Abstract
Cartilage defects are normally concomitant with posttraumatic inflammation and pose a major challenge in cartilage repair. Due to the avascular nature of cartilage and its inability to surmount an inflammatory response, the cartilage is easily attacked by proinflammatory factors and oxidative stress; if left untreated, osteoarthritis may develop. Suppression of inflammation has always been a crux for cartilage repair. Pharmacological drugs have been successfully applied in cartilage repair; however, they cannot optimally work alone. This review article will summarize current pharmacological drugs and their application in cartilage repair. The development of extracellular matrix-based scaffolds and preconditioned tissue-specific stem cells will be emphasized because both of these tissue engineering components could contribute to an enhanced ability not only for cartilage regeneration but also for anti-inflammation. These strategies could be combined to boost cartilage repair under inflammatory conditions.
Introduction
Joint injuries are common in the young and active population and often result in cartilage or osteochondral lesions. If left untreated, these defects might lead to joint swelling and pain, eventually progressing toward osteoarthritis (OA). Over 27 million Americans are affected by OA, introducing huge clinical and socioeconomic burdens.1 Traditional cartilage repair methods include the transplantation of osteochondral grafts,2 microfracturing, and autologous chondrocyte implantation3; however, none of these cartilage repair strategies have generated long-lasting hyaline cartilage that meets functional demands. The causes of cartilage impairment are diverse, including inflammation, hypertrophy, and senescence (Fig. 1).4,5 Excessive mechanical surface contact stress can directly damage articular cartilage and subchondral bone and adversely alter chondrocyte function,6 while the disruption of the homeostasis of chondrocytes may gradually develop into OA.7 In the early phase of OA, disease-modifying interventions targeting inflammatory processes might be most efficacious for the prevention and treatment of OA.8 To this end, many anti-inflammatory strategies have been discovered, such as growth factor applications, exertion of anticytokines or anti-inflammatory drugs, and stem cell-based therapies.
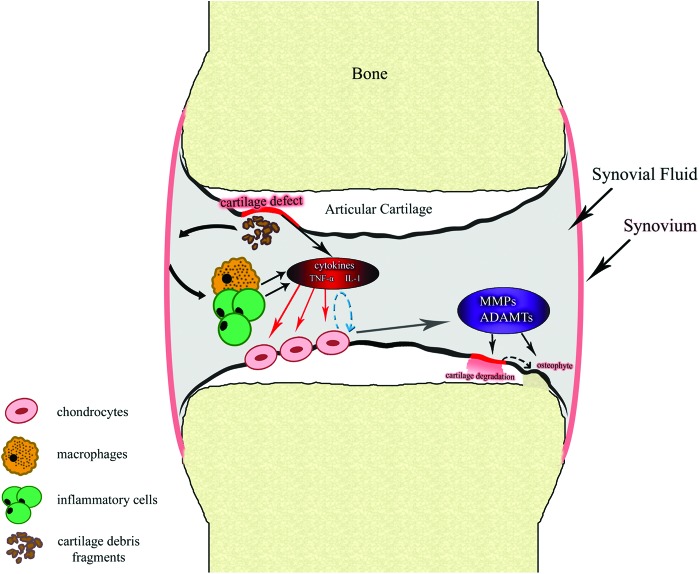
FIG. 1.
Inflammation in cartilage repair. Abnormally high contact stresses such as mechanical overload transmitted to focal areas of articular cartilage result in cartilage defects and release cartilage fragments. This process stimulates the synovial membrane, leading to the activation of macrophages and inflammatory cells such as T cells, which produce interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNFα). In an autocrine–paracrine manner, these activated inflammatory factors may stimulate chondrocytes to secrete degradative enzymes like proteinases, such as matrix metalloproteinases (MMPs) and a disintegrin and metalloprotease with thrombospondin motifs (ADAMTS), which are directly involved in degradation of type II collagen and aggrecans in cartilage matrix. In the meantime, chondrocytes can change phenotype and size in response to stimulation from inflammatory factors and undergo hypertrophy, which is an essential step in the endochondral ossification process. Color images available online at www.liebertpub.com/teb
Growth factors are utilized to improve clinical cartilage repair by altering the local biological environment at the site of cartilage damage. Inflammation at the damage site may disrupt the balance between catabolic and anabolic factors; growth factors that target specific catabolic proinflammatory mediators, such as cytokines or nitric oxide synthase (NOS), or affect anabolism are potential candidates in slowing down the structural progression of the disease. The production and activities of many proinflammatory factors, such as cytokines, chemokines, growth factors, and various immune response regulators, are controlled by different signaling systems, such as nuclear factor-kappa B (NF-κB), mitogen-activated protein kinases (MAPK), and Janus kinase/signal transducers and activators of transcription (JAK/STAT). Novel small-molecule regulators targeting specific signal pathways as well as related precursor molecules have received a great deal of attention as potential candidates for treatment of inflammatory diseases (Fig. 2).
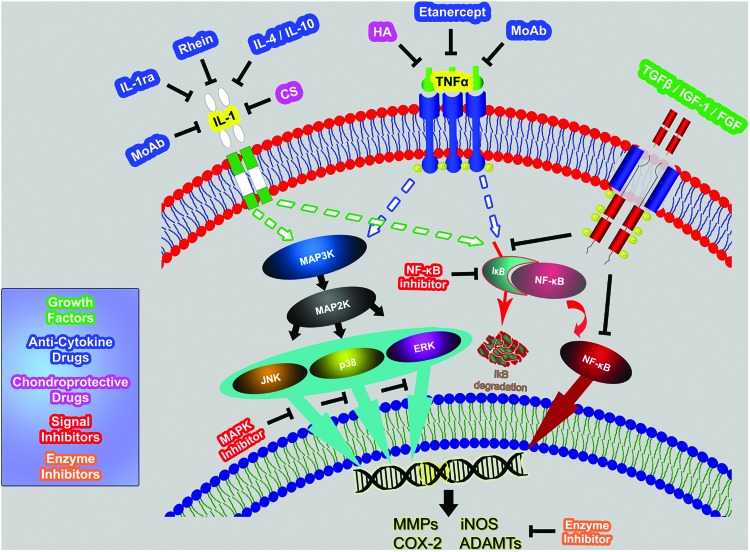
FIG. 2.
Schematic representation of key signaling pathways in the inflammatory process and potential strategies for inflammatory inhibition in cartilage repair. Use of anticytokine and chondroprotective drugs against IL-1, TNFα, or against their receptors is a direct and effective approach in the suppression of inflammation. Strategies targeting disturbance or intervention of key signal pathways in cytosol have been developed such as blocking the mitogen-activated protein kinases (MAPK) signal pathways or the nuclear factor-kappa B (NF-κB) pathway. Using inhibitors for downstream products of degradative enzymes, such as MMPs, cyclooxygenase 2 (COX-2), inducible nitric oxide synthase (iNOS), and ADAMTS, can also effectively protect cartilage from degradation and inflammation. Growth factors such as fibroblast growth factor (FGF)-2, insulin-like growth factor (IGF)-I, or transforming growth factor (TGF)-β may interfere with inflammation by blocking the associated signal pathways. Use of growth factors is a possible anti-inflammatory strategy in cartilage repair despite the fact that growth factors can promote neocartilage formation. Color images available online at www.liebertpub.com/teb
The development of stem cell technology provides the possibility of biotherapy for cartilage repair (Fig. 3). The availability of large quantities of mesenchymal stem cells (MSCs) and their multilineage differentiation potential, especially for chondrogenic differentiation, has made MSCs the ideal progenitor source for cartilage engineering and regeneration. Cell-based therapies using undifferentiated or prechondrogenic stem cells in biodegradable three-dimensional (3D) scaffolds for transplantation into focal lesions could regenerate hyaline-like cartilage.9,10 Since bioengineered cartilage constructs will eventually be transplanted into arthritic joints in which elevated levels of proinflammatory cytokines exist, it is especially important to select scaffolds that support the stability of bioengineered cartilage in an inflammatory environment. To this end, many extracellular matrix (ECM)-based scaffolds are hypothesized to meet the requirements of cartilage repair (Fig. 3).
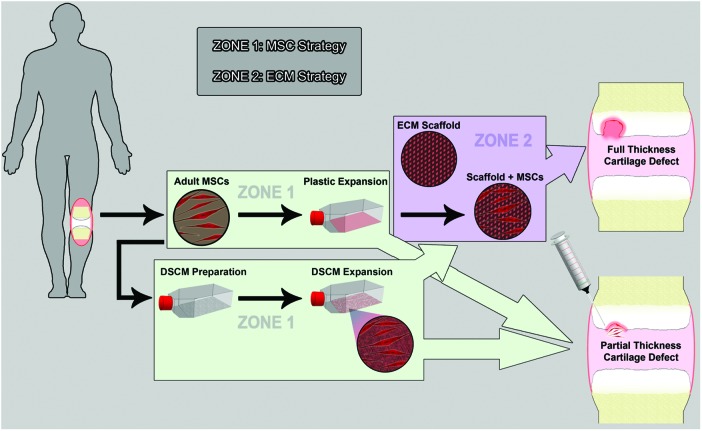
FIG. 3.
Therapeutic strategies for cartilage damage utilizing preconditioned stem cell and three-dimensional (3D) matrix. After ex vivo expansion of adult stem cells extracted from the human body, two strategies can be used for the treatment of cartilage defects: direct intra-articular injection or transplant of cells in a 3D scaffold. For full-thickness cartilage defects, the second strategy is normally used. Three-dimensional scaffolds can be made of extracellular matrix (ECM) materials. A structurally and mechanically stable scaffold allows for infiltration and attachment of bioactive molecules (IGF-I, FGF-2, and/or TGF-β), which can exert their anti-inflammatory effects on the surrounding environment. For partial-thickness cartilage defects, direct intra-articular injection of expanded stem cells is an option. Mesenchymal stem cells can secrete growth factors, which may exert their anti-inflammatory and antioxidative effects in the microenvironment where mesenchymal stem cells (MSCs) reside. These growth factors also benefit tissue regeneration. A recent finding suggests that decellularized stem cell matrix (DSCM) could rejuvenate expanded MSCs in proliferation and chondrogenic potential; this process may provide a large quantity of high-quality MSCs for cell-based cartilage regeneration. DSCM-expanded MSCs exhibiting an enhanced capacity against oxidation and inflammation may represent a promising anti-inflammatory strategy in the near future. Color images available online at www.liebertpub.com/teb
Recently, the decellularized ECM deposited by stem cells (DSCM) has attracted attention due to its excellent rejuvenation of expanded stem cells' chondrogenic potential, which has been reviewed.11 Furthermore, DSCM-expanded human synovium-derived stem cells (SDSCs) demonstrated in vitro antioxidant and anti-inflammatory capabilities.12 A study in mini pigs successfully proved its resurfacing effect on partial-thickness cartilage defects after intra-articular injection of DSCM-expanded allogeneic SDSCs.13 In this review article, the strategies using pharmacologic drugs, biomechanical stimulation, tissue-specific stem cells, and ECM-based scaffolds are summarized. An emerging strategy using a DSCM preconditioning approach to rejuvenate tissue-specific stem cells in both cell amount and chondrogenic potential is emphasized for its vital role in cartilage repair and anti-inflammation (Fig. 3). Future goals include the utilization of 3D ECM scaffolds, in conjunction with other combined strategies, including the use of preconditioned tissue-specific stem cells in a more favorable microenvironment to reduce the inflammation in cartilage repair.
Pharmacologic Strategy
Growth factors
In articular cartilage, numerous growth factors work in concert throughout life to regulate the development and homeostasis of articular cartilage.14 Disruption in the balance of regulatory factors may hinder tissue maintenance and repair, ultimately resulting in reduced synthesis of ECM, tissue degeneration, and consequently, an accelerated erosion of the articular surface.15 Bioactive growth factors are considered promising candidates for enhanced healing of chondral injuries and modification of the arthritic disease process. Members of the transforming growth factor beta (TGF-β)/bone morphogenetic protein family, insulin-like growth factor-I (IGF-I), and fibroblast growth factors (FGFs) are considered to be major anabolic factors for cartilage formation. They may stimulate chondrocyte synthesis of proteoglycans, aggrecan, and type II collagen, inducing cell proliferation, driving stem cell chondrogenic differentiation, and decreasing the catabolic effects of cytokines.16,17 Overexpression of the growth factor progranulin (PGRN), for example, has been implicated in the stimulation of chondrocyte proliferation; PGRN also acts as a physiological antagonist of tumor necrosis factor alpha (TNFα) signaling and disturbs the binding of TNFα and TNF receptor,18 potentially inhibiting cartilage degradation.19 Cytokines such as TNFα can stimulate vascular endothelial growth factor expression.20 Therefore, some anti-inflammatory strategies inhibit inflammation-induced angiogenesis although it remains unclear to what extent angiogenesis inhibition mediates their therapeutic effects.21
By exerting influence over inflammation, growth factors can modulate the microenvironment of chondrocytes to improve the local residence and provide a more ideal atmosphere for cartilage regeneration. For instance, growth factors such as platelet-derived growth factor (PDGF) and TGF-β obtained from platelet-rich plasma (PRP) decreased interleukin 1 beta (IL-1β)-induced NF-κB activation, a major pathway involved in the pathogenesis of OA (Fig. 2).22,23 Furthermore, in activated PRP, Bendinelli et al. observed increases in hepatocyte growth factor (HGF), IL-4, and TNFα; HGF and TNFα, by disrupting NF-κB-transactivating activity through the enhanced cellular IκBα expression, were important for the anti-inflammatory function of activated PRP.24 Paradoxically, platelet lysate (PL), a PRP derivative, was thought to play a role as a proinflammatory agent, acting synergistically with the canonical proinflammatory cytokines such as IL-1α, thus enhancing the initial inflammatory response; surprisingly, PL also contributes to the downregulation of the NF-κB signal pathway and cyclooxygenase 2 (COX-2) expression, thus triggering the resolution of the inflammation.25 The anti-inflammatory role of PRP was also demonstrated in an in vivo study. In the antigen-induced arthritis porcine model, the intra-articular injection of PRP attenuated the subsequent inflammatory response.26 The use of PRP may also improve the integration of an osteochondral graft at the cartilage interface and decreased degeneration in an in vivo rabbit model.27
The strategy combining growth factors and 3D scaffolds demonstrated a huge impact on tissue regeneration. Because of PRP's prominent role in anti-inflammation and fewer immunogenic as well as more biocompatible characteristics, the cell-free polyglycolic acid (PGA)-hyaluronan scaffold combined with PRP led to cartilage repair and improved patient-reported outcomes (the Knee injury and Osteoarthritis Score, KOOS) during 12 months of follow-up.28 Moreover, TGF-β, delivered together with calcium alginate to the sites of osteochondral defects, improved the repair of osteochondral defects in the rabbit knee.29 However, the local production of osteophytes has been observed in clinical trials; therefore, caution is advised because some growth factors favor the dedifferentiation of stem cells and promote the endochondral ossification process.30
Anticytokine therapy
Like growth factors, cytokines can also be produced in joint tissues and released into the synovial fluid; they influence the surrounding cells in an autocrine–paracrine manner (Fig. 1). Low levels of factors are necessary for normal homeostasis; however, inflammatory or oxidative stress conditions may disrupt normal homeostasis, driving the pathogenesis of OA. Among the vast number of cytokines, IL-1β and TNFα seem quite prominent and of major importance to cartilage destruction.31 Treatment strategies targeting major inflammatory factors have been developed (Fig. 2). Specific inhibitors of production/activity of IL-1, such as recombinant human IL-1 receptor antagonist (IL-1ra), can block the actions of IL-1 without any detectable agonist activity.32 The use of monoclonal antibodies against IL-1 or type I IL-1 receptor (IL-1RI) represents another possible approach in neutralization of the cytokine.33 Pharmacologic reagents, which downregulate the production and activity of active proinflammatory and procatabolic IL-1β, are also a feasible approach.34 One such example is to apply the rhein, an active metabolite of the semisynthetic anthraquinone derivative diacerein, to downregulate the production and activity of IL-1β in both the cartilage layer and synovial membrane.35 Etanercept, a recombinant soluble p75 TNF receptor, has a high affinity for TNFα, preventing it from binding with its receptor.36 A recent report indicates that etanercept enhanced preservation of osteochondral allograft viability.37 An in vivo study showed that subcutaneous injection of etanercept promoted repair of osteochondral defects in the rabbit knee.38 Application of the anti-TNFα monoclonal antibody in polyarthritic transgenic mice demonstrated reversal of cartilage degradation in the young mice.39
Besides IL-1β and TNFα, proinflammatory cytokines, such as IL-6,40 leukemia inhibitory factor,41 and the chemokine IL-8,42 may modulate the direct catabolic effects of some cytokines. They usually synergize with TNFα or IL-1β in the catabolic process of cartilage.43 Interference with those proinflammatory factors is another direction for anti-inflammatory therapy in cartilage repair. A number of anti-inflammatory cytokines, such as IL-4, IL-10, IL-11, and IL-13, were found in increased levels in the synovial fluid of OA patients.44 They decrease the production and/or activity of the proinflammatory cytokines in vitro45 and, thus, have been classified as anti-inflammatory cytokines. Endogenous IL-4 and IL-10 have been shown not only to reduce local IL-1 but may also have direct stimulatory or protective effects on chondrocyte metabolism.46,47 Since the early phase of OA is the most effective period to inhibit inflammation, administration of cytokine antagonists following cartilage repair is a safe strategy for not only promotion of cartilage integration but also the prevention of inflammation occurrence.
Chondroprotective drugs
Among pharmacological treatments, symptomatic slow-acting drugs have been largely studied over the last decade. Recently, there has been an increase in the use of symptomatic slow-acting/chondroprotective drugs such as glucosamine sulfate (GS), chondroitin sulfate (CS), hyaluronic acid (HA), and diacerein. Glucosamine decreased the activation of NF-κB in rat chondrocytes when treated with IL-1β.48 In normal human articular chondrocytes, GS inhibited IL-1β and TNFα-induced nitric oxide (NO) production, which is a major contributor for the inflammatory reaction in arthritis.49 Natural sulfated glycosaminoglycan (GAG), in particular CS, seemed to have beneficial effects on the pathophysiology of OA by reducing blood markers of inflammation and the activity of destructive proteases like matrix metalloproteinases (MMPs), diminishing pain as well as improving the function of the affected joint.50–52 In chondrocytes, CS diminished IL-1β-induced increases in p38 MAPK and extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation, and decreased NF-κB nuclear translocation and as a consequence, reduced the formation of proinflammatory cytokines, IL-1β and TNFα, and proinflammatory enzymes, such as phospholipase A2 (PLA2), COX-2, and inducible NOS (iNOS).53 However, other reports suggested that chondroitin-4-sulfate inhibited the enhanced expression of COX-2 and prostaglandin E (PGE) synthases 1, but had no effect on the IL-1β-induced decrease of IκBα and nuclear translocation of NF-κB54; the extent of sulfation influenced the responsiveness of inflammation.55
Synthetically sulfated HA was at first characterized as an inhibitor of TNFα.56 HA is an anionic nonsulfated GAG that acts as a crucial structural component of the ECM and as an important mediator of leukocyte adhesion and migration.57 HA is widely used in the treatment of OA and exerts significant chondroprotective effects. An in vitro report showed that HA altered the profile of inflammatory mediators, shifting the balance between cell matrix synthesis and degradation.58 An in vivo rabbit study showed that three weekly intra-articular injections of HA had a positive effect on the repair tissue that formed within the chondral defect at the early follow-up time point.59 CS, diacerein, GS, and HA demonstrated pain reduction and physical function improvement with very low toxicity in OA treatment and could be of potential interest for the symptomatic management of OA.60 Slow-acting drugs avoid interrupting the normal cellular function and toxicity to cells and, thus, could provide an anti-inflammatory strategy for cartilage repair (Fig. 2). However, high concentrations of chondroprotective drugs are not presumably attained through oral administration; intra-articular injections may represent a feasible and effective approach.61 With the application of tissue engineering in cartilage repair, slow-acting drugs serve as a basic unit of a 3D scaffold and, together with stem cells, demonstrated the potential of modulating inflammatory chemokines/receptors and catabolic/inhibiting factors.62
Small molecules targeting inflammation signals
Strategies for generating functional small molecules of synthetic and natural products targeting inflammatory signals have been considered effective in the suppression of inflammation.63 Primary proinflammatory factors IL-1β and TNFα have the capacity to activate a diverse array of intracellular signaling pathways; upon activation, they can induce phosphorylation-dependent signaling pathways, such as NF-κB, p38 MAPK, and c-Jun N-terminal kinase (JNK), which regulate the synthesis of several inflammatory cytokines and MMPs, many of which play major roles in the process of OA formation (Fig. 2).64
The NF-κB signaling pathway mediates critical events in the inflammatory response by chondrocytes, leading to progressive ECM damage and cartilage destruction. There are an increasing number of reports about NF-κB inhibitors, such as glucocorticoids, cyclosporine A, and tacrolinmus (FK-506).65 IκB kinase beta (IKKβ) has also become a particularly appealing target because of its crucial role in the activation of the NF-κB pathway.66 A recent report showed that a novel butanoylated GlcNAc derivative, 3,4,6-O-Bu3GlcNAc, an inhibitor of NF-κB activity, has the potential to stimulate new tissue production and reduce inflammation in IL-1β-induced chondrocytes with utility for OA and other forms of inflammatory arthritis.67 One concern about inhibiting several of these components of the NF-κB pathway is the specificity of such drugs. For example, the proteasome, which is responsible for IκB degradation, has many other important functions. Thus, inhibition of proteasome activity could potentially cause severe side effects. Also, it may not be feasible to block the NF-κB pathway for prolonged periods, since NF-κB plays an important role in the maintenance of host defense responses.68
Two MAPKs, p38 MAPK and JNK, are frequently activated by a wide range of environmental stresses and cytokines to induce inflammation and joint destruction, hence the name stress-activated protein kinases (SAPKs). p38 MAPK positively regulates the expression of many genes involved in inflammation, such as those coding for TNFα, IL-1β, IL-6, IL-8, COX-2, and collagenase-1 and -3.69 Many small-molecule inhibitors of p38 MAPK such as Cannabidiol,70 SB203580, Doramapimod (BIRB-796),71 VX-702,72 RO-3201195,73 and SB-24223574 have demonstrated positive anti-inflammatory effects. Among all these p38 MAPK inhibitors currently in clinical trials, SB203580 appears to be the most potent compound in terms of anti-inflammatory activity. For instance, inhibition of the p38 MAPK signaling pathway with SB203580 showed anti-inflammatory effects in both cartilage explants75 and animal models.76 However, there is a conflicting report showing that inhibition of the p38 MAPK pathway using SB203580 leads to OA-like changes in a rat animal model.77 Similarly, the JNKs are activated in macrophages after stimulation with lipopolysaccharides.78 Inhibition of the JNK signaling pathway has shown both preventive effects with regard to bone and cartilage destruction in rheumatoid arthritis79 and downregulation of IL-1-induced MMP-13 expression in OA chondrocytes.80
Small molecules targeting specific enzymes
Small molecules, which target specific enzymes involved in OA, have been developed as a possible way to alleviate inflammation in cartilage repair (Fig. 2). MMPs are synthesized and secreted by chondrocytes in response to stimulants such as IL-1 and TNF.81 Inappropriate expression of MMP activity constitutes part of the pathogenic mechanism associated with the destruction of cartilage and bone in OA. Possible strategies include impeding the production of MMPs, blocking the active site of MMPs, and increasing the endogenous production of tissue inhibitors of metalloproteinases (TIMPs).82
Another representative member of cyclooxygenase, the COX-2 enzyme, is primarily associated with inflammation. Cytokines and growth factors increase the expression of COX-2, mainly at inflammatory sites, producing prostaglandins that mediate inflammation, pain, and fever.83 Targeting cyclooxygenase, nonsteroidal anti-inflammatory drugs (NSAIDs) such as celecoxib play a major role in the management of inflammation and pain caused by arthritis.84,85 However, nonselective NSAIDs cause gastrointestinal complications in a significant number of patients, and COX-2 inhibitors have recently raised concerns regarding cardiovascular side effects/risks.86
The possible roles of NO in OA pathophysiology have been supported by a study showing that selective inhibition of the iNOS could reduce the progression of structural changes in experimental OA in dogs, partly related to a reduction in the levels of synovial inflammation.87 Many of these small-molecule compounds have already been launched on the market as drugs acting against inflammatory conditions. For example, leflunomide was found to significantly inhibit IL-4- and IL-13-enhanced production of chemokine (C-C motif) ligand (CCL)-26.88
Biomechanical Strategy
Biomechanical factors play an important role in the health of diarthrodial joints. Under normal physiological loading, moderate mechanical loading exhibits little or no wear over decades of use because articular cartilage provides a nearly frictionless surface for the transmission and distribution of joint loads. In reality, moderate loading is necessary to maintain healthy articular cartilage. Through a variety of mechanisms, including ion channels and integrin-mediated connections to the ECM that involve membrane, cytoskeletal, and intracellular deformation, chondrocytes in cartilage may perceive physical signals from the outside environment. Altered joint loading, which might be associated with obesity, malalignment, trauma, or joint instability, is a critical risk factor for joint degeneration and OA occurrence.
Biomechanical influences on cartilage are complex. Depending on the way cartilage is loaded, the effects on cartilage homeostasis can vary. An overload of mechanical stress may lead to acute joint injury such as posttraumatic OA, which is also caused by gradual onset of structural damage and cartilage compositional degradation due to chronic overloading of injured joints.89 Cartilage explants subjected to static compression exhibit a significant suppression of metabolic activity that is dependent on the magnitude of applied stress.90 A high-magnitude mechanical strain is proinflammatory and initiates cartilage destruction while inhibiting matrix synthesis, both of which are involved in the NF-κB-related signal pathway.91,92
It has been reported that a low-magnitude mechanical strain inhibits inflammation by suppressing IL-1β and TNFα-induced transcription of multiple proinflammatory mediators involved in cartilage degradation.91 It is noteworthy, however, that the influences of mechanical stress are not independent; many mechanical and physiochemical factors that are known to affect chondrocytes are inextricably coupled to one another within the cartilage ECM. Ramachandran et al. reported that treatment with C-type natriuretic peptide and dynamic compression increased anabolic activities and blocked catabolic effects induced by IL-1β.93 For cyclic loading, however, the cartilage reaction is different; low frequencies and amplitudes do not appear to affect biosynthesis rates. Some studies suggested that, above a certain threshold frequency, cyclic loading increases the synthesis of components of ECM, such as aggrecans, cartilage oligomeric matrix protein, and fibronectin.90
There is increasing evidence showing that muscle function is closely related to OA. The muscle, lying anatomically adjacent to cartilage, provides the cartilage with a biomechanical stimulation that promotes nutrient distribution and maintains homeostasis.92 Muscle loss94 and reduced muscle strength95 have been shown to be risk factors for knee OA. On the other hand, patients with knee OA were found to have impaired muscle function; quadriceps, hamstring, and hip muscles are significantly impaired in subjects with knee OA compared with age-matched controls.96 Recent studies showed that a rat chondrocyte cell line cocultured with muscle cells or cultured in muscle cell-conditioned medium in a monolayer demonstrated enhanced resistance to proinflammatory factors such as IL-1β and TNFα, suggesting that nonloading biochemical effects of muscle cells have a significant influence on cartilage homeostasis and a preventative role in OA formation.97 Mechanistically, it is known that the proinflammatory cytokines IL-1β and TNFα are major inflammation initiators in cartilage by inducing the expression of MMPs, promoting chondrocyte hypertrophy, and reducing the synthesis of cartilage matrix genes. However, muscle cell-derived factors or myokines inhibited the mRNA expression of MMPs as well as hypertrophic markers in bioengineered cartilage; it could also rescue IL-1β-induced chondrocyte growth arrest through regulating the cell cycle. The beneficial role of muscle cells on OA indicated that muscle strengthening exercises could be a potential intervention for OA.98
MSC Strategy
Inherent anti-inflammatory properties
Despite being the most advanced and promising approach for cartilage repair, chondrocyte-based cartilage repair has some disadvantages, such as morbidity caused by damage to the donor-site articular surface and cell senescence during ex vivo expansion.4 Furthermore, the inflammatory synovial fluid microenvironment triggers human chondrocytes to actively take part in inflammatory processes, particularly during the initiation and progression of inflammatory joint diseases and in the disruption of cartilage repair mechanisms resulting in cartilage degradation.99 Unlike chondrocytes, MSCs are not limited by an intrinsic tendency to lose their phenotype and dedifferentiate during expansion.100 MSCs also play an important role in immunomodulation and tissue regeneration by secretion of soluble factors.101,102 In an inflammatory environment, MSCs secrete factors which cause multiple anti-inflammatory effects and influence matrix turnover in synovium and cartilage explants.103 MSCs have also been revealed as robust sources of TIMP-mediated MMP-inhibition, capable of protecting the perivascular niche from high levels of destructive MMPs, even under pathological conditions.104
Due to their potential to modulate the local microenvironment via anti-inflammatory and immunosuppressive functions, MSCs have an additional advantage for allogeneic application (Fig. 3). Moreover, by secreting various bioactive soluble factors, MSCs can protect the cartilage from further tissue destruction and facilitate regeneration of the remaining progenitor cells in situ.105 The joint resurfacing function of MSCs was also demonstrated in in vivo studies. After intra-articular injection of autologous MSCs into a caprine OA model (complete excision of the medial meniscus and resection of the anterior cruciate ligament), there was evidence of marked regeneration of the medial meniscus and implanted cells were detected in the newly formed tissue. Degeneration of the articular cartilage, osteophytic remodeling, and subchondral sclerosis were reduced in cell-treated joints compared with joints treated with vehicle alone without cells.106 A clinical case report demonstrated that, 24 weeks after the injection of autologous MSCs into a knee with symptomatic and radiographic degenerative joint disease, there was significant cartilage growth, decreased pain, and increased joint mobility in the patient.107 As a result, MSCs could be used in cartilage repair as a potential anti-inflammatory strategy especially in the context of allogeneic transplantation.
Acquired anti-inflammatory capacity
Treatment strategies focused on both reducing inflammation and increasing tissue production are necessary to effectively treat OA from a tissue-engineering perspective. Despite their multilineage differentiation potential and immunomodulatory properties as well as anti-inflammatory abilities, MSC survival after transplantation is still very low, thereby hindering their therapeutic efficacy.108
Recent studies found that MSCs could be rejuvenated by preconditioning strategies, which enhance their post-transplantation survival and functionality.109 The concept of preconditioning was established in 1986 by Murry et al., who found that ischemic preconditioning of cardiomyocytes led to the activation of survival signaling.110 In theory, any factors that might influence the proliferation and differentiation of therapeutic cells can be an advantage. For instance, hypoxic preconditioning not only has prosurvival and cytoprotective effects, it also helps the cells maintain their stemness and promote proliferation and differentiation potential postengraftment.111 When referring to a cartilage engineering application, preconditioning of MSCs can be derived from microenvironment factors such as low oxygen,112 growth factors,113 or potentially pharmacological substances targeting specific signal pathways involved in MSC chondrogenesis such as the MAPK114,115 and Wnt signal pathways.114,116
DSCM-mediated stem cell preconditioning
The stem cell niche is a specialized microenvironment that helps sustain the stem cell pool within each tissue or organ system. It is hypothesized that stem cells can create their own microenvironment; in such a microenvironment, adult stem cells are expected to greatly expand, while retaining their stemness for a tissue-specific lineage. In 2009, He et al. utilized porcine SDSCs, tissue-specific stem cells for chondrogenesis,117 as a model to reconstruct an in vitro 3D stem cell microenvironment, in which expanded SDSCs produced a drastic increase in cell number and chondrogenic potential.118 Later on, this DSCM-mediated stem cell preconditioning was also demonstrated to be effective in rejuvenating human bone marrow stromal cells, in terms of enhanced proliferation and chondrogenic hypertrophy.119 Interestingly, this DSCM-mediated expansion system also works for primary chondrocytes, such as articular chondrocytes120,121 and nucleus pulposus cells.122,123
The regulation of intracellular reactive oxygen species (ROS) is crucial for cell survival in a harsh environment and guarantees successful cell therapy.124 DSCM preconditioning could decrease the expanded stem cell ROS level119,125 and protect human SDSCs from oxidative stress-induced cell senescence, as it applies to cell proliferation and differentiation capacity.12 Furthermore, our recent data showed that human SDSCs expanded on DSCM exhibited an increased chondrogenic potential as well as heightened protection against IL-1β-induced inflammation.126 The adaptive capacity in a harsh environment was also validated in a mini pig study, in which DSCM-expanded SDSCs exhibited an enhanced in vivo cartilage regeneration capacity postinjection, evidenced by intensely stained type II collagen and sulfated GAGs in the partial-thickness cartilage defects with negligible staining of type I collagen,13 indicating that DSCM preconditioned SDSCs have the ability to resist inflammation. In contrast, the existence of type I collagen in the regenerated cartilage from the plastic flask-expanded SDSC groups suggested fibrocartilage formation,13 which might be explained by dedifferentiation caused by inflammatory stress.127
Low oxygen and FGF-2 contribute to DSCM-mediated stem cell preconditioning
Low oxygen tension (hypoxia) maintains undifferentiated states of MSC phenotypes and also influences proliferation and cell fate commitment.128 There is increasing evidence suggesting that hypoxia (or, more appropriately, physiological hypoxia) can stimulate chondrogenesis.129,130 Hypoxic preconditioning can boost the expression of genes favoring MSC proliferation and growth prolongation by inducing the expression of prosurvival and proangiogenic markers in MSCs.131 Recently, hypoxia-inducible factors (HIFs) have been shown to activate specific signaling pathways such as Notch and the expression of transcription factors such as the octamer-binding transcription factor 4 (Oct4), which is responsible for controlling stem cell self-renewal and multipotency.132 This finding suggests that modulation of oxygen availability and HIF expression can influence stem cell fate. Our recent report suggested that the combination of hypoxia and FGF-2 significantly enhanced DSCM-expanded SDSC proliferation and chondrogenic potential.133 This microecosystem apparently reconciled the contradiction of Quantity versus Quality, a predicament often met in stem cell-based cartilage repair. In vivo, numerous growth factors and morphogens are immobilized by directly binding to the ECM through specific heparin-binding domains, by direct binding to ECM molecules such as collagen, or by direct anchoring to cell membranes.134 By immobilizing growth factors in a concentrated area, DSCM helps to amplify the effects of growth factors and reduce the inflammation reaction leading to improved proliferation and differentiation of stem cells.102
Small molecules targeting inflammation signals contribute to DSCM-mediated stem cell preconditioning
Widely used as an anti-inflammatory drug in cartilage repair, the p38 MAPK inhibitor also blocks chondrogenesis,135 indicating that the p38 MAPK inhibitor has some disadvantages in the treatment of cartilage damage with concomitant inflammation. Considering its adverse effects for clinical treatment of OA, MSCs pretreated by SB203580 in the DSCM expansion phase were assumed to avoid this dilemma. Preconditioning using SB203580 significantly enhanced DSCM-expanded human SDSC chondrogenic potential; this rejuvenation of chondrogenic capacity, instilled in SDSCs by SB203580, gave the stem cell the ability to resist the influence of IL-1β-induced inflammation.126
ECM Strategy
Compared to natural biomaterials, synthetic polymers are more controllable and predictable in their chemical and physical properties, holding some promise of success in tissue engineering and regenerative medicine. Of the synthetic polymers, those derived from poly(α-hydroxy esters),136 poly(propylene fumarates),137 polyurethanes,138 PGA,139 and poly(lactide-co-glycolide)140 have been used for cartilage regeneration; however, inflammatory reaction due to synthetic scaffolds that impaired the quality of regenerated cartilage has also been reported in some studies.141,142
Natural matrix scaffolds
Naturally occurring polymers as scaffolds offer options for cartilage tissue engineering due to biocompatibility, biodegradability, low toxicity of degradation by-products, and plasticity in processing into a variety of material formats.143 In an attempt to repair articular cartilage, allografted articular chondrocytes, embedded in collagen gel, were transplanted into full-thickness defects in rabbit articular cartilage; 24 weeks after implantation, the defects were filled with hyaline cartilage, synthesized type II collagen, and exhibited no signs of immunologic rejection and degeneration of the reparative tissue.144,145 Rahfoth et al. found that the transplantation of allograft chondrocytes embedded in agarose gel was a suitable method to repair articular cartilage defects in rabbits because there were no signs of graft-versus-host rejection or infiltration by immune cells.146 Other natural polymers that have been explored as bioactive scaffolds for cartilage tissue engineering include, but are not limited to silk,147 hyaluronan,148 and chitosan.149 In a recent study, the responses of articular chondrocytes under inflammatory conditions were compared after seeding within three polymeric scaffolding materials (silk, collagen, and polylactic acid [PLA]); Kwon et al. found that chondrocytes grown in the silk and collagen scaffolds exhibited higher levels of cartilage matrix gene expression than those in the PLA scaffolds.150 When using a PGA scaffold for treatment of full-thickness defects, however, there appears to be a moderate immunoreaction as evidenced by lymphocytes in transplanted joints.9,151
Chondrocyte sheets
A cell sheet technique developed in 1993152 shows promise in cartilage repair with concomitant inflammation. Hamahashi et al. reported that a layered chondrocyte sheet produced the most humoral factors, including PGE2, which plays a key role in its anti-inflammatory function.153 In chondrocyte sheets, catabolic factors such as MMP3, MMP13, and a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS5) were also observed to decrease, while the expression of TIMP1 with antagonistic actions against MMP3 increased.154 In a rabbit model with partial-thickness cartilage defects, layered chondrocyte sheets were able to maintain the cartilaginous phenotype and could be attached to the site of cartilage damage, acting as a barrier to prevent a loss of proteoglycan from these sites and to protect them from catabolic factors in the joint.154 This technique might have great potential for OA treatment and inflammatory prevention in cartilage repair.
Decellularized tissue matrix
In contrast to the DSCM described above, which attempts to rejuvenate expanded stem cells' chondrogenic potential as well as anti-inflammatory capacity, the decellularized tissue matrix influences tissue engineering directly, providing a carrier (or scaffold) and chondrogenically induced growth factors as well as an anti-inflammatory function. The use of ECM derived from decellularized tissue is increasingly common in regenerative medicine and tissue engineering.155 By virtue of physical, chemical, or enzymatic approaches, organ decellularization removes all cellular material without adversely affecting the composition, biologic activity, or mechanical integrity of the remaining 3D matrix.156 The molecules that constitute ECM are largely and highly conserved across species and are well tolerated even by xenogeneic recipients. The effects of xenogeneic ECM on the innate immune response, specifically the responding macrophages, may elicit a necessary M2 phenotypic profile to support a constructive remodeling response for the scaffold.157 In the field of cartilage regeneration, many decellularized tissue scaffolds showed positive effects and reparative capability for cartilage defects.158 For example, the combined use of cells with a decellularized aortic scaffold was able to prevent the generation of a strong inflammatory response and improve the overall tracheal cartilage regeneration.159 Compared with a PGA scaffold, an ECM scaffold derived from porcine cartilage not only strongly supported chondrogenic differentiation of rabbit MSCs but also helped maintain its phenotype in vivo.160
Summary
The treatment of cartilage injury remains a clinical challenge despite the advancements in surgical procedures and techniques. An important hurdle is the concomitant inflammation during cartilage repair. Pharmacological drugs have been successfully applied in cartilage repair; however, they cannot optimally work alone.161 With the development of ECM-based scaffolds and preconditioned tissue-specific stem cells, both tissue engineering components could not only contribute to an enhanced ability in anti-inflammation but also to cartilage regeneration. All these strategies could combine to boost cartilage repair under inflammatory conditions.
Disclosure Statement
The authors declare no potential conflicts of interest.
Acknowledgments
The authors thank Suzanne Danley for help in editing the article. This project was partially supported by Research Grants from the AO Foundation (S-12-19P) and the National Institutes of Health (NIH) (1 R03 AR062763-01A1 and 5 R03 DE021433-02).
References
- 1.Bitton R.The economic burden of osteoarthritis. Am J Manag Care, 15,S230, 2009 [PubMed] [Google Scholar]
- 2.Hangody L., and Füles P.Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am 85-A(Suppl 2),25, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Knutsen G., Engebretsen L., Ludvigsen T.C., Drogset J.O., Grøntvedt T., Solheim E, Strand T., Roberts S., Isaksen V., and Johansen O.Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am 86-A,455, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Li J.T., and Pei M.Cell senescence: a challenge in cartilage engineering and regeneration. Tissue Eng Part B 18,270, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Steinert A.F., Ghivizzani S.C., Rethwilm A., Tuan R.S., Evans C.H., and Nöth U.Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther 9,213, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckwalter J.A., Martin J.A., and Brown T.D.Perspectives on chondrocyte mechanobiology and osteoarthritis. Biorheology 43,603, 2006 [PubMed] [Google Scholar]
- 7.Heijink A., Gomoll A.H., Madry H., Drobnič M., Filardo G., Espregueira-Mendes J., and Van Dijk C.N.Biomechanical considerations in the pathogenesis of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 20,423, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sokolove J., and Lepus C.M.Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis 5,77, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pei M., He F., Boyce B.M., and Kish V.L.Repair of full-thickness femoral condyle cartilage defects using allogeneic synovial cell-engineered tissue constructs. Osteoarthritis Cartilage 17,714, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Pei M., He F., Kish V., and Vunjak-Novakovic G.Engineering of functional cartilage tissue using stem cells from synovial lining: a preliminary study. Clin Orthop Relat Res 466,1880, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pei M., Li J.T., Shoukry M., and Zhang Y.A review of decellularized stem cell matrix: a novel cell expansion system for cartilage tissue engineering. Eur Cell Mater 22,333, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Pei M., Zhang Y., Li J.T., and Chen D.Q.Antioxidation of decellularized stem cell matrix promotes human synovium-derived stem cell-based chondrogenesis. Stem Cells Dev 22,889, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pei M., He F., Li J., Tidwell J.E., Jones A.C., and McDonough E.B.Repair of large animal partial-thickness cartilage defects through intraarticular injection of matrix-rejuvenated synovium-derived stem cells. Tissue Eng Part A 19,1144, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Goldring M.B., Tsuchimochi K., and Ijiri K.The control of chondrogenesis. J Cell Biochem 97,33, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Alves H., Munoz-Najar U., De Wit J., Renard A.J., Hoeijmakers J.H., Sedivy J.M., Van Blitterswijk C., and De Boer J.A link between the accumulation of DNA damage and loss of multi-potency of human mesenchymal stromal cells. J Cell Mol Med 14,2729, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldring M.B.Osteoarthritis and cartilage: the role of cytokines. Curr Rheumatol Rep 2,459, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Montaseri A., Busch F., Mobasheri A., Buhrmann C., Aldinger C., Rad J.S., and Shakibaei M.IGF-1 and PDGF-bb suppress IL-1β-induced cartilage degradation through down-regulation of NF-κB signaling: involvement of Src/PI-3K/AKT pathway. PLoS One 6,e28663, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang W., Lu Y., Tian Q.Y., Zhang Y., Guo F.J., Liu G.Y., Syed N.M., Lai Y., Lin E.A., Kong L., Su J., Yin F., Ding A.H., Zanin-Zhorov A., Dustin M.L., Tao J., Craft J., Yin Z., Feng J.Q., Abramson S.B., Yu X.P., and Liu C.J.The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science 332,478, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng J.Q., Guo F.J., Jiang B.C., Zhang Y., Frenkel S., Wang D.W., Tang W., Xie Y., and Liu C.J.Granulin epithelin precursor: a bone morphogenic protein 2-inducible growth factor that activates Erk1/2 signaling and JunB transcription factor in chondrogenesis. FASEB J 24,1879, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pufe T., Harde V., Petersen W., Goldring M.B., Tillmann B., and Mentlein R.Vascular endothelial growth factor (VEGF) induces matrix metalloproteinase expression in immortalized chondrocytes. J Pathol 202,367, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Ashraf S., and Walsh D.A.Angiogenesis in osteoarthritis. Curr Opin Rheumatol 20,573, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Andia I., and Maffulli N.Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol 9,721, 2013 [DOI] [PubMed] [Google Scholar]
- 23.van Buul G.M., Koevoet W.L., Kops N., Bos P.K., Verhaar J.A., Weinans H., Bernsen M.R., and van Osch G.J.Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am J Sports Med 39,2362, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Bendinelli P., Matteucci E., Dogliotti G., Corsi M.M., Banfi G., Maroni P., and Desiderio M.A.Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: mechanisms of NF-κB inhibition via HGF. J Cell Physiol 225,757, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Pereira R.C., Scaranari M., Benelli R., Strada P., Reis R.L., Cancedda R., and Gentili C.Dual effect of platelet lysate on human articular cartilage: a maintenance of chondrogenic potential and a transient proinflammatory activity followed by an inflammation resolution. Tissue Eng Part A 19,1476, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Lippross S., Moeller B., Haas H., Tohidnezhad M., Steubesand N., Wruck C.J., Kurz B., Seekamp A., Pufe T., and Varoga D.Intraarticular injection of platelet-rich plasma reduces inflammation in a pig model of rheumatoid arthritis of the knee joint. Arthritis Rheum 63,3344, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Smyth N.A., Haleem A.M., Murawski C.D., Do H.T., Deland J.T., Kennedy J.G.The effect of platelet-rich plasma on autologous osteochondral transplantation: an in vivo rabbit model. J Bone Joint Surg Am 95,2185, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Siclari A., Mascaro G., Gentili C., Cancedda R., Boux E.A cell-free scaffold-based cartilage repair provides improved function hyaline-like repair at one year. Clin Orthop Relat Res 470,910, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mierisch C.M., Cohen S.B., Jordan L.C., Robertson P.G., Balian G., and Diduch D.R.Transforming growth factor-beta in calcium alginate beads for the treatment of articular cartilage defects in the rabbit. Arthroscopy 18,892, 2002 [DOI] [PubMed] [Google Scholar]
- 30.van der Kraan P.M., and van den Berg W.B.Osteophytes: relevance and biology. Osteoarthritis Cartilage 15,237, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Malemud C.J.Cytokines as therapeutic targets for osteoarthritis. Bio Drugs 18,23, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Pelletier J.P., Martel-Pelletier J., and Raynauld J.P.Most recent developments in strategies to reduce the progression of structural changes in osteoarthritis: today and tomorrow. Arthritis Res Ther 8,206, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pelletier J.P., Martel-Pelletier J., and Abramson S.B.Osteoarthritis, an inflammatory disease: potential implications for the selection of new therapeutic targets. Arthritis Rheum 44,1237, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Leeb B.F.Clinical efficacy and safety of diacerein in osteoarthritis-a review. Eur Musculoskelet Rev 5,23, 2010 [Google Scholar]
- 35.Martel-Pelletier J., Mineau F., Jolicoeur F.C., Cloutier J.M., and Pelletier J.P.In vitro effects of diacerhein and rhein on interleukin 1 and tumor necrosis factor-α systems in human osteoarthritic synovium and chondrocytes. J Rheumatol 25,753, 1998 [PubMed] [Google Scholar]
- 36.Scott D.L., and Kingsley G.H.Tumor necrosis factor inhibitors for rheumatoid arthritis. N Engl J Med 355,704, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Linn M.S., Chase D.C., Healey R.M., Harwood F.L., Bugbee W.D., and Amiel D.Etanercept enhances preservation of osteochondral allograft viability. Am J Sports Med 39,1494, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawaguchi A., Nakaya H., Okabe T., Tensho K., Nawata M., Eguchi Y., Imai Y., Takaoka K., and Wakitani S.Blocking of tumor necrosis factor activity promotes natural repair of osteochondral defects in rabbit knee. Acta Orthop 80,606, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shealy D.J., Wooley P.H., Emmell E., Volk A., Rosenberg A., Treacy G., Wagner C.L., Mayton L., Griswold D.E., and Song X.Y.Anti-TNF-alpha antibody allows healing of joint damage in polyarthritic transgenic mice. Arthritis Res 4,R7, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guerne P.A., Carson D.A., and Lotz M.IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J Immunol 144,499, 1990 [PubMed] [Google Scholar]
- 41.Villiger P.M., Geng Y., and Lotz M.Induction of cytokine expression by leukemia inhibitory factor. J Clin Invest 91,1575, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Recklies A.D., and Golds E.E.Induction of synthesis and release of interleukin-8 from human articular chondrocytes and cartilage explants. Arthritis Rheum 35,1510, 1992 [DOI] [PubMed] [Google Scholar]
- 43.Henrotin Y.E., De Groote D.D., Labasse A.H., Gaspar S.E., Zheng S.X., Geenen V.G., and Reginster J.Y.Effects of exogenous IL-1 beta, TNF alpha, IL-6, IL-8 and LIF on cytokine production by human articular chondrocytes. Osteoarthritis Cartilage 4,163, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Martel-Pelletier J., Alaaeddine N., and Pelletier J.P.Cytokines and their role in the pathophysiology of osteoarthritis. Front Biosci 4,694, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Alaaeddine N., Di Battista J.A., Pelletier J.P., Kiansa K., Cloutier J.M., and Martel-Pelletier J.Differential effects of IL-8, LIF (pro-inflammatory) and IL-11 (anti-inflammatory) on TNF-alpha-induced PGE(2)release and on signalling pathways in human OA synovial fibroblasts. Cytokine 11,1020, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Lubberts E., Joosten L.A., Helsen M.M., and van den Berg W.B.Regulatory role of interleukin 10 in joint inflammation and cartilage destruction in murine streptococcal cell wall (SCW) arthritis. More therapeutic benefit with IL-4/IL-10 combination therapy than with IL-10 treatment alone. Cytokine 10,361, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Lubberts E., Joosten L.A., van Den Bersselaar L., Helsen M.M., Bakker A.C., van Meurs J.B., Graham F.L., Richards C.D., and van Den Berg W.B.Adenoviral vector-mediated overexpression of IL-4 in the knee joint of mice with collagen-induced arthritis prevents cartilage destruction. J Immunol 163,4546, 1999 [PubMed] [Google Scholar]
- 48.Gouze J.N., Bianchi A., Becuwe P., Dauça M., Netter P., Magdalou J., Terlain B., and Bordji K.Glucosamine modulates IL-1-induced activation of rat chondrocytes at a receptor level, and by inhibiting the NF-kappa B pathway. FEBS Lett 510,166, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Shikhman A.R., Kuhn K., Alaaeddine N., and Lotz M.N-acetylglucosamine prevents IL-1 beta-mediated activation of human chondrocytes. J Immunol 166,5155, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Hochberg M., Chevalier X., Henrotin Y., Hunter D.J., and Uebelhart D.Symptom and structure modification in osteoarthritis with pharmaceutical-grade chondroitin sulfate: what's the evidence? Curr Med Res Opin 29,259, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Martel-Pelletier J., Kwan Tat S, and Pelletier J.P.Effects of chondroitin sulfate in the pathophysiology of the osteoarthritic joint: a narrative review. Osteoarthritis Cartilage 18 (Suppl 1),S7, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Volpi N.Anti-inflammatory activity of chondroitin sulphate: new functions from an old natural macromolecule. Inflammopharmacology 19,299, 2011 [DOI] [PubMed] [Google Scholar]
- 53.du Souich P., García A.G., Vergés J., and Montell E.Immunomodulatory and anti-inflammatory effects of chondroitin sulphate. J Cell Mol Med 13,1451, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kunze R., Hempel U., and Dieter P.Differential effect of chondroitin-4-sulfate on the immediate and delayed prostaglandin E2 release from osteoblasts. Prostaglandins Other Lipid Mediat 92,8, 2010 [DOI] [PubMed] [Google Scholar]
- 55.Kajahn J., Franz S., Rueckert E., Forstreuter I., Hintze V., Moeller S., and Simon J.C.Artificial extracellular matrices composed of collagen I and high sulfated hyaluronan modulate monocyte to macrophage differentiation under conditions of sterile inflammation. Biomatter 2,226, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang N.S., Intrieri C., Mattison J., and Armand G.Synthetic polysulfated hyaluronic acid is a potent inhibitor for tumor necrosis factor production. J Leukoc Biol 55,778, 1994 [DOI] [PubMed] [Google Scholar]
- 57.Tammi M., Day A.J., and Turley E.A.Hyaluronan and homeostasis: a balancing act. J Biol Chem 277,4581, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Moreland L.W.Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther 5,54, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strauss E., Schachter A., Frenkel S., and Rosen J.The efficacy of intra-articular hyaluronan injection after the microfracture technique for the treatment of articular cartilage lesions. Am J Sports Med 37,720, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Bruyère O., Burlet N., Delmas P.D., Rizzoli R., Cooper C., and Reginster J.Y.Evaluation of symptomatic slow-acting drugs in osteoarthritis using the GRADE system. BMC Musculoskelet Disord 9,165, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lotz M.K., and Kraus V.B.New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res Ther 12,211, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo Y., Yuan T., Xiao Z., Tang P., Xiao Y., Fan Y., and Zhang X.Hydrogels of collagen/chondroitin sulfate/hyaluronan interpenetrating polymer network for cartilage tissue engineering. J Mater Sci Mater Med 23,2267, 2012 [DOI] [PubMed] [Google Scholar]
- 63.Ivanenkov Y.A., Balakin K.V., and Tkachenko S.E.New approaches to the treatment of inflammatory disease: focus on small-molecule inhibitors of signal transduction pathways. Drugs R D 9,397, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Burrage P.S., Mix K.S., and Brinckerhoff C.E.Matrix metalloproteinases: role in arthritis. Front Biosci 11,529, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Lee J.I., and Burckart G.J.Nuclear factor kappa B: important transcription factor and therapeutic target. J Clin Pharmacol 38,981, 1998 [DOI] [PubMed] [Google Scholar]
- 66.Roman-Blas J.A., and Jimenez S.A.NF-κB as potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage 14,839, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Coburn J.M., Wo L., Bernstein N., Bhattacharya R., Aich U., Bingham C.O., 3rd, Yarema K.J., and Elisseeff J.H.Short-chain fatty acid-modified hexosamine for tissue-engineering osteoarthritic cartilage. Tissue Eng Part A 19,2035, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamamoto Y., and Gaynor R.B.Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest 107,135, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kyriakis J.M., and Avruch J.Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 81,807, 2001 [DOI] [PubMed] [Google Scholar]
- 70.El-Remessy A.B., Al-Shabrawey M., Khalifa Y., Tsai N.T., Caldwell R.B., and Liou G.I.Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am J Pathol 168,235, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuma Y., Sabio G., Bain J., Shpiro N., Márquez R., and Cuenda A.BIRB796 inhibits all p38 MAPK isoforms in vitro and in vivo. J Biol Chem 280,19472, 2005 [DOI] [PubMed] [Google Scholar]
- 72.Ding C.Drug evaluation: VX-702, a MAP kinase inhibitor for rheumatoid arthritis and acute coronary syndrome. Curr Opin Investig Drugs 7,1020, 2006 [PubMed] [Google Scholar]
- 73.Goldstein D.M., Alfredson T., Bertrand J., Browner M.F., Clifford K., Dalrymple S.A., Dunn J., Freire-Moar J., Harris S., Labadie S.S., La Fargue J., Lapierre J.M., Larrabee S., Li F., Papp E., McWeeney D., Ramesha C., Roberts R., Rotstein D., San Pablo B., Sjogren E.B., So O.Y., Talamas F.X., Tao W., Trejo A., Villasenor A., Welch M., Welch T., Weller P., Whiteley P.E., and Young K., Zipfel S.Discovery of S[5-amino-1-(4-fluorophenyl)-1H-pyrazol-4-yl]-[3-(2,3-dihydroxypropoxy)phenyl]methanone (RO3201195), an orally bioavailable and highly selective inhibitor of p38 MAP kinase. J Med Chem 49,1562, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Ward K.W., Proksch J.W., Salyers K.L., Azzarano L.M., Morgan J.A., Roethke T.J., McSurdy-Freed J.E., Levy M.A., and Smith B.R.SB-242235, a selective inhibitor of p38 mitogen-activated protein kinase. I: preclinical pharmacokinetics. Xenobiotica 32,221, 2002 [DOI] [PubMed] [Google Scholar]
- 75.Ridley S.H., Sarsfield S.J., Lee J.C., Bigg H.F., Cawston T.E., Taylor D.J., DeWitt D.L., and Saklatvala J.Actions of IL-1 are selectively controlled by p38 mitogen-activated protein kinase: regulation of prostaglandin H synthase-2, metalloproteinases, and IL-6 at different levels. J Immunol 158,3165, 1997 [PubMed] [Google Scholar]
- 76.Brown K.K., Heitmeyer S.A., Hookfin E.B., Hsieh L., Buchalova M., Taiwo Y.O., and Janusz M.J.P38 MAP kinase inhibitors as potential therapeutics for the treatment of joint degeneration and pain associated with osteoarthritis. J Inflamm (Lond) 5,22, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prasadam I., Mao X., Wang Y., Shi W., Crawford R., and Xiao Y.Inhibition of p38 pathway leads to OA-like changes in a rat animal model. Rheumatology (Oxford) 51,813, 2012 [DOI] [PubMed] [Google Scholar]
- 78.Chan E.D., Winston B.W., Jarpe M.B., Wynes M.W., and Riches D.W.Preferential activation of the p46 isoform of JNK/SAPK in mouse macrophages by TNF alpha. Proc Natl Acad Sci U S A 94,13169, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bogoyevitch M.A., Boehm I., Oakley A., Ketterman A.J., and Barr R.K.Targeting the JNK MAPK cascade for inhibition: basic science and therapeutic potential. Biochim Biophys Acta 1697,89, 2004 [DOI] [PubMed] [Google Scholar]
- 80.Ahmed S., Rahman A., Hasnain A., Goldberg V.M., and Haqqi T.M.Phenyl N-tertbutylnitronedown-regulates interleukin-1 β stimulated matrix metalloproteinase-13 gene expression in human chondrocytes: suppression of c-Jun NH2-terminal kinase, p38-mitogen-activated protein kinase and activating protein-1. J Pharmacol Exp Ther 305,981, 2003 [DOI] [PubMed] [Google Scholar]
- 81.Tanaka S., Hamanishi C., Kikuchi H., and Fukuda K.Factors related to degradation of articular cartilage in osteoarthritis: a review. Semin Arthritis Rheum 27,392, 1998 [DOI] [PubMed] [Google Scholar]
- 82.Jackson C., Nguyen M., Arkell J., and Sambrook P.Selective matrix metalloproteinase (MMP) inhibition in rheumatoid arthritis—targetting gelatinase A activation. Inflamm Res 50,183, 2001 [DOI] [PubMed] [Google Scholar]
- 83.Crofford L.J.COX-1 and COX-2 tissue expression: implications and predictions. J Rheumatol 24,15, 1997 [PubMed] [Google Scholar]
- 84.Jiang D., Zou J., Huang L., Shi Q., Zhu X., Wang G., and Yang H.Efficacy of intra-articular injection of celecoxib in a rabbit model of osteoarthritis. Int J Mol Sci 11,4106, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Noble S.L., King D.S., and Olutade J.I.Cyclooxygenase-2 enzyme inhibitors: place in therapy. Am Fam Physician 61,3669, 2000 [PubMed] [Google Scholar]
- 86.Funk C.D., and FitzGerald G.A.COX-2 inhibitors and cardiovascular risk. J Cardiovasc Pharmacol 50,470, 2007 [DOI] [PubMed] [Google Scholar]
- 87.Pelletier J-P., Jovanovic D., Fernandes J.C., Manning P., Connor J.R., Currie M.G., Di Battista J.A., and Martel-Pelletier J.Reduced progression of experimental osteoarthritis in vivo by selective inhibition of inducible nitric oxide synthase. Arthritis Rheum 41,1275, 1998 [DOI] [PubMed] [Google Scholar]
- 88.Kagami S., Saeki H., Komine M., Kakinuma T., Tsunemi Y., Nakamura K., Sasaki K., Asahina A., and Tamaki K.Interleukin-4 and interleu kin-13 enhance CCL26 production in a human keratinocyte cell line, HaCaT cells. Clin Exp Immunol 141,459, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Buckwalter J.A., Anderson D.D., Brown T.D., Tochigi Y., and Martin J.A.The roles of mechanical stresses in the pathogenesis of osteoarthritis: implications for treatment of joint injuries. Cartilage 4,286, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guilak F., Sah R.L., and Setton L.A.Physical regulation of cartilage metabolism. In: Mow V.C., and Hayes. W.C., eds. Basic Orthopaedic Biomechanics. 2nd ed. Philadelphia, Lippincott-Raven, 1997 [Google Scholar]
- 91.Deschner J., Hofman C.R., Piesco N.P., and Agarwal S.Signal transduction by mechanical strain in chondrocytes. Curr Opin Clin Nutr Metab Care 6,289, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Knobloch T.J., Madhavan S., Nam J., Agarwal S, Jr., and Agarwal S.Regulation of chondrocytic gene expression by biomechanical signals. Crit Rev Eukaryot Gene Expr 18, 139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ramachandran M., Achan P., Salter D.M., Bader D.L., and Chowdhury T.T.Biochemical signals and the C-type natriuretic peptide counteract catabolic activities induced by IL-1β in chondrocyte/agarose constructs. Arthritis Res Ther 13,R145, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scott D., Blizzard L., Fell J., and Jones G.Prospective study of self-reported pain, radiographic osteoarthritis, sarcopenia progression, and falls risk in community-dwelling older adults. Arthritis Care Res 64,30, 2012 [DOI] [PubMed] [Google Scholar]
- 95.Slemenda C., Heilman D.K., Brandt K.D., Katz B.P., Mazzuca S.A., and Braunstein E.M.Reduced quadriceps strength relative to body weight: a risk factor for knee osteoarthritis in women? Arthritis Rheum 41,1951, 1998 [DOI] [PubMed] [Google Scholar]
- 96.Alnahdi A.H., Zeni J.A., and Snyder-Mackler L.Muscle impairments in patients with knee osteoarthritis. Sports Health 4,284, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rainbow R.S., Kwon H., Foote A.T., Preda R.C., Kaplan D.L., and Zeng L.Muscle cell-derived factors inhibit inflammatory stimuli-induced damage in hMSC-derived chondrocytes. Osteoarthritis Cartilage 21,990, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roos E.M., Herzog W., Block J.A., and Bennell K.L.Muscle weakness, afferent sensory dysfunction and exercise in knee osteoarthritis. Nat Rev Rheumatol 7,57, 2011 [DOI] [PubMed] [Google Scholar]
- 99.Röhner E., Matziolis G., Perka C., Füchtmeier B., Gaber T., Burmester G.R., Buttgereit F., and Hoff P.Inflammatory synovial fluid microenvironment drives primary human chondrocytes to actively take part in inflammatory joint diseases. Immunol Res 52,169, 2012 [DOI] [PubMed] [Google Scholar]
- 100.Chen F.H., Rousche K.T., and Tuan R.S.Technology Insight: adult stem cells in cartilage regeneration and tissue engineering. Nat Clin Pract Rheumatol 2,373, 2006 [DOI] [PubMed] [Google Scholar]
- 101.Caplan A.I., and Dennis J.E.Mesenchymal stem cells as trophic mediators. J Cell Biochem 98,1076, 2006 [DOI] [PubMed] [Google Scholar]
- 102.Pei M., Li J.T., Zhang Y., Liu G.H., Wei L., and Zhang Y.Y.Expansion on matrix deposited by nonchondrogenic urine stem cells strengthens repeated passage bone marrow stromal cells' chondrogenic capacity. Cell Tissue Res 356,391, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Buul G.M., Villafuertes E., Bos P.K., Waarsing J.H., Kops N., Narcisi R, Weinans H, Verhaar JA, Bernsen MR, and van Osch GJ.Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthritis Cartilage 20,1186, 2012 [DOI] [PubMed] [Google Scholar]
- 104.Lozito T.P., and Tuan R.S.Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J Cell Physiol 226,385, 2011 [DOI] [PubMed] [Google Scholar]
- 105.Gupta P.K., Das A.K., Chullikana A., and Majumdar A.S.Mesenchymal stem cells for cartilage repair in osteoarthritis. Stem Cell Res Ther 3,25, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Murphy J.M., Fink D.J., Hunziker E.B., and Barry F.P.Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum 48,3464, 2003 [DOI] [PubMed] [Google Scholar]
- 107.Centeno C.J., Busse D., Kisiday J., Keohan C., Freeman M., and Karli D.Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician 11,343, 2008 [PubMed] [Google Scholar]
- 108.Toma C., Wagner W.R., Bowry S., Schwartz A., and Villanueva F.Fate of culture-expanded mesenchymal stem cells in the microvasculature: in vivo observations of cell kinetics. Circ Res 104,398, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu S.P., Wei Z., and Wei L.Preconditioning strategy in stem cell transplantation therapy. Transl Stroke Res 4,76, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Murry C.E., Jennings R.B., and Reimer K.A.Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74,1124, 1986 [DOI] [PubMed] [Google Scholar]
- 111.Rosová I., Dao M., Capoccia B., Link D., and Nolta J.A.Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 26,2173, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Portron S., Merceron C., Gauthier O., Lesoeur J., Sourice S., Masson M., Fellah B.H., Geffroy O., Lallemand E., Weiss P., Guicheux J., and Vinatier C.Effects of in vitro low oxygen tension preconditioning of adipose stromal cells on their in vivo chondrogenic potential: application in cartilage tissue repair. PLoS One 8,e62368, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Holland T.A., Bodde E.W., Cuijpers V.M., Baggett L.S., Tabata Y., Mikos A.G., and Jansen J.A.Degradable hydrogel scaffolds for in vivo delivery of single and dual growth factors in cartilage repair. Osteoarthritis Cartilage 15,187, 2007 [DOI] [PubMed] [Google Scholar]
- 114.Li J., Hansen K.C., Zhang Y., Dong C., Dinu C.Z., Dzieciatkowska M., and Pei M.Rejuvenation of chondrogenic potential in a young stem cell microenvironment. Biomaterials 35,642, 2014 [DOI] [PubMed] [Google Scholar]
- 115.Stanton L.A., Underhill T.M., and Beier F.MAP kinases in chondrocyte differentiation. Dev Biol 263,165, 2003 [DOI] [PubMed] [Google Scholar]
- 116.Im G.I., and Quan Z.The effects of Wnt inhibitors on the chondrogenesis of human mesenchymal stem cells. Tissue Eng Part A 16,2405, 2011 [DOI] [PubMed] [Google Scholar]
- 117.Jones B., and Pei M.Synovium-derived stem cells: a tissue-specific stem cell for cartilage tissue engineering and regeneration. Tissue Eng Part B Rev 18,301, 2012 [DOI] [PubMed] [Google Scholar]
- 118.He F., Chen X.D., and Pei M.Reconstruction of an in vitro tissue-specific microenvironment to rejuvenate synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A 15,3809, 2009 [DOI] [PubMed] [Google Scholar]
- 119.Pei M., He F., and Kish V.L.Expansion on extracellular matrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue-specific lineage potential. Tissue Eng Part A 17,3067, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pei M., He F., and Wei L.Three dimensional cell expansion substrate for cartilage tissue engineering and regeneration: a comparision in decellularized matrix deposited by synovium-derived stem cells and chondrocytes. J Tissue Sci Eng 2,104, 2011 [Google Scholar]
- 121.Pei M., and He F.Extracellular matrix deposited by synovium-derived stem cells delays chondrocyte dedifferentiation and enhances redifferentiation. J Cell Physiol 227,2163, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.He F., and Pei M.Rejuvenation of nucleus pulposus cells using extracellular matrix deposited by synovium-derived stem cells. Spine 37,459, 2012 [DOI] [PubMed] [Google Scholar]
- 123.Pei M., Shoukry M., Li J.T., Daffner S., France J., and Emery S.E.Modulation of in vitro microenvironment facilitates synovium-derived stem cell-based nucleus pulposus tissue regeneration. Spine 37,1538, 2012 [DOI] [PubMed] [Google Scholar]
- 124.Dhalla N.S., Golfman L., Takeda S., Takeda N., and Nagano M.Evidence for the role of oxidative stress in acute ischemic heart disease: a brief review. Can J Cardiol 15,587, 1999 [PubMed] [Google Scholar]
- 125.He F., and Pei M.Extracellular matrix enhances differentiation of adipose stem cells from infrapatellar fat pad toward chondrogenesis. J Tissue Eng Regen Med 7,73, 2013 [DOI] [PubMed] [Google Scholar]
- 126.Zhang Y., Li J.T., and Pei M.Decellularized Matrix Benefits Expanded Human Stem Cell Chondrogenesis in Resistance to an Inflammatory Environment. Trans ORS 38,470, 2013 [Google Scholar]
- 127.Gelse K., Mühle C., Franke O., Park J., Jehle M., Durst K., Göken M., Hennig F., von der Mark K., and Schneider H.Cell-based resurfacing of large cartilage defects: long-term evaluation of grafts from autologous transgene-activated periosteal cells in a porcine model of osteoarthritis. Arthritis Rheum 58,475, 2008 [DOI] [PubMed] [Google Scholar]
- 128.Mohyeldin A., Garzón-Muvdi T., and Quiñones-Hinojosa A.Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 7,150, 2010 [DOI] [PubMed] [Google Scholar]
- 129.Lafont J.E.Lack of oxygen in articular cartilage: consequences for chondrocyte biology. Int J Exp Pathol 91,99, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li J.T., He F., and Pei M.Creation of an in vitro microenvironment to enhance human fetal synovium-derived stem cell chondrogenesis. Cell Tissue Res 345,357, 2011 [DOI] [PubMed] [Google Scholar]
- 131.Chacko S.M., Ahmed S., Selvendiran K., Kuppusamy M.L., Khan M., and Kuppusamy P.Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Cell Physiol 299,1562, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pistollato F., Rampazzo E., Persano L., Abbadi S., Frasson C., Denaro L., D'Avella D., Panchision D.M., Della Puppa A., Scienza R., and Basso G.Interaction of hypoxia-inducible factor-1α and Notch signaling regulates medulloblastoma precursor proliferation and fate. Stem Cells 28,1918, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li J.T., and Pei M.Optimization of an in vitro three-dimensional microenvironment to reprogram synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A 17,703, 2011 [DOI] [PubMed] [Google Scholar]
- 134.Rider C.C.Heparin/heparan sulphate binding in the TGF-beta cytokine superfamily. Biochem Soc Trans 34,458, 2006 [DOI] [PubMed] [Google Scholar]
- 135.Oh C.D., Chang S.H., Yoon Y.M., Lee S.J., Lee Y.S., Kang S.S., and Chun J.S.Opposing role of mitogen-activated protein kinase subtypes, erk-1/2 and p38, in the regulation of chondrogenesis of mesenchymes. J Biol Chem 275,5613, 2000 [DOI] [PubMed] [Google Scholar]
- 136.Li W.J., Cooper J.A., Jr, Mauck R.L., and Tuan R.S.Fabrication and characterization of six electrospun poly(alpha-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater 2,377, 2006 [DOI] [PubMed] [Google Scholar]
- 137.Liao E., Yaszemski M., Krebsbach P., and Hollister S.Tissue-engineered cartilage constructs using composite hyaluronic acid/collagen I hydrogels and designed poly(propylene fumarate) scaffolds. Tissue Eng 13,537, 2007 [DOI] [PubMed] [Google Scholar]
- 138.Werkmeister J.A., Adhikari R., White J.F., Tebb T.A., Le T.P., Taing H.C., Mayadunne R., Gunatillake P.A., Danon S.J., and Ramshaw J.A.Biodegradable and injectable cure-on-demand polyurethane scaffolds for regeneration of articular cartilage. Acta Biomater 6,3471, 2010 [DOI] [PubMed] [Google Scholar]
- 139.Pei M., Seidel J., Vunjak-Novakovic G., and Freed L.E.Growth Factors for sequential cellular de- and redifferentiation in tissue engineering. Biochem Biophys Res Commun 294,149, 2002 [DOI] [PubMed] [Google Scholar]
- 140.Sittinger M., Reitzel D., Dauner M., Hierlemann H., Hammer C., Kastenbauer E., Planck H., Burmester G.R., and Bujia J.Resorbable polyesters in cartilage engineering: affinity and biocompatibility of polymer fiber structures to chondrocytes. J Biomed Mater Res 33,57, 1996 [DOI] [PubMed] [Google Scholar]
- 141.Britt J.C., and Park S.S.Autogenous tissue-engineered cartilage: evaluation as an implant material. Arch Otolaryngol Head Neck Surg 124,671, 1998 [DOI] [PubMed] [Google Scholar]
- 142.Cao Y., Rodriguez A., Vacanti M., Ibarra C., Arevalo C., and Vacanti C.A.Comparative study of the use of poly(glycolic acid), calcium alginate and pluronics in the engineering of autologous porcine cartilage. J Biomater Sci Polym Ed 9,475, 1998 [DOI] [PubMed] [Google Scholar]
- 143.Velema J., and Kaplan D.Biopolymer-based biomaterials as scaffolds for tissue engineering. Adv Biochem Eng Biotechnol 102,187, 2006 [DOI] [PubMed] [Google Scholar]
- 144.Masuoka K., Asazuma T., Ishihara M., Sato M., Hattori H., Ishihara M., Yoshihara Y., Matsui T., Takase B., Kikuchi M., and Nemoto K.Tissue engineering of articular cartilage using an allograft of cultured chondrocytes in a membrane-sealed atelocollagen honeycomb-shaped scaffold (ACHMS scaffold). J Biomed Mater Res B Appl Biomater 75,177, 2005 [DOI] [PubMed] [Google Scholar]
- 145.Wakitani S., Kimura T., Hirooka A., Ochi T., Yoneda M., Yasui N., Owaki H., and Ono K.Repair of rabbit articular surfaces with allograft chondrocytes embedded in collagen gel. J Bone Joint Surg Br 71,74, 1989 [DOI] [PubMed] [Google Scholar]
- 146.Rahfoth B., Weisser J., Sternkopf F., Aigner T., von der Mark K., and Bräuer R.Transplantation of allograft chondrocytes embedded in agarose gel into cartilage defects of rabbits. Osteoarthritis Cartilage 6,50, 1998 [DOI] [PubMed] [Google Scholar]
- 147.Altman G.H., Diaz F., Jakuba C., Calabro T., Horan R.L., Chen J., Lu H., Richmond J., and Kaplan D.L.Silk-based biomaterials. Biomaterials 24,401, 2003 [DOI] [PubMed] [Google Scholar]
- 148.Pei M., Solchaga L.A., Seidel J., Zeng L., Caplan A.I., Vunjak-Novakovic G., and Freed L.E.Bioreactors mediate the effectiveness of tissue engineering scaffolds. FASEB J 16,1691, 2002 [DOI] [PubMed] [Google Scholar]
- 149.Suh J.K., and Matthew H.W.Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials 21,2589, 2000 [DOI] [PubMed] [Google Scholar]
- 150.Kwon H., Sun L., Cairns D.M., Rainbow R.S., Preda R.C., Kaplan D.L., and Zeng L.The influence of scaffold material on chondrocytes under inflammatory conditions. Acta Biomater 9,6563, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schreiber R.E., Ilten-Kirby B.M., Dunkelman N.S., Symons K.T., Rekettye L.M., Willoughby J., and Ratcliffe A.Repair of osteochondral defects with allogeneic tissue engineered cartilage implants. Clin Orthop Relat Res S382,367, 1999 [DOI] [PubMed] [Google Scholar]
- 152.Okano T., Yamada N., Sakai H., and Sakurai Y.A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J Biomed Mater Res 27,1243, 1993 [DOI] [PubMed] [Google Scholar]
- 153.Hamahashi K., Sato M., Yamato M., Kokubo M., Mitani G., Ito S., Nagai T., Ebihara G., Kutsuna T., Okano T., and Mochida J.Studies of the humoral factors produced by layered chondrocyte sheets. J Tissue Eng Regen Med 2012[Epub ahead of print]; DOI: 10.1002/term.1610 [DOI] [PubMed] [Google Scholar]
- 154.Kaneshiro N., Sato M., Ishihara M., Mitani G., Sakai H., and Mochida J.Bioengineered chondrocyte sheets may be potentially useful for the treatment of partial thickness defects of articular cartilage. Biochem Biophys Res Commun 349,723, 2006 [DOI] [PubMed] [Google Scholar]
- 155.Badylak S.F.The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol 13,377, 2002 [DOI] [PubMed] [Google Scholar]
- 156.Gilbert T.W., Sellaro T.L., and Badylak S.F.Decellularized of tissue and organs. Biomaterials 27,3675, 2006 [DOI] [PubMed] [Google Scholar]
- 157.Badylak S.F., Valentin J.E., Ravindra A.K., McCabe G.P., and Stewart-Akers A.M.Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A 14,1835, 2008 [DOI] [PubMed] [Google Scholar]
- 158.Benders K.E., van Weeren P.R., Badylak S.F., Saris D.B., Dhert W.J., and Malda J.Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol 31,169, 2013 [DOI] [PubMed] [Google Scholar]
- 159.Paz A.C., Kojima K., Iwasaki K., Ross J.D., Canseco J.A., Umezu M., and Vacanti C.A.Tissue engineered trachea using decellularized aorta. J Bioeng Biomed Sci S2,001, 2011 [Google Scholar]
- 160.Choi K.H., Choi B.H., Park S.R., Kim B.J., and Min B.H.The chondrogenic differentiation of mesenchymal stem cells on an extracellular matrix scaffold derived from porcine chondrocytes. Biomaterials 31,5355, 2010 [DOI] [PubMed] [Google Scholar]
- 161.Chevalier X., Florent Eymard F., and Richette P.Biologic agents in osteoarthritis: hopes and disappointments. Nat Rev Rheumat 9,400, 2013 [DOI] [PubMed] [Google Scholar]