Abstract
The creation of decellularized organs for use in regenerative medicine requires the preservation of the organ extracellular matrix (ECM) as a means to provide critical cues for differentiation and migration of cells that are seeded onto the organ scaffold. The purpose of this study was to assess the influence of varying pH levels on the preservation of key ECM components during the decellularization of rat lungs. Herein, we show that the pH of the 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS)-based decellularization solution influences ECM retention, cell removal, and also the potential for host response upon implantation of acellular lung tissue. The preservation of ECM components, including elastin, fibronectin, and laminin, were better retained in the lower pH conditions that were tested (pH ranges tested: 8, 10, 12); glycosaminoglycans were preserved to a higher extent in the lower pH groups as well. The DNA content following decellularization of the rat lung was inversely correlated with the pH of the decellularization solution. Despite detectible levels of cyotoskeletal proteins and significant residual DNA, tissues decellularized at pH 8 demonstrated the greatest tissue architecture maintenance and the least induction of host response of all acellular conditions. These results highlight the effect of pH on the results obtained by organ decellularization and suggest that altering the pH of the solutions used for decellularization may influence the ability of cells to properly differentiate and home to appropriate locations within the scaffold, based on the preservation of key ECM components and implantation results.
Introduction
The prospect of using decellularized organs that have been recellularized by patient-specific progenitor cells for organ and tissue replacement opens the possibility for future clinical applications wherein an essentially autologous transplant occurs.1–3 Retention of extracellular matrix (ECM) components within the decellularized organ is crucial in influencing the behavior of cells that are subsequently placed on the decellularized scaffold.4–8 ECM components play a major role in the proper migration, protein expression, and active signaling pathways of the donor cells.9–13 We have previously shown that rat lungs decellularized by an alkaline detergent-based decellularization solution retain key ECM components including collagens, laminin, and fibronectin; other matrix components such as elastin and glycosaminoglycans (GAGs) are significantly diminished.4,14 This work also demonstrated that recellularization of these lungs was supported by the remaining ECM. This was demonstrated by reseeding the decellularized lung ECM scaffold with a heterogeneous pool of neonatal rat lung cells, which appropriately populated the respiratory compartment of the lung with a variety of epithelial cell types, including type 1 and type 2 alveolar epithelial cells. While our previous work has shown that several ECM components such as collagen are retained to a detectable level by using a decellularization solution at pH 12, here we extended those findings by testing a range of pHs on the retention of ECM components.
While an immediate goal of decellularization is to preserve the structure of the lung and its function as a substrate for cell growth, several ECM component proteins are of particular importance because of their abundance in the basement membrane or because of the role that they play in maintaining the mechanical integrity of the organ. For re-population of the decellularized lung ECM scaffolds, basement membrane proteins such as fibronectins and laminins play a direct role in the appropriate attachment and differentiation of seeded cells.8,9 For the maintenance of tissue architecture and to ultimately support breathing, critical ECM components including collagens, elastin, and proteoglycans are required.8 Retention of both the integrity of the basement membrane and mechanical function must be taken into account for optimization of the tissue engineering process.
We have previously compared two detergent-based methods of lung decellularization, one based on 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS, 8 mM in phosphate buffered saline [PBS] with 1 M NaCl and 25 mM EDTA) and the other based on sodium dodecyl sulfate (SDS; 1.8 mM SDS in PBS with 1 M NaCl and 25 mM EDTA).14 These findings indicated that decellularization with 8 mM CHAPS resulted in better collagen retention and, as a consequence, produced lungs with greater mechanical integrity when compared with the 1.8 mM SDS-based decellularization procedure. Both methods of decellularization, however, resulted in large losses of other ECM components including loss of elastin and sulfated glycosacminoglycans. Other methods of organ decellularization include the use of chemical methods that rely on alkaline conditions to decellularize tissues as a means to disrupt cellular membranes and remove nucleic acids.2,15 Given that the pH of the decellularization solution plays a role in the loss or retention of ECM components in other tissues, notably pericardium,16 we tested various CHAPS-based decellularization solutions varying only in pH in an effort to understand what role pH plays in the maintenance of the lung ECM. CHAPS solutions ranged in pH from 8 to 12. We found that lungs decellularized with the lower pH solutions retained more elastic fibers, fibronectin, and laminin. GAGs were also retained to a greater extent in the more neutral pH ranges. Histological analysis also confirms that the more mild pH decellularization conditions favor better preservation of the ECM. However, while DNA content was significantly decreased in all pH groups tested, there was more DNA retained in the lungs within the lower pH groups when compared with the pH 12 condition. Taken together, these data implicate the importance of pH levels on the preservation of the ECM.
Materials and Methods
Organ harvest and decellularization
Lung tissue was obtained from young adult (3-month-old) male Sprague Dawley rats (Charles River). All animal work was performed with approval from the Yale University Institutional Animal Care and Use Committee, and all animal care complied with the Guide for the Care and Use of Laboratory Animals. Organ harvest was performed as previously described.4 Briefly, animals were euthanized via intraperitoneal injection of sodium pentobarbital (140 mg/kg; Sigma-Aldrich) and heparin (250 U/kg; Sigma-Aldrich). The lungs were perfused via the right ventricle with PBS containing 50 U/mL heparin (Sigma-Aldrich) and 1 μg/mL sodium nitroprusside (Fluka) and the heart, lungs, and trachea were dissected and removed en bloc. Tracheal and pulmonary artery cannulae were inserted and sutured into place, to provide access for perfusion and decellularization. Per our previously published methods, lungs were decellularized via perfusion of the pulmonary artery while maintaining physiologically appropriate pulmonary arterial pressures (i.e., below 25 mmHg).4 In each case, a total of 500 mL of decellularization solution (8 mM CHAPS, 1 M NaCl, 25 mM EDTA in 1×PBS) was infused via the pulmonary artery; pH was adjusted prior to decellularization using hydrochloric acid to final pH values of 8.0, 10.0, and 12.0.
After decellularization, tissues were extensively rinsed via vascular perfusion with 1×PBS. The decellularized tissues were further rinsed with PBS containing 10% fetal bovine serum, 10% pen-strep solution, and 2% gentamicin for 48 h to remove any remnant cellular debris as described previously.17
Histological analysis and immunohistochemistry
Tissues were formalin-fixed, paraffin-embedded, and sectioned at a thickness of 5 μm. Analysis was performed with standard hematoxylin and eosin (H&E) staining, elastic van Gieson (for elastin), and Alcian blue at pH 2.5 (for proteoglycans). For immunohistochemistry, tissue sections were deparaffinized, rehydrated, and rinsed in PBS with 0.2% Triton X-100 for 15 min. Antigen retrieval was performed with 20 ng/mL proteinase K in TE buffer (pH 8.0), at 37°C for 10 min. Sections were then blocked with 5% bovine serum albumin and 0.75% glycine in PBS for 1 h at room temperature. Primary antibodies for fibronectin (ab6328; Abcam) and laminin (ab11575; Abcam) were applied 1:100 in blocking buffer overnight at 4°C, followed by secondary antibodies at 1:500 dilution for 1 h at room temperature. Primary antibody for β-actin (ab8226; Abcam) was applied at 1:200 in blocking buffer. Species appropriate secondary antibodies were used at 1:500 (Invitrogen). Slides were mounted using DAPI-containing mounting media.
Western blot
Tissues were digested in RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% [v/v] Triton X-100, 0.5% [w/v] sodium deoxycholate, and 0.1% [w/v] SDS) with freshly added protease inhibitors and homogenized at 15,000 rpm for 30 s. After incubation for 1 h at 4°C, insoluble particles were removed by centrifugation at 14,000 g for 25 min. Protein concentration was quantified via Bradford assay (Bio-Rad), and then placed in Laemmli's reducing buffer for 25 min at 65°C. Protein was run on polyacrylamide gels, transferred to a nitrocellulose membrane, and blocked for 2 h in 5% nonfat dry milk (NFDM) in TBS with 0.05% tween-20 (TBS-T). Primary antibodies of monoclonal anti-rat β-actin (ab8226; Abcam) at a dilution of 1:2000 and monoclonal anti-rat MHC class I (55917; Becton Dickinson) antibody of RT1A at a dilution of 1:500 were applied overnight in 1.5% NFDM in TBS-T, followed by horseradish peroxidase-conjugated goat secondary antibodies (sc-2020; Santa Cruz) for 1 h at room temperature at a dilution of 1:2000. Protein was detected using substrate from Supersignal West Pico (Thermo Scientific).
Collagen assay
Collagen was quantified with a colorimetric assay to detect hydroxyproline using a modification of Grant's method.18 Lung samples were lyophilized and weighed, then incubated in papain (10 U/mL; 25 mg/mL) at 60°C overnight (Sigma). Papain-digested samples were incubated in 6 N HCl at 115°C for 18 h, neutralized, oxidized with chloramine-T, and then reacted with p-dimethylaminobenzaldehyde. Absorbance was measured at a wavelength of 550 nm and a 1: 10 w/w ratio of hydroxyproline to collagen was used to calculate the collagen content of the tissue.
Sulfated GAG assay
Sulfated GAGs were quantified using the Blyscan GAG assay kit (Biocolor). Lung samples were lyophilized and weighed, then incubated in papain (25 mg/mL) at 60°C overnight (Sigma). Papain-digested samples (prepared as described for the collagen assay, above) were assayed according to the manufacturer's instructions. Absorption was measured at a wavelength of 650 nm, and GAG content was quantified using a standard curve.
DNA assay
DNA content of tissues was quantified using the Quant-iT PicoGreen dsDNA assay kit (Invitrogen), following the manufacturer's instructions. Briefly, tissue samples were weighed and lyophilized, diluted in TE buffer, and mixed with the Quant-iT PicoGreen reagent. Fluorescence was measured at 535 nm with excitation at 485 nm, and DNA content was quantified using a standard curve.
Transmission electron microscopy
Samples were fixed using 4% paraformaldehyde in PBS and then placed in 2% glutaraldehyde and 2.5% paraformaldehyde in 0.1 M sodium cacodylate buffered fixative for 2 h at room temperature. The samples were rinsed in 0.1 M sodium cacodylate buffer and postfixed in 1% osmium tetroxide for 1 h, then stained en bloc in 2% uranyl acetate in maleate buffer pH 5.2 for a further hour. The samples were rinsed, dehydrated through a graded ethanol series, and infiltrated with epon resin and baked overnight at 60°C. Hardened blocks were cut using a Leica UltraCut UCT and 60 nm sections were collected on nickel grids and stained using 2% uranyl acetate and lead citrate. Samples were viewed on a FEI Tecnai Biotwin transmission electron microscopy (TEM) at 80 kV. Images were taken using a Morada CCD digital camera using iTEM (Olympus) software.
Subcutaneous implant of decellularized lungs
The upper right (cranial) lobe from either native tissues or lungs decellularized with CHAPS solutions at pH 8, 10, and 12 were subcutaneously implanted into recipient Sprague Dawley rats. The animals were anesthetized via an IP injection of ketamine (100 mg/kg) and xylazine (10 mg/kg), followed by the creation of an abdominal incision through the skin of ∼1 cm. A pocket was created between the skin and the abdominal wall, into which half of a decellularized lobe was placed. Two lung pieces inserted into this subcutaneous pocket were ∼5 mm in length, and had an average decellularized weight of 197 mg (SD±72 mg). The skin was sutured and the implants were left in place for 7 days. After 7 days, the implants were explanted and photographed to document infiltration of host cells into the lung implant.
Results
Effectiveness of decellularization is dependent on pH
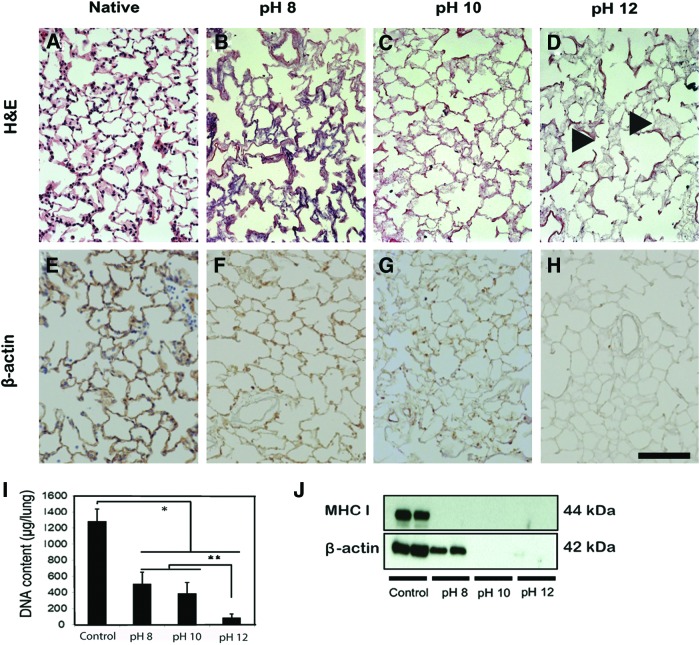
The gross morphological appearance of lungs decellularized at pH 12 differed from native tissue in that they were smaller, white, and semi-translucent. Lungs decellularized at pH 8 and at pH 10 appeared discolored (brown) and slightly less translucent than those decellularized at pH 12 (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tec). Unlike native tissue (Fig. 1A) all CHAPS-treated lungs were completely devoid of intact cells by standard H&E histological staining (Fig. 1B–D), though some deep blue remnants (DNA smears) were occasionally noted throughout the lung. These smears of DNA were more prevalent in the pH 8 group than in the pH 10 or 12 groups (Fig. 1B). Additionally, though all decellularized lungs retained the overall structure of both the proximal and distal regions of the lung, the general architecture of the alveolar septae was more intact in the lower (i.e., pH 8, 10) rather than higher (i.e., pH 12) group, (Fig. 1B, C, compared to D damage indicated by arrows) suggesting more damage to the matrix in the higher pH group.
FIG. 1.
Effectiveness of CHAPS-based decellularization is pH dependent. Hematoxylin and eosin (H&E) (A–D) and β-actin immunostaining (E–H, in brown) of lungs decellularized at various pHs. When compared to native lung tissue (A), no intact nuclei are observed in any decellularized tissue (B–D). There are, however, substantial DNA remnants in lung decellularized at pH 8 (B, F) and to a much lesser extent when decelled at pH 10 (C, G). Most of the cell DNA and cytoskeletal staining is negative in pH 12 group (D, H), although there is evidence of damage to the matrix at pH 12 (D, as indicated by arrowheads). DNA quantification in native and decellularized lungs (I) indicates that DNA decreases with increasing pH of decellularization solution. Western blot analysis for MHC class I and β-actin in decellularized lungs (J). Cell membrane protein MHC class I is not detected in any decellularized groups, and β-actin is only detected in the native and pH 8 group. Scale bar=100 μm. Asterisk indicates significant difference between the groups (*p<0.0001, **p<0.01) Data are expressed as mean values±SD, n=5. CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate. Color images available online at www.liebertpub.com/tec
Immunostaining for β-actin demonstrated an increase in the effectiveness of cytoskeletal protein removal with increasing pH. The alveolar septae remained strongly positive in the pH 8 group when compared to native tissue (Fig. 1E, F, respectively). In the pH 10 group, the β-actin staining of the alveolar septae was relatively weak (Fig. 1G). In the pH 12 group, the majority of the lung tissue was negative for β-actin staining (Fig. 1H); the alveolar septae were immune-negative for β-actin and denatured cell components were not evident (Fig. 1H). When quantifying DNA by the Quant-iT PicoGreen assay, it appears that the DNA content of all decellularized scaffolds were significantly lower than that of native lung (p<0.0001) (Fig. 1I). However, the contents were significantly higher in the pH 8 and pH 10 groups than that in pH 12 group (p<0.001). Western blot analysis revealed that MHC class I was not detected in any of the decellularized groups (Fig. 1J). However, beta actin was detected in the pH 8 group, suggesting insufficient decellularization when using a CHAPS-based solution at lower pH (Fig. 1J).
Protein content and organization after decellularization is pH dependent
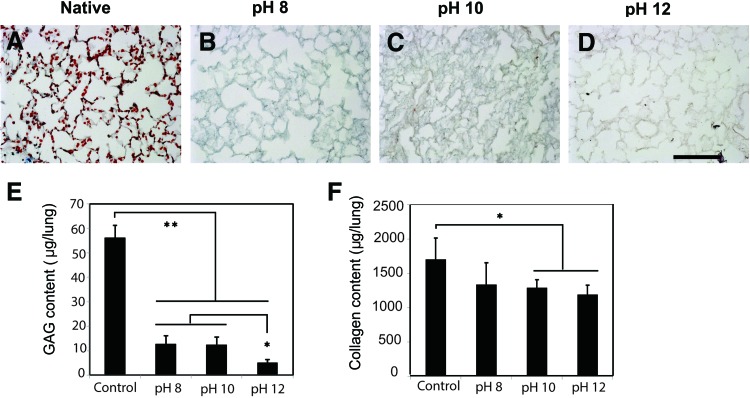
The principal determinants of both the tissue integrity and mechanical behavior of lung tissue are collagen, elastin, and GAGs. All three of these matrix components were analyzed for organization and depletion as a function of decellularization pH. Alcian blue histologic staining was utilized to evaluate the presence of GAGs, a matrix component responsible for the retention of water in the tissue. GAGs were noticeably diminished compared to native lung (Fig. 2A–E, in blue) in decellularized tissues, especially in the groups decellularized at pH 10 and pH 12 (Fig. 2C–E). To examine how the collagen content is effected by detergent pH, a hydroxyproline collagen assay was performed. The assay showed that total collagen content was diminished by high pH (Fig. 2F). Although the content was significantly lower in pH 10 and pH 12 groups than that of native lung (p<0.05), the remaining collagen was 70% of native lung, whereas the pH 8 group not significantly less than native lung.
FIG. 2.
Retention of GAGS and collagen in decellularized lungs is pH dependent. Immunostaining for GAGs in native (A) and decellularized lungs (B–D) at various pHs. Alcian blue staining reveals alveolar septae are strongly positive for GAGs in native lung (A), and dramatically decrease in GAG content when treated with increasing pH of decelluar solution. Sulfated GAG and collagen quantification (E, F) in native and decellularized lungs. GAGs were significantly lower in all decellularized lungs as compared with native. Collagen content was decreased in all decellularized groups, with a significant loss at pHs 10 and 12. Scale bar=100 μm. Asterisk indicates a significant difference between the groups (*p<0.05, **p<0.005). Data are expressed as mean values±SD, n=5. GAG, glycosaminoglycan. Color images available online at www.liebertpub.com/tec
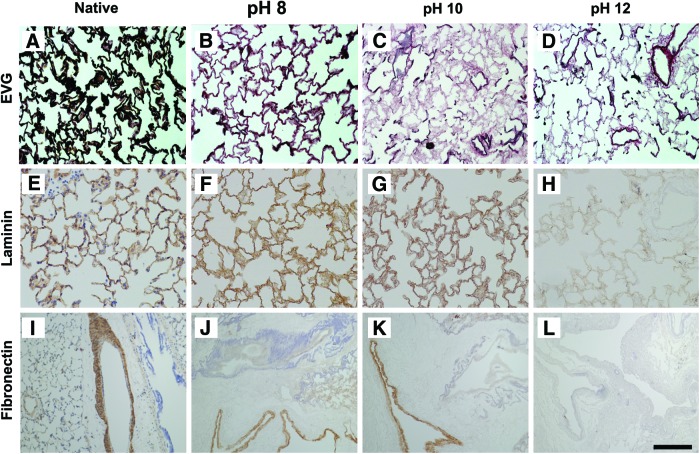
Verhoff von-Gieson staining was utilized to evaluate the presence and organization of elastic fibers, a critical component for elastic recoil of lung tissue. The septae of lower pH groups appeared thicker and possessed a greater abundance of dark, wavy elastic fibers (in black) than those of the higher pH groups (Fig. 3B, compared to C, D). Elastin content appeared to be less than that of native lung by staining, however (Fig. 3A). Identifiable fibers were largely absent in the lungs decellularized at pH 12. These data suggest that elastic fibers are only preserved when using decellularization solution around pH=8 or lower. Laminin was also strongly positive in the alveolar septa in both the low pH groups, but was markedly reduced in the pH=12 group (Fig. 3F–H). Immunohistochemical staining of fibronectin revealed that large blood vessels remained immunopositive for fibronectin after decellularization in both of the low pH groups, but that fibronectin was largely absent at pH=12 (Fig. 3I–L). With respect to the airways, the alveolar septa were strongly immunopositive for fibronectin in the pH 8 and 10 group and greatly diminished in the pH 12 groups.
FIG. 3.
Maintenance of elastin, laminin, and fibronectin in decellularized lungs is pH dependent. Elastic-Van Gieson (EVG) staining for elastin shows highly concentrated elastic fibers in native lung (A), reduced amounts in pH 8 group (B) and severely diminished levels in the pH 10 and 12 groups (C, D). Alveolar septae are strongly positive for laminin and fibronectin in native lung (E, I). Laminin and fibronectin are generally preserved in pH 8 and pH 10 groups (F and G; J and K), but not in pH 12 group (H, L). Scale bar=100 μm. Color images available online at www.liebertpub.com/tec
Transmission electron microscopy
Decellularization of lungs at pH 8, 10, and 12 resulted in the removal of intact cells and preservation of alveolar architecture and capillary structures (Fig. 4B–D), though the presence of cell debris appears to be inversely correlated with pH, as cellular remnants appear at pH 8 and 10, but not 12 (Fig. 4B, C, compared to D). In all cases, separation of compartments was maintained by intact basement membranes. The presence of collagen fibrils within the septae further illustrates the structural integrities of the decellularized scaffolds.
FIG. 4.
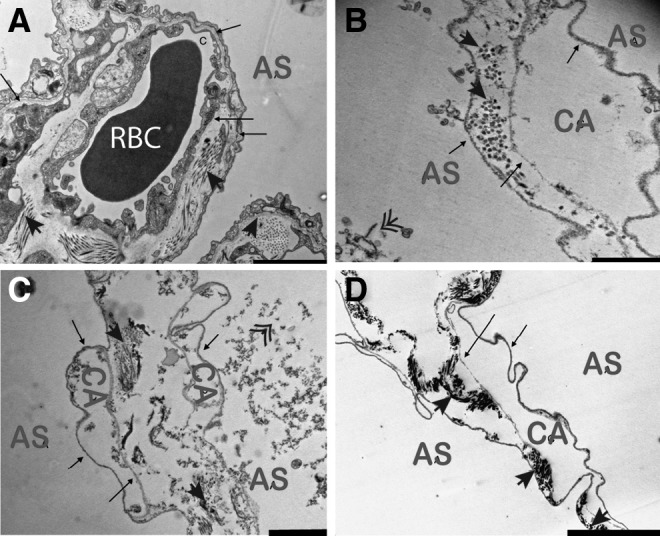
Transmission electron microscopy demonstrates maintenance of alveolar architecture and intact alveolar-capillary barriers. Native (A) and decellularized tissue at pH 8 (B), 10 (C), and 12 (D) illustrate maintenance of basement membranes (arrows) and overall alveolar architecture. Distinctions can be made between air spaces (AS), capillaries (CA), and red blood cells (RBC); collagen fibrils are present in the alveolar septae (solid arrow heads). While some cell debris is noted at pH 8 and 10 (double arrow heads); pH 12 is devoid of cellular components. Scale bar in A=5 μm, B=1 μm, C=2 μm, D=5 μm.
Subcutaneous implant of decellularized lungs
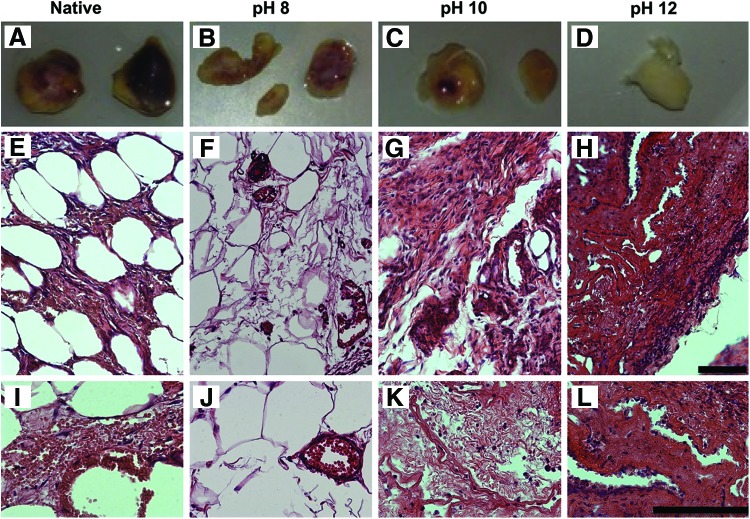
Decellularized lungs were subcutaneously implanted to assess whether host-derived immune response varied depending on the pH of the CHAPS decellularization solution (Fig. 5A–D). Unsurprisingly, after 7 days the implanted native lungs elicited the greatest host response (Fig. 5A), as indicated by the blackened regions of the tissue and several stiff nodules through the matrix. Similar to native tissue, all decellularized tissues remained intact during the implantation (Fig. 5B–D). The decellularized lungs appear to have varying degrees of color (red), decreasing with increasing pH. Upon examining H&E images of the native (Fig. 5E, I) lungs, it is clear that these tissues elicited an immune response as made evident by the thickening of the alveolar wall (Fig. 5I), the infiltration of host cells, and the general collapse of the tissue. Decellularized tissues also elicited host response; the amount of fibrotic tissue formation and host cell infiltration increased with increasing decellularization pH. Tissues decellularized at pH 8 (Fig. 5F, J) maintained the majority of alveolar structure, and sustained very few infiltrating cells (Fig. 5F). Of the cells that are present in the pH 8 scaffold, the majority are located in the vasculature and appear to be red blood cells (Fig. 5J). The pH 10 lungs demonstrated almost a complete fibrosis of the tissue (Fig. 5G, K), with very little alveolar regions remaining (Fig. 5G). The pH 12 group underwent the most severe host response of all the decellularized tissue by developing into a thick, fibrotic matrix.
FIG. 5.
Subcutaneous implants of lungs decellularized with pH 8, pH 10, and pH 12. CHAPS reveal differences in host immune reactivity, and concomitant infiltrating cells, when implanted into immunocompetent rats. Gross images of native (A) and acellular lung tissues (B–D) that were decellularized at pH 8 (B), 10 (C), or 12 (D) and subsequently subcutaneously implanted into immunocompetent rats for 7 days. Explanted lungs were visually inspected for evidence of immune reaction from the donor. A strong immune reaction was observed with the native implant as evidenced by blackened tissue, and the decellularized tissue demonstrated a loss in color (primarily red) with increasing pH. H&E images of native (E, l), and acellular lung tissues decellularized at pH 8 (F, J), 10 (G, K), or 12 (H, L). The pH 8 lungs largely maintained tissue architecture maintenance, and of the tissues demonstrated the lowest evidence of fibrotic tissue formation. In addition, it appears that the majority of infiltrating cells are red blood cells found in the vasculature (J). After 7 days, both pH 10 and 12 tissue samples were comprised of very dense tissue, with little evidence of alveolar structure retention. Decellularized lung implants had an average weight of 197 mg (SD±72 mg). Scale bars=50 μm. Color images available online at www.liebertpub.com/tec
Discussion
Matrix scaffolds fabricated from decellularized donor organs provide a means to tissue engineer custom-tailored and fully functional tissue for use in regenerative medicine. The goal of the present study was to further optimize a CHAPS-based decellularization process by adjusting the pH of the solution to minimize damage to the ECM scaffold, while maintaining the effective removal of cellular and nuclear material. Toward this goal, we examined the effectiveness of CHAPS as a decellularization agent at three different pH levels while examining the retention and organization of collagen, elastin, GAGs, laminin, and fibronectin in lung tissue. We found that decreasing the pH of the CHAPS solution resulted in a dramatic increase in global matrix preservation; however, this decrease in detergent alkalinity also resulted in a concomitant decrease in decellularization efficiency. Interestingly, implant studies revealed that this decrease in decellularization efficiency did not appear to induce as severe a host response as the more thoroughly decellularized and matrix-depleted tissues (i.e., tissues decelled at higher pHs).
When examining the gross morphological consequence of CHAPS-based decellularization, lungs exposed to detergents at pH 12 decreased in volume and resulted in a viscous effluent. In the lower pH groups (pH 8 and pH 10), the lung appeared very swollen and the entirety of the lung was filled with excessively viscous liquid. The observed swelling was still present in lungs treated with decellularization solution at pH 11 (data not shown). The observed difference in lung distension and effective decellularization between groups is likely due, in part, to pH-dependent swelling of liberated DNA.19 Specifically, the viscosity of DNA is very high at lower pHs (below 11.7), and at pH 11.7 is dramatically reduced to 10.9% of the intrinsic viscosity, and finally plateaus around a pH of 12.20 At pH 12, DNA denatures to a single strand (each single strand is free to adopt different random coiled conformations), each with a lower intrinsic viscosity than the double-stranded DNA. Therefore, it is possible that insufficient DNA removal observed in lower pHs might simply be due to the combination of high viscosity of DNA and the complex architecture of a whole organ. Therefore the “handle-ability” of the organ that is conferred by high pH could compromise the gains in matrix retention that is observed with lower pH solutions, depending on the application.
In our previous studies, we determined that CHAPS-based decellularization (at pH 12) resulted in decreased GAG and elastin content in decellularized tissues but not in total collagen content.14 GAGs are found on cell surfaces, within intracellular vesicles, and incorporated into the ECM.21 They help control macromolecular and cellular movement across the basal lamina, bind growth factors and cytokines, and contribute to the properties of the ECM by creating repulsive forces to sequester water using negatively charged “tails”.22 Because GAGS are intrinsically part of the cell surface, removal of cells and cell components will ultimately result in the depletion of cell-bound GAGs. Additionally, the solubility of GAGs embedded within the ECM increases in alkaline solutions.2 Accordingly, GAG levels were significantly lower in the higher pH groups (as evident by histology and the quantitative GAG assay) in the experiments described herein. Previously, we have demonstrated that the decrease in GAG content our CHAPS-treated decellularized scaffold does retain an ultimate tensile stress similar to normal lung.14 Although not measured in this study, this increase in GAG content due to a decreased detergent pH would likely assist in creating a more robust “native-like structure” scaffold with enhanced mechanical integrity. Further, the increase in GAG content would presumably assist in promoting desirable cell behaviors upon recellularization of the scaffolds.
Elastin is also an important ECM component in lung parenchyma, as it contributes to tissue elasticity, extensibility, and the intrinsic tissue recoil property essential for breathing.23 Additionally, elastic fibers endow the pulmonary vasculature with resilience and are critical to the function of arteries.24 Without sufficient elastin content, tissue degradation and calcification could occur upon implantation.25 Therefore, it would be highly desirable to preserve elastin during decellularization. As seen previously, elastic fibers were mostly retained in the high pH group.14 Elastin retention was, however, enhanced in groups decelled at a lower pH, indicating a pH-dependent preservation of the matrix.
Fibronectin and laminin are both important ECM basement membrane proteins for cell adhesion. They serve a prominent role in the normal biological function and development of the lung and promote the formation and maintenance of the vasculature.12 Additionally, the various laminin isoforms (α, β, and γ) contribute to the proliferation, differentiation, and migration of lung epithelial cells.26,27 In addition, there appears to be a contrasting relationship between the presence of certain isoforms of laminin (laminin-322) and fibronectin in the promotion of cell migration and adhesion in bronchial epithelial cells.28 Fibronectin, particularly isoforms containing the EIIIA segment, has also been shown to play a role in the proliferation of lung epithelial cells.29 Together these prior studies highlight the important role that the ECM plays in the proper maintenance of lung epithelium. These prior studies also demonstrate the importance of maintaining an adequate ECM structure in tissue-engineered lungs. As evidenced by histological examination, using the decellularization solution at a lower pH preserves the majority of the vascular and alveolar basement membranes. Specifically, immunostaining for fibronectin and laminin indicates that there is a greater retention of these ECM components in the lower pH decellularized lungs, pH 8 and pH 10 compared with lungs decellularized at pH 12. The blood vessels retained fibronectin at pH 10 but these structures were immunonegative at pH 12. The matrix retention exhibited at lower pHs may provide an additional advantage for cell attachment and adhesion in the recellularization process. In addition to the role that the ECM components play in the maintenance of lung epithelium, laminin and collagen have been shown to promote the differentiation and adhesion of endothelial cells.30
In terms of decellularization effectiveness, immunoblotting showed that MHC class I is negative in all decellularized groups (at all pHs) indicating that the components of 8 mM CHAPS, 1 M NaCl, and 25 mM EDTA are sufficient to lyse cells and remove cell membranes at all pH values examined. However, β-actin was detected via immunoblot and immunofluorescence in the group decellularized at pH 8, suggesting that CHAPS-based cytoskeleton removal is compromised at a lower pH. Further, when examining total DNA content (Fig. 1I, J), the removal of DNA was significantly decreased at lower pHs, as compared with higher pHs. Taken together, these data indicate that decellularization by the solution described is partially compromised when utilized at lower pHs.
A potential problem that may be associated with incomplete removal of DNA from the lung scaffold is the potential for the host to elicit an enhanced immune response to the implanted lung.31 To test this possibility, we performed subcutaneous implants of each of the lungs decellularized with different pH conditions. Within the constraints of our study, we found that the residual DNA within the lung scaffolds decellularized at lower pHs was not sufficient to induce a severe host response. Specifically, although the pH 12 lungs retained the least amount of remaining DNA as per our assays described above, these tissues had the greatest amount of infiltrating cells and the worst fibrotic response. In contrast, the pH 8 lungs had the least amount of infiltration, the greatest maintenance of tissue architecture, and interestingly, an apparent infiltration of blood into the vasculature of the implanted tissue. Although this finding is preliminary in terms of sample size and implant duration, our results suggest that within the range of residual DNA that is present after our decellularization methods, minimizing host response is more dependent on an intact ECM scaffold than was previously considered. Although not examined in this study, it is possible that remaining ECM available postalkaline decellularization is fragmented, and that the damaged elements of the ECM are inducing an even more severe host response than the residual DNA found in tissue decellularized at lower pH.32
The use of CHAPS under strong alkaline conditions has previously been considered a “double-edged sword”: it allows for complete removal of cells and cellular debris, but in exchange for substantial matrix damage including loss of GAGs, elastin, fibronectin, and laminin. If the goal of decellularization is total removal of DNA and immunogenic molecules (i.e., prioritizing removing donor immunogenicity),31 it seems that higher alkaline treatment detergent would be a better choice as a decellularization solution. Macchiarini et al. reported that although decellularized tissue-engineered trachea still retained some pre-existing cellular elements in the cartilaginous regions, it did not induce inflammation and did not require immunosuppressive drugs. They speculate that retained elements may even provide helpful signals to both graft and host cells.3 However, the preservation of key matrix components is important for successful clinical use to restore functionality to the organ of interest.
Therefore, our data indicate that a near physiological, reduced pH CHAPS-based solution may be a suitable compromise (i.e., at pH 8). ECM components are better preserved with this pH condition, and after 7 days post subcutaneous implant the host response appears to be less than at other pH conditions. There is, however, potential that these organs may elicit a greater amount of host cellular infiltrate in response to the residual cell debris over time, and further evaluation will be needed to determine both longer-term reactions and recellularization capacity of these tissues. Additional evaluation may be beneficial to optimize the most suitable decellularization protocol for generating an appropriate acellular ECM for preclinical and clinical use.
Conclusions
This study contributes to the growing body of literature dedicated to producing a decellularized scaffold for use in regenerative medicine. Our comparison of the decellularization capacity of a CHAPS-based decellularization solution at different pHs clearly shows differential effects in terms of matrix preservation, cellular removal, and host response. The lower pH solution suppressed ECM damage, most notably the loss of GAG and elastin content, but could not effectively remove all DNA. Despite the residual DNA, however, lower pH acellular matrices also elicited the lowest host response in short-term implant studies. Although recelluarization and eventual clinical effect remains to be seen, the present pH-based study adds important information in the field of tissue engineering and scaffold generation via decellularization.
Supplementary Material
Acknowledgments
We wish to thank Dr. Liqiong Gui and Dr. Sumati Sundaram for their invaluable information and advice. We are also grateful to Angela Huang, Sashka Dimitrievska, and Michael Boyle for technical assistance.
Disclosure Statements
This work was funded by NIH R01 HL098220-01 (L.E. Niklason), and in part by an award from United Therapeutics, Inc. United Therapeutics did not affect the content or conclusions of this article. J. Mendez is supported by NIH T32 GM086287-01 (L.E. Niklason). L.E. Niklason has a financial interest in Humacyte, Inc., a regenerative medicine company. Humacyte did not fund these studies, and Humacyte did not affect the design, interpretation, or reporting of any of the experiments herein.
References
- 1.Schmidt C,. and Baier J.Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials 21,2215, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Gilbert T., Sellaro T., and Badylak S.Decellularization of tissues and organs. Biomaterials 27,3675, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Macchiarini P., et al. Clinical transplantation of a tissue-engineered airway. Lancet 372,2023, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Petersen T.H., et al. Tissue-engineered lungs for in vivo implantation. Science 329,538, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ott H.C., et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med 14,213, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Ott H.C., et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med 16,927, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Song J.J., et al. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med 19,646, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunsmore S., and Eugene Rannels D.Extracellular matrix biology in the lung. Am J Physiol 270, L3-27, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Brown B., Lindberg K., Reing J.E., Beer Stolz D., and Badylak S.The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng 12,519, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Cortiella J., et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A 16,2565, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Cavalcante F., et al. Mechanical interactions between collagen andproteoglycans: implications for the stability of lung tissue. J Appl Physiol 98,672, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Badylak S.F., Taylor D., and Uygun K.Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng 13,27, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoshiba T., Lu H., Kawazoe N., and Chen G.Decellularized matrices for tissue engineering. Expert Opin Biol Ther 10,1717, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Petersen T.H., Calle E.A., Colehour M.B., and Niklason L.E.Matrix composition and mechanics of decellularized lung scaffolds. Cells Tissues Organs 195,222, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falke G., Yoo J., Gyun Kwon T., and Moreland R.Formation of corporal tissue architecture in vivo using human cavernosal muscle and endothelial cells seeded on collagen matrices. Tissue Eng 9,871, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Mendoza-Novelo B., et al. Decellularization of pericardial tissue and its impact on tensile viscoelasticity and glycosaminoglycan content. Acta Biomater 7,1241, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Gui L., chan S.A., Breuer C.K., and Niklason L.E.Novel utilization of serum in tissue decellularization. Tissue Eng Part C Methods 16,173, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant R.A.Estimation of hydroxyproline by the autoanalyser. J Clin Pathol 17,685, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song J.J., et al. Enhanced in vivo function of bioartificial lungs in rats. ATS 92,998, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Ehrlich P., and Doty P.The alkaline denaturation of deoxyribose nucleic acid. J Am Chem Soc 80,4251, 1958 [Google Scholar]
- 21.Ferdous Z., and Grande-Allen J.G.Utility and control of proteoglycans in tissue engineering. Tissue Eng 13,1893, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Badylak S.Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transplant Immunol 12,367, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Yuan H., et al. Effects of collagenase and elastase on the mechanical properties of lung tissue strips. J Appl Physiol 89,3, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Kielty C.M., Sherratt M.J., and Adrian Shuttleworth C.Elastic fibres. J Cell Sci 115(Pt 14),2817, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Isenburg J.C., Simionescu D.T., and Vyavahare N.R.Tannic acid treatment enhances biostability and reduces calcification of glutaraldehyde fixed aortic wall. Biomaterials 26,1237, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Nguyen N.M., and Senior R.M.Laminin isoforms and lung development: all isoforms are not equal. Dev Biol 294,271, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Miner J.H., and Yurchenco P.D.Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol 20,255, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Kligys K., et al. Laminin-332 and alpha3beta1 integrin-supported migration of bronchial epithelial cells is modulated by fibronectin. Am J Respir Cell Mol Biol 49,731, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kikuchi W., Arai H., Ishida A., Takahashi Y., and Takada G.Distal pulmonary cell proliferation is associated with the expression of EIIIA+fibronectin in the developing rat lung. Exp Lung Res 29,135, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Filova E., et al. Improved adhesion and differentiation of endothelial cells on surface attached fibrin structures containing extracellular matrix proteins. J Biomed Mater Res A 102,698, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Wallis J.M., et al. Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng Part C Methods 18,420, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Badylak S.F.Decellularized allogenic and xenogeneic tissue as a bioscaffold for regenerative medicine:factors that influence the host response. Ann Biomed Eng 2014[Epub ahead of print]; DOI 10.1007/s10439-013-0963-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.