Abstract
Due to increasing interest in the removal of immunosuppressive pathways in cancer, the combination of IL2 with antibodies to neutralize TGFβ, a potent immunosuppressive cytokine, was assessed. Combination immunotherapy resulted in significantly greater anti-tumor effects. These were correlated with significant increases in the numbers and functionality of NK cells, NK progenitors and activated CD8 T cells resulting in the observed anti-tumor effects. Combination immunotherapy was also accompanied with lesser toxicities than IL2 therapy alone. Additionally, we observed a dual competition between NK and activated CD8 T cells such that after immunotherapy, the depletion of either effector population resulted in the increased total expansion of the other population and compensatory anti-tumor effects. This study demonstrates the efficacy of this combination immunotherapeutic regimen as a promising cancer therapy and illustrates the existence of potent competitive regulatory pathways between NK and CD8 T cells in response to systemic activation.
INTRODUCTION
NK-based immunotherapy is a promising treatment against multiple cancers due to the ability of NK cells to eliminate tumor cells without prior immunization(1). IL2 is used widely to activate NK cells both in vivo and in vitro and it is currently approved for treatment in metastatic melanoma and renal cell carcinoma (1, 2). However, as a cancer therapeutic, benefits in survival have been hampered (1, 3) in part because of limitations in systemic IL2 administration and associated toxicities(4, 5) as well as potential expansion of regulatory T cells (Tregs) by engaging the high-affinity IL2-receptor (CD25)(6).
Secretion of immunosuppressive cytokines such as TGFβ by Tregs and/or tumor cells results in NK cell suppression. TGFβ inhibits IFNγ production, impairs degranulation, and decreases expression of activating receptors such as NKG2D and/or NKp30 on NK cells resulting in diminished tumor lysis(7, 8) and allogeneic bone marrow (BM) rejection(9). Furthermore NK cell homeostasis(8) and ontogeny(10) is negatively controlled by TGFβ. Therapeutically, neutralization of TGFβ using monoclonal antibodies (mAb), TGFβ-receptor kinase inhibitors, or antisense TGFβ-oligonucleotides have led to promising results in several cancers by preventing tumor-sensitized Treg expansion, augmenting anti-tumor responses in a NK and/or CD8 T cell-manner, and suppressing tumor progression and metastasis (6, 11–18). TGFβ blockade also restored NKG2D expression and IFNγ secretion by NK cells(7).
Despite these promising results, immunotherapeutic strategies that favor NK cells by promoting immune activation and preventing immune suppression could lead to greater anti-tumor efficacy. We have previously shown that the combination of anti-CD25 and IL2 improved NK cell anti-tumor responses due to elimination of Tregs(19). Additionally, the development of nanolipogels that allows sustained delivery of IL2 combined with TGFβ-receptor inhibitor resulted in delayed tumor growth due to increased presence of NK cells and effector CD8 T cells at the tumor site(20). Here, we investigated the efficacy of using anti-TGFβ (clone 1D11), which neutralizes the three isoforms of TGFβ; combined with low dose (LD) IL2 in NK and T cell expansion and function. We report here that combination immunotherapy allows for greater expansion and activation of NK and CD8 T cells, increased anti-tumor effects and diminished toxicities. Furthermore, mechanistic assessment revealed a dual regulatory role between NK and T cells limiting each other’s expansion and effects which can account for the immunotherapeutic success of NK cell and CD8 T cell-based cancer therapies
MATERIAL AND METHODS
Mice
The UC-Davis IACUC approved all studies and protocols. Female C57BL/6 mice were purchased from the Animal Production Area, NCI (Frederick, MD). Perforin-deficient (C57BL/6-Prf1tm1sdz), B6Smn.C3-Faslgld (FasL−/−) and wild type (WT) counterparts were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were used at 8–12 weeks of age and housed under specific pathogen-free conditions.
Immunotherapy Treatment
Mice were treated intraperitoneally with 240ug of anti-TGFβ clone 1D11 (NCCC) every other day and/or 0.2–1 million IU of recombinant-human IL2 (NCI repository, Frederick, MD) daily for 7-days. Rat-IgG (rIgG, Jackson Immunoresearch) and/or PBS were used as controls. Some mice received 200ug of anti-NK1.1 (clone PK136) (NCCC) or anti-CD8 (cloneYTS169.4) intraperitoneally (Taconic) two-days prior to anti-TGFβ and IL2 administration. Organs were collected one-day (24h) or three-days (72h) after end of seven-day treatment with IL2.
Flow Cytometry
Antibody staining of single-cell suspensions was performed as previously described(21). Foxp3 intracellular kit (eBioscience) was used following manufacturer’s instructions(19). For intracellular staining, PE anti-Granzyme B or isotype control (Invitrogen, Grand Island, NY) was used. Stained cells were analyzed with an LSR-Fortessa cytometer (Becton Dickinson, San Jose, CA) and FlowJo software (TreeStar) was used for data analysis.
Cytotoxic Assays
NK cell cytotoxic function was determined by a standard 4-hour 51Cr-release assay against the NK-sensitive tumor cell line YAC-1 (ATCC: Manassas, VA)(22) using treated splenocytes or purified NK cells (NK negative selection kit (StemCell technology, Vancouver, Canada)) as effector cells. CD8 T cell cytotoxic function was determined by a redirected assay as previously described(23).
In vitro assessment of NK expansion
2 millions of splenocytes from C57BL/6 mice were cultured with 1000 IU/mL of rhIL-2 and 20–80ug/ml of anti-TGFβ in 6-well plates by triplicate at 37C and 5% CO2. Rat-IgG was used as control (80ug/mL). At day 7, cells were collected and viability was determined by trypan blue staining. Flow cytometry was used to determine the percentage of NK cells (CD45+CD3−NK1.1+). 2 millions of in vitro T-cell depleted splenocytes using anti-Thy1.2 and rabbit-complement as previously described(24) were also cultured in the same conditions. At day 7, adherent lymphokine-activated killer cells (ALAKs) were collected and viability was measured by trypan blue.
Toxicity assessment
IL6 cytokine-bead-array (CBA) and liver enzyme alanine transaminase (ALT, IDToxTM ALT Enzyme Assay Kit, ID Labs Inc, Ontario, Canada) levels were quantified in serum samples collected at 24h post-treatment according manufacture instructions and as previously described(25). Absorbance of each well was determined at 340 nm on a plate reader (VERSAma turntable plate reader). Each sample was run in triplicate Histology analysis was also performed from liver, lung, and gut collected 24h after end of treatment and pathology assessed. Lungs, livers and guts were flushed and fixed in 10% neutral-buffered formalin. Samples were then embedded in paraffin, cut into 5-μm-thick sections, and stained with hematoxylin and eosin (H&E) at the Histology Consultation Services in Everson, WA. All slides were coded and read in a blinded fashion. The scoring was determined by a board certified Pathologist using the previously described criteria(25)
Survival Studies
2×105 cells of 3LL Lewis lung carcinoma cell line (ATCC) were intravenously injected into mice 4 days prior to initiation of CT. Some mice also received 200ug anti-CD8 and/or anti-NK1.1 or rIgG on the same day of tumor injection and once a week until the end of the experiment. Mice were monitored for survival and euthanized when moribund.
Statistical Analysis
Each experiment was performed at least two times with 3–8 mice per group with the exception of FasL−/− experiment that was done one time. Student’s two-tailed t-test, one-way ANOVA (Tukey post-test analysis), two-way ANOVA (Bonferroni post-test analysis) or Log-rank test was used when appropriate for statistic significance (Graphpad Prism 4, La Jolla, CA). P-values were considered statistically significant when p<0.05.
RESULTS
Anti-TGFβ and IL2 treatment increases NK cell numbers and function and promotes NK cell maturation
We first investigated the effects of administration of LD IL2 and anti-TGFβ (1D11 clone) combination therapy (CT) on NK cell responses in vivo looking 24h or 72h after cessation of therapy. As expected, both NK cell numbers and percentages were significantly increased after CT compared to IL2 alone (Fig. 1A, data not shown), whereas anti-TGFβ treatment alone did not have any effect.).
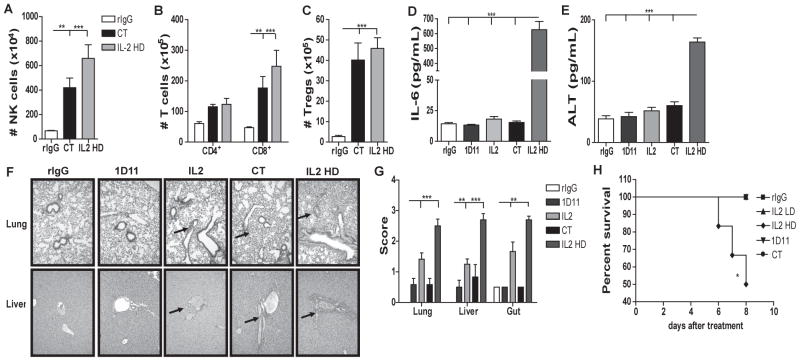
Fig 1. Combination of IL2 and anti-TGFβ significantly increases NK cell population, improves NK function in mice and promotes NK cell maturation.
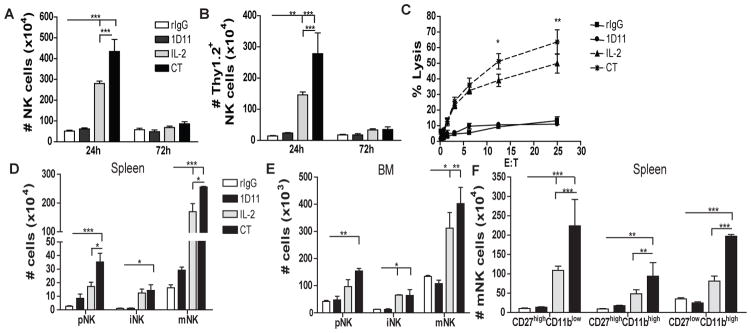
24h or 72h after the end of treatment spleen or BM of C57BL/6 mice were collected and single cell suspensions were stained for NK cell phenotypic analysis by flow cytometry. (A) Total number of NK cells (CD45+CD3−NK1.1+) is shown for mice treated with rIgG/PBS (white bars), 1D11 (dark gray bars), LD IL2 (IL2: light gray bars) or combination of both (CT: black bars). (B) Total number of Thy1.2+ NK cells. (C) Percentage of tumor lysis of purified NK cells cultured at different effector:target (E:T) ratios was assessed. (D–E) Total number of cells that are precursor NK (pNK:CD3−CD122+NKG2D+NK1.1−DX5−), immature NK (iNK:CD3−CD122+NKG2D+NK1.1+DX5−) and mature NK (mNK:CD3−CD122+NKG2D+NK1.1+DX5+) cells for spleen and BM at 24h. (C) Distribution of CD27 and CD11b at 24h from spleen is shown. Data are representative of two or three experiments with 3 mice per group (mean ± SEM). One-Way Anova or Two-Way Anova was used to assess significance. Significant differences are displayed for comparisons with rIgG control group as well as between IL2 and CT groups (*p<0.05, **p<0.01, ***p<0.001).
More importantly, after CT, NK cell activation was significantly augmented as evidenced by the higher expression of the activation marker Thy1.2 and enhanced tumor-cell lytic capability compared to mice receiving IL2 alone (Fig. 1B–C). These results correlated with significantly higher levels of granzyme B (data not shown). Additionally slight upregulation of the NK cell receptors NKG2D, NKG2A and Ly49G2 was also detected that can also be correlated with activation (data not shown) (21).
However, the effect of CT on the NK cell population was not observed 72h after treatment-cessation (Fig. 1A). Analysis of NK cells 48h after end of treatment showed an intermediate number of NK cells between results obtained at 24h and 72h post-treatment suggesting an NK cell contraction (data not shown). This significant reduction of the total number of NK cells after cessation of treatments is likely due to the short in vivo half-life of IL2 and 1D11as well as the need of NK cells for exogenous cytokines stimulation due to the NK cell “cytokine addiction” phenomenon indicating that continued presence of the therapy is likely needed for sustained effects (26–28).
Given the reported role of TGFβ in NK cell ontogeny (10), we hypothesized that the benefit of CT treatment could be the consequence of not only the expansion from the mature NK cell compartment, but also increased NK cell development and maturation, In fact, higher presence of NK cell progenitors after NK cell stimulation was observed. CT resulted in a significant increase of numbers of precursor (pNK: CD3−CD122+NKG2D+NK1.1−DX5−), immature (iNK: CD3−CD122+NKG2D+NK1.1+DX5−), and mature (mNK: CD3−CD122+ NKG2D+NK1.1+DX5+) NK cell subsets in both spleen and BM (Fig. 1D–E). Expression of CD27 and CD11b has also been used to further differentiate mNK (29). In the spleen, an increase of both immature-like phenotype (CD27highCD11blow, CD27highCD11bhigh) and mature-like phenotype (CD27lowCD11bhigh) was observed (Fig. 1F) whereas in the liver and BM caused primarily increase of the immature-like phenotype (data not shown). These data indicate a possible de novo generation of NK cells in the BM and liver due to CT treatment that could repopulate other organs such as the spleen and LN. It does also revealed a higher NK cell developmental rate that possibly contributes to the expansion of mNK cells after CT administration.
The effect of anti-TGFβ on NK cells was indirect as in vitro co-incubation of splenocytes with IL2 and anti-TGFβ induced higher percentage and number of NK cells in a dose-dependent manner; whereas no effect was observed when splenocytes were T cell depleted, indicating that T cells may directly regulate and inhibit NK cell expansion via TGFβ (Fig. S1). These data indicates that CT not only results in expansion of mNK but also improves NK cell cytotoxic capabilities and accelerates NK cell ontogeny.
Anti-TGFβ plus IL2 increases CD4 and CD8 T cells but not regulatory T cells
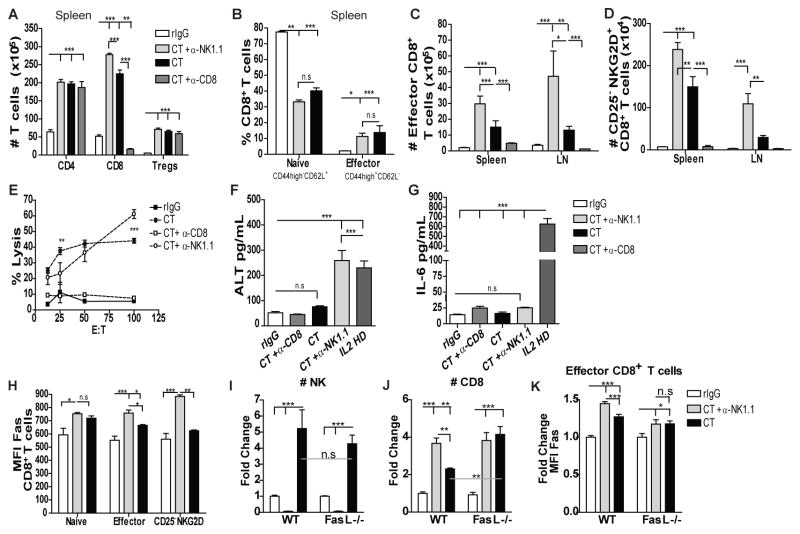
Previous studies have reported a positive impact of IL2 and TGFβ neutralization on the adaptive immune cell compartment (15, 20, 30). Thus, as expected, CT also significantly enhanced CD4 and CD8 T cell numbers, whereas Tregs were not significantly affected compared to IL2 alone (Fig. 2A-C). The impact of CT on T cell expansion can be the consequence of neutralizing the levels of TGFβ produced by IL2-stimulated Tregs in the presence of anti-TGFβ (Fig. 2D). Similar to NK cells, the effect of CT on the T cell compartment was not observed 72h post-treatment suggesting a T cell contraction (Fi2.B). The loss of T cells after stimulation can be a consequence of the role of IL2 in activation-induced cell death (AICD) (31),
Fig 2. IL2 and TGFβ blockade results in T cell expansion with improved CD8 T cell function.
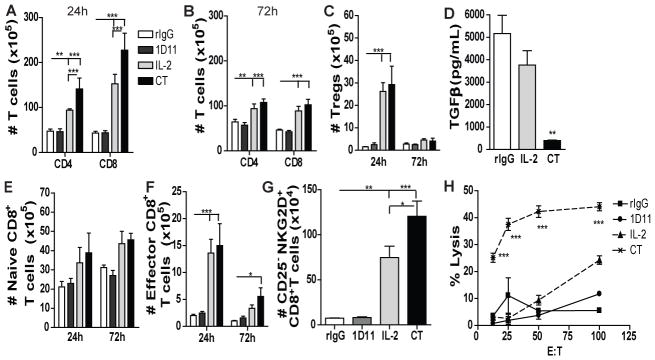
Single cell suspension from spleen were stained for CD45, CD3, CD8, CD4, CD44, CD62L or CD45, CD3, CD4, CD25 and Foxp3 to determine the distribution of CD4 T cell, CD8 T cell and Tregs. (A–C) Total number of CD4 T cells (CD45+CD3+CD4+), CD8 T cells (CD45+CD3+CD8+) and Tregs (CD45+CD3+CD4+CD25+Foxp3+) at 24h and 72h. (D) TGFβ Levels in the serum of treated mice at 24h. (E–F) Total number of naïve CD8 T cells (CD45+CD3+CD8+CD44−CD62L−) and effector CD8 T cells (CD45+CD3+CD8+CD44+CD62L−) at 24h and 72h. (G) Total number of bystander memory-activated CD8 T cells (CD45+CD3+CD8+CD44+CD25−NKG2D+) at 24h. ((H) CD8 T cell-specific lysis of P815 tumor cells from splenocytes of treated mice assessed by redirected killing assay. Data are representative of 3 independent experiments with 3 mice per group (mean ± SEM). One-Way Anova or Two-Way Anova was used to assess significance. Significant differences are displayed for comparisons with rIgG control group as well as between IL2 and CT groups (*p<0.05, **p<0.01, ***p<0.001).
Further analysis of the CD8 T cell population, a cell of interest that contribute to the anti-tumor benefits reported for CT, revealed a significant expansion of total effector memory (CD44+CD62L−) CD8 T cells and in particular, cells with the phenotype of non-antigen specific bystander-activated memory (CD44+CD25−NKG2D+) CD8 T cells after CT treatment in the spleen (Fig. 2E–G) which correlated with a markedly increased lytic ability indicative of bystander T cell expansion in response to cytokine alone stimulation (23) (Fig. 2H). Therefore, CT treatment also affected the CD8 T cell compartment resulting in bystander and memory cell expansion and enhanced lytic capabilities.
Combination of anti-TGFβ and LD IL2 leads to comparable immunological effects to high dose (HD) IL2 without induction of toxicities
We next determined the safety and efficacy of CT using LD IL2 (0.2×106 IU) and compared it to treatment with HD IL2 (1 million IU) monotherapy that induces significant toxicities in mice. The treatment with CT or HD IL2 resulted in comparable expansion of Tregs, NK, CD4 and CD8 T cells (Fig. 3A–C) and similar NK and CD8 T cell activity determined by granzyme B expression and cytolytic capability (Fig. S2A–B). The NK cells expanded after CT or HD IL2 displayed similar phenotypic profile (Fig. S2). However, CT did not lead to systemic toxicities whereas HD IL2 did as evidenced by high serum levels of IL6 and the liver enzyme ALT, as well as significant liver, lung and gut damage (Fig. 3D–G). Histological examination showed HD IL2 resulted in higher periportal lymphocytic aggregates in the liver and significant peribronchial and perivascular infiltrates and interstitial lymphocytic pneumonitis in the lung (Fig 3F). More importantly, approximately 50% of the mice that received HD IL2 did not survive the duration of the treatment compared to 100% of the CT group (Fig. 3H). Additionally, no differences were observed when anti-TGFβ was combined with HD IL2 (Fig. S2D) indicating that the additive effect of anti-TGFβ to IL2 treatment is IL2 dose-dependent as high levels of IL2 are sufficient to induce maximal immune responses. These data suggest that the use of anti-TGFβ and LD IL2 can achieve comparable NK and T cell augmentation compared to HD IL2 without the marked toxicities.
Fig 3. Combination therapy results in similar expansion of NK and T cells without induction of toxicity observed in HD IL2-treated mice.
(A–C) Total number of NK, CD4, CD8 T cells and Tregs in the spleen at 24h post-treatment. rIgG (white bars), CT (LD IL2 + 1D11) (black bars) or IL2 HD (1 million IU) (gray bars). (D–E) Serum IL6 and alanine transaminase (ALT) levels at 24h. (F) Representative images of histopathology slides of liver and lungs. (G) Scoring of H&E histology slides of livers, gut, and lungs at 24h. (H) Percentage of survival during treatment. Black arrows indicate periportal lymphocytic aggregates that are more significant in HD IL2 treated mice. Data is representative of at least two independent experiments with 3 mice per group (mean ± SEM). One-Way Anova or logrank test (H) was used to assess significance. Significant differences are displayed for comparisons with rIgG control group. (*p<0.05, **p<0.01, ***p<0.001).
The combination of anti-TGFβ and IL2 significantly improves anti-tumor effects in an NK and CD8 T cell dependent manner
We then evaluated the anti-tumor efficacy of systemic CT in a murine lung metastatic carcinoma model (3LL). Similar of what has been described for other mouse tumor models (20, 30), CT administration significantly increased the survival of metastatic tumor-bearing mice compared to IL2 alone (Fig. 4A). The anti-tumor responses of CT were both CD8 and NK cell-dependent as only the depletion of both populations abrogated the survival effects mediated by CT (Fig. 4B). These data indicate that CT results in enhanced anti-tumor responses by improving both NK cell and CD8 T cell compartments similar to what was observed by Park et al(20).
Fig 4. Prolonged tumor survival after treatment with IL2 and anti-TGFβ is NK and CD8 T cell-dependent.
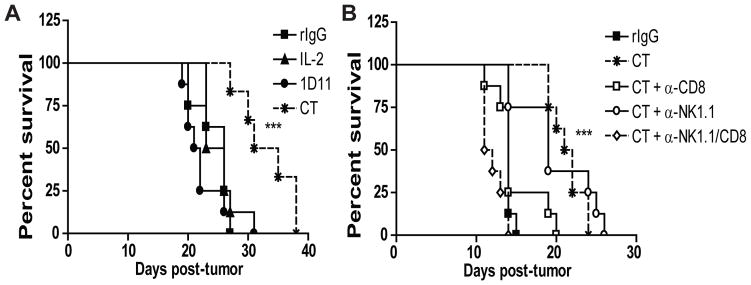
(A–B) Percent survival of mice treated with either rIgG, LD IL2, 1D11, or CT with or without NK and/or CD8 T cell depletion. Data are representative of two independent experiments with 8 mice per group. Statistical analysis was performed using logrank test. Significance for IL2 single therapy versus CT therapy treated mice (A) and between CT therapy and CT plus anti-NK1.1/CD8 treated mice (B) is shown (***p<0.001)
The minimal effect of either single NK or CD8 T cell depletion on the survival of tumor bearing mice was initially surprising; particularly in the case of NK depletion due to the importance of NK cells in the initial stages of anti-tumor responses (Fig. 4B). When NK or CD8 T cells were depleted in tumor-bearing rIgG-treated mice without prior stimulation, depletion of NK cells resulted in slightly accelerated tumor progression and mouse death compared to control or CD8 depleted groups (data not shown) which demonstrated the need for NK cells in the initial phase of the anti-tumor responses. These data also indicated that after CT, NK cells and CD8 T cells could compensate for each other to mount strong anti-tumor effects.
NK cell and CD8 T cells exhibit dual regulation after systemic activation by immunotherapy
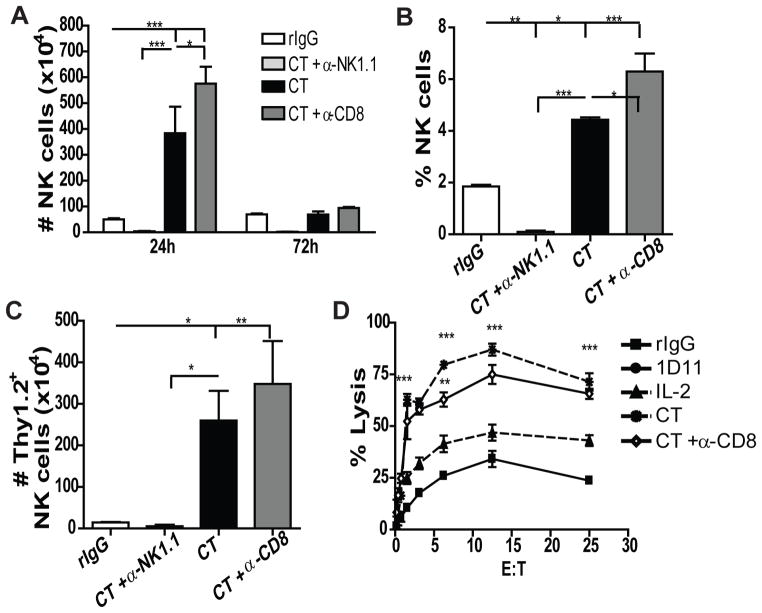
In multiple infection models, it has been postulated that regulation between NK and CD8 T cells exists indirectly through elimination of antigen presenting cells (APC), CD4 T cells or by direct effects (32–34). Furthermore, both populations respond to the same cytokines(35), such as IL2, which could result in their direct competition. To investigate the possible existence of dual regulation between NK and CD8 T cells after immunotherapy, we depleted mice of either cell-type two days prior to CT treatment (Fig. S3A) and determined the impact of these depletions on the cell expansion of the remaining population. CD8 depletion resulted in a significantly higher expansion of NK cells in the spleen that was characterized by having both immature-like and mature phenotypes and similar receptor expression patterns as the non-depleted group (Fig. 5 and data not shown). NK cytotoxic function was comparable (Fig. 5D) suggesting the primary effects of CD8 T cells were with regard to NK cell expansion. Interestingly, prior NK depletion induced a higher expansion of CD8 T cells whereas, CD4 T cells and Tregs were not affected following CT (Fig. 6A). Particularly, higher numbers of effector and bystander memory-activated CD8 T cells were observed (Fig. 6B–D) which also correlated with elevated cytotoxic function (Fig. 6E). Similar effects were also observed in LN (data not shown). A stimulatory environment seems to be required because depletion of NK or CD8 T cells in resting mice did not increase CD8 T or NK cells, respectively (Fig. S3B–C). Moreover, this regulation was independent of TGFβ as similar effects were observed in IL2-treated mice (Fig. S3D–H). These results demonstrate a dual regulation between NK and CD8 T cells that could be a consequence of cytokine competition for IL2, homeostatic proliferation or direct elimination of CD8 T cells by NK cells.
Fig 5. CD8 T cell depletion improves NK cell expansion during NK stimulation.
C57BL/6 mice were treated with rIgG (white bars) or LD IL2 (0.2 million IU) and 1D11 (CT: black bars) as previously described. Some groups additionally received anti-NK1.1 (light gray bars), anti-CD8 (dark gray bars) or rIgG (black bars) two days prior to treatment. (A) Total number of NK cells is shown at 24h and 72h in the spleen. (B) Percentage of NK cells at 24h after end of treatment in the spleen. (C) Number of Thy1.2+ NK cells at 24h in the spleen. (D) NK-dependent tumor lysis of purified NK cells from the spleen against Yac-1 tumor cells. Data are representative of three independent experiments with 3 mice per group (mean ± SEM). One-Way Anova or Two-Way Anova was used to assess significance.(*p<0.05, **p<0.01, ***p<0.001).
Fig 6. NK cells negatively regulate CD8 T cell expansion after LD IL2 and anti-TGFβ stimulation in a Fas-FasL dependent manner to reduce CD8 T cell dependent toxicity.
Single cell-suspension from spleens and LN were stained as previously described to determine T cell distribution at 24 and 72h. (A) Total number of CD4, CD8 T cells, and Tregs 24h after end of treatment from spleens (B) Percentage of effector and naive CD8 T cells 24h post-treatment. (C–D) Total number of effector and bystander CD8 T cells for the spleen and LN at 24h post-treatment. (E) CD8 T cell-specific tumor lysis. (F–G) ALT and IL6 serum levels at 24h. (H) MFI expression of Fas for naive, effector and bystander CD8 T cells is shown. WT or FasL-deficient mice were treated as previously and spleens were collected 24h after treatment. (I–J) Fold increase of NK and CD8 T cells 24h after treatment in WT and FasL-deficient mice. (K) Fold change in MFI expression of Fas for effector CD8 T cells in WT and FasL-deficient mice. Data are representative of 2–5 independent experiments (with 3 mice per group (mean ± SEM). One-Way Anova or Two-Way Anova was used to assess significance (n.s: no significance, *p<0.05, **p<0.01, ***p<0.001).
Because a role of NK cells in preventing CD8 T cell-dependent toxicity during infection has been previously suggested (32, 33), we also determined the impact of NK cell depletion on the induction of toxicity. Higher levels of ALT but not IL6 were detected in serum in NK depleted group compared to the non-NK depleted groups after CT (Fig. 6F–G). These data point to the role of NK cells in not only restraining T cell numbers and activity but also in limiting pathology.
The NK cell-dependent regulation of CD8 T cells involves the Fas-FasL pathway
There are multiple mechanisms that can be involved in this dual NK-CD8 T cell regulation. Some studies from infection mouse models have shown a direct or indirect impact on CD8 T cells by NK cells in a perforin-dependent manner (32, 34) or CD4 T cell-dependent manner (33), respectively. However, despite the impaired NK cell function, NK depletion of IL2-treated perforin-deficient mice (Pfn-KO) still resulted in CD8 T cell expansion comparable to WT mice (Fig. S4A-E). To exclude the CD8+ T-cell regulation via CD4+ T cell elimination that was previously suggested by Waggoner et al, anti-CD4 was used with or without anti-NK1.1 prior CT treatment. Anti-TGFβ was used in these experiments as to minimize the effect of Treg depletion on CD4-depleted mice. Anti-CD4 administration did not alter CD8+ T-cell expansion after CT treatment unless NK cells were also depleted (Fig. S4F-G) suggesting a direct regulation of CD8+ T cells by NK cells.
Interestingly, upregulation of Fas expression was also observed in CT-treated CD8 T cells after NK depletion, particularly in the effector and bystander memory-activated CD8 T cell subsets, whereas no differences were found in naive CD8 T cells (Fig. 6H) and in effector CD4 T cell (Fig. S4H). High levels of Fas were also induced by IL2 treatment alone (Fig. S3I), which was previously reported (36). In contrast, no differences were found for PD1 or Rae1δ (data not shown) that could indicate a CD8 T cell elimination through PD1-PDL1 and NKG2DL-NKG2D pathways respectively. Therefore, these results suggest the Fas-FasL pathway as a regulatory mechanism between NK and CD8 T cells after exogenous stimulation with IL2 and/or anti-TGFβ. To determine the impact of Fas-FasL in the regulation of CD8 T cells by NK cells after immunotherapeutic stimulation, we administered CT treatment to mice lacking FasL expression. CT resulted in a comparable NK cell expansion (Fig. 6I) while negatively affecting NK cell function (data not shown). However, a higher expansion of CD8 T cells was accomplished by CT treatment in FasL-deficient mice compared to WT, with no differences if mice where NK cell depleted prior immunotherapy treatment (Fig. 6J). Additionally, Fas expression was not altered by NK cell depletion prior to CT treatment in FasL-deficient mice (Fig. 6K) whereas it was increased in WT mice (Fig. 6H, K). All together, these data suggest a direct elimination of CD8 T cells by NK cells in a Fas-FasL-dependent manner.
In conclusion, this study provides evidence for the existence of dual NK-CD8 T-cell regulation and suggests a regulatory role of NK cells to prevent exacerbated CD8+ T-cell responses during non-antigen specific stimulatory models similar to what has been already suggested for virus or antigen-specific stimulatory models
DISCUSSION
The concurrent blockade of inhibitory signals while providing positive stimuli has ushered in a new era in cancer immunotherapy. We demonstrated that the combination of LD IL2 (stimulatory) with anti-TGFβ (suppressive) resulted in significant increases in both activated NK and effector CD8 T cells correlating with longer survival in tumor-bearing mice. More importantly, CT did not induce appreciable toxicities yet resulted in comparable effects on NK and CD8 T cells in comparison to HD IL2. The use of this CT immunotherapy of IL2 and anti-TGFβ also resulted in the generation of de novo NK cells confirming a role of TGFβ in the maturation of NK cells (10). In contrast to Marcoe et al., anti-TGFβ alone did not improve NK cell maturation or numbers. The accumulation of mNK observed in adult CD11cdnR mice is likely the consequence of the lack of response to TGFβ over time due to deficiency in TGFβ-receptor signaling(14). Additionally, in our study, the use of anti-TGFβ with short half-life may not be sufficient or complete enough to observe effects on NK cell development without prior stimulation or unless myelosuppressive therapies, which promote NK ontogeny, are applied. Nevertheless, the impact of TGFβ neutralization in NK cell development during IL2-dependent stimulation of NK cells provides another mechanism of action for this immunotherapeutic strategy that makes it more attractive than others because of its multilayered effect. Blockade of TGFβ not only prevents the immuosuppressive effects of IL2-induced Tregs allowing for greater expansion and improved functionality of NK cells; but also generates new mNK cells that will benefit from the therapy that might result in enhanced anti-tumor responses compared to other therapies that only target existing NK cells without generation of de novo NK cells.
A dramatic reduction of immune cell expansion was observed short after immunotherapy treatment cessation. Previously, our group described a CD4 conventional T cell lost following immunotherapy treatment with agonistic CD40 and IL2 that was associated with IFNγ-dependent upregulation of PDL1 (37, 38), IFNγ was also showed to negatively regulate influenza virus-specific CD8 T cell responses (39). It is possible that IL2 dependent IFNγ production can lead to immune cell loss following treatment cessation. Furthermore, cytokine deprivation is also important for antigen-stimulated T cell contraction (40). Due to the loss of effect after immunotherapy, it is clear that approaches similar to the one developed by Park et al might be necessary to achieve prolonged anti-tumor effects (20). However, it is important to take into account the induction of immune cell exhaustion as it was showed NK cell anergy after sustained stimulation with IL15 (41) and CD8 T cell exhaustion has been described after chronic virus infection (42).
In this study, we have also demonstrated for the first time after immunotherapy the existence of a dual regulation between NK and CD8 T cells, which seems to have a stronger regulation towards CD8 T cells by NK cells. NK cell inhibition of CD8 T cells has been previously shown in various infection models where the regulation can be done by directly eliminating CD8 T cells in a perforin, IL10- and/or NKG2D- depending manner or indirectly by affecting the levels of mature DCs and CD4 T cells (32, 33, 43–46). More importantly, this regulatory effect by NK cells of the adaptive immune response is not limited to infection models and it has been suggested for autoimmune disorders by limiting proliferation of autoreactive T cells (47). For example, depletion of NK cells resulted in an increase of encephalitis and demyelization after Theiler’s murine encephalitis virus (48) and in various experimental autoimmune encephalomyelitis models (47). Additionally, Barber et al suggested a negative regulation of tumor infiltrated CD8 T cell by NK cells in a low immunogenic lymphoma mouse model (49). Our study along with these studies suggests a general role of NK cells in the regulation and inhibition of the adaptive immune response that seems to occur as a mechanism to most likely prevent strong immune response that otherwise could lead to autoimmunity.
Similarly, and in agreement with our study, CD8 depletion prior to MCMV infection has also been shown to induce enhanced NK cell numbers and activity (50). Because both NK and CD8+ T-cells use the same cytokines for development, maintenance, and activation such as IL2 and IL15 (35). It is possible that this dual regulation is in part due to competition for cytokines by both immune cells limiting the ability of each population to proliferate after stimulation. Homeostatic proliferation could also partially explain our results. However, the lack of CD8+ T-cells expansion after CD4+ T-cell depletion, which creates a bigger niche for their proliferation, suggest otherwise. Because NK cells are able to mount a faster immune response compared to CD8+ T-cells, the use of cytokines by NK cells could restrict, to a greater extent, the expansion of CD8+ T-cells. Additionally, we have observed a higher expansion of both effector and bystander memory-activated CD8+ T-cell subsets when NK cells were not present in both spleen and LN. We have previously demonstrated that after systemic administration of cytokines, antigen-nonspecific bystander CD8 T cell expansion occurs primarily in the memory (CD44high) population which highly expresses CD122, the low affinity IL2R, compared with naive cells, highlighting the need for heightened cytokine levels in their expansion and maintenance of their activated state(23). This requirement for IL2 would explain the differences observed in this population when NK cells are absent indicating a cytokine competition between NK and CD8 T cells. Moreover, the effect of immunotherapy on the CD4 T cell compartment was minimal as approximately 30% of this compartment after treatment represents Tregs. This lack of effect on the CD4 T cell compartment could be explained by induction of apoptosis via PD1/PDL1 pathway after immunotherapy as was previously suggested (37, 38).
NK-CD8 T cell regulation can also be explained by direct elimination of activated CD8 T cells by NK cells. In a MCMV infection model, perforin- and IL-10-dependent regulation of CD8 T cell responses by NK cells prevented animal death (32). In our model, perforin did not seem to have a role in the NK-CD8 T cell regulation as no differences were observed in perforin deficient mice. NKG2D could also play a role in CD8 T cell regulation as expression of NKG2D ligands is observed 24–48h after activation of CD8 T cells and was also suggested in a CD8 T cell-priming model (34), but we did not observe upregulation of Rae1-δ expression on CD8 T cells after treatment suggesting that other ligands may be involved. Upregulation of polivirus receptor (PVR) was also observed on antigen specific T cells after staphylococcus aureus enterotoxin B treatment that render elimination by NK cells in a NKG2D-DNAM1 dependent manner (51), but again no differences in PVR expression was detected on effector CD8 T cells. In contrast, our model suggests that the FasL-Fas pathway is involved in the direct regulation of CD8 T cells by NK cells. Upregulation of Fas is observed after IL2 treatment and has been involved in the activation induce cell death of T cells (31, 36). Additionally, Fas-FasL induced cell death has proved to be important in the elimination of alloreactive CD8 T cells after hematopoietic stem cell transplantation (52, 53).
There are significant differences between mouse and human NK cells, including their tissue location. In contrast to humans whose NK cells can be found in the LNs in steady state conditions, mouse NK cells rapidly accumulate in the LN only during inflammation, immunization or infections (29, 54). It is likely that this dual regulatory ability of NK and T cells may occur at earlier stages in activation in human LN compared to mouse models.
In conclusion, this study demonstrated for the first time the existence of a dual regulation between NK and CD8 T cells in a stimulatory non-antigen-specific model that can have tremendous impact on the efficacy of the immunotherapeutic strategy chosen. It also demonstrates the critical protective role of NK cells in regulating T cell function during not only during infection but also in other types of stimulation to prevent exacerbated responses that would result in detrimental toxicities. Finally, combination immunotherapy targeting positive and negative regulators may lead to greater anti-tumor efficacy.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant R01-HL089905.
We thank Weihong Ma and Monja Metcalf for their technical assistance.
Footnotes
The authors disclose no conflict of interest.
References
- 1.Sutlu T, Alici E. Natural killer cell-based immunotherapy in cancer: current insights and future prospects. Journal of internal medicine. 2009;266:154–181. doi: 10.1111/j.1365-2796.2009.02121.x. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. Jama. 1994;271:907–913. [PubMed] [Google Scholar]
- 3.Buyse M, Squifflet P, Lange BJ, Alonzo TA, Larson RA, Kolitz JE, George SL, Bloomfield CD, Castaigne S, Chevret S, Blaise D, Maraninchi D, Lucchesi KJ, Burzykowski T. Individual patient data meta-analysis of randomized trials evaluating IL-2 monotherapy as remission maintenance therapy in acute myeloid leukemia. Blood. 2011;117:7007–7013. doi: 10.1182/blood-2011-02-337725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peace DJ, Cheever MA. Toxicity and therapeutic efficacy of high-dose interleukin 2. In vivo infusion of antibody to NK-1.1 attenuates toxicity without compromising efficacy against murine leukemia. The Journal of experimental medicine. 1989;169:161–173. doi: 10.1084/jem.169.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steel JC, Waldmann TA, Morris JC. Interleukin-15 biology and its therapeutic implications in cancer. Trends in pharmacological sciences. 2012;33:35–41. doi: 10.1016/j.tips.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annual review of immunology. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 7.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nature immunology. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 8.Ghiringhelli F, Menard C, Martin F, Zitvogel L. The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunological reviews. 2006;214:229–238. doi: 10.1111/j.1600-065X.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 9.Barao I, Hanash AM, Hallett W, Welniak LA, Sun K, Redelman D, Blazar BR, Levy RB, Murphy WJ. Suppression of natural killer cell-mediated bone marrow cell rejection by CD4+CD25+ regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5460–5465. doi: 10.1073/pnas.0509249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcoe JP, Lim JR, Schaubert KL, Fodil-Cornu N, Matka M, McCubbrey AL, Farr AR, Vidal SM, Laouar Y. TGF-beta is responsible for NK cell immaturity during ontogeny and increased susceptibility to infection during mouse infancy. Nature immunology. 2012;13:843–850. doi: 10.1038/ni.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith AL, Robin TP, Ford HL. Molecular pathways: targeting the TGF-beta pathway for cancer therapy. Clin Cancer Res. 2012;18:4514–4521. doi: 10.1158/1078-0432.CCR-11-3224. [DOI] [PubMed] [Google Scholar]
- 12.Penafuerte C, Galipeau J. TGF beta secreted by B16 melanoma antagonizes cancer gene immunotherapy bystander effect. Cancer Immunol Immunother. 2008;57:1197–1206. doi: 10.1007/s00262-008-0453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrausch U, Jensen SM, Twitty C, Poehlein CH, Haley DP, Walker EB, Fox BA. Disruption of TGF-beta signaling prevents the generation of tumor-sensitized regulatory T cells and facilitates therapeutic antitumor immunity. J Immunol. 2009;183:3682–3689. doi: 10.4049/jimmunol.0900560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nam JS, Terabe M, Mamura M, Kang MJ, Chae H, Stuelten C, Kohn E, Tang B, Sabzevari H, Anver MR, Lawrence S, Danielpour D, Lonning S, Berzofsky JA, Wakefield LM. An anti-transforming growth factor beta antibody suppresses metastasis via cooperative effects on multiple cell compartments. Cancer research. 2008;68:3835–3843. doi: 10.1158/0008-5472.CAN-08-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perry K, Wong L, Liu V, Park I, Zhang Q, Rejen V, Huang X, Smith ND, Jovanovic B, Lonning S, Teicher BA, Lee C. Treatment of transforming growth factor-beta-insensitive mouse Renca tumor by transforming growth factor-beta elimination. Urology. 2008;72:225–229. doi: 10.1016/j.urology.2007.11.091. [DOI] [PubMed] [Google Scholar]
- 16.Ogasawara K, Lanier LL. NKG2D in NK and T cell-mediated immunity. Journal of clinical immunology. 2005;25:534–540. doi: 10.1007/s10875-005-8786-4. [DOI] [PubMed] [Google Scholar]
- 17.Hulper P, Schulz-Schaeffer W, Dullin C, Hoffmann P, Harper J, Kurtzberg L, Lonning S, Kugler W, Lakomek M, Erdlenbruch B. Tumor localization of an anti-TGF-beta antibody and its effects on gliomas. International journal of oncology. 2011;38:51–59. [PubMed] [Google Scholar]
- 18.Terabe M, Ambrosino E, Takaku S, O’Konek JJ, Venzon D, Lonning S, McPherson JM, Berzofsky JA. Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-beta monoclonal antibody. Clin Cancer Res. 2009;15:6560–6569. doi: 10.1158/1078-0432.CCR-09-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallett WH, Ames E, Alvarez M, Barao I, Taylor PA, Blazar BR, Murphy WJ. Combination therapy using IL-2 and anti-CD25 results in augmented natural killer cell-mediated antitumor responses. Biol Blood Marrow Transplant. 2008;14:1088–1099. doi: 10.1016/j.bbmt.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, Jay SM, Demento SL, Agawu A, Licona Limon P, Ferrandino AF, Gonzalez D, Habermann A, Flavell RA, Fahmy TM. Combination delivery of TGF-beta inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nature materials. 2012;11:895–905. doi: 10.1038/nmat3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barao I, Alvarez M, Ames E, Orr MT, Stefanski HE, Blazar BR, Lanier LL, Anderson SK, Redelman D, Murphy WJ. Mouse Ly49G2+ NK cells dominate early responses during both immune reconstitution and activation independently of MHC. Blood. 2011;117:7032–7041. doi: 10.1182/blood-2010-11-316653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raziuddin A, Bennett M, Winkler-Pickett R, Ortaldo JR, Longo DL, Murphy WJ. Synergistic effects of in vivo depletion of Ly-49A and Ly-49G2 natural killer cell subsets in the rejection of H2(b) bone marrow cell allografts. Blood. 2000;95:3840–3844. [PubMed] [Google Scholar]
- 23.Tietze JK, Wilkins DE, Sckisel GD, Bouchlaka MN, Alderson KL, Weiss JM, Ames E, Bruhn KW, Craft N, Wiltrout RH, Longo DL, Lanier LL, Blazar BR, Redelman D, Murphy WJ. Delineation of antigen-specific and antigen-nonspecific CD8(+) memory T-cell responses after cytokine-based cancer immunotherapy. Blood. 2012;119:3073–3083. doi: 10.1182/blood-2011-07-369736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallett WH, Ames E, Motarjemi M, Barao I, Shanker A, Tamang DL, Sayers TJ, Hudig D, Murphy WJ. Sensitization of tumor cells to NK cell-mediated killing by proteasome inhibition. J Immunol. 2008;180:163–170. doi: 10.4049/jimmunol.180.1.163. [DOI] [PubMed] [Google Scholar]
- 25.Bouchlaka MN, Sckisel GD, Chen M, Mirsoian A, Zamora AE, Maverakis E, Wilkins DE, Alderson KL, Hsiao HH, Weiss JM, Monjazeb AM, Hesdorffer C, Ferrucci L, Longo DL, Blazar BR, Wiltrout RH, Redelman D, Taub DD, Murphy WJ. Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy. The Journal of experimental medicine. 2013;210:2223–2237. doi: 10.1084/jem.20131219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ling H, Li X, Jha S, Wang W, Karetskaya L, Pratt B, Ledbetter S. Therapeutic role of TGF-beta-neutralizing antibody in mouse cyclosporin A nephropathy: morphologic improvement associated with functional preservation. Journal of the American Society of Nephrology : JASN. 2003;14:377–388. doi: 10.1097/01.asn.0000042168.43665.9b. [DOI] [PubMed] [Google Scholar]
- 27.Melder RJ, Osborn BL, Riccobene T, Kanakaraj P, Wei P, Chen G, Stolow D, Halpern WG, Migone TS, Wang Q, Grzegorzewski KJ, Gallant G. Pharmacokinetics and in vitro and in vivo anti-tumor response of an interleukin-2-human serum albumin fusion protein in mice. Cancer Immunol Immunother. 2005;54:535–547. doi: 10.1007/s00262-004-0624-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller JS. Therapeutic applications: natural killer cells in the clinic. Hematology/the Education Program of the American Society of Hematology. American Society of Hematology. Education Program. 2013;2013:247–253. doi: 10.1182/asheducation-2013.1.247. [DOI] [PubMed] [Google Scholar]
- 29.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 30.Wojtowicz-Praga S, Verma UN, Wakefield L, Esteban JM, Hartmann D, Mazumder A. Modulation of B16 melanoma growth and metastasis by anti-transforming growth factor beta antibody and interleukin-2. Journal of immunotherapy with emphasis on tumor immunology : official journal of the Society for Biological Therapy. 1996;19:169–175. doi: 10.1097/00002371-199605000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Schmitz I, Krueger A, Baumann S, Schulze-Bergkamen H, Krammer PH, Kirchhoff S. An IL-2-dependent switch between CD95 signaling pathways sensitizes primary human T cells toward CD95-mediated activation-induced cell death. J Immunol. 2003;171:2930–2936. doi: 10.4049/jimmunol.171.6.2930. [DOI] [PubMed] [Google Scholar]
- 32.Lee SH, Kim KS, Fodil-Cornu N, Vidal SM, Biron CA. Activating receptors promote NK cell expansion for maintenance, IL-10 production, and CD8 T cell regulation during viral infection. The Journal of experimental medicine. 2009;206:2235–2251. doi: 10.1084/jem.20082387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2012;481:394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soderquest K, Walzer T, Zafirova B, Klavinskis LS, Polic B, Vivier E, Lord GM, Martin-Fontecha A. Cutting edge: CD8+ T cell priming in the absence of NK cells leads to enhanced memory responses. J Immunol. 2011;186:3304–3308. doi: 10.4049/jimmunol.1004122. [DOI] [PubMed] [Google Scholar]
- 35.Fehniger TA, Cooper MA, Caligiuri MA. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine & growth factor reviews. 2002;13:169–183. doi: 10.1016/s1359-6101(01)00021-1. [DOI] [PubMed] [Google Scholar]
- 36.Refaeli Y, Van Parijs L, London CA, Tschopp J, Abbas AK. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 1998;8:615–623. doi: 10.1016/s1074-7613(00)80566-x. [DOI] [PubMed] [Google Scholar]
- 37.Alderson KL, Zhou Q, Berner V, Wilkins DE, Weiss JM, Blazar BR, Welniak LA, Wiltrout RH, Redelman D, Murphy WJ. Regulatory and conventional CD4+ T cells show differential effects correlating with PD-1 and B7-H1 expression after immunotherapy. J Immunol. 2008;180:2981–2988. doi: 10.4049/jimmunol.180.5.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berner V, Liu H, Zhou Q, Alderson KL, Sun K, Weiss JM, Back TC, Longo DL, Blazar BR, Wiltrout RH, Welniak LA, Redelman D, Murphy WJ. IFN-gamma mediates CD4+ T-cell loss and impairs secondary antitumor responses after successful initial immunotherapy. Nature medicine. 2007;13:354–360. doi: 10.1038/nm1554. [DOI] [PubMed] [Google Scholar]
- 39.Prabhu N, Ho AW, Wong KH, Hutchinson PE, Chua YL, Kandasamy M, Lee DC, Sivasankar B, Kemeny DM. Gamma interferon regulates contraction of the influenza virus-specific CD8 T cell response and limits the size of the memory population. Journal of virology. 2013;87:12510–12522. doi: 10.1128/JVI.01776-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diaz-Guerra E, Vernal R, del Prete MJ, Silva A, Garcia-Sanz JA. CCL2 inhibits the apoptosis program induced by growth factor deprivation, rescuing functional T cells. J Immunol. 2007;179:7352–7357. doi: 10.4049/jimmunol.179.11.7352. [DOI] [PubMed] [Google Scholar]
- 41.Elpek KG, Rubinstein MP, Bellemare-Pelletier A, Goldrath AW, Turley SJ. Mature natural killer cells with phenotypic and functional alterations accumulate upon sustained stimulation with IL-15/IL-15Ralpha complexes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21647–21652. doi: 10.1073/pnas.1012128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wherry EJ. T cell exhaustion. Nature immunology. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 43.Narni-Mancinelli E, Jaeger BN, Bernat C, Fenis A, Kung S, De Gassart A, Mahmood S, Gut M, Heath SC, Estelle J, Bertosio E, Vely F, Gastinel LN, Beutler B, Malissen B, Malissen M, Gut IG, Vivier E, Ugolini S. Tuning of natural killer cell reactivity by NKp46 and Helios calibrates T cell responses. Science (New York, NY. 2012;335:344–348. doi: 10.1126/science.1215621. [DOI] [PubMed] [Google Scholar]
- 44.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nature immunology. 2003;4:175–181. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 45.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. The Journal of experimental medicine. 2002;195:335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dowdell KC, Cua DJ, Kirkman E, Stohlman SA. NK cells regulate CD4 responses prior to antigen encounter. J Immunol. 2003;171:234–239. doi: 10.4049/jimmunol.171.1.234. [DOI] [PubMed] [Google Scholar]
- 47.Shi FD, Van Kaer L. Reciprocal regulation between natural killer cells and autoreactive T cells. Nature reviews. 2006;6:751–760. doi: 10.1038/nri1935. [DOI] [PubMed] [Google Scholar]
- 48.Paya CV, Patick AK, Leibson PJ, Rodriguez M. Role of natural killer cells as immune effectors in encephalitis and demyelination induced by Theiler’s virus. J Immunol. 1989;143:95–102. [PubMed] [Google Scholar]
- 49.Barber MA, Zhang T, Gagne BA, Sentman CL. NK cells negatively regulate antigen presentation and tumor-specific CTLs in a syngeneic lymphoma model. J Immunol. 2007;178:6140–6147. doi: 10.4049/jimmunol.178.10.6140. [DOI] [PubMed] [Google Scholar]
- 50.Salem ML, Hossain MS. In vivo acute depletion of CD8(+) T cells before murine cytomegalovirus infection upregulated innate antiviral activity of natural killer cells. International journal of immunopharmacology. 2000;22:707–718. doi: 10.1016/s0192-0561(00)00033-3. [DOI] [PubMed] [Google Scholar]
- 51.Ardolino M, Zingoni A, Cerboni C, Cecere F, Soriani A, Iannitto ML, Santoni A. DNAM-1 ligand expression on Ag-stimulated T lymphocytes is mediated by ROS-dependent activation of DNA-damage response: relevance for NK-T cell interaction. Blood. 2011;117:4778–4786. doi: 10.1182/blood-2010-08-300954. [DOI] [PubMed] [Google Scholar]
- 52.Priyadharshini B, Thornley TB, Daniels KA, Cuthbert A, Welsh RM, Greiner DL, Brehm MA. Alloreactive CD8 T cells rescued from apoptosis during co-stimulation blockade by Toll-like receptor stimulation remain susceptible to Fas-induced cell death. Immunology. 2013;138:322–332. doi: 10.1111/imm.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li JH, Rosen D, Sondel P, Berke G. Immune privilege and FasL: two ways to inactivate effector cytotoxic T lymphocytes by FasL-expressing cells. Immunology. 2002;105:267–277. doi: 10.1046/j.1365-2567.2002.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watt SV, Andrews DM, Takeda K, Smyth MJ, Hayakawa Y. IFN-gamma-dependent recruitment of mature CD27(high) NK cells to lymph nodes primed by dendritic cells. J Immunol. 2008;181:5323–5330. doi: 10.4049/jimmunol.181.8.5323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.