantly limited by a lack of biomarkers sensitive to early treatment effect. Several studies demonstrate that clinical scales and NCS are insensitive to change in early diabetic neuropathy.18 IENFD as an objective, direct measure of small diameter axon integrity that can be performed in a blinded fashion and is responsive to intervention.1,9,19 IENFD specifically evaluates distal unmyelinated axons and thus does not directly assess large myelinated fibers. However, IENFD is correlated to large fiber surrogates, including NCS, among patients with diabetic neuropathy.20 Longitudinal studies are required to determine if early IENFD decline presages future large fiber loss. Demonstration of significant improvement in IENFD in the exercise group, and cessation of progression in the counseling group, suggest IENFD may be particularly responsive to treatment effect. Overall, these findings support use of IENFD as a surrogate measure in future early treatment or prevention studies for diabetic neuropathy.
Acknowledgments
The study was performed through a grant from the National Institute for Diabetes and Digestive and Kidney Diseases, R01-DK064814. Research reported for this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award number 1ULTR001067. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author Contributions
J. Robinson Singleton and A. Gordon Smith were responsible for study concept and design, acquisition of data, analysis and interpretation, writing and critical revision of the manuscript. Robin Marcus assisted with the study design and supervised the exercise intervention. Justin Jackson and Margaret Lessard assisted acquisition of data, its analysis/interpretation and provided critical revision of the manuscript. Timothy Graham provided assistance with the metabolic endpoints of the intervention, evaluated data, and provided critical revision of the manuscript.
Conflict of Interest
None declared.
References
- Smith AG, Howard JR, Kroll R, et al. The reliability of skin biopsy with measurement of intraepidermal nerve fiber density. J Neurol Sci. 2005;228:65–69. doi: 10.1016/j.jns.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Lauria G, Bakkers M, Schmitz C, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst. 2010;15:202–207. doi: 10.1111/j.1529-8027.2010.00271.x. [DOI] [PubMed] [Google Scholar]
- Callaghan B, Feldman E. The metabolic syndrome and neuropathy: therapeutic challenges and opportunities. Ann Neurol. 2013;74:397–403. doi: 10.1002/ana.23986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AG, Singleton JR. Obesity and hyperlipidemia are risk factors for early diabetic neuropathy. J Diabetes Complications. 2013;27:436–442. doi: 10.1016/j.jdiacomp.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilford BL, Ryals JM, Wright DE. Phenotypic changes in diabetic neuropathy induced by a high-fat diet in diabetic C57BL/6 mice. Exp Diabetes Res. 2011;2011:848307. doi: 10.1155/2011/848307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenkamp-Strahm CM, Kappmeyer AJ, Schmalz JT, et al. High-fat diet ingestion correlates with neuropathy in the duodenum myenteric plexus of obese mice with symptoms of type 2 diabetes. Cell Tissue Res. 2013;354:381–394. doi: 10.1007/s00441-013-1681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupachyk S, Watcho P, Hasanova N, et al. Triglyceride, nonesterified fatty acids, and prediabetic neuropathy: role for oxidative-nitrosative stress. Free Radic Biol Med. 2012;52:1255–1263. doi: 10.1016/j.freeradbiomed.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AM, Hayes JM, McLean LL, et al. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes. 2009;58:2376–2385. doi: 10.2337/db09-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluding PM, Pasnoor M, Singh R, et al. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complications. 2012;26:424–429. doi: 10.1016/j.jdiacomp.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AG, Russell J, Feldman EL, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006;29:1294–1299. doi: 10.2337/dc06-0224. [DOI] [PubMed] [Google Scholar]
- Feldman EL, Stevens MJ, Thomas PK, et al. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281–1289. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- Singleton JR, Bixby B, Russell JW, et al. The Utah Early Neuropathy Scale: a sensitive clinical scale for early sensory predominant neuropathy. J Peripher Nerv Syst. 2008;13:218–227. doi: 10.1111/j.1529-8027.2008.00180.x. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine. Guidelines for exercise testing and prescription. Philadelphia: Lea and Febiger; 1991. [Google Scholar]
- Beriault K, Carpentier AC, Gagnon C, et al. Reproducibility of the 6-minute walk test in obese adults. Int J Sports Med. 2009;30:725–727. doi: 10.1055/s-0029-1231043. [DOI] [PubMed] [Google Scholar]
- Bassey EJ, Short AH. A new method for measuring power output in a single leg extension: feasibility, reliability and validity. Eur J Appl Physiol Occup Physiol. 1990;60:385–390. doi: 10.1007/BF00713504. [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- Dyck PJ, Norell JE, Tritschler H, et al. Challenges in design of multicenter trials: end points assessed longitudinally for change and monotonicity. Diabetes Care. 2007;30:2619–2625. doi: 10.2337/dc06-2479. [DOI] [PubMed] [Google Scholar]
- Boyd AL, Barlow PM, Pittenger GL, et al. Topiramate improves neurovascular function, epidermal nerve fiber morphology, and metabolism in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2010;3:431–437. doi: 10.2147/DMSOTT.S13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura A, Deguchi T, Sugimoto K, et al. Intraepidermal nerve fiber density and nerve conduction study parameters correlate with clinical staging of diabetic polyneuropathy. Diabetes Res Clin Pract. 2013;99:24–29. doi: 10.1016/j.diabres.2012.09.026. [DOI] [PubMed] [Google Scholar]


