Abstract
We present a premature male neonate with confirmed Factor V Leiden deficiency diagnosed prenatally with cardiac and abdominal calcifications. Our patient’s findings suggest that clinicians consider thromboembolic conditions when multiple fetal calcifications are visualized.
Keywords: factor V Leiden, thromboembolic disorders, hypercoagulation disorder, abdominal calcifications, cardiac calcifications, fetal calcifications
Introduction
Factor V Leiden (FVL) deficiency is the most common cause of inherited thrombophilia (1). The prevalence of heterozygosity of FVL is 5% in Caucasians, 2.2% in Hispanic Americans, 1.2% in African Americans and 0.45% in Asian Americans (1). Homozygotes account for approximately 1% of all patients with FVL gene mutation (1). Presentation of thromboembolic events during childhood are rare with an estimated annual incidence of 0.14 per 10,000 (2). Most events occur during the neonatal period and first year of life with an incidence reported as 0.51 per 10,000 (3).
Thromboembolic events occur commonly in association with both venous and arterial indwelling catheters (3). Perinatal ischemic stroke due most frequently to arterial or venous thrombosis is estimated to occur in 1 in 2300 to 5000 births (4). Prenatal dural sinus thromboses have been described particularly among infants with vascular malformations (5). Other postnatal presentations include thrombocytopenia and purpura fulminans.
We found no reports to date of a thromboembolic event presenting as a fetal myocardial calcification. This case report describes a premature neonate with a prenatal diagnosis of cardiac, abdominal and placental calcifications who was found to be homozygous for FVL. We propose that prothrombotic disorders should be considered among patients with multiple calcifications.
Case Presentation
A 40 year-old G2 P0 mother with a history of one previous miscarriage presented at 18 weeks gestational age (GA) when a fetal ultrasound revealed myocardial and abdominal echogenicities and partial placental abruption. A fetal echocardiogram performed at 19 weeks GA at our regional referral center demonstrated a 4.5 mm echogenic myocardial focus located at the left ventricular apex. No other structural or functional defects were noted. High-resolution fetal ultrasound showed an abdominal echogenicity along the contour of the right diaphragm. The fetal brain was normal.
Preliminary maternal work-up included an amniocentesis at 18 weeks GA that demonstrated a normal 46 XY karyotype. Infectious work-up for HIV, rubella, syphilis, cytomegalovirus and toxoplasma also yielded negative results. Subsequent testing for inherited thrombophilia revealed that both parents were heterozygous for FVL.
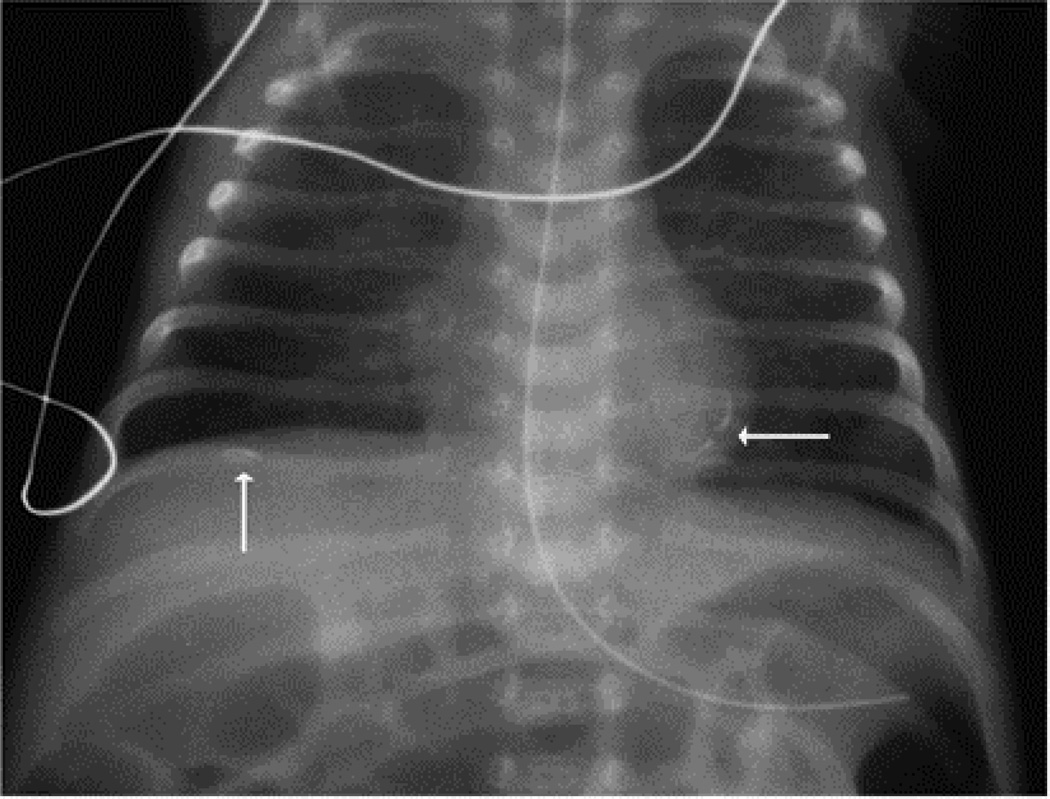
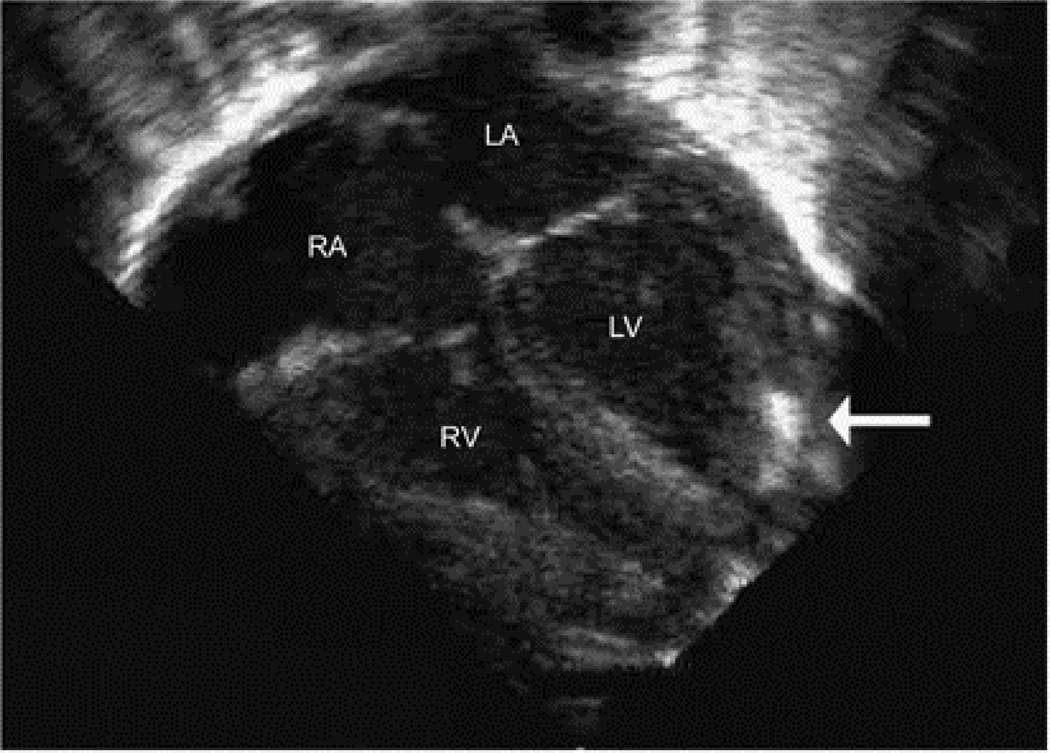
This Caucasian male infant was born 29 3/7 weeks GA, 930 g (25%) via caesarian section for non-reassuring fetal heart tracings. Radiograph on DOL 1 showed cardiac and left subdiaphragmatic calcifications (Figure 1). Echocardiogram on DOL 1 showed an isolated echogenic 5 mm × 1.5 mm focus in the apical aspect of the left ventricular free wall with normal ventricular function (Figure 2). Follow-up abdominal ultrasound revealed a small echogenicity near the left lobe of the liver with no appreciable thromboses on Doppler flow studies. No thromboses were appreciated in the kidneys or other abdominal organs. Evaluation for tuberous sclerosis was negative. This patient had an umbilical venous line placed at birth that was removed by postnatal day 10. No other central lines were placed during his hospitalization.
Figure 1.
Chest X-ray/KUB revealing cardiac and left subdiaphragmatic calcifications.
Figure 2.
Still frame from a transthoracic echocardiogram on DOL 1 demonstrating a 5 × 1.5 mm echogenic focus in the apical aspect of the left ventricular (LV) free wall.
RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle
Placental pathology demonstrated a small, immature placenta with diffuse choriamnionic hemosiderosis and retromembranous hemorrhage consistent with chronic placental abruption. In addition, fetal vascular thromboses were seen. Testing for FVL revealed that our patient was homozygous for the gene mutant (G1691A).
Due to the risk for thromboembolic events in the brain, the patient underwent serial head ultrasounds and brain MRI, which were normal. We did not pursue coronary angiography because the infant was clinically stable and the potential findings seemed unlikely to change management. Anti-coagulation therapy was not started because of the risk of bleeding in a premature neonate.
This patient was discharged on DOL 67 at 2150 g. Repeat echocardiogram at three months of age showed an unchanged left ventricular echogenic focus with normal cardiac function, consistent with a stable calcification.
Discussion
Fetal echogenic foci in the heart and abdomen are diagnostically challenging because both findings can represent benign pathology or can signify in-utero infections or chromosomal abnormalities. Echogenic cardiac foci occur in 3–4% of routine second trimester ultrasounds (6). Although most isolated echogenic foci represent normal cardiac development (7), association with anueploidy has been reported (8). Diffuse cardiac echogenic foci accompanied by pericardial effusions and impaired heart function have been described in patients with infectious myocarditis (9). Cardiac tumors can present as single or multiple fetal echogenic foci (10). Rhabdomyoma is the most common type of neonatal cardiac tumor. Approximately 40% of rhabdomyomas present in fetal life are associated with tuberous sclerosis (10).
Fetal echogenic abdominal foci are common and can resolve spontaneously without intervention. Other differential diagnoses include meconium peritonitis, infection, neoplasms, and echogenic bowel. Diffuse abdominal echogenicity can represent calcified areas of meconium peritonitis (11). Echogenic bowel is associated with aneuploidy or cystic fibrosis (11). Hepatic infection can present with isolated right upper quadrant abdominal calcifications (12).
As seen with our patient, infectious and genetic etiologies should first be considered when echogenic foci are visualized in multiple organs. However, inherited thrombophilia can lead to diffuse thromboses in the fetus and placenta. In this setting, we suggest that clinicians consider evaluation of inherited thrombophilia. Initial prenatal testing can include evaluation of the most common inherited thrombophilas (FVL and Prothrombin gene mutation) among the parents. Further testing of other rare defects such as protein C, S, and antithrombin deficiency should be considered. Coagulation studies should be evaluated within the context of normal physiologic hemostasis of pregnancy.
Prenatal genetic testing should guide the postnatal evaluation of infants with suspected inherited thrombophilia. Testing for other coagulation abnormalities should be considered among patients with hetero- or homozygosity for FVL or Prothrombin gene mutation because the risk of recurrent thromboses with combined inherited defects is significantly higher than with a single gene defect (13). Nowak- Gottl et al. reported an odds ratio for recurrent thrombosis of 4.6 (95% C.I. 2.3–9.0) for a single defect versus an odds ratio of 24.0 (95% C.I. 5.3–108.7) for combined defect (13). Finally, coagulation levels should be compared against published normal value ranges for premature (14) or full term neonates (15). Identification of prothrombotic disorders in the neonatal period is important for genetic counseling for subsequent pregnancies and alerting parents to risk factors that may predispose children to future thromboembolic events.
Anti-coagulation treatment for infants with inherited thrombophilia is controversial. To our knowledge, there have been no randomized trials among infants to test anti-platelet or thrombolytic medications as treatment or prophylaxis for thrombosis. Clot formation associated with indwelling catheters involves removal of central catheters or use of recombinant tissue plasminogen activator (16). Some clinicians support thrombolytic therapy with unfractionated heparin infusions acutely following large thromboembolic events (16). Because of the short half-life of unfractionated heparin, anitcoaguation can be reversed quickly in the event of bleeding (16). More recently clinicians have chosen low molecular weight heparin (LMWH) to treat newborn thromboembolism due to the lower risk of acute bleeding (16). A systematic review of 8 studies by Malowany et al demonstrated complete or partial resolution of clot formation in 59–100% and major bleeding in 0–19% of patients (17).
Limited data supports prolonged prophylactic therapy because of the need for drug level monitoring and bleeding risk. A prospective study of over 200 newborns with homozygosity and double heterozygosity of FVL and prothrombin gene mutation showed no difference in neurologic or physical development at 24 months of age compared to heterozygote controls (18). To date the administration of prophylactic anti-coagulation therapy has unclear benefit.
We propose that the increased risk of in-utero thrombosis due to FVL homozygosity likely explains the placental, abdominal and cardiac calcifications in our patient. Although fetal evaluation frequently identify benign echogenicities, our patient’s findings suggest that clinicians consider thromboembolic conditions when prenatal testing reveal multiple fetal calcifications.
Acknowledgements
The authors would like to thank Jonathon Rhodes, M.D. for his help in preparing this manuscript. This work was supported by the National Institutes of Health under award number: T32HL007572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ridker, et al. Ethnic distribution of factor V Leiden in 4047 men and women. Implications for venous thromboembolism screening. JAMA. 1997;255:1305. [PubMed] [Google Scholar]
- 2.Van Ommen CH, Heijboer H, Buller HR, et al. Venous thromboembolism in childhood: A prospective two-year registry in the Netherlands. J Pediatr. 2001;139(5):676–681. doi: 10.1067/mpd.2001.118192. [DOI] [PubMed] [Google Scholar]
- 3.Nowak-Gottl U, von Kries R, Gobel U. Neonatatal symptomatic thromboembolism in Germany: two year survey. Arch Dis Child Fetal Neonatal Ed. 1997;76:F163–F167. doi: 10.1136/fn.76.3.f163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raju TNK, Nelson KB, Ferriero D, Lynch JK. Ischemic Perinatal Stroke: Summary of a Workshop Sponsored by the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke. Pediatrics. 2007 Sep;120(3):609–616. doi: 10.1542/peds.2007-0336. [DOI] [PubMed] [Google Scholar]
- 5.Spampinato MV, et al. Thrombosed Fetal Dural Sinus Malformation Diagnosed with Magnetic Resonance Imaging. Obstet and Gynecol. 2008;111:569–572. doi: 10.1097/01.AOG.0000289227.12531.03. [DOI] [PubMed] [Google Scholar]
- 6.Coco C, Jeanty P, Jeanty C. An isolated echogenic heart focus is not an indication for amniocentesis in 12,672 unselected patients. J Ultrasound Med. 2004 Aprl;23(4):489–496. doi: 10.7863/jum.2004.23.4.489. [DOI] [PubMed] [Google Scholar]
- 7.Levy D, Mintz M. The Left Ventricular Echogenic Focus: A Normal Finding. AJR. 1988;150:85–86. doi: 10.2214/ajr.150.1.85. [DOI] [PubMed] [Google Scholar]
- 8.Manning J, et al. Significance of Fetal Intracardiac Echogenic Foci in Relation to Trisomy 21: A Prospective Sonographic Study of High-Risk Pregnant Women. AJR. 1998;170:1083–1084. doi: 10.2214/ajr.170.4.9530064. [DOI] [PubMed] [Google Scholar]
- 9.Konstantinidou A, et al. Transplacental infection of Coxsackievirus B3 pathological findings in the fetus. J Med Virol. 2007;79:754–757. doi: 10.1002/jmv.20887. [DOI] [PubMed] [Google Scholar]
- 10.Zhou QC, et al. Prenatal echocardiographic differential diagnosis of fetal cardiac tumors. Ultrasound Obstet Gynecol. 2004;23:165–171. doi: 10.1002/uog.979. [DOI] [PubMed] [Google Scholar]
- 11.Lince DM, et al. The clinical significance of increased echogenicity in the fetal abdomen. AJR. 1985;145(5):683–686. doi: 10.2214/ajr.145.4.683. [DOI] [PubMed] [Google Scholar]
- 12.Smichen MJ, et al. Fetal hepatic calcifications: prenatal diagnosis and outcome. Am J Obstet Gynecol. 2002;187(6):1617–1622. doi: 10.1067/mob.2002.127899. [DOI] [PubMed] [Google Scholar]
- 13.Nowak-Gottle U, et al. Risk of recurrent venous thrombosis in children with combine prothrombotic risk factors. Blood. 2001;97(4):858–862. doi: 10.1182/blood.v97.4.858. [DOI] [PubMed] [Google Scholar]
- 14.Reverdiau-Moalic P, et al. Evolution of Blood Coagulation Activators and Inhibitors in the Health Human Fetus. Blood. 1996;88(6):900–906. [PubMed] [Google Scholar]
- 15.Monagle P, et al. Developmental haemostasis impact for clinical haemostasis laboratories. Thromb Haemost. 2006;95:362–372. doi: 10.1160/TH05-01-0047. [DOI] [PubMed] [Google Scholar]
- 16.Beardsley DS. Venous Thromboembolism in the Neonatal Period. Seminars in Perinatology. 2007;31:250–253. doi: 10.1053/j.semperi.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Malowany JI, Monagle P, Knoppert DC, Lee DSC, Wu J, McCusker P, et al. Enoxaparin for neonatal thrombosis: A call for a higher dose for neonates. Thrombosis Research. 2008;122(6):826–830. doi: 10.1016/j.thromres.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Hundsdoerfer P, et al. Homozygous and double heterozygous Factor V Leiden and Factor II G20210A genotypes predispose infants to thromboembolism but are not associated with an increase of fetal loss. Thromb Haemost. 2003;90:628–635. doi: 10.1160/TH03-02-0096. [DOI] [PubMed] [Google Scholar]