Abstract
Macromolecular crowding (MMC) is a biophysical effect that governs biochemical processes inside and outside of cells. Since standard cell culture media lack this effect, the physiological performance of differentiated and progenitor cells, including extracellular matrix (ECM) deposition, is impaired in vitro. To bring back physiological crowdedness to in vitro systems, we have previously introduced carbohydrate-based macromolecules to culture media and have achieved marked improvements with mixed MMC in terms of ECM deposition and differentiation of mesenchymal stem cells (MSCs). We show here that although this system is successful, it is limited, due to viscosity, to only 33% of the fractional volume occupancy (FVO) of full serum, which we calculated to have an FVO of approximately 54% v/v. We show here that full-serum FVO can be achieved using polyvinylpyrrolidone (PVP) 360 kDa. Under these conditions, ECM deposition in human fibroblasts and MSCs is on par, if not stronger than, with original MMC protocols using carbohydrates, but with a viscosity that is not significantly changed. In addition, we have found that the proliferation rate for bone marrow-derived MSCs and fibroblasts increases slightly in the presence of PVP360, similar to that observed with carbohydrate-based crowders. A palette of MMC compounds is now emerging that enables us to tune the crowdedness of culture media seamlessly from interstitial fluid (9% FVO), in which the majority of tissue cells might be based, to serum environments mimicking intravascular conditions. Despite identical FVO's, individual crowder size effects play a role and different cell types appear to have preferences in terms of FVO and the crowder that this is achieved with. However, in the quest of crowders that we have predicted to have a smoother regulatory approval path, PVP is a highly interesting compound, as it has been widely used in the medical and food industries and shows a novel promising use in cell culture and tissue engineering.
Introduction
The success of stem cell therapy for regenerative medicine and tissue engineering hinges on the culture conditions in vitro that enable optimal growth and differentiation. However, contemporary cell culture on polystyrene and similar surfaces represents a significant departure from the microenvironment in which cells normally grow in vivo. One such deficiency of cell culture is the lack of macromolecular crowding (MMC). MMC is a very important physiological effect that is known to drive biochemical processes.1,2 The interior of cells, whether bacterial, animal, or plant, and the exterior of most cells of multicellular organisms, are highly crowded by macromolecules such as proteins, carbohydrates, lipids, and nucleic acids (reviewed in Chen et al.3). MMC acts via the excluded volume effect (EVE), which arises from the mutual impenetrability of soluble macromolecules.
According to EVE theory, the volume of solution that is excluded to a particular molecule is dependent on the sum of nonspecific steric hindrances (governed by size and shape) and electrostatic repulsions (governed by electrical charge) between the background macromolecules.4 Crowding is a result of the reduction of the available solvent volume by a macromolecule, which can be mobile or fixed.5 Crowding hinders solute diffusion, thereby increasing the effective solute concentration. This, in turn, increases the chemical potential of the solute. Crowding can, thus, shift reaction equilibria and accelerate the rates of chemical reactions as well as affect various processes such as intracellular transport, signal transduction, and metabolism.4 It enhances biochemical reactions such as enzymatic catalysis, supramolecular aggregation, and receptor-ligand interactions (reviewed in Chen et al.3).
We previously introduced MMC into culture media to reduce the disparity between the in vivo and in vitro stem cell microenvironment. MMC empowers stem cells to build their own extracellular matrix (ECM) microenvironment through the process of ECM deposition, which is regulated by MMC by means of accelerating extracellular proteolytic trimming of pro-collagen to collagen and fibrillogenesis of collagen and other ECM components, thus intensifying ECM cross-linking and remodeling.6 This system works for a variety of cell types3 and establishes a positive feedback loop of dynamic cell-matrix reciprocity that modulates differentiation and proliferation.6 To assess the power of crowders in solution, a surrogate marker known as fractional volume occupancy (FVO), which is dependent on the hydrodynamic radii (RH) of the crowders, is used.3
Our current protocol uses a mixture consisting of Ficoll (Fc) 70 kDa and Ficoll 400 kDa3,6 with a total FVO of 18% v/v. (The chemical structure of Ficoll is shown in Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tec.) This FVO had been modeled after the albumin content in bone marrow.3 It has been successful thus far, but here, we challenge Ficoll's crowding potential in enhancing the deposition of ECM proteins collagen I, collagen IV, and fibronectin, by testing a range of physiological FVOs. These FVOs have been determined by calculating the total FVO of albumin, fibrinogen, and globulin in blood plasma and interstitial fluid. We show that the FVOs lies within a physiological range of 9% v/v to 54% v/v. We have discovered that reducing the FVO of our Ficoll mixture from 18% v/v to 9% v/v (the latter corresponding to the FVO of interstitial fluid) strengthened the crowding effect significantly. We also demonstrate for the first time that a noncarbohydrate single crowder species (as opposed to a mixture), namely polyvinylpyrrolidone (PVP), when used at a physiological FVO of 18% v/v for PVP 40 kDa and 54% v/v for PVP 360 kDa, can also exert a crowding effect, which is comparable or even superior to that of the Ficoll mixture at an FVO of 18% v/v. (The chemical structure of PVP is shown in Supplementary Fig. S2.) Differences in the crowding effect among the crowders that were tested at various physiological FVOs could, in part, be explained by the interplay between the viscosity of the crowded solution and the EVE. PVP can now be viewed as both a macromolecular crowder and a biomaterial for future use in tissue engineering and expansion of progenitor cells in vitro.
Materials and Methods
Calculation of FVO
FVO is the fraction of the total volume occupied by macromolecules. Calculations for estimating the FVO were carried out as follows3:
(1) We calculated the volume of each macromolecule, which is considered a sphere with RH as the hydrodynamic radius:
Volume of a sphere,

[The hydrodynamic radii (RH) used in our calculations are as follows: Ficoll 70 kDa=4 nm; Ficoll 400 kDa=8 nm; PVP 40 kDa=5.1 nm; PVP 360 kDa=19 nm3,7].
(2) We calculated the number of macromolecules in mass m:
The molecular weight (MW) of the macromolecule contains Avogadro number of molecules.
Number of molecules in mass m,
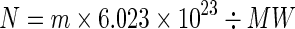
(3) We calculated the FVO,

where NV is the number of particles per total volume.
Plasma and interstitial serum comprises the following major serum proteins: albumin, globulins, and fibrinogen. Globulins are divided into four sub-groups: alpha-1, alpha-2, beta, and gamma. Each globulin subgroup further comprises a variety of proteins (Supplementary Table S1). Based on the known MWs and hydrodynamic radii (RH) of the various proteins in each globulin subgroup, we obtained an average MW and RH for each of the four globulin subgroups. The hydrodynamic radius, MW, and percentage concentration of fibrinogen and albumin in plasma serum have been previously reported.7 The percentage concentration of the individual globulin subgroups in plasma is derived from the concentration ranges of the globulins in blood and the theoretical proportion of albumin: globulin, that is, 60:35 (Table 1). It has been previously reported that the total concentration of proteins in plasma is estimated to be five times more than that in interstitial fluid.8 Hence, the percentage concentration of the individual serum proteins in interstitial fluid is assumed to also be five times less than that in plasma (Table 2).
Table 1.
FVO of Proteins in Blood Plasma
| Protein | % in plasma | Protein concentration (mg/mL) | FVO (% v/v) |
|---|---|---|---|
| Albumin | 60.00 | 80.00 | 18.72 |
| α1 Globulin | 1.81 | 2.41 | 1.32 |
| α2 Globulin | 9.04 | 12.05 | 7.77 |
| β Globulin | 10.84 | 14.45 | 3.29 |
| γ Globulin | 13.25 | 17.67 | 8.35 |
| Fibrinogen | 4.00 | 5.33 | 5.20 |
Total FVO: 44.65% v/v≈45% v/v.
FVO, fractional volume occupancy.
Table 2.
FVO of Proteins in Interstitial Fluid
| Protein | % in interstitial fluid | Protein concentration (mg/mL) | FVO (% v/v) |
|---|---|---|---|
| Albumin | 60.00 | 16.00 | 3.74 |
| α1 Globulin | 1.81 | 0.48 | 0.26 |
| α2 Globulin | 9.04 | 2.40 | 1.55 |
| β Globulin | 10.84 | 2.88 | 0.66 |
| γ Globulin | 13.25 | 3.52 | 1.67 |
| Fibrinogen | 4.00 | 1.06 | 1.04 |
Total FVO: 8.93% v/v≈9% v/v.
Preparation of crowder solutions
Solutions of macromolecules spanning a wide range of concentrations were prepared in Hanks balanced salt solution (HBSS; Gibco). HBSS was the buffer of choice, because it mimics physiological electrolyte concentrations and pH. The macromolecules used were Ficoll 70 kDa (GE Healthcare), Ficoll 400 kDa (GE Healthcare), PVP 40 kDa (Sigma-Aldrich), and PVP 360 kDa (Sigma-Aldrich). The Ficoll crowding mixture was prepared fresh by dissolving Ficoll 70 and Ficoll 400 in HBSS according to the concentrations shown in Table 3, in order to achieve the desired FVOs of 9% v/v, 18% v/v, 36% v/v, and 54% v/v. PVP solutions were prepared fresh by dissolving either PVP 40 kDa or PVP 360 kDa in HBSS according to the concentrations shown in Table 3 so as to achieve the desired FVOs of 9% v/v, 18% v/v, 36% v/v, and 54% v/v.
Table 3.
Concentration of Ficoll and PVP Crowders at Various FVOs
| FVO\crowder | 9% v/v | 18% v/v | 36% v/v | 54% v/v |
|---|---|---|---|---|
| Ficoll (Fc) mixture (mg/mL) | Fc70: 18.75 | Fc70: 37.5 | Fc70: 75 | Fc70: 112.5 |
| Fc400: 12.5 | Fc400: 25 | Fc400: 50 | Fc400: 75 | |
| PVP360 (mg/mL) | 1.89 | 3.78 | 7.56 | 11.34 |
| PVP40 (mg/mL) | 10.75 | 21.5 | 43 | 64.5 |
PVP, polyvinylpyrrolidone.
Collagen aggregation assay
To quantify the rate of collagen I fibril formation, a turbidity assay was performed. The turbidity read-out system is dependent on the concentration of the collagen I solution. A standard noncrowded bovine collagen I solution was prepared at 1.5 mg/mL (Koken) in phosphate-buffered saline (PBS) at room temperature (25°C). The crowded collagen I solution was prepared by adding the Ficoll mixture, PVP 40 kDa, or PVP 360 kDa at their FVOs of 9% v/v, 18% v/v, 36% v/v, and 54% v/v to the standard noncrowded solution. The amount of collagen I fibril formation was measured using a Tecan microplate plate reader (Magellan) at an absorbance of 313 nm over a period of 120 min. The rate of collagen I fibril formation was calculated as the gradient of the steep portion of the absorbance-time graph and the fold change obtained by dividing each rate with the rate of the control (absence of crowding). PVP 40 kDa at 54% FVO, PVP 360 kDa at 54% FVO, and the Ficoll mixture at 18% FVO in the absence of collagen did not affect the absorbance reading over time (Supplementary Table S2).
Cell culture
Human bone marrow mesenchymal stem cells (MSCs) (passage 5–8; Lonza) and human dermal fibroblasts (HDFs, passage 5–8; Lonza) were seeded on 24-well plates at 10,000 cells/well in high-glucose Dulbecco's modified Eagle's medium (DMEM; Gibco) that was supplemented with 10% fetal bovine serum, 100 units/mL penicillin, and 100 μL/mL streptomycin, at 5% CO2 and 37°C.9 After 24 h of attachment, the culture medium was changed to crowding media containing Ficoll mixture, PVP40, or PVP360 with FVOs of 0% v/v, 9% v/v, 18% v/v, 36% v/v, and 54% v/v (concentrations stated in Table 1) in high-glucose DMEM that was supplemented with 10% FBS, 100 units/mL penicillin, 100 μL/mL streptomycin, and 100 μM of l-ascorbic acid 2-phosphate10). To observe changes in fibronectin deposition, cells were fixed and stained after a total of 3 days of crowding. To observe changes in proliferation and collagen I and IV deposition, cells were fixed and stained after 6 days of crowding (with one medium change after 3 days).
Immunocytochemistry
Cell layers were fixed with methanol, blocked with 3% bovine serum albumin in PBS for an hour, and then incubated with mouse anti-human collagen I monoclonal primary antibody at 500×dilution (Sigma-Aldrich) or rabbit anti-human fibronectin polyclonal primary antibody at 100×dilution (Dako) or rabbit anti-human collagen IV polyclonal primary antibody (Abcam) at 500×dilution for 90 min. The cell layer was washed thrice with PBS and incubated with the AlexaFluor 594 goat anti-mouse or AlexaFluor 488 goat anti-rabbit secondary antibody at 400×dilution for 60 min (Invitrogen). Cell nuclei were stained with 4′,6-diamidino-2-phenylindoldilactate (DAPI, 0.5 μg/mL) for 60 min.
Method of quantification of matrix deposition and nuclei count
Adherent fluorescent cytometry was based on a montage of nine sites per well taken by a coolSNAP HQ camera attached to a Nikon TE2000 microscope at 2×magnification, covering 83% of the total well area. AlexaFluor 594 was viewed under a single rhodamine filter (Ex 572 nm/Em 630 nm), while DAPI fluorescence was obtained with a single DAPI filter (Ex 350 nm/Em 465 nm). Images of DAPI-stained monolayers were scored under the “count nuclei” module with the parameters: 10 min, 15 max and 10 diameter and were thresholded, and protein deposition was measured by an image analysis software (MetaMorph 6.3v3). The extent of collagen I, collagen IV, and fibronectin deposition was quantified by area of AlexaFluor 594 or 488 fluorescence from thresholded events, then normalized to nuclei count based on detected DAPI fluorescence. The area measurements were imported into Microsoft Excel, and the mean±SD of the areas was calculated (3).
Sodium dodecylsulphate–polyacrylamide gel electrophoresis
Protein samples were separated under nonreducing conditions using 5% resolving/3% stacking polyacrylamide gels as outlined in.11 Protein bands were stained with the SilverQuest™ kit (Invitrogen).
Viscosity measurements
The viscosity of the crowder solutions was measured using a Brookfield DV-II+cone-and-plate viscometer (Brookfield Engineering Laboratories, Inc.) at room temperature.
Determination of the polydispersity index of PVP crowder solutions
The polydispersity index (PdI) was measured by means of dynamic light scattering (DLS). DLS was performed using the Zetasizer nano ZS (Malvern Instruments). PdI is a dimensionless measure of the broadness of the size distribution calculated from the cumulants analysis. In the Zetasizer software, it ranges from 0 to 1. PdI values can be interpreted as follows (Malvern): <0.08: Nearly monodisperse sample. Usually, DLS can only give a monomodal distribution within this range; 0.08–0.7: Mid-range value of PdI. It is the range over which the distribution algorithms operate best; and >0.7: Indicates a very broad distribution of particle sizes. Temperature was set to a range of 5°C–40°C, and sample temperature was equilibrated for 5 min before measurements. PdIs were calculated from three series of repeated measurements, with each series containing at least 14 individual measurements (as determined by the Zetasizer software). Dispensable cuvettes used for the measurements were rinsed with the corresponding buffer before use, and samples were thoroughly vortexed before loading.
Statistical analysis
Quantitative assays were analysed using ANOVA and the post hoc Bonferroni (Origin Pro 8.0) in order to compare mean values at a significance level of 95% (p=0.05). The data were presented as mean±SD with a p<0.05.
Results
Calculation of FVO; PdI of PVP crowder solutions
We have calculated the FVO for blood plasma (Table 2) and interstitial fluid (Table 3). The calculated physiological FVOs range from 9% v/v in interstitial fluid to 45% v/v in blood plasma, thus yielding a total FVO of 54% v/v in the extracellular environment. In this study, the crowding effect of the single crowders PVP40 and PVP360 and the previously established Ficoll mixture (i.e., Ficoll 70 kDa+Ficoll 400 kDa)3,12 have been tested at (physiological) FVOs 9%, 18%, 36%, and 54%.
We have also determined the PdI for solutions of PVP 40 kDa and PVP 360 kDa to be 0.304 and 0.368, respectively (Table 4).
Table 4.
The Polydispersity Index of Solutions of PVP 40 kDa and PVP 360 kDa
| Crowder | Polydispersity index |
|---|---|
| PVP40 | 0.304±0.004 |
| PVP360 | 0.368±0.002 |
| Fc70 | 0.224±0.014 |
| Fc400 | 0.240±0.011 |
Crowding drives collagen aggregation in the absence of cells
In the presence of the Fc mix, the rate of collagen fibrillogenesis peaked at 9% v/v FVO (2.15±0.06-fold increase; Table 5). Thereafter, it decreased as the FVO of the Fc mix increased from 18% to 54%. In the presence of PVP40, the rate of collagen fibrillogenesis peaked at a higher FVO of 18% (3.78±0.50-fold increase) and subsequently decreased with increasing FVO. In contrast, a dose-dependent increase in rate of collagen fibrillogenesis was observed throughout the physiological FVO range with PVP360, reaching a peak at an FVO of 54% (2.76±0.35-fold increase).
Table 5.
The Rate of Collagen Fibrillogenesis Under Crowding Relative to Uncrowded Conditions (FVO 0% v/v)
| MMC\FVO (% v/v) | 0 | 9 | 18 | 36 | 54 |
|---|---|---|---|---|---|
| Ficoll mixture | 1 | 2.15±0.06 | <1 | <1 | <1 |
| PVP 360 kDa | 1 | 1.78±0.13 | 1.85±0.35 | 2.06±0.46 | 2.76±0.35 |
| PVP 40 kDa | 1 | 1.97±0.15 | 3.78±0.50 | 3.12±0.38 | <1 |
MMC, macromolecular crowding.
Ficoll mixture and PVP increase collagen I, IV, and fibronectin deposition in ECM
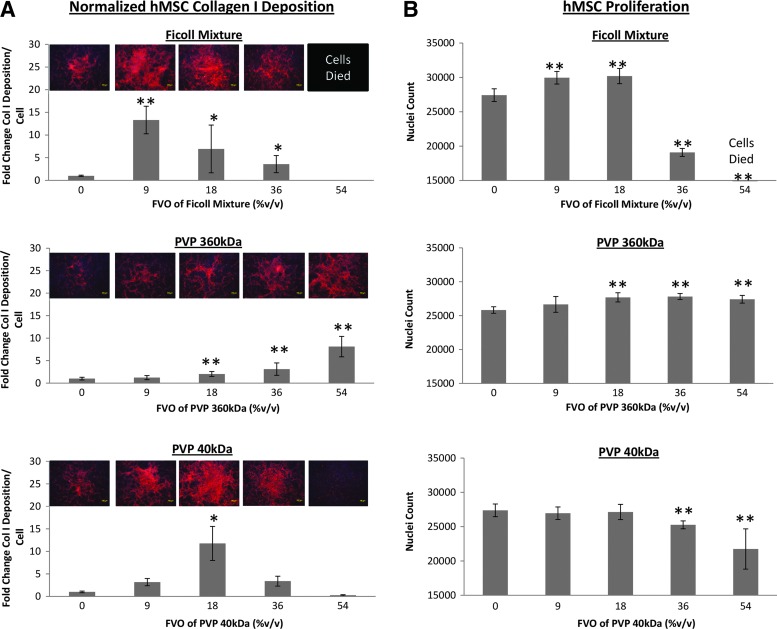
With Ficoll mixture, maximal collagen I and IV deposition per cell was achieved at an interstitial fluid FVO of 9% v/v. At this FVO, collagen deposition per cell is observed to be >200% better than the current crowding concentration of FVO 18% v/v (Figs. 1A and 2A and Supplementary Figs. S3 and S4). Interestingly, we observed differential effects of Ficoll crowders on collagen and fibronectin deposition. For fibronectin deposition, both 9% and 18% FVOs performed similarly well (Supplementary Fig. S4), with the Fc mix at 18% FVO providing a 10% increase in fibronectin deposition compared to 9% FVO.
FIG. 1.
The effect of fractional volume occupancies (FVOs) from 0% v/v to 54% v/v of the Ficoll mixture, polyvinylpyrrolidone (PVP) 360 kDa and PVP 40 kDa on (A) collagen I deposition and (B) proliferation of human dermal fibroblasts (HDFs). *p<0.05, **p<0.01 versus controls. Data are expressed as mean±SD, calculated from triplicates. Corresponding immunocytochemistry (ICC) images in (A) show collagen I deposition in red counterstained with 4′,6-diamidino-2-phenylindoldilactate (DAPI) for cell nuclei in blue. Color images available online at www.liebertpub.com/tec
FIG. 2.
The effect of FVOs from 0% v/v to 54% v/v of the Ficoll mixture, PVP 360 kDa and PVP 40 kDa on (A) collagen I deposition, and (B) proliferation of human mesenchymal stem cells (hMSCs). *p<0.05, **p<0.01 versus controls. Data are expressed as mean±SD, calculated from triplicates. Corresponding ICC images in (A) show collagen I deposition in red counterstained with DAPI for cell nuclei in blue. Color images available online at www.liebertpub.com/tec
Differential effects of PVP40 crowders on matrix deposition have also been observed. With PVP40, maximal collagen I deposition per cell was achieved at an FVO of 18% (Figs. 1A and 2A); collagen IV deposition per cell at an even higher 54% FVO; and fibronectin deposition per cell at 36% FVO; (Supplementary Figs. S4 and S5). However, using PVP40 at 54% FVO, collagen I deposition was significantly inhibited (Figs. 1A and 2A) for both cell types, which may, in part, contribute to the significant decrease in cell proliferation.13
Interestingly, differential effects of PVP360 crowders on matrix deposition were not apparent within the physiological 0–54% FVO range. Collagen I, collagen IV, and fibronectin deposition increased in a dose-dependent manner, achieving maximal matrix deposition at an FVO of 54% v/v (Figs. 1A and 2A and Supplementary Figs. S3–S5).
A sodium dodecylsulphate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis of the cell layer that had been treated with Fc mix 9%, PVP40 18%, and PVP360 54% revealed cross-linked collagen footprints with higher MW collagen I bands, that is, β and γ bands, thus indicating the formation of a stable collagen matrix (Fig. 3).
FIG. 3.
Silver staining after pepsin digest of collagen secreted by hMSC in media and cell layers after (A) 2 days and (B) 7 days of crowding: No crowding; Ficoll mixture 9% v/v (i.e., Ficoll mixture of 70 and 400 kDa at a combined FVO of 9% v/v), PVP 360 kDa at an FVO of 54% v/v, and PVP 40 kDa at an FVO of 18% v/v. Accumulation of high amounts of PVP 360 kDa and PVP 40 kDa in the media was observed to impair migration of proteins in the gel.
Cell proliferation under crowding with the Ficoll mixture and PVP solutions
With the Fc mix, both HDFs and human mesenchymal stem cells (hMSCs) proliferation rates were significantly higher at 9% and 18% FVO. At 36% FVO, the Fc mix appeared to inhibit cell proliferation and 54% caused cell death after the first day of crowding (Supplementary Fig. S6). In the presence of PVP40, a significant increase in the proliferation rate of HDFs was observed under crowding with 9%, 18%, and 36% (Figs. 1B and 2B). However, 54% of PVP40 appeared to inhibit HDF proliferation. The proliferative effects of PVP40 appeared to be cell-type dependent, with no enhanced proliferation rate of hMSCs observed throughout the physiological FVO range. In fact, a decrease in hMSC proliferation rate at FVO 36% and 54% was observed. In contrast, the proliferation rates of both cell types increased throughout the physiological FVO range under crowding with PVP360 (Figs. 1B and 2B). No cell death was observed throughout the 7-day incubation period at 54% of PV40 and PVP360 (Supplementary Fig. S6).
Viscosity measurements for the Ficoll mixture and PVP solutions
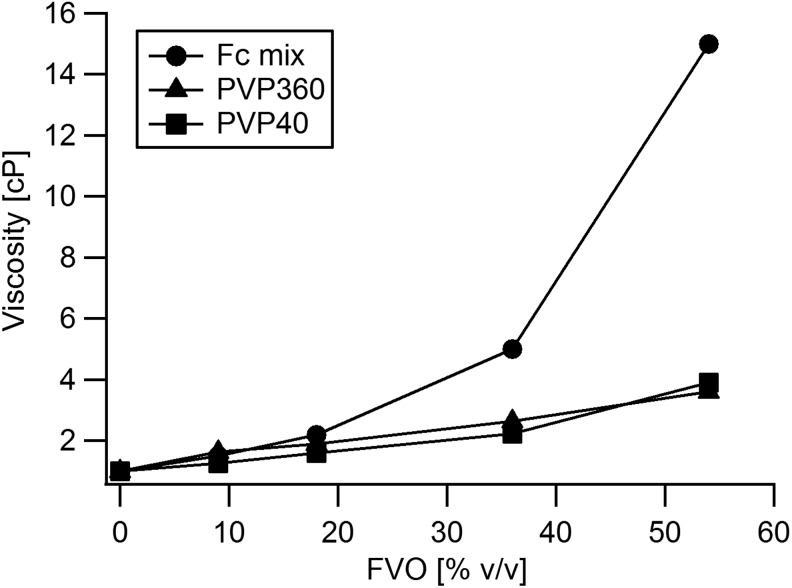
Increasing the concentration of crowders increases the viscosity of the solution. Figure 4 shows that the viscosity of PVP40 and PVP360 solutions increases linearly with increasing FVO concentration, whereas that of the Ficoll mixture was observed to increase exponentially. The viscosities of PVP40 and PVP360 solutions with FVOs of 9% v/v to 54% v/v and of Ficoll mixture with FVOs from 9% v/v to 36% v/v were observed to fall within physiological blood viscosity of healthy adults, that is, <5.5 cP.14 However, the viscosity of the Fc mix at 54% v/v was 15 cP and is thrice higher than the physiological blood viscosity.
FIG. 4.
Viscosities of solutions of PVP and the Ficoll mixture. Viscosities were measured in an FVO range of 9–54% v/v. Within this range, the viscosity of the Ficoll mixture differed considerably from that of PVP 40 kDa and PVP 360 kDa. The Ficoll mixture viscosity was higher than that of PVP40 and PVP360 at FVOs of 18% and higher.
Discussion
MMC is emerging as a valuable biophysical tool in tissue engineering that facilitates microenvironment formation by cultured cells3 and stabilizes or drives differentiation.6 An important parameter that is used to assess the degree of crowding is the FVO. Ideally, a fractional occupancy should be chosen that corresponds to the natural environment of the cell type which is to be cultured. Here, we used the FVO of interstitial fluid and blood plasma, respectively, as reference points. We had originally reported effective FVOs for in vitro crowding ranging from 5.2% v/v for dextran sulphate (DxS) 500 kDa to 18% v/v for a mixture of Ficoll 70 kDa and Ficoll 400 kDa (called the “Fc mix”). Negatively charged DxS is a potentially ideal crowder, because it combines the effects of both steric repulsion and electrostatic repulsion. However, its uses are limited, as it interferes with the action of growth factors due to direct binding3 (unpublished data). As an alternative, we developed the concept of mixed MMC with neutral components,3,15 which we found to be efficacious at an FVO of 18% v/v, which corresponds to the FVO of albumin in bone marrow,3,6 and so was designed to partially model the perfused bone marrow niche.
Here, we present new FVO calculations which suggest that the Fc mix at an FVO of 18% v/v represents only half of a (theoretical) total FVO of plasma but overshoots that of interstitial fluid by 100%. When we attempted to emulate plasma FVOs with the Fc mix, both microenvironment formation and cell proliferation were compromised due to the high viscosity of the Fc mix. Interestingly, reducing FVO of the Fc mix to that of interstitial fluid at 9% v/v potentiated the MMC effect and resulted in enhanced collagen I deposition. Considering that most cell types are not in direct contact with plasma but with interstitial fluid, these findings explain the particularly beneficial effects on fibroblasts which we have observed. In addition, we report the novel finding that the formerly clinically used plasma expander PVP360 shows a broad bandwidth as a neutral crowder to achieve FVOs in the physiological range of 9–54% (which agrees with plasma and interstitial FVOs) while keeping the viscosity low. The amplified ECM deposition under PVP360 crowding was scalable to 54%, where it showed a maximum and surpassed deposition amounts seen with the Fc mixture. The efficacy of PVP40 peaked at 18% FVO. The results of the cell-free collagen I fibrillogenesis assays were in good agreement with the collagen I deposition results. PVP40, PVP360, and the Fc mix at respective FVOs of 18%, 54%, and 9% caused the greatest increase in the rate of spontaneous collagen I fibril assembly (Table 5).
The individual PdIs of PVP40 and PVP360 are higher than the individual PdIs of Fc70 and Fc400. The Fc mix, however, contains two different sizes of Ficoll and, as a result, the effect on ECM deposition is more pronounced because of mixed MMC.3 The increased ECM deposition that we have observed in our study with PVP is likely due to the moderate polydispersity of PVP40 and PVP360. It has been recently suggested that excluded volume depends on polydispersity and that the greater the polydispersity, the greater will be the ECM deposition.16
The difference in the effects on collagen I, IV, and fibronectin matrix deposition between PVP40 and PVP360 could be due to the interplay between EVE and viscosity. Viscosity is known to play a major role in protein association. The higher the viscosity, the slower the diffusion rate and this would decrease the kinetics of diffusion-controlled reactions, such as collagen aggregation and deposition. The overall effect on matrix deposition would, thus, depend on the net effect of the presence of crowders, which itself depends on the tradeoff between EVE and viscosity. High viscosity can also limit cytoskeleton assembly, cell mobility, and cellular proliferation.17 We have found that the viscosity of Fc mix at 54% FVO is approximately four times higher than that of PVP360 at the same FVO (15 cP vs. 3.6 cP). With the Fc mix at 54% FVO, HDFs and hMSCs were not able to survive. This could be due to the extremely high viscosity of the Fc mix, which is approximately thrice that of the blood viscosity in a healthy normal adult. According to EVE theory, the excluded volume is dependent on the hydrodynamic radii (RH) of the macromolecules.4 The RH of PVP40 is 5.1 nm and that of PVP360 is 19 nm,7 which translates to an approximate 50 times difference in volume. PVP360 would exclude more volume to collagen I than PVP40, leading to more deposition. If higher physiological FVOs (e.g., 36% and 54%) are required in a particular in vitro system, PVP360 would be the crowder of choice based on our analysis. At these higher FVOs, the EVE of PVP360 would be expected to outweigh the corresponding increase in its viscosity. With PVP40 and Fc mix, however, the increase in viscosity would appear to outweigh EVE. These observations confirm predictions made by theoretical studies1).
EVE has been reported to enhance the formation of ECM by increasing lysyl oxidase activity, resulting in an enhanced presence of collagen crosslinks.9 We have observed in our SDS-PAGE analysis that the addition of PVP40 or PVP360 within the first 2 days during the culture of hMSC was able to reduce the amount of pro-collagen I in the culture medium and enhance the amount of cross-linked (i.e., processed) collagen molecules deposited within the cell layer.
Among the three types of MMCs used in our 2D in vitro culture, the synthetic carbohydrate Ficoll had the most significant overall efficacy in enhancing matrix deposition at low FVOs of 9% v/v or 18% v/v. However, crowding using Ficoll has its limitations due to (1) high cost and (2) a limited range of physiological FVOs with physiological viscosities. The existing crowdedness of a 2D and 3D in vitro culture system can be different. Using the existing 2D-crowding protocol with Ficoll 18% v/v may not be the optimum crowding method to maximize ECM deposition in a 3D environment.18 These limitations may be addressed with our proposed low-cost PVP360 system. PVP360 offers (1) a physiological viscosity in the entire range of physiological FVOs tested; (2) a strong EVE due to its large hydrodynamic radius, which (3) increases matrix deposition in a dose-dependent manner. These three factors could explain the enhanced proliferation of both HDFs and hMSCs that we have observed. We have previously observed that MSCs proliferate faster on an enriched matrix which had been laid down under MMC conditions3 Using a low molar concentration of PVP360, we are able to achieve a high FVO and a physiological viscosity. When applying MMC to cell culture, we would aim at minimizing the molar concentration of the crowder and its viscosity while maximizing the FVO. We have shown here that this is possible with PVP360.
In comparison with Ficoll, PVP is not biodegradable. PVP40 has been shown to be uptaken and released via pinocytosis by macrophages,19 but the fate of PVP in other cell types such as hMSC and HDF has yet to be reported. Nonetheless, PVP of approximately Mw 360 kDa (Povidone K90) has been approved by FDA for use in both the food and pharmaceutical industry.20–22 Although PVP360 is not known to be excreted by kidneys,23 FDA has considered PVP as generally safe for human's daily intake since 196620 with wide ranging uses from tablet binders for daily supplements, formulations of antibiotics, chemotherapeutic drugs, and ophthalmic and topical solutions, used as a detoxifying agent in many toxic compounds to form nontoxic complexes, to its presence in various processed food. We envision that it can now take on a new role as a macromolecular crowder. It is nontoxic, stable, able to fulfill good manufacturing practice requirements, and cost effective.
Conclusions
The formerly clinically used plasma expander PVP is a novel addition to the toolbox of MMC in tissue culture. It is efficacious as a polydisperse crowder and enables a broad bandwidth of FVOs to emulate different extracellular environments. Since MMC effects are not fully understood at this point of time, a certain degree of titration is advisable to obtain the maximum performance of specific cell types. However, current data suggest the application of Ficoll mixed MMC at 9% to 18% FVO, PVP40 for approximately 18% FVO, and PVP360 for approximately 54%.
Supplementary Material
Acknowledgments
The authors thank Namgung Bumseok, Department of Biomedical Engineering, National University of Singapore, for help with the viscosity measurements. They also thank Jean-Yves Dewavrin, NUS Graduate School for Integrative Sciences and Engineering, National University of Singapore, for help with some polydispersity measurements. The authors acknowledge support from the NUS Tissue Engineering Program (Life Sciences Institute NUS). They also gratefully acknowledge support from the National University of Singapore (NUS) Graduate School for Integrative Sciences and Engineering (R.R.), Department of Biomedical Engineering (L.N.S.J.), and NUS Faculty Research Committee Grants: Engineering in Medicine, R397-000-082-112 to D.T. and M.R., R391-000-032-112 to M.R., and R-154-000-543-112 to T.W.
Disclosure Statement
No competing financial interests exist.
References
- 1.Ellis R.J.Macromolecular crowding: obvious but underappreciated. Trends Biochem Sci 26,597, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Minton A.P.The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J Biol Chem 276,10577, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Chen C., Loe F., Blocki A., Peng Y., and Raghunath M.Applying macromolecular crowding to enhance extracellular matrix deposition and its remodeling in vitro for tissue engineering and cell-based therapies. Adv Drug Deliv Rev 63,277, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Zimmerman S., and Minton A.Macromolecular crowding: biochemical, biophysical, and physiological consequences. Annu Rev Biophys Biomol Struct 22,27, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Dix J., and Verkman A.Crowding effects on diffusion in solutions and cells. Annu Rev Biophys 37,247, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Ang X., Lee M., Blocki A., Chen C., Ong L., Asada H., et al. Macromolecular crowding amplifies adipogenesis of human bone marrow-derived MSCs by enhancing the pro-adipogenic environment. Tissue Eng Part A 20,966, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong J., Wenby R., Meiselman H., and Fisher T.The hydrodynamic radii of macromolecules and their effect on red blood cell aggregation. Biophys J 87,4259, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marieb E., and Hoehn K.Human Anatomy & Physiology. Boston, MA: Pearson, 2013 [Google Scholar]
- 9.Lareu R.R., Arsianti I., Subramhanya H.K., Yanxian P., Raghunath M.In vitro enhancement of collagen matrix formation and crosslinking for applications in tissue engineering: a preliminary study. Tissue Eng 13,385, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Hata R., and Senoo H.L-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissue-like substance by skin fibroblasts. J Cell Physiol 138,8, 1989 [DOI] [PubMed] [Google Scholar]
- 11.Raghunath M., Steinmann B., DeLozier-Blanchet C., Extermann P., and Superti-Furga A.Prenatal diagnosis of collagen disorders by direct biochemical analysis of chorionic villus biopsies. Pediatr Res 36,441, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Zeiger A., Loe F., Li R., Raghunath M., and Van Vliet K.Macromolecular crowding directs extracellular matrix organization and mesenchymal stem cell behavior. PLoS One 7,e37904, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fassett J., Tobolt D., and Hansen L.Type I collagen structure regulates cell morphology and EGF Signaling in primary rat hepatocytes through cAMP-dependent protein kinase A. Mol Biol Cell 17,345, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenson R., Mccormick A., and Uretzr E.Distribution of blood viscosity values and biochemical correlates in healthy adults. Clin Chem 42,e1189, 1996 [PubMed] [Google Scholar]
- 15.Zhou H.Effect of mixed macromolecular crowding agents on protein folding. Proteins 72,1109, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satyam A., Kumar P., Fan X., Gorelov A., Rochev Y., Joshi L., et al.. Macromolecular crowding meets tissue engineering by self-assembly: a paradigm shift in regenerative medicine. Adv Mater 2014. DOI: 10.1002/adma.201304428 [DOI] [PubMed] [Google Scholar]
- 17.Khorshid F.The Effect of the Medium Viscosity on the Cells Morphology in Reaction of Cells to Topography—I. Abstract presented at the Proc 2nd Saudi Sci Conl, Fac Sci, KAU, 2005, pp. 67–98 [Google Scholar]
- 18.Chen B., Wang B., Zhang W., Zhou G., Cao Y., and Liu W.Macromolecular crowding effect on cartilaginous matrix production: a comparison of two-dimensional and three-dimensional models. Tissue Eng Part C 19,586, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pratten M., Williams K., and Lloyd J.A quantitative study of pinocytosis and intracellular proteolysis in rat peritoneal macrophages. Biochem J 168,365, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.International Programme on Chemical Safety. http://www.inchem.org/documents/jecfa/jecmono/v15je08.htm accessed January31, 2014 (2014)
- 21.Codex Alimentarius. http://www.codexalimentarius.net/gsfaonline/additives/details.html?id=358 accessed January31, 2014 (2013)
- 22.Folttmann H., and Quadir A.Polyvinylpyrrolidone (PVP)—one of the most widely used excipients in pharmaceuticals: an overview. Drug Deliv Technol 8,22, 2008 [Google Scholar]
- 23.Robinson B., Sullivan F., Borzelleca J., and Schwartz S.PVP: A Critical Review of the Kinetics and Toxicology of Polyvinylpyrrolidone (Povidone). Chelsea, MI: CRC Press, 1990 [Google Scholar]
- 24.Gershengorn M., Cheng S., Lippoldt R., Lord R., and Robbins J.Characterization of human thyroxine-binding globulin. Evidence for a single polypeptide chain. J Biol Chem 252,8713, 1977 [PubMed] [Google Scholar]
- 25.Hong S., and Pedersen P.ATP synthase and the actions of inhibitors utilized to study its roles in human health, disease, and other scientific areas. Microbiol Mol Biol Rev 72,590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.