Abstract
In the tissue engineering field dynamic culture systems, such as spinner flasks, are widely used due to their ability to improve mass transfer in suspension cell cultures. However, this culture system is often unsuitable to culture cells in three-dimensional (3D) scaffolds. To address this drawback, we designed a multicompartment holder for 3D cell culture, easily adaptable to spinner flasks. Here, the device was tested with human mesenchymal stem cells (MSCs) seeded in 3D porous chitosan scaffolds that were maintained in spinner flasks under dynamic conditions (50 rpm). Standard static culture conditions were used as control. The dynamic conditions were shown to significantly increase MSCs proliferation over 1 week (approximately 6-fold) and to improve cell distribution within the scaffold. Moreover, they also promoted osteogenic differentiation of MSCs, inducing an earlier peak in alkaline phosphatase (ALP) activity, and a more homogenous ALP staining and matrix mineralization in the whole scaffolds, but particularly in the center. Overall, this study shows a new multicompartment holder to culture 3D scaffolds that can broaden the application of spinner flasks.
Introduction
In the tissue engineering field, dynamic culture conditions are known to recreate a more physiological environment, characteristic of living tissues.1,2 Spinner flasks are probably the simplest dynamic culture systems commercially available. They have been used for long times in cell culture, and they are the first step when it comes to scale up the classical two-dimensional (2D) cell culture. This culture system environment is externally controlled by the incubator environment (O2, pH, and humidity) but agitation is controlled in terms of velocity and type of impeller.2,3 It has been widely explored for the culture of suspension cells, and in some cases for attachment-dependent cells in the form of cell aggregates4,5 or cell-loaded microcarriers.3,6–9 However, only a few studies described the use of spinner flasks for cell cultures in three-dimensional (3D) scaffolds. In those cases, the 3D constructs have been cultured in different ways: (1) maintained in free floating,10,11 (2) threaded in stacks onto needles embedded in the stoppers of the spinner flask12–15 or (3) directly fixed with clamps to the spinner's wall.16 However, these solutions can damage both the scaffolds and the attached cells and can contribute to an heterogeneous cell distribution. An approach where the constructs were maintained in a vertically suspended position, closed with stainless steel screws showed that the holding molds cannot be easily handled for sampling collection over time.17
The use of adequate dynamic conditions is critical to implement successful 3D cultures and boost the use of 3D scaffolds as a more physiological approach in tissue engineering. In view of the prior art, a multicompartment holder, adaptable to standard spinner flasks, was designed to overcome the lack of appropriate solutions for dynamic cell cultures in 3D scaffolds.18 The proposed device provides a means of protecting fragile matrices from the turbulent environment generated by the conventional stirring process, inappropriate for free-floating cultures or exposed matrices. It also allows the easy individual handling of the samples during the course of an experiment, expanding the versatility of spinner flask systems.
This study explores the use of this multicompartment container in spinner flasks to culture human mesenchymal stem cells (MSCs) in 3D scaffolds of chitosan (Ch), a well-known polymer. Cell viability, metabolic activity, proliferation, and spatial distribution within the 3D matrix were analyzed in both, dynamic and static, conditions. Moreover, MSCs osteogenic differentiation and the production of endogenous extracellular matrix (ECM) were analyzed in the culture system here proposed.
Materials and Methods
Preparation of Ch 3D scaffolds
High-molecular weight (Mw) Ch (France Chitine) was purified as previously described.19 Porous scaffolds of purified Ch (Mw of 366±47×103; degree of acetylation of 13.5±0.8) were prepared by freeze-drying as described in previous works from our team.20 Briefly, Ch powder was first dried for 48 h at 60°C. Ch viscous solution (2% w/v in 0.2 M acetic acid) was casted in a 48-well plate mold and frozen at −20°C for 24 h. The frozen plates were lyophilized at −80°C for 48 h, under vacuum. Ch scaffolds were removed from the plate and cut in a cylindrical shape with a trephine blade (4 mm diameter×3 mm height) to fit in the holders of the multicompartment holder. The scaffolds were individually weighted and stored, protected from light and humidity until further use. Ch scaffolds were characterized by scanning electron microscopy as described in Supplementary Materials and Methods (Supplementary Data are available online at www.liebertpub.com/tec).
Routine culture of human MSCs
MSCs (Lonza) were routinely expanded in basal medium (BM) composed of low-glucose Dulbecco's modified Eagle's medium (DMEM; Gibco), supplemented with 10% v/v fetal bovine serum (FBS, MSCs qualified; Gibco) and 1% v/v penicillin/streptomycin (Pen/Strep; Gibco).21 Cells were seeded at a concentration of 3000 cells/cm2 and expanded in T-flasks at 37°C, under a humidified atmosphere of 5% v/v CO2 in air, with culture medium being changed twice a week and trypsinized when reaching 70% confluence. All experiments were performed with MSCs at passage 7.
MSCs seeding in Ch 3D scaffolds
Lyophilized Ch scaffolds were hydrated through a series of graded decreasing ethanol solutions (70%, 50%, and 25% v/v) and in phosphate-buffered saline, as described.20 To promote cell adhesion, Ch scaffolds were incubated overnight with human fibronectin (FN, 40 μg/mL; Invitrogen), at 250 rpm and 37°C. MSCs seeding in Ch scaffolds was performed in a 96-well tissue culture polystyrene plate previously coated with poly(2-hydroxyethyl methacrylate) (0.8 mg/cm2).22 Ten microliters of cell suspension (5×105 MSCs) were dropped in scaffolds' top. After 4 h, the scaffolds were immersed in 150 μL of culture medium. After 24 h, the percentage of cell adhesion was determined as follows:
 |
MSCs culture in 3D Ch scaffolds in static and dynamic conditions in the multicompartment holder for spinner flask
MSCs-loaded Ch scaffolds were transferred either to six-well culture plates (BD Falcon) (static culture) or to a multicompartment holder (Fig. 1)23 in a 25 mL spinner flask (Bellco Biotechnologies) (dynamic conditions). Each multicompartment holder has six independent compartments (6 mm diameter×6 mm height) to house disc-shaped samples with a maximum size of 5 mm diameter×5 mm height. The compartments have 1 mm perforations (top, bottom, and side) to permit fluid perfusion through the 3D cell-material constructs. The container has a perforated lid to retain free-floating samples. All the components can be sterilized in an autoclave, and then assembled under sterile conditions.
FIG. 1.
Details of the multicompartment holder system for three-dimensional (3D) dynamic cell culture in spinner flask. (A) Perforated container and lid. (B) Stirring bar made of stainless steel. (C) Assembly of the multicompartment holder with the lid; the impeller support was modified to accommodate the container. (D) Scheme of the impeller shaft (1) with a magnet support (2) and a magnet (3), holding two containers (4) and a lid (5). (E) Scheme of two multicompartment holders with a lid in the spinner flask (6). Color images available online at www.liebertpub.com/tec
In dynamic conditions, spinner flasks were maintained under continuous agitation (50 rpm) 24 h after transference of six cell-seeded constructs, in a total volume of 24 mL, 50% of the medium being renewed after 1 week. The entire apparatus, including a magnetic stir plate, was placed in a standard incubator (37°C, 95% humidified air and 5% v/v CO2).
Static cultures in six-well plates were performed with one scaffold per well in 4 mL of culture medium, to maintain the same volume-to-disc ratio used under dynamic conditions. Medium feeding regimen was also identical to the one used for dynamic conditions. In both cases, cultures were maintained during 14 days. Periodically, the samples were collected and analyzed for cell viability (LIVE/DEAD assay), cell proliferation (DNA quantification by PicoGreen assay and Ki-67 immunostaining), cell metabolism (Glucose and Lactic Acid quantification), and cell spatial distribution (hematoxylin&eosin [H&E] staining). All the assays are extensively described in Supplementary Materials and Methods.
MSCs differentiation into osteogenic lineage in Ch 3D scaffolds in static and dynamic conditions in the multicompartment holder for spinner flask
MSCs seeded onto Ch 3D scaffolds were cultured in osteoinductive medium (OM), as previously described.24 The OM includes low-glucose DMEM supplemented with 10% v/v FBS (preselected batch from PAA), 1% v/v Pen/Strep, 100 nM dexamethasone (Sigma), 10 mM β-glycerophosphate (Sigma), and 0.05 mM 2-phospho-L-ascorbic acid (Fluka). The MSCs cultured in OM were maintained during 28 days, under static and dynamic conditions, with 50% of the culture medium being exchanged every 7 days. MSCs differentiation was analyzed by quantification of alkaline phosphatase (ALP) activity, ALP and von Kossa staining, and production of collagen type I (Col I). Detailed protocols for each analysis are described in Supplementary Materials and Methods.
Statistical analysis
The experimental results are presented as the mean±standard deviation. Statistical analysis was performed using GraphPad Prism vs. 6.0 for Windows (vs. 6.01). The nonparametric Mann–Whitney test was used to compare samples of nonrelated conditions. A significance value of at least p<0.05 (*) was considered.
Results
Cell viability of MSCs 3D cultures in the multicompartment holder for spinner flask
Ch scaffolds prepared and characterized as described in Supplementary Data, were previously incubated with FN (40 μg/mL), as FN adsorption was shown to promote MSCs adhesion in preliminary assays (Supplementary Fig. S2). The cell seeding efficiency after 24 h was about 97.2%±1.6%. Representative images of cell viability analysis of the matrices' surfaces are depicted in Figure 2.
FIG. 2.
Viability of mesenchymal stem cells (MSCs) cultured in chitosan (Ch) 3D scaffolds in static and dynamic conditions. Representative CSLM images (z-stacks) from LIVE/DEAD cytotoxicity/viability assay performed at days 7 and 14 of culture (Calcein AM stains live cells in green; EthD-1 stains dead cells in red; scale bar, 200 μm). Color images available online at www.liebertpub.com/tec
It could be observed that MSCs remained viable for 14 days in culture, in both static and dynamic conditions. When looking in more detail, cell viability appears to be higher in the scaffolds cultured in the spinner flask, within the multicompartment holder, particularly at day 7. After 14 days of culture, a lower cell density was detected in the surface of the scaffold kept under dynamic conditions. Moreover, cells are better distributed throughout the scaffolds' surface, forming a monolayer, while in the static control more cell clusters were formed.
MSCs proliferation and metabolic activity in 3D cultures in the multicompartment holder for spinner flask
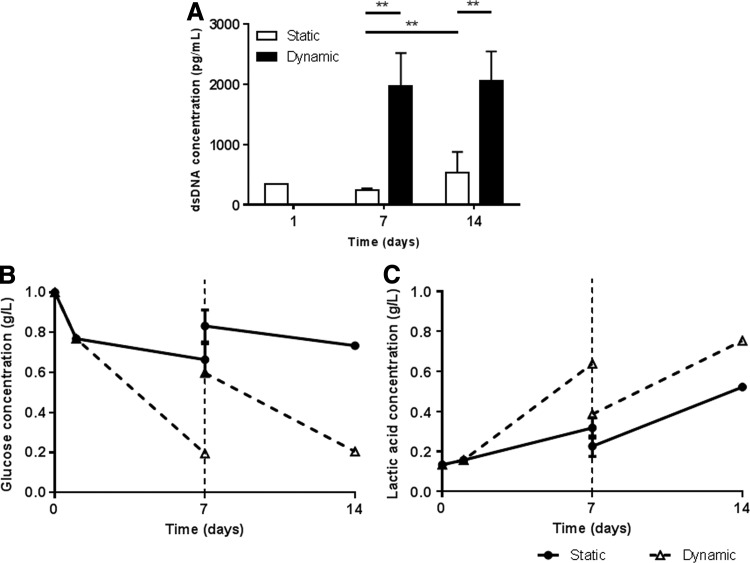
The amount of DNA determined at days 1, 7, and 14 in each scaffold is presented in Figure 3A. In general, it was possible to observe an increase in DNA content along time in culture for both conditions, suggesting that cells were able to proliferate. Under static conditions, the DNA content slightly decreases from days 1 to 7 (about 0.7±0.3-fold), after which it significantly increases (**p<0.025), about 2.3±1.6-fold after 14 days in culture, suggesting cell recovery. On the other hand, under dynamic conditions, DNA content rapidly increases, reaching ∼5.8±2.4-fold after 7 days in culture, after which it stabilizes (6.1±1.8-fold after 14 days in culture). Dynamic conditions induce significantly higher (*p<0.05) increase in MSCs proliferation in 3D cultures.
FIG. 3.
Proliferation and metabolic activity of MSCs cultured in Ch 3D scaffolds under static and dynamic conditions. (A) DNA content of cell-loaded scaffolds was quantified using PicoGreen assay. DNA content is significantly higher (**p<0.025) in dynamic versus static cultures. In static, DNA content significantly increases (**p<0.025) from day 7 to 14. Results are shown as mean±standard deviation (SD) (n=5). (B) Glucose and (C) lactic acid concentration (g/L) in culture supernatants collected during time in culture. Results are shown as mean±SD (n=3 replicates of one from three independent experiments).
In addition, glucose and lactic acid levels were quantified in the collected supernatants during culture. The results obtained show higher glucose consumption and higher lactic acid production under dynamic conditions, when compared with the static control (Fig. 3C, D), which is in accordance with the trend in DNA content. Importantly, glucose concentration in the medium was always higher than 0.2 g/L, indicating that these conditions did not lead to glucose starvation. Also, the lactic acid production did not exceed 0.8 g/L, which is far from the lactic acid level known to be inhibitor for MSCs cultures (3.2 g/L).25 These observations also corroborate the cell viability (LIVE/DEAD) results, in which cell density appeared to be higher after 7 days of culture in dynamic conditions.
MSCs spatial distribution within 3D scaffolds cultured in the multicompartment holder for spinner flask
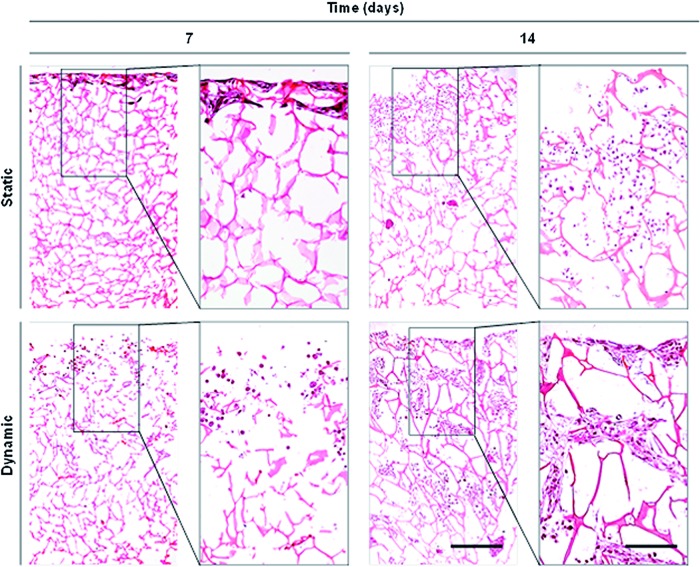
To compare cell distribution within 3D scaffolds cultured in static and in dynamic conditions, cross sections of paraffin-embedded scaffolds were stained with H&E (Fig. 4). The images clearly showed the scaffolds' architecture (in pink) and the presence of MSCs (in purple). In static conditions, MSCs were found only in the scaffolds' periphery, with a more round shape, occasionally forming multicellular aggregates. Contrarily, in dynamic conditions, MSCs present a more spread-out shape and are better distributed throughout the depth of the porous matrix, particularly after 14 days of culture. This observation clearly shows that cells are able to proliferate and/or migrate toward scaffolds' center, only when cultured under dynamic conditions within the multicompartment holder in the spinner flasks.
FIG. 4.
Comparison of MSCs distribution in Ch 3D scaffolds in static and dynamic cultures at days 7 and 14. Hematoxylin&eosin (H&E) staining of scaffolds' cross sections (7 μm photomicrographs, scale bars: 500 and 200 μm) show cells (in purple) distributed along periphery and center of the scaffolds (in pink). Color images available online at www.liebertpub.com/tec
MSCs osteogenic differentiation in 3D cultures in the multicompartment holder for spinner flask
MSCs cultured in Ch 3D scaffolds were able to survive, actively proliferate, and migrate through the scaffold's depth, when cultured in dynamic conditions. Therefore, the potential of these cells to undergo osteogenic differentiation was further explored by analyzing ALP expression and mineralization. As depicted in Figure 5A, samples from dynamic 3D cultures visibly presented higher ALP expression until day 14. From day 21 to 28, ALP staining was particularly intense in the static conditions, showing multicellular aggregates, with most cells being positive at day 28. Nevertheless, the analysis of transversal sections of the scaffolds clearly shows that a higher number of positive ALP cells are observed in scaffolds' center in the dynamic system, when compared with the static, in which almost no staining was detected. These observations are in agreement with the previous results that showed increased homogeneous distribution of cells within the 3D structures. ALP specific activity was also quantified as depicted in Figure 5B, confirming the results observed for the staining (also, low ALP activity was detected in basal conditions, as observed in Supplementary Fig. S3). The major observed difference seemed to be an early peak of ALP in dynamic conditions, when compared with the static control. However, it was only possible to do the analysis until day 14, since for longer time periods the digestion of the scaffolds was impaired. We hypothesize that this should be due to the high amount of matrix present in the scaffolds at days 21 and 28. To assess matrix mineralization, von Kossa staining was performed at day 28. The staining of the whole scaffolds demonstrated that matrix mineralization occurred at both culture conditions, being higher when scaffolds were cultured under dynamic conditions. This was evident at both the periphery and center of the scaffolds (Fig. 5C).
FIG. 5.
MSCs differentiation into osteogenic lineage when cultured in Ch 3D scaffolds in static and dynamic conditions. (A) Representative images of alkaline phosphatase (ALP) staining in whole scaffolds (scale bar, 1 mm). (B) Quantification of ALP activity (nmol/min/mg total protein). Results are shown as mean±SD (n=4); (*p<0.05; ALP activity is significantly higher in dynamic versus static conditions). (C) Representative images of von Kossa staining after 28 days of culture (scale bar, 1 mm). (D) Immunostaining of collagen type I (Col I) produced by MSCs after 28 days of culture (7-μm scaffolds' cross sections; scale bars: 200 and 50 μm). OM, osteoinductive medium. Color images available online at www.liebertpub.com/tec
The presence of Col I, a common bone phenotypic marker, was also detected in both 3D culture systems, confirming that MSCs were able to secrete endogenous ECM components, corroborating the previous results with ALP and von Kossa that evidenced the benefic effect of dynamic system (Fig. 5D).
MSC spatial distribution and metabolic activity in osteogenic 3D cultures in the multicompartment holder for spinner flask
To confirm that the observed pattern of MSCs distribution within 3D structures was maintained when moving from basal to osteoinductive conditions, scaffolds were likewise cross sectioned and analyzed by H&E staining (Fig. 6A). Representative images of the sections corroborate the findings observed under basal conditions. As depicted, even at day 28, cells were essentially present at the scaffolds' periphery on the static system, in contrast with the dynamic conditions, where cells exhibited a more homogeneous distribution throughout the scaffolds' transversal section.
FIG. 6.
Comparison of MSCs distribution and metabolic activity in Ch 3D scaffolds in static and dynamic cultures under osteogenic conditions. (A) H&E staining of scaffolds' cross-sections (7-μm photomicrographs; scale bar, 200 μm) show cells (in purple) distributed along periphery and center of the scaffolds (in pink). (B) Glucose and (C) lactic acid concentrations (g/L) were quantified in culture supernatants. Results are shown as mean±SD (n=3 replicates from n=1 representative experiment). Color images available online at www.liebertpub.com/tec
Glucose and lactic acid concentrations were also monitored to indirectly evaluate cell viability (Fig. 6B). Once more, higher glucose consumption was observed in the dynamic system, in particular in the first week of culture. This observation was confirmed by the lactic acid production (Fig. 6C), in which the higher production was observed in the first week of culture, decreasing until the last week. These results support the hypothesis that MSCs remained viable in the scaffolds cultured under osteogenic and dynamic conditions. However, as long as cell differentiation progressed, the metabolic activity was reduced.
Discussion
This study describes a method of culture 3D scaffolds under dynamic conditions in spinner flasks, using a simple and easy handling multicompartment holder.23 In the literature, cell culture in 3D scaffolds within spinner flasks has been generally performed in free-floating conditions.10,11 In specific cases, scaffolds were held by needles12–15,26 or clamps16 to the spinner's cap or wall. However, these approaches do not protect the cells from the stirring process and can also damage the scaffolds. Maintaining the samples within closed molds has also been described,17 but this system cannot be easily handled during the culture period.
The proposed multicompartment holder has been specially designed to fit into standard spinner flasks and can be used to protect delicate samples from the turbulent environment generated by the stirring process. It is characterized by its simplicity, since it can be easily adapted to different spinner flasks. It is versatile, due to its multicompartments, allowing for a large number of experimental conditions to be simultaneously tested in the same media conditions (e.g., different types of scaffolds), since each compartment can be accessed separately. Therefore, samples can be individually handled at specific times during the course of an experiment. This device is expected to expand the field of application of spinner flasks, considering there is a lack of adequate solutions for 3D cell cultures in scaffolds (e.g., hydrogel-like) in dynamic conditions. This product could also lead to sizeable saving on culture media. Although it has been designed for small-scale spinner flasks, it can be easily scaled up to large-scale industrial applications.
Here, to validate the proposed device, we cultured MSCs in Ch 3D scaffolds. These scaffolds have been widely used by our team20,27–29 as Ch is considered to be one of the most promising biomaterials for tissue-engineering applications, in particular for bone regeneration. This material induces a controlled foreign body reaction, has antibacterial properties, can be easily molded into porous structures, which are suitable for cell ingrowth, and it is also known to be osteoconductive.30 In this study, Ch scaffolds were precoated with bovine FN (40 μg/mL) to improve cell adhesion, as previously described.27
Dynamic conditions were established using spinner flasks with agitation speed of 50 rpm. Typically, low stirring speeds from 50 to 80 rpm on small-scale spinner flasks are used among the literature and have proven to be efficient to reduce the concentration gradients of oxygen and nutrients, particularly for MSCs culture in microcarrier-based systems31–34 and polymeric scaffolds.12,15,35 Of notice is the importance of maintaining an initial static period of 24 h to promote robust cell seeding onto the scaffolds, before starting stirring. This time was essential to prevent cell detachment and death (data not shown), and has been also referred by other authors like Hewitt et al.36 To prevent initial cell loss, other authors have opted to use initial low agitation speed, which was afterward increased.7
MSCs 3D cultures in the multicompartment holder in spinner flasks were analyzed in what concerns cell viability, metabolic activity, proliferation, and spatial distribution. Although a high number of viable cells was observed, at day 14, the number of cells in the scaffolds' surface appears to be lower in dynamic than in static conditions, which lead us to hypothesize that cells could have migrated toward the center of the scaffolds or removed by the agitation, although this last hypothesis was less probable since the holder is expected to protect samples from stirring. Cell proliferation confirmed the good performance of the dynamic conditions with the multicompartment holder, since an increase in DNA content (6.1±1.8-fold) was observed after 14 days, which was not observed in static conditions (2.3±1.6-DNA fold increase). These values are in the range of the ones observed with other MSCs expansion systems, using microcarriers in spinner flasks, although do not present the microcarriers-associated disadvantages as cell detachment from microcarriers' surface or aggregates formation.3,7,33,36 Accordingly, in terms of metabolic activity, higher glucose consumption accompanied by higher lactic acid production was observed in dynamic vs. static conditions, although lactic acid values were always below growth-inhibitory concentrations.3,7,25 Other authors have correlated MSCs growth with glucose and lactic concentrations, concluding that increasing cell numbers indicate result in lower glucose and higher lactic acid concentrations in the medium.37
One of the most remarkable features of this dynamic culture system is its clear effect on spatial cell distribution within 3D structures. This effect of spinner flask cultures has been described by Stiehler et al.35 and Mygind et al.15 in 3D scaffolds of PLGA and hydroxyapatite, respectively. However, in both studies the scaffolds had to be punctured by needles supported by the spinners' lid.
Due to the good outcome in basal conditions, the culture in the multicompartment holder in the spinner flask was conducted in osteogenesis-inducing conditions to evaluate cell differentiation in this system. Previous work from our group has already shown that FN adsorption on Ch scaffolds per se induces osteogenic differentiation of rat bone marrow stromal cells.38
Along 28 days, cell differentiation was periodically evaluated by ALP expression, matrix mineralization, and production of Col I. The results indicate that the dynamic culture system proposed here clearly promoted an earlier and more homogenous MSCs differentiation along the osteoblastic lineage, in accordance to literature using other dynamic culture systems.35,39,40 It is known that MSCs differentiation is highly dependent on cell density and the establishment of cell–cell contact,7,41 which might be favored when scaffold-colonization is more effective. We cannot exclude that MSCs osteogenic differentiation was also promoted by shear-stress related with the use of dynamic conditions. Previous studies demonstrated that shear stress increased ALP activity and other osteogenic gene markers in MSCs cultures under dynamic conditions.42
This increased cell differentiation was accompanied by a decrease in cell metabolism, which was also already reported for MSCs culture in 2D.43 Of relevance is the fact that when we look into more detail of ALP and von Kossa stainings it is evident that the holder in the spinner flask contributed to increased cell differentiation and mineralization in the scaffolds center. Our results clearly show that this is due to an enhanced cell distribution within the 3D structure observed in these conditions.
The dynamic conditions used in this study may offer a more favorable hydrodynamic environment providing the necessary physical stimuli and nutrient transport to support tissue development.44,45 Future studies should be performed to investigate the fluid dynamics in the chambers of the multicompartment holder.
Overall, this study presents a novel method to culture 3D scaffolds in spinner flasks with the advantage of providing a dynamic environment in 3D while protecting the structures from mechanical damage, without requiring complex approaches for holding and securing the samples.
Conclusions
This study proposes a new method to culture 3D scaffolds in spinner flasks. For that, a multicompartment holder, adapted to standard spinner flasks, was designed and its ability to support the culture of MSCs in Ch scaffolds under dynamic conditions was investigated here. The approach proposed here shows that MSCs remained viable, presented an enhanced growth rate and, more interestingly, became more homogenously distributed within 3D scaffolds in dynamic conditions. Moreover, MSCs differentiation along the osteoblastic lineage was also favored with this strategy. The dynamic environment provided by this simple and easy-handling system improved human MSCs behavior similar to other more complex dynamic systems. Therefore, the multicompartment holder proposed can extend the application of spinner flasks to cell culture in 3D matrices, by an easy modification. This may lead to a controlled, reproducible, cost-effective and easy-handling system for 3D cultures as more reliable in vitro cell culture models or in the preparation of implantable engineered tissues.
Supplementary Material
Acknowledgments
The research described was financially supported by FEDER funds through the “Programa Operacional Factores de Competitividade” (COMPETE) and by Portuguese funds through “Fundação para a Ciência e a Tecnologia” (FCT) in the framework of the project BIOMATRIX refa PTDC/SAU-BEB/101235/2008 and FCOMP-01-0124-FEDER-010915. Raquel Gonçalves and Graciosa Teixeira are grateful to FCT for their postdoctoral (SFRH/BPD/85651/2012) and PhD (SFRH/BD/88429/2012) grants, respectively.
Disclosure Statement
No competing financial interests exist.
References
- 1.Rungarunlert S., Techakumphu M., Pirity M.K., and Dinnyes A.Embryoid body formation from embryonic and induced pluripotent stem cells: Benefits of bioreactors. World J Stem Cells 1,11, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeatts A.B., Choquette D.T., and Fisher J.P.Bioreactors to influence stem cell fate: augmentation of mesenchymal stem cell signaling pathways via dynamic culture systems. Biochim Biophys Acta 1830,2470, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.dos Santos F., Andrade P.Z., Abecasis M.M., Gimble J.M., Chase L.G., Campbell A.M., Boucher S., Vemuri M.C., Silva C.L., and Cabral J.M.Toward a clinical-grade expansion of mesenchymal stem cells from human sources: a microcarrier-based culture system under xeno-free conditions. Tissue Eng Part C Methods 17,1201, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gigout A., Buschmann M.D., and Jolicoeur M.Chondrocytes cultured in stirred suspension with serum-free medium containing pluronic-68 aggregate and proliferate while maintaining their differentiated phenotype. Tissue Eng Part A 15,2237, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Lee T.J., Bhang S.H., La W.G., Yang H.S., Seong J.Y., Lee H., Im G.I., Lee S.H., and Kim B.S.Spinner-flask culture induces redifferentiation of de-differentiated chondrocytes. Biotechnol Lett 33,829, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Chen M., Wang X., Ye Z., Zhang Y., Zhou Y., and Tan W.A modular approach to the engineering of a centimeter-sized bone tissue construct with human amniotic mesenchymal stem cells-laden microcarriers. Biomaterials 32,7532, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Goh T.K., Zhang Z.Y., Chen A.K., Reuveny S., Choolani M., Chan J.K., and Oh S.K.Microcarrier culture for efficient expansion and osteogenic differentiation of human fetal mesenchymal stem cells. Biores Open Access 2,84, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pörtner R., Nagel-Heyer S., Goepfert C., Adamietz P., and Meenen N.M.Bioreactor design for tissue engineering. J Biosci Bioeng 100,235, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Turner A.E., and Flynn L.E.Design and characterization of tissue-specific extracellular matrix-derived microcarriers. Tissue Eng Part C Methods 18,186, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Chen K.Y., Chung C.M., Chen Y.S., Bau D.T., and Yao C.H.Rat bone marrow stromal cells-seeded porous gelatin/tricalcium phosphate/oligomeric proanthocyanidins composite scaffold for bone repair. J Tissue Eng Regen Med 7,708, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee D.P., Smith D.F., Rogers S.H., Emmanual J.E., Jadin K.D., and Hayes B.K.Effect of 3D-microstructure of bioabsorbable PGA:TMC scaffolds on the growth of chondrogenic cells. J Biomed Mater Res B Appl Biomater 88,92, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Çetin D., Kahraman A.S., and Gümüşderelioğlu M.Novel pHEMA-gelatin SPHs as bone scaffolds in dynamic cultures. J Mater Sci Mater Med 23,2803, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Kim H.J., Kim U.J., Leisk G.G., Bayan C., Georgakoudi I., and Kaplan D.L.Bone regeneration on macroporous aqueous-derived silk 3-D scaffolds. Macromol Biosci 7,643, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Meinel L., Karageorgiou V., Fajardo R., Snyder B., Shinde-Patil V., Zichner L., Kaplan D., Langer R., and Vunjak-Novakovic G.Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng 32,112, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Mygind T., Stiehler M., Baatrup A., Li H., Zou X., Flyvbjerg A., Kassem M., and Bünger C.Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials 28,1036, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Song K., Liu T., Cui Z., Li X., and Ma X.Three-dimensional fabrication of engineered bone with human bio-derived bone scaffolds in a rotating wall vessel bioreactor. J Biomed Mater Res A 86,323, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Janjanin S., Li W.J., Morgan M.T., Shanti R.M., and Tuan R.S.Mold-shaped, nanofiber scaffold-based cartilage engineering using human mesenchymal stem cells and bioreactor. J Surg Res 149,47, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrias C., and Goncalves R.M.Container, useful in spinner flask assembly and kit for culturing cell samples in three-dimensional matrix, comprises chambers for containing cell samples, where one of the walls of chambers are perforated to allow culture media and a lid; Patent Number(s): W02013043072-A1 (2011)
- 19.Antunes J.C., Pereira C.L., Molinos M., Ferreira-da-Silva F., Dessì M., Gloria A., Ambrosio L., Gonçalves R.M., and Barbosa M.A.Layer-by-layer self-assembly of chitosan and poly(γ-glutamic acid) into polyelectrolyte complexes. Biomacromolecules 12,4183, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Amaral I.F., Sampaio P., and Barbosa M.A.Three-dimensional culture of human osteoblastic cells in chitosan sponges: the effect of the degree of acetylation. J Biomed Mater Res A 76,335, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Bidarra S.J., Barrias C.C., Barbosa M.A., Soares R., Amédée J., and Granja P.L.Phenotypic and proliferative modulation of human mesenchymal stem cells via crosstalk with endothelial cells. Stem Cell Res 7,186, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Folkman J., and Moscona A.Role of cell shape in growth control. Nature 273,345, 1978 [DOI] [PubMed] [Google Scholar]
- 23.Barrias C.C., and Gonçalves R.M.Multi-compartment holder for 3D cell culture under dynamic conditions. Provisional patent application UK no.11163979, 2011
- 24.Bidarra S.J., Barrias C.C., Barbosa M.A., Soares R., and Granja P.L.Immobilization of human mesenchymal stem cells within RGD-grafted alginate microspheres and assessment of their angiogenic potential. Biomacromolecules 11,1956, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Patel S.D., Papoutsakis E.T., Winter J.N., and Miller W.M.The lactate issue revisited: novel feeding protocols to examine inhibition of cell proliferation and glucose metabolism in hematopoietic cell cultures. Biotechnol Prog 16,885, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Zhu B., Bailey S.R., and Mauli Agrawal C.Calcification of primary human osteoblast cultures under flow conditions using polycaprolactone scaffolds for intravascular applications. J Tissue Eng Regen Med 6,687, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amaral I.F., Unger R.E., Fuchs S., Mendonça A.M., Sousa S.R., Barbosa M.A., Pêgo A.P., and Kirkpatrick C.J.Fibronectin-mediated endothelialisation of chitosan porous matrices. Biomaterials 30,5465, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Barbosa J.N., Amaral I.F., Aguas A.P., and Barbosa M.A.Evaluation of the effect of the degree of acetylation on the inflammatory response to 3D porous chitosan scaffolds. J Biomed Mater Res A 93,20, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Santos S.G., Lamghari M., Almeida C.R., Oliveira M.I., Neves N., Ribeiro A.C., Barbosa J.N., Barros R., Maciel J., Martins M.C., Gonçalves R.M., and Barbosa M.A.Adsorbed fibrinogen leads to improved bone regeneration and correlates with differences in the systemic immune response. Acta Biomater 9,7209, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Di Martino A., Sittinger M., and Risbud M.V.Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 26,5983, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Eibes G., dos Santos F., Andrade P.Z., Boura J.S., Abecasis M.M., da Silva C.L., and Cabral J.M.Maximizing the ex vivo expansion of human mesenchymal stem cells using a microcarrier-based stirred culture system. J Biotechnol 146,194, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Ferrari C., Balandras F., Guedon E., Olmos E., Chevalot I., and Marc A.Limiting cell aggregation during mesenchymal stem cell expansion on microcarriers. Biotechnol Prog 28,780, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Rafiq Q.A., Brosnan K.M., Coopman K., Nienow A.W., and Hewitt C.J.Culture of human mesenchymal stem cells on microcarriers in a 5 l stirred-tank bioreactor. Biotechnol Lett 35,1233, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Weber C., Pohl S., Pörtner R., Wallrapp C., Kassem M., Geigle P., and Czermak P.Expansion and harvesting of hMSC-TERT. Open Biomed Eng J 1,38, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stiehler M., Bünger C., Baatrup A., Lind M., Kassem M., and Mygind T.Effect of dynamic 3-D culture on proliferation, distribution, and osteogenic differentiation of human mesenchymal stem cells. J Biomed Mater Res A 89,96, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Hewitt C.J., Lee K., Nienow A.W., Thomas R.J., Smith M., and Thomas C.R.Expansion of human mesenchymal stem cells on microcarriers. Biotechnol Lett 33,2325, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Schop D., Janssen F.W., Borgart E., de Bruijn J.D., and van Dijkhuizen-Radersma R.Expansion of mesenchymal stem cells using a microcarrier-based cultivation system: growth and metabolism. J Tissue Eng Regen Med 2,126, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Lamghari M., Amaral I.F., Sousa S.R., Sampaio P., and Barbosa M.A.Rat bone marrow stromal cell osteogenic differentiation and fibronectin adsorption on chitosan membranes: the effect of the degree of acetylation. J Biomed Mater Res A 75,387, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Holtorf H.L., Sheffield T.L., Ambrose C.G., Jansen J.A., and Mikos A.G.Flow perfusion culture of marrow stromal cells seeded on porous biphasic calcium phosphate ceramics. Ann Biomed Eng 33,1238, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z.Y., Teoh S.H., Teo E.Y., Khoon Chong M.S., Shin C.W., Tien F.T., Choolani M.A., and Chan J.K.A comparison of bioreactors for culture of fetal mesenchymal stem cells for bone tissue engineering. Biomaterials 31,8684, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Fekete N., Rojewski M.T., Lotfi R., and Schrezenmeier H.Essential components for ex vivo proliferation of mesenchymal stromal cells. Tissue Eng Part C Methods 20,129, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Yourek G., McCormick S.M., Mao J.J., and Reilly G.C.Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regen Med 5,713, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pattappa G., Heywood H.K., de Bruijn J.D., and Lee D.A.The metabolism of human mesenchymal stem cells during proliferation and differentiation. J Cell Physiol 226,2562, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Bilgen B., and Barabino G.A.Location of scaffolds in bioreactors modulates the hydrodynamic environment experienced by engineered tissues. Biotechnol Bioeng 98,282, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Bilgen B., Chang-Mateu I.M., and Barabino G.A.Characterization of mixing in a novel wavy-walled bioreactor for tissue engineering. Biotechnol Bioeng 92,907, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.