Abstract
BACKGROUND
Previous studies have found no association between graft ischemic time (IT) and survival in pediatric heart transplant (HTx) recipients. However, previous studies were small or analyzed risk only at the extremes of IT, where observations are few. We sought to determine whether graft IT is independently associated with graft survival in a large cohort of children with no a priori assumptions about where the risk threshold may lie.
METHODS
All children aged <18 years in the U.S. undergoing primary HTx (1987 to 2008) were included. The primary end point was graft loss (death or retransplant) within 6 months. Multivariate analysis was performed to analyze the association between graft IT and graft loss within 6 months after transplant. A secondary end point of longer-term graft loss was assessed among recipients who survived the first 6 months after transplant.
RESULTS
Of 4,716 pediatric HTxs performed, the median IT was 3.5 hours (interquartile range, 2.7–4.3 hours). Adjusted analysis showed that children with an IT > 3.5 hours were at increased risk of graft loss within 6 months after transplant (hazard ratio, 1.3; 95% confidence interval, 1.1–1.5; p = 0.002). Among 6-month survivors, IT was not associated with longer-term graft loss.
CONCLUSIONS
IT beyond 3.5 hours is associated with a 30% increase in risk of graft loss within 6 months in pediatric HT recipients. Although the magnitude of risk associated with IT is small compared with the risk associated with recipient factors, these findings may be important during donor assessment for high-risk transplant candidates.
Keywords: ischemic time, pediatric heart transplant, graft survival, follow-up studies, risk factors
In both adult and pediatric heart transplantation (HTx), surgeons strive to minimize graft ischemic time (IT), despite conflicting reports of the importance of graft IT in adult HTx1–11 and several pediatric reports that have demonstrated no association between graft IT and HTx survival in children.12–14 A previous single-center pediatric study reported an IT of up to 8 hours was associated with excellent outcomes after transplant.13 Because wait-list mortality is higher among children awaiting HTx than in any other solid organ transplant population,15 these findings suggest that it may be safe to expand the pediatric donor pool by accepting hearts from farther geographic distances.
Previous pediatric reports have been limited to small single-center experiences or studies exploring the effect of prolonged IT (eg, ≥8 hours) where observations are relatively sparse and a negative study may be caused by a loss of statistical power. Because larger adult studies have tended to find an association between longer IT and reduced early survival, we sought to examine the association between IT and graft loss using a large, multicenter cohort of children undergoing HTx with no a priori assumption about where the time threshold may fall. Therefore, the specific objectives of this study were:
to test the hypothesis that longer donor IT is associated with early graft loss (6 months) in pediatric HTx recipients, after adjusting for patient factors, and
to test the hypothesis that graft IT is not associated with long-term graft loss conditional upon survival to 6 months.
A better understanding of this relationship will not only assist transplant physicians in the evaluation of donor offers for their candidates but may also provide insights into need for further improvement in protocols for current donor management and organ preservation.
Methods
Study population
All children aged <18 years who underwent their first HTx in the United States (U.S.) between October 15, 1987, and March 31, 2008, were identified using Organ Procurement and Transplantation Network (OPTN) data. The OPTN database includes data on all transplant recipients in the U.S. The Health Resources and Services Administration, U.S. Department of Health and Human Services, provides oversight to the activities of the OPTN contractor, the United Network of Organ Sharing (UNOS). The study excluded children who received a re-HTx or multivisceral transplantation and those with missing data for graft ischemic time.
Study design and definitions
The primary study hypothesis was that increasing graft IT would be associated with increased risk of graft loss within the first 6 months after transplant in pediatric HTx recipients. The primary study end point was time to early graft loss, defined as time to death or re-HTx during the first 6 months. The secondary study end point was time to graft loss conditional upon surviving the first 6 months (longer-term, conditional survival). Transplant recipients were divided into quartiles of graft IT. All demographic and clinical variables were defined at the time of transplant, unless stated otherwise.
The level of hemodynamic support at the time of transplant was defined as a categoric variable. A child was defined to be on extracorporeal membrane oxygenation (ECMO) if supported on ECMO, on a ventilator if supported by a ventilator but not on ECMO, and on inotropes if supported by inotropes but not a ventilator or ECMO. Renal function at the time of transplant was estimated using serum creatinine levels and the Schwartz formula.16 No patients had any missing data for the variables of age, sex, race/ethnicity, diagnosis, hemodynamic support, dialysis, or dates of transplant, death, or repeat transplant. For children with missing data on any other variable, we created the indicator variable “variable not reported” to allow these patients to contribute their other risk factors in adjusted models. All children were monitored from the time of transplant until death, re-HTx, or the day of last observation on February 25, 2009.
Statistical analysis
Summary statistics are presented as median (interquartile range [IQR], 25th, 75th percentile) or number (%). The association of graft IT with survival after transplant was first assessed with graft IT as a continuous variable using a restricted cubic spline to allow for the most flexible relationship with outcome. Because this analysis demonstrated worse outcomes in children with graft IT above the median, we divided them into 4 groups by quartiles of IT for describing patient factors and association of IT with outcomes. Baseline characteristics were compared among groups using the Kruskal-Wallis test for continuous variables and the chisquare test for categoric variables. Unadjusted survival curves were computed using the Kaplan-Meier method. A multivariable Cox model without IT data for early (6 months) survival after transplant was developed using forward selection; all variables in Table 1 were considered, and continuous variables were fitted with a restricted cubic spline. Variables significant at the 0.10 level, based on a likelihood ratio test, were retained, and IT quartiles were added to the model. The interaction of IT with recipient or donor age or recipient diagnosis was also assessed. A similar method was used for analyzing long-term survival, limiting analysis to recipients who survived the first 6 months after HTx. The groups were compared for the distribution of causes of death using Fisher’s exact test. Analyses were performed using SAS 9.1 software (SAS Institute Inc, Cary, NC) and STATA 10.0 software (StataCorp LP, College Station, TX).
Table 1.
Baseline Patient Characteristics Among Patient Groups According to Quartiles of Ischemic Timea
| Variablesb | Total | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
|---|---|---|---|---|---|---|
| (n = 4,716) | (n = 1,204) | (n = 1,169) | (n = 1,164) | (n = 1,179) | p-valuec | |
| Recipient age, years | 3 (0–12) | 7 (0–14) | 5 (0–13) | 3 (0–11) | 1 (0–9) | <0.001 |
| Donor age, years | 4 (0–14) | 8 (1–17) | 5 (1–16) | 4 (0–12) | 3 (0–9) | <0.001 |
| Female sex | 2034 (43) | 497 (41) | 505 (43) | 511 (44) | 521 (44) | 0.47 |
| Race | <0.001 | |||||
| White | 3055 (65) | 747 (62) | 772 (66) | 761 (65) | 775 (66) | |
| Black | 832 (18) | 257 (21) | 216 (18) | 207 (18) | 152 (13) | |
| Hispanic | 596 (13) | 138 (11) | 126 (11) | 145 (12) | 187 (16) | |
| Other | 233 (5) | 62 (5) | 55 (5) | 51 (4) | 65 (6) | |
| Era of transplant | <0.001 | |||||
| 1987–1992 | 970 (21) | 306 (25) | 231 (20) | 214 (18) | 219 (19) | |
| 1993–1996 | 983 (21) | 274 (23) | 255 (22) | 209 (18) | 245 (21) | |
| 1997–2000 | 858 (18) | 203 (17) | 188 (16) | 237 (20) | 230 (20) | |
| 2001–2004 | 982 (21) | 213 (18) | 255 (22) | 267 (23) | 247 (21) | |
| 2005–2008 | 923 (20) | 208 (17) | 240 (21) | 237 (20) | 238 (20) | |
| Medicaid insurance | 1357 (29) | 324 (27) | 344 (29) | 358 (31) | 331 (28) | 0.19 |
| Diagnosis | <0.001 | |||||
| CHD | 2297 (49) | 424 (35) | 482 (41) | 597 (51) | 794 (67) | |
| Cardiomyopathy | 1907 (40) | 603 (50) | 569 (49) | 444 (38) | 291 (25) | |
| Other | 512 (11) | 177 (15) | 118 (10) | 123 (11) | 94 (8) | |
| Support at HTx | <0.001 | |||||
| ECMO | 239 (5) | 51 (4) | 61 (5) | 68 (6) | 59 (5) | |
| Ventilator | 764 (16) | 199 (17) | 162 (14) | 199 (17) | 204 (17) | |
| Inotropes | 1265 (27) | 333 (28) | 362 (31) | 320 (27) | 250 (21) | |
| None of above | 2448 (52) | 621 (52) | 584 (50) | 577 (50) | 666 (56) | |
| VAD | 297 (6) | 90 (7) | 78 (7) | 75 (6) | 54 (5) | 0.03 |
| Prostaglandin | 346 (7) | 61 (5) | 83 (7) | 88 (8) | 114 (10) | <0.001 |
| Prior sternotomy | 469 (41) | 96 (38) | 103 (34) | 125 (41) | 145 (52) | <0.001 |
| PRA >10% | 379 (13) | 119 (17) | 79 (11) | 89 (13) | 92 (13) | 0.01 |
| Dialysis after listing | 67 (1) | 18 (2) | 17 (1) | 18 (2) | 14 (1) | 0.89 |
| Creatinine clearance | ||||||
| <40 ml/min/1.732 | 319 (9) | 69 (8) | 74 (9) | 72 (8) | 104 (12) | |
| ≥40 ml/min/1.732 | 3103 (91) | 747 (92) | 789 (91) | 800 (92) | 767 (88) | |
| Bilirubin at HTx, mg/dl | 0.8 (0.5–1.5) | 0.8 (0.5–1.4) | 0.7 (0.4–1.4) | 0.8 (0.5–1.6) | 0.9 (0.5–1.8) | <0.001 |
CHD, congenital heart disease; ECMO, Extracorporeal membrane oxygenation, HTx, heart transplant; PRA, panel reactive antibodies, VAD, ventricular assist device.
Graft ischemic time (in hours) in quartile 1 <2.73; quartile 2, 2.73–3.51; quartile 3, 3.52– 4.30; quartile 4, >4.30.
Values are summarized as number (%) or median (interquartile range).
The p-values reflect a comparison of the 4 quartiles.
Results
Study population
During the study duration, 5,297 children aged <18 years underwent HTx in the U.S. Of these, 267 were excluded for missing IT, 288 were excluded for re-HTx, and 26 were excluded for multiorgan transplantation. The data for the remaining 4,716 children were analyzed. The baseline demographic and clinic characteristics of the study population by IT quartiles are summarized in Table 1. The median IT was 3.5 hours (IQR, 2.7–4.3 hours). The median recipient age was 3 years, and median donor age was 4 years.
Children with longer ITs were more likely to be younger, supported with prostaglandin E, and to have a cardiac diagnosis of congenital heart disease and a prior sternotomy (p <0.001 for distribution of all, Table 1). Recipients with longer ITs were also more likely to have renal dysfunction (p = 0.02) and higher bilirubin levels (p <0.001). There were no differences among the groups with respect to the type of medical insurance.
IT and early (6-month) post-HTx survival
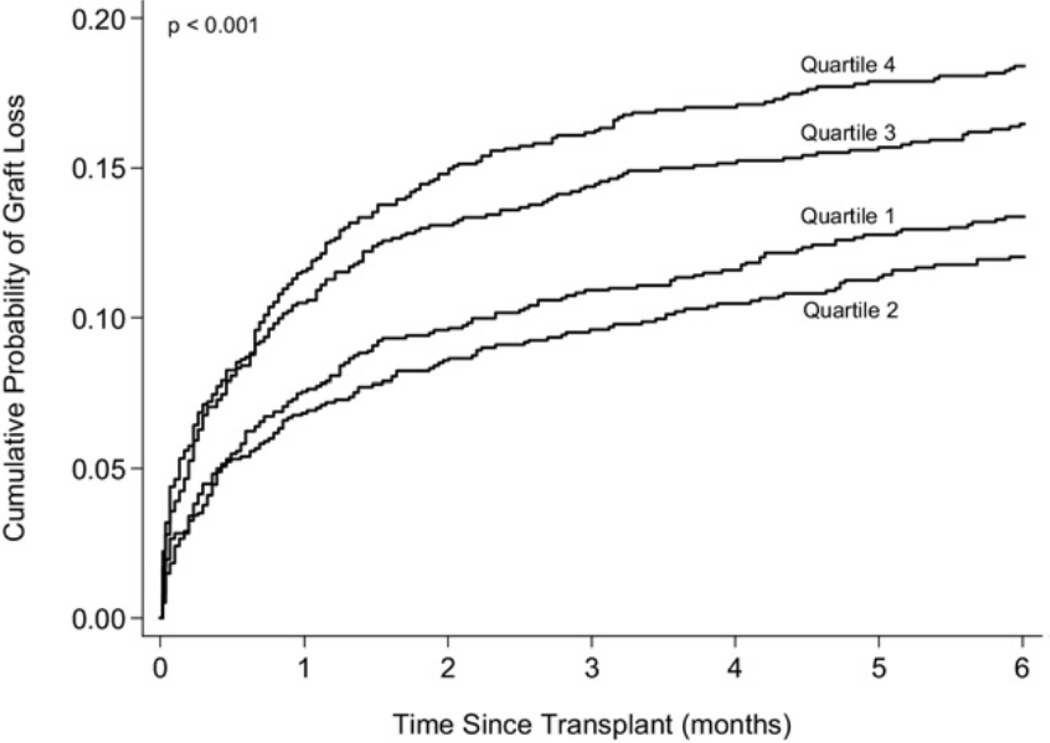
Of 4,716 children in the study, 707 children (15%) reached the primary end point of graft loss within the first 6 months (664 deaths, 43 re-HTx). Graft loss occurred in 13%, 12%, 16%, and 18% of HTx recipients in the 4 groups, respectively. Figure 1 illustrates the observed cumulative graft loss within 6 months after transplant among the 4 groups of children.
Figure 1.
Observed graft loss (death or retransplantation) during the first 6 months after transplant in the 4 groups of heart transplant recipients by quartiles of ischemic time (in hours): quartile 1, < 2.73; quartile 2, 2.73–3.51; quartile 3, 3.52–4.30; quartile 4, >4.30.
Unadjusted analysis (first quartile being the reference group) showed time to graft loss in the second quartile children (graft IT, 2.73–3.51 hours) was similar to those in the first quartile (hazard ratio [HR], 0.9; 95% confidence interval [CI], 0.7–1.2; p = 0.34). However, children in the third quartile (graft IT, 3.52–4.30 hours) were at higher risk of early graft loss (HR, 1.3; 95% CI 1.0 –1.6; p = 0.03), as were those in the fourth quartile (HR, 1.4; 95% CI 1.2–1.8, p = 0.001). Multivariable analysis (Table 2) showed children in the third quartile (HR, 1.3; 95% CI 1.1–1.6; p = 0.009) and fourth quartile (HR, 1.3; 95% CI 1.1–1.5; p = 0.01) were at a higher risk of death or retransplant within 6 months after HTx than children with an IT that was lower the median. Other factors associated with increased risk of early graft loss in adjusted analysis were female sex, donor age <1 year, congenital heart disease vs cardiomyopathy, prior sternotomy, ECMO support, ventilator support, renal dysfunction, and earlier era of HTx (Table 2). There was no interaction of IT with recipient or donor age, or with cardiac diagnosis.
Table 2.
Multivariable Predictors of Time to Graft Loss During the First 6 Months After Transplant
| Predictor | HR (95% CI) | p-value |
|---|---|---|
| Ischemic timea | ||
| Quartile 3 | 1.3 (1.1–1.6) | 0.009 |
| Quartile 4 | 1.3 (1.1–1.5) | 0.01 |
| Age at HTx (recipient) | N/Ab | 0.02 |
| Female sex | 1.2 (1.0–1.4) | 0.009 |
| Donor age < 1 year | 1.4 (1.1–1.8) | 0.005 |
| Diagnosis vs cardiomyopathy | ||
| Congenital | 1.7 (1.4–2.1) | <0.001 |
| Other | 1.4 (1.0–1.8) | 0.02 |
| Type of support vs none | ||
| ECMO | 3.3 (2.5–4.3) | <0.001 |
| Ventilator | 1.8 (1.5–2.2) | <0.001 |
| Inotropes | 1.1 (0.9–1.4) | 0.45 |
| Dialysis after listing | 2.1 (1.4–3.1) | 0.001 |
| Creatinine clearance vs ≥ 40c | ||
| <40 | 1.9 (1.5–2.5) | <0.001 |
| Not reported | 1.5 (1.1–2.0) | 0.01 |
| Bilirubin at HTx (recipient) | N/Ab | 0.08 |
| Prior sternotomy | ||
| Yes | 1.8 (1.2–2.7) | 0.004 |
| Not reported | 1.7 (1.2–2.6) | 0.006 |
| Era vs 1987–1992 | ||
| 1993–1996 | 1 (0.8–1.3) | 0.62 |
| 1997–2000 | 0.8 (0.6–1.1) | 0.13 |
| 2001–2004 | 0.6 (0.4–0.9) | 0.01 |
| 2005–2008 | 0.5 (0.4–0.7) | <0.001 |
CI, confidence interval; ECMO, extracorporeal membrane oxygenation; HR, hazard ratio; HTx, heart transplantation.
Reference group = ischemic time less than the median; quartile 3, 3.52–4.30 hours; quartile 4, >4.30 hours.
No hazard ratios are associated with these continuous variables because restricted cubic splines were used to model them.
Calculated as ml/min/1.732.
Long-term conditional survival
In children who survived the first 6 months, IT was not associated with long-term survival. Adjusted analysis showed children in the third quartile (HR, 0.9; 95% CI, 0.8 –1.0; p = 0.12) and the fourth quartile (HR, 1.0; 95% CI, 0.8 –1.2; p = 0.91) had long-term survival similar to those with an IT below the median (Table 3). Patient factors associated with a higher risk of long-term graft loss included older age, female sex, human leukocyte antigen sensitization (panel reactive antibodies >10%), and black race. Prostaglandin support at transplant was associated with a decreased risk of long-term graft loss.
Table 3.
Multivariable Predictors of Time to Graft Loss in 6-Month Survivors
| Predictor | HR (95% CI) | p-value |
|---|---|---|
| Ischemic timea | ||
| Quartile 3 | 0.9 (0.8–1.0) | 0.18 |
| Quartile 4 | 1 (0.9–1.2) | 0.82 |
| Age at Htx >5 years | 1.2 (1.1–1.2) | <0.001 |
| Female sex | 1.3 (1.1–1.4) | <0.001 |
| PGE support at HTx | 0.7 (0.5–0.9) | 0.006 |
| PRA vs ≤10% | ||
| >10% | 1.3 (1.1–1.5) | 0.007 |
| Not reported | 0.9 (0.8–1.0) | 0.16 |
| Racial group | ||
| Black | 2.1 (1.9–2.4) | <0.001 |
| Hispanic | 1.1 (0.9–1.4) | 0.18 |
| Other | 1.5 (1.1–1.9) | 0.009 |
CI, confidence interval; HR, hazard ratio; HTx, heart transplantation; PGE, prostaglandin; PRA, panel reactive antibodies.
Reference group = ischemic time less than the median; quartile 3, 3.52–4.30 hours; quartile 4, >4.30 hours.
Cause of death
Of the 707 graft loss events (664 deaths and 43 retransplants) within the first 6 months, patients with graft IT >3.5 hours had 407 events (17.4%) vs 300 (12.6%) events (p <0.001). Causes of death were recorded in 661, including cardiac rejection in 125, cardiac death not related to rejection in 307, non-cardiac death in 193, and other causes in 36. Despite 386 deaths (16.5%) in patients with graft IT >3.5 hours vs 275 (11.6%; p <0.001), the distribution of causes of death was similar across both groups. The association of IT quartiles to graft loss from causes other than rejection was similar to that described with overall graft loss.
Discussion
In this study of a large cohort of children undergoing HTx in the U.S., we found that IT > 3.5 hours was associated with a 30% increase in the risk of graft loss by 6 months after transplant, after adjusting for patient factors and era of transplant. There was no association between IT and longterm survival in patients who survived the first 6 months. Although multivariable analysis showed the magnitude of the increased risk was not as high as the risk associated with congenital heart disease (HR, 1.7), patient support (HR, 3.3 for ECMO; 2.1 for ventilator), renal dysfunction (HR, 1.9) or prior sternotomy (HR, 1.8), it was statistically significant. A 30% increase in the risk of graft loss in a low-risk candidate represents a minimal additional risk, but it may be clinically important in an otherwise high-risk transplant candidate.
Although several multicenter analyses in adult HTx recipients have reported an association between longer IT and worse outcomes after transplant,5, 6, 9, 10 others, particularly those reporting single-center experiences, have not found such an association.1–3, 7, 11, 17, 18 A recent OPTN data analysis suggested that the association of IT with outcomes may be limited to children or adults recipients who received a heart from a donor aged >19 years.4 However, pediatric analyses were not presented. Relatively few studies, all single-center, have focused on this association in children.
In one study from Loma Linda University with predominantly infant recipients and overall excellent results, 14 children with donor ITs >8 hours had outcomes similar to 14 recipients with ITs <90 minutes. Morgan et al14 reported that among children who underwent HTx at Columbia University, those with ITs >4 hours had outcomes similar to recipients with ITs <4 hours. These results speak to the best possible outcomes that may be achieved by a single center and are valuable to other centers looking to adopt the clinical practices of highly successful centers. Yet, the small sample populations examined and low frequency of graft loss events increases the chances that a negative finding may be attributable to type II statistical error and that the results may not apply to other centers—problems that could be overcome by testing the hypothesis in a sufficiently large multicenter cohort.
Our study included >4,000 children during 2 decades who received allografts at centers throughout the U.S., thus providing sufficient power to adequately test the hypothesis while adjusting simultaneously for a variety of patient factors. The potential mechanisms for this association include a higher risk of graft dysfunction in patients with longer Its due to cardiac myocyte damage19 and an increased risk of multiorgan dysfunction if also associated with prolonged cardiopulmonary bypass time, although cardiopulmonary bypass time is not specifically reported in the UNOS data set and is reflected only as a component of total IT.
These findings suggest that the distance between the donor and the recipient and, thus, the duration of organ transport between the two hospitals, is important in evaluating donor offers for high-risk pediatric candidates. Furthermore, because IT is also affected by the complexity of transplant surgery, the importance of minimizing IT by closely coordinating the recipient’s operation with organ arrival from the donor hospital cannot be overstated. There is increasing recognition that the presence of multiple risk factors in transplant recipients confers at least an additive risk for early death after transplant.20, 21 Because the association described in our study was present after adjusting for recipient risk factors, long IT likely represents additional risk for the transplant candidate. Our recent analysis suggests that the risk of post-transplant in-hospital mortality in pediatric HT recipients varies between 1% and 60% based on recipient factors alone.21
Although the magnitude of the additional risk from prolonged IT is small and not of clinical importance in an otherwise low-risk candidate, it may increase the risk of early post-transplant graft loss significantly in a high-risk transplant recipient. Because graft IT is affected by the transport distance as well as the complexity of the transplant surgery, these factors may be important during donor assessment for high-risk candidates, as are the urgency of transplant, position on the wait-list, and the likelihood of future offers for the candidate being considered.
These findings also suggest that the emerging extracorporeal perfusion devices for organ transport may stand to benefit the pediatric patients as well as adult patients. Devices such as the Organ Care System (Transmedics, Andover, MA), designed to support the procured heart in a warm, functioning state during transport, have demonstrated safety and efficacy in adults in Europe and are currently under investigation in the U.S. In theory, such devices may prove particularly useful in high-risk pediatric recipients where the technical complexity of the transplant operation frequently results in extended ischemic times independent of donor distance.
Although not an a priori hypothesis of the study, we anticipated that the association of a longer IT with all-cause early graft loss might be mediated by a higher likelihood of primary graft failure amongst patients with a longer IT. However, we did not find a specific cause of death to be more common in recipients with longer IT. This may be due to a long list of options available to centers for coding the cause of death after transplant. Furthermore, assigning a primary cause of death in a patient with poor graft function supported in the intensive care unit, who develops secondary or sequential complications such as infection or multiorgan failure, may be a challenging task with heterogeneous responses.
This study has limitations: First, this was a retrospective analysis of national registry data. However, selection bias is unlikely because OPTN captures all transplants in the U.S., and data collection is performed prospectively for real-time patient listing, organ allocation, and transplant, and certain safeguards to data quality are to be expected.
Second, we were unable to identify the mechanism for increased early death with higher IT, despite analyzing the frequency distribution of causes of death. This may reflect the difficulty in assigning the primary cause of death and differentiating it from secondary complications in transplant recipients.
Third, recipients in the higher IT quartiles were younger and more likely to have congenital heart disease and prior sternotomy, factors also associated with a worse early post-transplant outcome. Transplant teams may also accept donor hearts from long distances for their sickest patients. The large sample size provided the necessary statistical power to adjust for multiple factors simultaneously. However, the possibility that the observed association may be partly due to residual confounding cannot be excluded.
Lastly, although the risk of graft loss appeared flat after 3.5 hours, we cannot exclude some rise in risk in Its >3.5 hours given the relative small number of observations at the extreme IT in the study cohort.
In conclusion, graft IT < 3.5 hours is associated with a 30% increase in risk of graft loss within 6 months in pediatric HTx recipients, after adjusting for patient factors, but not with long-term graft survival. These findings may be important in donor assessment and in the development of solid organ preservation strategies for high-risk transplant candidates.
Acknowledgments
This work was supported by the National Institutes of Health under award number: T32HL007572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The work was partly supported by Health Resources and Services Administration contract 234-2005-370011C. The data were supplied by the UNOS as the contractor for the OPTN. The interpretation and reporting of these data are the responsibility of the authors and not an official policy of or interpretation by the OPTN or the U.S. Government.
This study was partly supported by the Transplant Research and Education Fund, Department of Cardiology, Children’s Hospital Boston, Boston, Massachusetts.
Footnotes
Disclosure Statement
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript or other conflicts of interest to disclose.
References
- 1.Pflugfelder PW, Thomson D, Singh NR, Menkis AH, McKenzie FN, Kostuk WJ. Cardiac allograft ischemic time. Relation to graft survival and cardiac function. Circulation. 1989;80:III116–III121. [PubMed] [Google Scholar]
- 2.Mullen JC, Bentley MJ, Modry DL, Koshal A. Extended donor ischemic times and recipient outcome after orthotopic cardiac transplantation. Can J Cardiol. 2001;17:421–426. [PubMed] [Google Scholar]
- 3.Pflugfelder PW, Singh NR, McKenzie FN, Menkis AH, Novick RJ, Kostuk WJ. Extending cardiac allograft ischemic time and donor age: effect on survival and long-term cardiac function. J Heart Lung Transplant. 1991;10:394–400. [PubMed] [Google Scholar]
- 4.Russo MJ, Chen JM, Sorabella RA, et al. The effect of ischemic time on survival after heart transplantation varies by donor age: an analysis of the United Network for Organ Sharing database. J Thoracic Cardiovasc Surg. 2007;133:554–559. doi: 10.1016/j.jtcvs.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Young JB, Naftel DC, Bourge RC, et al. Matching the heart donor and heart transplant recipient. Clues for successful expansion of the donor pool: a multivariable, multiinstitutional report. The Cardiac Transplant Research Database Group. J Heart Lung Transplant. 1994;13:353–364. [PubMed] [Google Scholar]
- 6.Banner NR, Thomas HL, Curnow E, Hussey JC, Rogers CA, Bonser RS. The importance of cold and warm cardiac ischemia for survival after heart transplantation. Transplantation. 2008;86:542–547. doi: 10.1097/TP.0b013e31818149b9. [DOI] [PubMed] [Google Scholar]
- 7.Briganti EM, Bergin PJ, Rosenfeldt FL, Esmore DS, Rabinov M. Successful long-term outcome with prolonged ischemic time cardiac allografts. J Heart Lung Transplant. 1995;14:840–845. [PubMed] [Google Scholar]
- 8.Del Rizzo DF, Menkis AH, Pflugfelder PW, et al. The role of donor age and ischemic time on survival following orthotopic heart transplantation. J Heart Lung Transplant. 1999;18:310–319. doi: 10.1016/s1053-2498(98)00059-x. [DOI] [PubMed] [Google Scholar]
- 9.Ganesh JS, Rogers CA, Banner NR, Bonser RS. Donor cause of death and medium-term survival after heart transplantation: a United Kingdom national study. J Thoracic Cardiovasc Surg. 2005;129:1153–1159. doi: 10.1016/j.jtcvs.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 10.Kirklin JK, Naftel DC, Bourge RC, et al. Evolving trends in risk profiles and causes of death after heart transplantation: a ten-year multi-institutional study. J Thoracic Cardiovasc Surg. 2003;125:881–890. doi: 10.1067/mtc.2003.168. [DOI] [PubMed] [Google Scholar]
- 11.Morgan JA, John R, Weinberg AD, et al. Prolonged donor ischemic time does not adversely affect long-term survival in adult patients undergoing cardiac transplantation. J Thoracic Cardiovasc Surg. 2003;126:1624–1633. doi: 10.1016/s0022-5223(03)01026-2. [DOI] [PubMed] [Google Scholar]
- 12.Bailey LL, Razzouk AJ, Hasaniya NW, Chinnock RE. Pediatric transplantation using hearts refused on the basis of donor quality. Ann Thorac Surg. 2009;87:1902–1908. doi: 10.1016/j.athoracsur.2009.03.090. [DOI] [PubMed] [Google Scholar]
- 13.Scheule AM, Zimmerman GJ, Johnston JK, Razzouk AJ, Gundry SR, Bailey LL. Duration of graft cold ischemia does not affect outcomes in pediatric heart transplant recipients. Circulation. 2002;106:I163–I167. [PubMed] [Google Scholar]
- 14.Morgan JA, John R, Park Y, et al. Successful outcome with extended allograft ischemic time in pediatric heart transplantation. J Heart Lung Transplant. 2005;24:58–62. doi: 10.1016/j.healun.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Almond CS, Thiagarajan RR, Piercey GE, et al. Waiting list mortality among children listed for heart transplantation in the United States. Circulation. 2009;119:717–727. doi: 10.1161/CIRCULATIONAHA.108.815712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 17.Mitropoulos FA, Odim J, Marelli D, et al. Outcome of hearts with cold ischemic time greater than 300 minutes. A case-matched study. Eur J Cardiothorac Surg. 2005;28:143–148. doi: 10.1016/j.ejcts.2005.01.067. [DOI] [PubMed] [Google Scholar]
- 18.Marasco SF, Esmore DS, Richardson M, et al. Prolonged cardiac allograft ischemic time—no impact on long-term survival but at what cost? Clinical Transpl. 2007;21:321–329. doi: 10.1111/j.1399-0012.2007.00644.x. [DOI] [PubMed] [Google Scholar]
- 19.Kawauchi M, Gundry SR, Beierle F, Alonso de Begona J, Bailey LL. Myosin light chain efflux after heart transplantation in infants and children and its correlation with ischemic preservation time. J Thoracic Cardiovasc Surg. 1993;106:458–462. [PubMed] [Google Scholar]
- 20.Davies RR, Russo MJ, Mital S, et al. Predicting survival among high-risk pediatric cardiac transplant recipients: an analysis of the United Network for Organ Sharing database. J Thoracic Cardiovasc Surg. 2008;135:147–155. doi: 10.1016/j.jtcvs.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 21.Almond C, Gauvreau K, Canter C, Piercey G, Singh T. Validation of a risk prediction model for in-hospital mortality following pediatric heart transplantation. J Heart Lung Transplant. 2010;29:S36. [Google Scholar]